Abstract
Acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT), secondary to cardiovascular disease and sepsis, is associated with high in-hospital mortality. Although studies have examined cardiovascular disease and sepsis in AKI, the association between AKI and hepatic functional impairment remains unclear. We hypothesized that hepatic function markers would predict mortality in patients undergoing CRRT. We included 1,899 CRRT patients from a multi-centre database. In Phase 1, participants were classified according to the total bilirubin (T-Bil) levels on the day of, and 3 days after, CRRT initiation: T-Bil < 1.2, 1.2 ≤ T-Bil < 2, and T-Bil ≥ 2 mg/dL. In Phase 2, propensity score matching (PSM) was performed to examine the effect of a T-Bil cutoff of 1.2 mg/dL (supported by the Sequential Organ Failure Assessment score); creating two groups based on a T-Bil cutoff of 1.2 mg/dL 3 days after CRRT initiation. The primary endpoint was total mortality 90 days after CRRT initiation, which was 34.7% (n = 571). In Phase 1, the T-Bil, aspartate transaminase (AST), alanine transaminase (ALT), and AST/ALT (De Ritis ratio) levels at CRRT initiation were not associated with the prognosis, while T-Bil, AST, and the De Ritis ratio 3 days after CRRT initiation were independent factors. In Phase 2, T-Bil ≥1.2 mg/dL on day 3 was a significant independent prognostic factor, even after PSM [hazard ratio: 2.41 (95% CI; 1.84-3.17), p < 0.001]. T-Bil ≥1.2 mg/dL 3 days after CRRT initiation predicted 90-day mortality. Changes in hepatic function markers in acute renal failure may enable stratification of high-risk patients.
Introduction
Acute kidney injury (AKI) manifests as a variety of pathological states, necessitating differentiation of the etiology to treat reversible factors [Citation1]. An epidemiological study conducted with patients with AKI who underwent continuous renal replacement therapy (CRRT), using the Diagnosis Procedure Combination database in Japan [Citation2], reported that medical disorders, including cardiovascular disease, accounted for approximately half of the etiology of AKI, followed by sepsis, and post-cardiac surgery, where only post-cardiac surgery yielded a lower mortality rate than the others. Renal replacement therapy (RRT), which is essential for life support in patients with AKI, can be classified into continuous or intermittent, based on the treatment time. However, currently, the choice of RRT for septic AKI at each facility is based on each individual case, as it depends not only on the pathological state of the injury, but also on the experience and medical infrastructure of the treatment facility [Citation3]. Respiratory failure requiring ventilation management, heart failure, hepatic failure, and haematological abnormalities can increase the mortality rate in patients with AKI, and epidemiological studies have suggested that AKI in the intensive care unit (ICU) is part of multiple organ failure [Citation4].
Therefore, it is clinically important to identify prognostic factors in patients requiring CRRT among the AKI population.
The Sequential Organ Failure Assessment (SOFA) score [Citation5] is a good predictor of in-hospital mortality in patients with sepsis; in critically ill patients with AKI undergoing CRRT, the SOFA score has shown greater mortality prediction accuracy than the acute physiology and chronic health evaluation II (APACHE-II) score [Citation6]. A systematic review of septic shock, that used the Delphi method and other techniques, reported that a combination of persistent hypotension’ vasopressor treatment, and blood lactate levels can be used as a new diagnostic criterion for septic shock [Citation7].
A study on patients with sepsis indicated that the aspartate transaminase (AST)/alanine transaminase (ALT) ratio (De Ritis ratio) can determine the 30-day total mortality rate [Citation8], while hepatic function markers have garnered attention in critically ill patients. Hepatic functional impairment (total bilirubin [T-Bil] level >2 mg/dL within 48 h of hospitalization) was reported to be associated with prognosis in patients in ICU [Citation9]. T-Bil levels and life prognosis have been examined in a wide range of disease groups, but the results have been inconsistent. An observational study showed that the mortality rate was significantly higher in patients whose blood bilirubin level peaked 3.5 days after cardiovascular surgery than that in patients whose blood bilirubin level peaked earlier [Citation10]. Similar findings have been reported for the blood bilirubin level following surgery for biliary tract cancer [Citation11]. AKI and liver dysfunction have recently been linked to increased risk of bleeding and death. Patients with acute liver failure requiring renal replacement therapy have been reported to have impaired thrombus formation and stability as measured by ROTEM, indicating a bleeding tendency [Citation12, Citation13]. Thus, previous studies have reported the utility of capturing the gradual and time-sensitive changes in hepatic functional impairment in critically ill patients and the association between AKI and liver dysfunction. In contrast, only a few studies have examined the relationship between AKI and changes in hepatic functional impairment over time.
Therefore, the present study aimed to examine changes in hepatic function markers and their effects on prognosis in patients with AKI undergoing CRRT.
Materials and methods
Study design and data collection
This observational cohort study used data from an administrative claims database linked to the medical records of Nippon Medical School Hospital and Nippon Medical School Hokusoh Hospital. The following patient data were collected from the administrative claims database: age, sex, treatment methods, medication, length of hospital stay, discharge status, and post-discharge outcome. The clinical data in the medical records, including the laboratory data, were electronically added to the database. Data were anonymized and could not be used to identify specific individuals. This study was approved by the institutional review board of Nippon Medical School (Document No. M-2021-022) and conducted in accordance with the revised Declaration of Helsinki. Consent was obtained by the opt-out method.
Patient selection and endpoints
The data of patients who had been hospitalized between January 2016 and December 2021 and underwent CRRT treatment were extracted from the database. The exclusion criteria of the study were as follows: age under 12 years [Citation14]; estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 at the start of CRRT; death within 48 h of the start of CRRT; hepatic cirrhosis, acute alcoholic hepatitis, toxic hepatitis, acute pancreatitis, or a history of pancreatic, gallbladder, biliary, or hepatic surgery between admission and the start of CRRT.
In this study, shock is defined as the need for catecholamine drugs on the day of CRRT initiation (CRRT Day 0). The catecholamine drugs considered in this definition include adrenaline, noradrenaline, dopamine, and dobutamine. Additionally, the anti-MRSA (Methicillin-resistant Staphylococcus aureus) medications evaluated in this research comprise vancomycin hydrochloride, teicoplanin, linezolid, and daptomycin.
We applied the mode of continuous venovenous haemofiltration to all cases. During ICU stay, all patients were switched from maintenance hemo and peritoneal dialysis to CRRT.Criteria for CRRT initiation included medically refractory or persistent electrolyte imbalance, metabolic acidosis, decreased urine output with urinary overload and/or progressive azotemia, and hemodynamic instability. In general, vascular access for CRRT was established using the femoral or internal jugular vein, and a post-dilution technique of continuous venous haemodiafiltration was employed. After CRRT initiation, to maintain adequate CRRT, the attending physician and an experienced nurse were responsible for monitoring each patient’s weight, urine output, laboratory results, actual dose, and haemodynamics; the results discussed with the nephrologist. CRRT prescriptions were determined in consultation with the attending physician and nephrologist. Our ICU consists of a physician, a nurse, a clinical engineer, and a pharmacist.
In Phase 1, participants were classified into the following three groups based on the T-Bil level on the day of, and 3 days after, the start of CRRT (day 0 and day 3, respectively): group 1, T-Bil < 1.2; group 2, 1.2 ≤ T-Bil < 2; and group 3, T-Bil ≥ 2 mg/dL. The clinical characteristics of the three groups, and mortality 90 days after commencement of CRRT were examined. Because of the different severity levels among the groups in Phase 1, Phase 2 was the next step. In Phase 2, propensity score matching (PSM) was used to examine the effect of a T-Bil cutoff of 1.2 mg/dL, which was supported by the SOFA score [Citation5], on prognosis in actual clinical practice. The SOFA score was reported to be a good predictor of 30-day and in-hospital mortality in patients with sepsis in the ICU, with an AUC of 0.88 [Citation15]. Participants were divided into two groups based on the T-Bil cutoff of ≥1.2 mg/dL on day 3, and the clinical characteristics, and mortality 90 days after commencement of CRRT, were examined.
Statistical analysis
Categorical variables were expressed as numerical values and percentages and compared using the chi-squared test. Continuous values were expressed as medians and interquartile ranges and analyzed using the Mann–Whitney U test. For survival analysis, the cumulative incidence rate of the outcome was estimated using the Kaplan–Meier method, and differences among the three groups stratified according to the T-Bil level were compared using the log-rank test. Bonferroni’s correction was employed to control for multiple comparisons. Cox regression analysis was performed using the forced input method to identify the independent factors associated with the outcome. The following variables were examined on the day of (day 0), and 3 days after (day 3), CRRT initiation and included in the multivariate analysis: age, sex, creatinine level, platelet count, number of catecholamines, anti-methicillin resistant Staphylococcus aureus (anti-MRSA) drug use, ventilator use, circulation device use, cancer, baseline platelet transfusion, red blood cell transfusion, hemoglobin and hepatic function markers (T-Bil, AST, ALT, and the De Ritis ratio). In the Cox regression analysis, the T-Bil level, creatinine, and platelet count were categorized based on the SOFA score [Citation5] (Table S1), and the ALT, AST, and De Ritis ratio were also categorized [Citation16] (Table S2).
PSM analysis was performed to adjust for potential selection bias in treatment allocation. A propensity score was created using a multivariate logistic regression model. The following variables were included in this model: age, sex, baseline creatinine level, platelet count, T-Bil, AST, ALT, De Ritis ratio, number of catecholamines, anti-MRSA drugs, ventilator use, and circulation device. The one-to-one and nearest-neighbour methods, with calipers set at 0.2 of the standard deviation of the logit of the propensity score, were used. A two-sided P-value <0.05 was considered statistically significant. Statistical analysis was performed using R software version 4.2.2 Patched (R Foundation for Statistical Computing, Vienna, Austria).
Results
Non-PSM
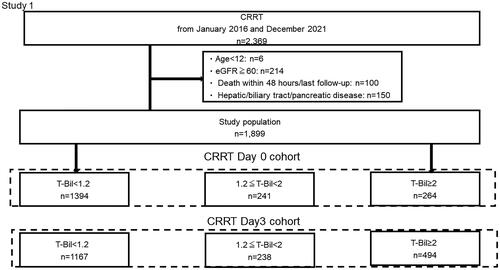
Of the 2,369 patients who underwent CRRT between January 2016 and December 2021, the present study excluded six patients aged under 12 years, 214 patients with baseline eGFR ≥60 mL/min/1.73 m2, 100 patients who died within 48 h of undergoing CRRT, and 150 patients diagnosed with hepatic cirrhosis, acute alcoholic hepatitis, toxic hepatitis, or acute pancreatitis; had undergone pancreatic/biliary tract surgery between hospital admission and the initiation of CRRT, or had undergone hepatic surgery. Thus, 1899 participants were included in the final analysis. Based on the baseline T-Bil level on the CRRT start date (day 0), participants were divided into the following three groups: T-Bil < 1.2, n = 1394; 1.2 ≤ T-Bil < 2, n = 241; and T-Bil ≥ 2 mg/dL, n = 264. Thereafter, based on the day 3 T-Bil level, participants were similarly divided into the following three groups: T-Bil < 1.2, n = 1167; 1.2 ≤ T-Bil < 2, n = 238; and T-Bil ≥ 2 mg/dL, n = 494 (). The most common diagnosis on hospitalization was cardiovascular disease (682 patients), followed by specific infectious and parasitic diseases (406 patients), many of whom were diagnosed with sepsis (Table S3).
Figure 1. Patient flowchart for Phase 1.
A total of 2,369 patients were identified from the database, and the application of the exclusion criteria resulted in a study population of 1,899 patients. Participants were classified into three groups based on the T-Bil levels on day 0 and day 3 after CRRT.
CRRT, continuous renal replacement therapy; T-Bil, total bilirubin
Comparison of the characteristics of the three groups classified using the T-Bil level revealed greater severity in the T-Bil ≥ 1.2 group than that in the T-Bil < 1.2 group, such as in the number of catecholamine drugs and the proportions of patients requiring platelet transfusion, red blood cell transfusion, fresh-frozen plasma, anti-MRSA drugs, and circulation devices at baseline on CRRT Day 0 (). shows the background characteristics of all participants on CRRT Day 3, classified into three groups based on the T-Bil level, which shows the same trends as that in .
Table 1. Patient characteristics stratified according to T-Bil on the day of CRRT initiation.
Table 2. Patient characteristics stratified according to the T-Bil level on day 3 post-CRRT initiation.
The 90-day mortality rate was 34.7% (571 patients). On CRRT Day 0, 90-day mortality was significantly lower in the T-Bil < 1.2 group than in the T-Bil ≥ 1.2 group (p = 0.002), but not significantly different between the 1.2 ≤ T-Bil < 2 and T-Bil ≥ 2 groups (p = 0.213). On CRRT Day 3, 90-day mortality was significantly lower in the T-Bil < 1.2 group than in the T-Bil ≥ 1.2 group (p < 0.001) and in the 1.2 ≤ T-Bil < 2 group than in the T-Bil ≥ 2 group (p = 0.002) (). Cox proportional hazards regression for all participants revealed that age, number of catecholamines, anti-MRSA drugs, artificial ventilation, and platelet count were significant factors affecting mortality on CRRT Day 0, while T-Bil, AST, ALT, and the De Ritis ratio were not independent factors at this time-point. T-Bil, AST, and the De Ritis ratio were independent factors affecting mortality on CRRT Day 3, unlike on CRRT Day 0 ().
Figure 2. Kaplan–Meier curve for 90-day total mortality of the participants classified into three groups based on the T-Bil levels on day 0 and day 3 after CRRT.
On both day 0 and day 3 after CRRT initiation, the mortality rate was significantly higher in groups 2 and 3 than that in group 1.
CRRT, continuous renal replacement therapy; T-Bil, total bilirubin
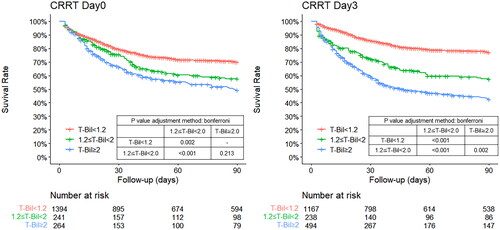
Table 3. Cox regression analysis: Factors associated with 90-day mortality.
PSM
Because of the different severity levels among the groups in Phase 1, Phase 2 was the next step. Participants were divided into the following groups based on the T-Bil cutoff of 1.2 on day 3: T-Bil < 1.2 (n = 1167) and T-Bil ≥ 1.2 (n = 732). Using PSM, 338 matched cohorts were created, and the C-statistic was set at 0.89 (). After PSM, the following patient background items showed a standardized mean difference greater than 0.20 between the two groups classified based on the T-Bil level of 1.2 on day 3: cancer, baseline platelet transfusion, red blood cell transfusion, and hemoglobin (Table S4). The 90-day mortality rate on day 3 was significantly lower in the T-Bil < 1.2 group than that in the T-Bil ≥ 1.2 group (p < 0.001) (). T-Bil ≥1.2 mg/dL on day 3 showed a high hazard ratio in Cox regression analysis after adjustment for group differences in cancer, hemoglobin, baseline platelet transfusion, and red blood cell transfusion after PSM (hazard ratio: 2.31, 95% CI: 1.76-3.04, p < 0.001) ().
Figure 3. Patient flowchart for Phase 2.
After applying the exclusion criteria, the remaining 1,899 patients were classified based on the T-Bil cutoff of 1.2 mg/dL on day 3 after CRRT. After matching, 354 pairs were examined.
CRRT, continuous renal replacement therapy; T-Bil, total bilirubin
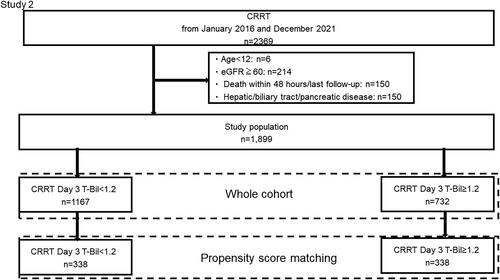
Figure 4. Kaplan–Meier curve for 90-day total mortality of the participants classified into two groups based on the T-Bil cutoff of 1.2 on day 3 after CRRT.
The cohort matched with the whole cohort. The T-Bil ≥ 1.2 group had a significantly higher mortality rate, even after propensity-score matching.
CRRT, continuous renal replacement therapy; T-Bil, total bilirubin
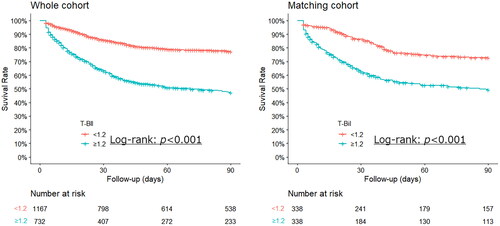
Table 4. Cox regression analysis: Factors associated with 90-day mortality in cases after propensity score matching.
Discussion
In the present study, we hypothesized that hepatic function markers would serve as prognosticators of mortality in patients undergoing CRRT. Hepatic function markers measured at the start of CRRT were not identified as factors associated with the 90-day mortality rate. However, hepatic function markers measured 3 days after the start of CRRT were found to be independent prognostic factors for the 90-day mortality rate. The T-Bil cutoff of ≥1.2 mg/dL defined by the SOFA score, and the De Ritis ratio, were confirmed to be useful prognostic factors for patients undergoing CRRT for AKI. These results suggest that evaluation of delayed hepatic functional impairment may enable prediction of the short-term prognosis.
T-BIL
The effects of hyperbilirubinemia have been examined in the field of intensive care and sepsis, but not in patients undergoing CRRT. The present study is the first to demonstrate that elevation in the bilirubin level over time reflects the prognosis of patients undergoing CRRT. The bilirubin level is a general marker of hepatic function, and we believe its significance lies in the fact that it can also be used in routine clinical practice for patients undergoing CRRT. However, the bilirubin level is not a superior index for identifying the complexly interconnected factors of hepatic disorders, and it is difficult to determine clear cutoff values for each disease [Citation17]. In fact, the clinical symptoms of sepsis-associated hepatic functional impairment include hypoxic hepatitis, sepsis-induced cholestasis, and protein synthesis dysfunction, accompanied by coagulopathy and other conditions; the bilirubin level reportedly rises to reflect these symptoms. Furthermore, it has been reported that elevation in the bilirubin level (>1 mg/dL) within 72 h is associated with the risk of mortality in patients with severe sepsis and septic shock [Citation18] and that hyperbilirubinemia on admission is associated with the mortality risk in patients with sepsis who have developed acute respiratory distress syndrome [Citation19]. A bilirubin concentration of 1-6 mg/dL exhibited a linear correlation with the mortality rate, but the correlation disappeared at higher concentrations [Citation20]. The present study excluded patients whose primary diagnosis was biliary-pancreatic disease, and thus, we considered a bilirubin level of 1.2 mg/dL a suitable cutoff value.
Cardiac disease and bilirubin were reported to cause hepatic functional impairment via cardiogenic shock, reflecting tissue hypoperfusion and haemostasis due to heart failure and excessive volume/pressure load [Citation21]. A study has also reported that heart failure with reduced ejection fraction is associated with a worsening of outcomes, even after adjusting for other predictive variables, including N-terminal pro b-type natriuretic peptide and troponin T [Citation22].
An association between higher cumulative fluid balance and higher risk of death [Citation23] has been reported in older patients with AKI requiring CRRT.
Studies investigating patients undergoing cardiovascular surgery have found an association between the survival rate and development of hyperbilirubinemia in the late postoperative period, rather than in the early postoperative period [Citation10, Citation24].
De Ritis ratio
The De Ritis ratio is an independent diagnostic discriminant of septic shock and mortality rate [Citation8, Citation25]. Furthermore, a study reported an association between the De Ritis ratio and total and cardiovascular mortality in patients with hypertension [Citation26, Citation27]. This ratio is also reportedly correlated with brain natriuretic peptide elevation and may be a predictive factor of the cardiovascular mortality rate in the general population [Citation26, Citation27]. Acute cardiogenic liver injury (ACLI) is considered to be one of the factors underlying hepatic functional impairment [Citation21]. ACLI is associated with acute heart failure, respiratory failure, and septic shock. However, these studies also showed that 39%–70% of patients with ACLI had an underlying diagnosis of chronic heart failure [Citation28–30]. These findings suggest that ACLI arises from a combination of hepatic haemostasis due to elevated hepatic venous pressure and perfusion disorder, rather than a single hemodynamic impairment entity [Citation30]. Moreover, cardiovascular disease was the most common comorbidity in the present study, which might have led to the identification of a delayed increase in the De Ritis ratio as a prognostic factor.
Lactate is an important marker of sepsis, and a delayed increase in lactate levels was reported to be independently associated with in-hospital mortality rate [Citation31]. However, the lactate levels in the participants of the present study were unknown.
Thus, based on the above-mentioned results, focusing on the changes in the hepatic function of patients undergoing CRRT over time as prognostic factors may lead to early stratification of high-risk patients with AKI.
Study limitations
First, this was a retrospective observational study based on an administrative claims database. Second, the lack of information on the Glasgow Coma Scale and lactate levels prevented us from assessing their association with the prognosis of the participants in this study. The lack of lactate levels may create a gap in our understanding of the pathophysiology and severity of AKI, especially since lactate levels are an indicator of tissue perfusion failure and metabolic disturbances in patients with AKI [Citation32]. Third, it does not apply to the prediction of short-term mortality because it excludes death within 48 h. Fourth, we excluded patients with an eGFR ≥ 60 to focus on worsening chronic renal failure. This provides some bias in the study population. Fifth, T-BilDay0 to T-BilDay3 values were each classified and evaluated, but the association with the magnitude of change was not evaluated. Sixth, post-discharge treatment was unknown. Thus, future studies analyzing data from validation cohorts with large prospective databases are justified to overcome these limitations.
Conclusions
In this study, T-Bil ≥ 1.2 mg/dL on day 3 after CRRT initiation was strongly associated with the 90-day mortality rate. The results suggest that changes in hepatic function markers in patients with renal failure over time could serve as prognostic factors that would enable early stratification of high-risk patients in the AKI population.
Authors’ contributions
This study concept was proposed by TN. Material preparation, and data collection and analysis, were performed by TN and YK. The first draft of the manuscript was written by TN, and all authors commented on versions of the manuscript. All the authors read and approved the final manuscript.
Supplemental Material
Download MS Word (29.2 KB)Acknowledgements
The authors extend their sincere thanks to all the people who have been involved in patient care, including emergency staff, technicians, medical engineers, nurses, pharmacists, physicians, and surgeons at Nippon Medical School Hospital and Nippon Medical School Chiba Hokusoh Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Doi K, Nishida O, Shigematsu T, et al. The Japanese clinical practice guideline for acute kidney injury 2016. J Intensive Care. 2018;6(1):48. doi: 10.1186/s40560-018-0308-6.
- Iwagami M, Yasunaga H, Noiri E, et al. Current state of continuous renal replacement therapy for acute kidney injury in Japanese intensive care units in 2011: analysis of a national administrative database. Nephrol Dial Transplant. 2015;30(6):988–995. doi: 10.1093/ndt/gfv069.
- Egi M, Ogura H, Yatabe T, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). J Intensive Care. 2021;9(1):53. doi: 10.1186/s40560-021-00555-7.
- Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-Related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751.
- Wang H, Kang X, Shi Y, et al. SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail. 2020;42(1):638–645. doi: 10.1080/0886022X.2020.1788581.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Schupp T, Weidner K, Rusnak J, et al. Diagnostic and prognostic value of the AST/ALT ratio in patients with sepsis and septic shock. Scand J Gastroenterol. 2023;58(4):392–402. doi: 10.1080/00365521.2022.2131331.
- Kramer L, Jordan B, Druml W, et al. Incidence and prognosis of early hepatic dysfunction in critically ill patients–a prospective multicenter study. Crit Care Med. 2007;35(4):1099–1104. doi: 10.1097/01.CCM.0000259462.97164.A0.
- Farag M, Veres G, Szabó G, et al. Hyperbilirubinaemia after cardiac surgery: the point of no return. ESC Heart Fail. 2019;6(4):694–700. doi: 10.1002/ehf2.12447.
- Sawangkajohn W, Luvria V, Leeratanakachorn N, et al. Re-rising of total bilirubin level after postoperative day 3 (The V pattern) predicting liver failure and survival of patients who underwent hepatectomy for cholangiocarcinoma. Asian Pac J Cancer Prev. 2020;21(12):3573–3578. doi: 10.31557/APJCP.2020.21.12.3573.
- Zanetto A, Rinder HM, Campello E, et al. Acute kidney injury in decompensated cirrhosis is associated with both hypo-coagulable and hyper-coagulable features. Hepatology. 2020;72(4):1327–1340. doi: 10.1002/hep.31443.
- Zanetto A, Northup P, Roberts L, et al. Haemostasis in cirrhosis: understanding destabilising factors during acute decompensation. J Hepatol. 2023;78(5):1037–1047. doi: 10.1016/j.jhep.2023.01.010.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813.
- Khwannimit B, Bhurayanontachai R, Vattanavanit V. Comparison of the accuracy of three early warning scores with SOFA score for predicting mortality in adult sepsis and septic shock patients admitted to intensive care unit. Heart Lung. 2019;48(3):240–244. doi: 10.1016/j.hrtlng.2019.02.005.
- Botros M, Sikaris KA. The De Ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117–130.
- Woźnica EA, Inglot M, Woźnica RK, et al. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27(4):547–551. doi: 10.17219/acem/68363.
- Patel JJ, Taneja A, Niccum D, et al. The association of serum bilirubin levels on the outcomes of severe sepsis. J Intensive Care Med. 2015;30(1):23–29. doi: 10.1177/0885066613488739.
- Zhai R, Sheu CC, Su L, et al. Serum bilirubin levels on ICU admission are associated with ARDS development and mortality in sepsis. Thorax. 2009;64(9):784–790. doi: 10.1136/thx.2009.113464.
- Pierrakos C, Velissaris D, Felleiter P, et al. Increased mortality in critically ill patients with mild or moderate hyperbilirubinemia. J Crit Care. 2017;40:31–35. doi: 10.1016/j.jcrc.2017.01.017.
- Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61(24):2397–2405. doi: 10.1016/j.jacc.2013.03.042.
- Adamson C, Cowan LM, de Boer RA, et al. Liver tests and outcomes in heart failure with reduced ejection fraction: findings from DAPA-HF. Eur J Heart Fail. 2022;24(10):1856–1868. doi: 10.1002/ejhf.2649.
- Jhee JH, Park JY, An JN, et al. Cumulative fluid balance and mortality in elderly patients with acute kidney injury requiring continuous renal-replacement therapy: a multicenter prospective cohort study. Kidney Res Clin Pract. 2020;39(4):414–425. doi: 10.23876/j.krcp.20.089.
- Chen X, Bai M, Zhao L, et al. Time to peak bilirubin concentration and advanced AKI were associated with increased mortality in rheumatic heart valve replacement surgery patients with severe postoperative hyperbilirubinemia: a retrospective cohort study. BMC Cardiovasc Disord. 2021;21(1):16. doi: 10.1186/s12872-020-01830-5.
- Zhao PY, Yao RQ, Ren C, et al. De Ritis ratio as a significant prognostic factor in patients with sepsis: a retrospective analysis. J Surg Res. 2021;264:375–385. doi: 10.1016/j.jss.2021.03.018.
- Liu H, Ding C, Hu L, et al. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Medicine (Baltimore). 2021;100(31):e26693. doi: 10.1097/MD.0000000000026693.
- Yokoyama M, Watanabe T, Otaki Y, et al. Association of the aspartate aminotransferase to alanine aminotransferase ratio with BNP level and cardiovascular mortality in the general population: the Yamagata study 10-year follow-up. Dis Markers. 2016;2016:4857917–4857919. doi: 10.1155/2016/4857917.
- Raurich JM, Llompart-Pou JA, Ferreruela M, et al. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth. 2011;25(1):50–56. doi: 10.1007/s00540-010-1058-3.
- Fuhrmann V, Kneidinger N, Herkner H, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35(8):1397–1405. doi: 10.1007/s00134-009-1508-2.
- Henrion J, Descamps O, Luwaert R, et al. Hypoxic hepatitis in patients with cardiac failure: incidence in a coronary care unit and measurement of hepatic blood flow. J Hepatol. 1994;21(5):696–703. doi: 10.1016/s0168-8278(94)80226-2.
- He M, Huang J, Li X, et al. Risk factors for mortality in sepsis patients without lactate levels increasing early. Emerg Med Int. 2023;2023:6620157. doi: 10.1155/2023/6620157.
- Kim SG, Lee J, Yun D, et al. Hyperlactatemia is a predictor of mortality in patients undergoing continuous renal replacement therapy for acute kidney injury. BMC Nephrol. 2023;24(1):11. doi: 10.1186/s12882-023-03063-y.
