Abstract
Diabetic kidney disease (DKD) is a serious complication of diabetes mellitus (DM) and has become the main cause of end-stage renal disease worldwide. In recent years, with the increasing incidence of DM, the pathogenesis of DKD has received increasing attention. The pathogenesis of DKD is diverse and complex. Extracellular vesicles (EVs) contain cell-derived membrane proteins, nucleic acids (such as DNA and RNA) and other important cellular components and are involved in intercellular information and substance transmission. In recent years, an increasing number of studies have confirmed that EVs play an important role in the development of DKD. The purpose of this paper is to explain the potential diagnostic value of EVs in DKD, analyze the mechanism by which EVs participate in intercellular communication, and explore whether EVs may become drug carriers for targeted therapy to provide a reference for promoting the implementation and application of exosome therapy strategies in clinical practice.
1. Introduction
Diabetic kidney disease (DKD) is the most common microvascular complication of diabetes mellitus (DM) and the main cause of end-stage renal disease (ESRD). It is associated with high morbidity and mortality and seriously affects the quality of life of patients [Citation1]. Studies have shown that approximately 30%-40% of DM patients will develop DKD [Citation2]. It is estimated that the number of DM patients will increase from 537 million to 783 million in the next 24 years, which will lead to an increase in the prevalence of DKD worldwide [Citation3]. The development of DKD includes glomerular hyperfiltration, progressive albuminuria, decreased renal function, and eventually ESRD. The main pathogenic factors of DKD include abnormal glucose metabolism, haemodynamic changes, oxidative stress, inflammatory responses, autophagy and impaired immunity, which eventually lead to glomerular hypertrophy, glomerulosclerosis, tubulointerstitial inflammation and fibrosis [Citation4]. In clinical practice, urinary microalbumin is a predictor of early DKD. However, studies have shown that renal function is impaired before the detection of urinary microalbumin [Citation5]. Therefore, more specific and sensitive biomarkers are needed for the diagnosis of DKD. At present, the treatments for DKD mainly include dietary intervention, control of blood glucose and blood pressure, improvement of albuminuria, and the use of renin-angiotensin-aldosterone system (RAAS) inhibitors. In recent years, a variety of new hypoglycemic drugs, including sodium-dependent glucose transporter-2 (SGLT-2) inhibitors [Citation6] and glucagon-like peptide-1 receptor agonists (GLP-1 RAs), have been reported to have renal protective effects [Citation7]. These therapies have certain limitations and side effects, and clinical application can cause dry cough, abnormal blood potassium and other adverse reactions. Therefore, it is still highly important to explore the pathogenesis, diagnosis and treatment of DKD. An increasing number of studies have shown that extracellular vesicles (EVs) are closely related to the occurrence and development of DKD [Citation8]. In this review, we summarize recent advances in the diagnosis, pathogenesis and potential therapeutic role of EVs in DKD.
2. The formation and function of EVs
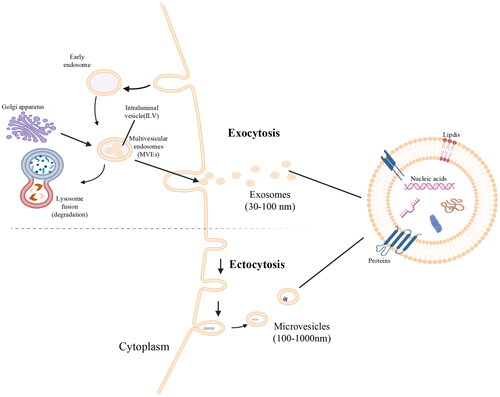
EVs are extracellular lipid bilayer vesicles that are actively secreted by almost all eukaryotic cells and prokaryotic cells [Citation9]. EVs are mainly divided into two categories, namely, exosomes and ectosomes. The difference between the two is mainly due to their different sources. Exosomes begin with invagination of the plasma membrane and form early endosomes. Early endosomes are invaginated with the help of the Golgi apparatus to form multiple lumen vesicles, which are transformed into multivesicular bodies (MVBs). MVBs can be degraded by fusion with lysosomes or autophagosomes and can also fuze with the plasma membrane and be secreted into the extracellular microenvironment to form exosomes [Citation10]. See . Ectosomes are vesicles that clamp the surface of the plasma membrane by sprouting outward. They include microvesicles, microparticles and large vesicles with a diameter of 50 nm ∼ 1 µm [Citation11]. In the process of exosome formation, Ras-related protein GTPase regulates the fusion of MVBs with the cell membrane and the spatiotemporal transport of vesicles [Citation12]. Endosomal-sorting complex required for transport (ESCRT) is a tetrameric polyprotein complex that plays an important role in the biosynthesis of EVs [Citation13]. The cargo sorting and biogenesis of EVs involve ESCRT-dependent or ESCRT-independent pathways [Citation14]. ESCRT pathway proteins include ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, TSG101, Alix, etc [Citation15]. The ESCRT-independent pathway may involve four transmembrane proteins (namely, CD63, CD9, CD81, and CD82), ceramide, heat shock proteins (HSPs), phosphatidic acid, ceramide and cholesterol [Citation16].
EVs contain proteins, DNA fragments, circular RNA (circRNA), mRNA, microRNA (miRNA), transcription factors, etc., which are involved in the exchange of information between cells and have the advantages of small size, good stability, high safety and long half-life [Citation17]. Researchers have identified 9769 proteins, 3408 mRNAs, 2838 miRNAs and 1116 lipids in EVs [Citation18]. Exosome proteins are mainly cargo or membrane proteins that are involved in antigen presentation, membrane transport and fusion, and immune defence [Citation19]. Lipids are an important part of the exosome membrane and are used to stabilize exosome contents and participate in many processes; thus, EVs can be used as biomarkers and drug delivery carriers [Citation20]. EVs exist in biological fluids, including plasma and urine. There are three main ways for EVs to participate in cell communication [Citation21]: first, exosome membrane proteins bind to target cell membrane proteins; second, exosome membrane proteins are cleaved by proteases, and the cleaved fragments are used as ligands to bind to receptors on the cell membrane; and third, the exosome membrane is directly fused with the target cell membrane. EVs interfere with a variety of physiological and pathological regulatory processes by participating in intercellular information exchange. EVs can not only participate in physiological processes such as nervous system signaling [Citation22], reproduction and development [Citation23], and cell proliferation and maturation [Citation24] but also activate the immune system by releasing proinflammatory factors and tumor necrosis factor (TNF) and enhancing NK cell activity [Citation25], thereby participating in pathological processes such as tumor progression, inflammation, and pathogen infection and transmission.
EVs begin with invagination of the plasma membrane and form early endosomes. Early endosomes are invaginated with the help of the Golgi apparatus to form multiple lumen vesicles, which are transformed into multivesicular bodies (MVBs). MVBs can be degraded by fusion with lysosomes or autophagosomes and can also fuse with the plasma membrane and be secreted into the extracellular microenvironment to form EVs.
3. EVs as potential biomarkers for DKD diagnosis
At present, the clinical use of the albumin excretion rate and glomerular filtration rate to evaluate renal function can only achieve partial diagnosis. Although renal biopsy is the gold standard for the diagnosis of DKD, this method is invasive and has certain contraindications. The glomerular structure of DM patients is irreversibly damaged before the appearance of microalbumin [Citation26]. Therefore, urinary microalbumin has certain limitations as an early predictor of DKD. Moreover, early renal injury in DM patients has no obvious clinical manifestations [Citation27]. Above all, it is necessary to find more accurate potential diagnostic biomarkers for DKD and provide better methods for clinical diagnosis and treatment. Urine is a common source for detecting biomarkers of renal disease. Urinary EVs, namely, nanovesicular entities, are released by each epithelial cell of the nephron [Citation28].
The RNA carried by urine EVs can be transmitted to distant target cells and participate in intercellular communication. Free urinary RNA is unstable and easily decomposed by endogenous RNase; urinary exosomal RNA is protected from endogenous RNase activity by microbubbles [Citation29]. Therefore, urinary exosomal RNA is more stable. Many studies have shown that urinary exosomal RNA may provide valuable insights into renal pathophysiology, participate in the regulation of protein transcription and translation, and play an important role in the pathogenesis of DKD [Citation30]. Lv et al. [Citation31] reported that compared with that in the healthy control group, the expression level of podocyte-associated CD2AP mRNA in the urinary EVs of patients with kidney disease was significantly downregulated, and it was negatively correlated with the serum creatinine level, fibrosis score, glomerulosclerosis and proteinuria. This finding suggested that the CD2AP mRNA expression in urinary EVs can be used as a noninvasive indicator for monitoring renal function and fibrosis in patients with kidney disease. Zhao et al. [Citation32] reported that the expression of miR-4491, miR-2117, miR-5088-5P, miR-219a-3p, miR-4687-3p, miR-516b-5p, miR-4534 and miR-5007-3 was upregulated more than 2-fold in the urinary EVs of DKD patients with type 2 diabetes mellitus (T2DM), and the expression of miR-4534 and miR-516b-5p was correlated with the level of proteinuria. Moreover, miR-4534 may affect the FoxO signaling pathway by targeting BNIP3 and promoting renal injury. Feng et al. [Citation33] reported that urinary EV-derived CCL21 mRNA was significantly increased in DKD patients compared with DM patients and healthy controls and was associated with the proteinuria levels and eGFR. Compared with that in DM patients, the expression of CCL21 mRNA in the urinary EVs of DKD patients with normal renal function is increased, which can more effectively distinguish early DKD patients from DM patients. Therefore, CCL21 mRNA can be used as a potential marker for the early diagnosis of DKD. A recent study [Citation34] revealed that the expression level of urinary exosomal miRNA-615-3p in DKD patients was significantly greater than that in healthy controls and patients with T2DM, and exosomal miRNA-615-3p was positively correlated with plasma TGF-β1 levels, serum creatinine levels, 24-h urine protein levels, etc., negatively correlated with the eGFR and albumin levels, and was involved in DKD inflammation and fibrosis. Therefore, urinary exosomal miRNA-615-3p is also expected to be a biomarker for evaluating the functional status of DKD patients. Similarly, Han et al. [Citation35] reported that the expression of miR-145-5p and miR-27a-3p in the urinary exosomes of DKD patients was greater than that in the urinary exosomes of DM patients and was positively correlated with albuminuria and serum creatinine levels and negatively correlated with the eGFR. Bioinformatics analysis revealed that the target genes of miR-145-5p are located in actin filaments, the cytoskeleton and exosomes and are involved in the pathological processes of DKD, including apoptosis, inflammation and fibrosis.
The proteins carried by EVs can also be used as biomarkers for the diagnosis of DKD. Wilms’ tumor-1 (WT1), a transcription factor with a zinc finger structure, plays an important role in maintaining mature podocyte homeostasis and is often used as a molecular marker of differentiated podocytes [Citation36]. Kalani et al. [Citation37] detected WT1 in the urine of 33 of 48 T1DM patients and only 1 of 23 healthy controls. The WT1 protein level in the urinary EVs of patients with T1DM-related proteinuria was significantly greater than that in patients without proteinuria, and the expression level of the WT1 protein in urinary EVs increased with decreasing renal function. Therefore, this study confirmed that the WT1 protein in urinary EVs is expected to be a biomarker for early diabetic nephropathy. In addition, a recent study revealed that the expression of calcium signaling pathway-related proteins in urinary EVs can affect the progression of DKD. Calcium is a rich ion in the human body and is the most important source of cations in the blood. Calcium imbalance can decrease islet β-cell function and induce various complications of diabetes, such as neuropathy, nephropathy, cardiovascular disease, and retinopathy [Citation38]. For the first time, Li et al. [Citation39] reported that the expression of urinary exosomal protein calmodulin-1 (CALM1) was significantly greater in DKD patients than in healthy controls and patients with T2DM, and the expression of CALM1 increased with decreasing eGFR. This study suggests that CALM1 may be used as an early noninvasive biomarker for the diagnosis of DKD.
In addition, serum EVs may also be a diagnostic biomarker for DKD. Regmi et al. [Citation40] reported significant differences in the expression levels of 38 serum miRNAs between DM patients and DKD patients, and levels of miR-99b, miR122-5p, miR-20a, and miR-486 in serum EVs were significantly correlated with albuminuria, the eGFR, blood glucose levels and blood lipid levels. Bioinformatics analysis revealed that the above four miRNAs may affect genes related to oxidative stress, inflammation and apoptosis in DKD through the PI3K/Akt, p53, AMPK, FOXO and other signaling pathways, thus interfering with the pathological development of DKD. However, this conclusion still needs to be further verified to provide a more solid basis for the use of miRNAs as biomarkers for minimally invasive blood diagnosis of DKD. Moreover, in one paper, a panel of 11 miRNAs loaded onto CD31+ EVs was associated with the presence of diabetic complications as a whole [Citation41], and miR-21-5p levels were strongly correlated with renal function. In this respect, EV-shuttled miR-21-5p has been largely studied in the context of diabetes complications [Citation42], and it has been shown to be a promising biomarker for the diagnosis of T2DM, cardiovascular disease and neurodegenerative diseases [Citation43]. The level of miR-21 can be regulated by oestrogen-based hormone replacement therapy [Citation44] ().
Table 1. EVs As potential biomarkers for DKD diagnosis.
4. The pathogenesis of EVs in DKD
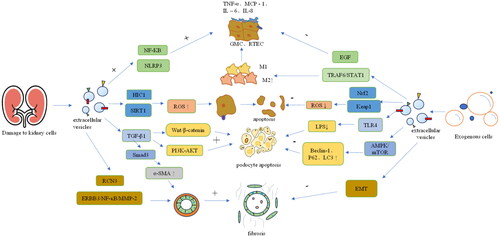
The basic pathological features of DKD include increased glomerular mesangial matrix, basement membrane thickening, glomerular sclerosis, and tubulointerstitial inflammation and fibrosis. The main initiating mechanism of DKD is hyperglycemia-induced vascular dysfunction, but its progression is due to different pathological mechanisms, including inflammation, oxidative stress, autophagy, podocyte injury and renal fibrosis [Citation45]. Although modern medicine has many methods for the treatment of DKD, it is still poor at delaying decreases in renal function. Therefore, we still need to explore the pathogenesis of DKD to identify better treatment methods. Compared with normal mesangial cells, mesangial cells stimulated with high glucose release more EVs after incubation for 16 and 24 h and can change the information exchange between cells, damage cells, and promote renal fibrosis [Citation46]. This finding suggests that EVs may be closely related to the pathogenesis of DKD. See .
NF-κB: nuclear factor kappa-B; TNF-α: tumor necrosis factor-α; MCP-1: monocyte chemoattractant protein-1; IL-6: interleukin-6; IL-1β: interleukin-1β; NLRP3: NOD-like receptor thermal protein domain associated protein 3; HIC1: hypermethylated in cancer 1; SIRT1: silent information regulator 1; TGF-β1: transforming growth factor-β1; α-SMA: α-smooth muscle actin; RCN3: reticulocalbin-3; EGF: epidermal growth factor; TRAF6: TNF receptor associated factor 6; STAT1: signal transducer and activator of transcription 1; Nrf2: nuclear factor erythroid 2-related factor 2; EMT: epithelial–mesenchymal transition.
4.1. EVs mediate the inflammatory response
An important pathological basis of DKD is the continuous microinflammation of renal tissue. Inflammatory factors such as cytokines, chemokines, growth factors, and cell adhesion molecules play key roles in diabetes-induced renal injury [Citation47]. Microinflammation induces glomerular endothelial cell injury through the protein kinase C (PKC) signaling pathway, advanced glycation end products (AGEs) pathway and polyol pathway, leading to macrophage infiltration of renal tissue, causing renal injury and promoting the development of DKD [Citation48]. There are two types of macrophages: M1 and M2. M1 macrophages release proinflammatory cytokines, such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-6 (IL-6), C-X-C motif chemokine ligand 9 (CXCL9) and C-X-C motif chemokine ligand 10 (CXCL10), while M2 macrophages secrete anti-inflammatory mediators, including interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [Citation49].
On one hand, EVs can induce inflammation. Nuclear factor kappa-B (NF-κB) is considered to be an important transcription factor that controls the expression of proinflammatory genes. Liu et al. [Citation50] first conducted in vitro studies and found that compared with those in the control group, high glucose-stimulated macrophage-derived EVs had higher concentrations of the exosome proteins iNOS and IL-1β, which can activate macrophages through the NF-κB signaling pathway. High glucose-stimulated macrophage-derived EVs promoted the expression of TNF-α, monocyte chemoattractant protein-1 (MCP-1), IL-6 and IL-1β inflammatory factors in mesangial cells and significantly upregulated the expression of NLRP3, ASC, caspase-1 and IL-1β in mesangial cells, indicating that high glucose-stimulated macrophage-derived EVs promote the inflammatory response of mesangial cells and the activation of the NLRP3 inflammasome. Similarly, in vivo studies revealed that the mRNA expression levels of inflammatory factors and the expression of the inflammasome-related proteins NLRP3 and ASC were significantly increased in DKD mice treated with high glucose-stimulated macrophage-derived EVs. Therefore, high glucose stimulates macrophage-derived EVs to accelerate glomerulosclerosis by promoting NLRP3 inflammasome activation, activating mesangial cell inflammation, and accelerating glomerulosclerosis. In addition, macrophage-derived EVs can affect renal tubular epithelial cells (RTECs). Zhao et al. [Citation51], in in vitro and in vivo studies, showed that miR-7002-5p from high glucose-induced macrophage-derived EVs can inhibit autophagy of RTECs by targeting Atg9b, which can significantly increase the expression of inflammatory factors such as TNF-α, IL-1β and MCP-1 in renal tubular epithelial cells, promote tubulointerstitial inflammation, and ultimately leading to renal tubular dysfunction and affecting renal function. They found that reducing EV miR-7002-5p expression can alleviate RTEC inflammation and dysfunction while activating autophagy. In summary, the important role of macrophage-derived EVs in DKD has been elucidated, which is expected to provide new insights for the prevention and treatment of DKD.
On the other hand, EVs can inhibit inflammation. Mesenchymal stem cells (MSCs) have powerful anti-inflammatory and immunomodulatory properties, which make them attractive therapeutic tools for tissue damage and inflammation. Zhang et al. [Citation52] reported that miR-146a-5p derived from human umbilical cord-derived mesenchymal stem cells (UC-MSCs) promotes M2 macrophage polarization by inhibiting the TRAF6/STAT1 signaling pathway, thereby increasing the secretion of anti-inflammatory factors, reducing inflammatory cell infiltration into renal tissue, and significantly improving renal function in DKD rats. Studies have shown that MSCs can upregulate the serum levels of the anti-inflammatory cytokines interleukin-10 (IL-10) and epidermal growth factor (EGF) [Citation53]. In vitro, MSCs can downregulate inflammation-related cytokines (such as IL-6, MCP-1, TNF-α and IL-1β) to inhibit lipopolysaccharide (LPS)-stimulated rat peritoneal macrophage activation. The above studies have shown that mesenchymal stem cells can prevent renal injury in diabetic rats through immune regulation, thereby restoring the homeostasis of the immune microenvironment and helping to prevent renal dysfunction and glomerulosclerosis.
4.2. EVs and oxidative stress
Increased oxidative stress is caused by the excessive production of reactive oxygen species (ROS) in the absence of the accompanying antioxidant pathway. There is increasing evidence that oxidative stress is a key cause of DKD-related pathological changes [Citation54]. Hyperglycemia induces excessive accumulation of ROS, resulting in increased expression levels of IL-6, TNF-α, and NF-κB, thereby promoting mesangial cell proliferation and extracellular matrix expansion, increasing endothelial cell permeability and inducing damage and apoptosis of endothelial cells and podocytes, which eventually leads to structural damage of the glomerular filtration membrane and proteinuria [Citation55]. Therefore, DKD-related kidney injury can be improved by inhibiting oxidative stress.
Recent studies have shown that serum EVs can affect oxidative stress in DKD patients. Hypermethylated in cancer 1 (HIC1) is a transcriptional repressor that regulates oxidative stress through silent information regulator 1 (SIRT1), a protein whose target genes have antioxidant activities [Citation56]. Hyperglycemia can increase the expression of HIC1, which in turn promotes oxidative stress by inhibiting the expression of the antioxidant gene SIRT1, leading to inflammation and apoptosis [Citation57]. Gao [Citation58] demonstrated that miR-4449 in serum EVs from DKD patients can promote oxidative stress and increase ROS levels by targeting HIC1 to inhibit the expression of the antioxidant gene SIRT1, thereby promoting the expression of proinflammatory cytokines such as IL-1β and IL-18 in RTECs and promoting apoptosis.
4.3. EVs and renal fibrosis
Renal fibrosis is a common pathological process in chronic kidney disease. Fibrosis is also considered to be the key to the final progression of DKD to renal failure in type 1 and type 2 diabetes patients [Citation59]. The renal interstitial fibrosis grade in DKD patients is positively correlated with the serum creatinine concentration at biopsy. The main cause of renal interstitial fibrosis is the excessive accumulation of extracellular matrix (ECM) proteins in the renal interstitium [Citation60]. In recent years, an increasing number of studies have shown that renal fibrosis is a sign of various chronic kidney diseases and that EVs can participate in renal fibrosis in DKD patients. TGF-β1 is a fibrogenic factor secreted by glomerular endothelial cells, glomerular mesangial cells and renal tubular epithelial cells and can lead to mesangial cell hypertrophy, glomerular basement membrane thickening, renal tubular interstitial fibrosis and renal function decline [Citation61].
The interaction between glomerular endothelial cells (GECs) and glomerular mesangial cells (GMCs) is an important aspect of DKD. Studies [Citation62] have shown that compared with NG-treated GECs, HG-treated GECs secrete more EV-derived TGF-β1 mRNA, which can increase the expression of α-smooth muscle actin (α-SMA) in GMCs through the TGF-β1/Smad3 signaling pathway, eventually leading to GMC proliferation and excessive accumulation of extracellular matrix proteins. This study showed that EVs from HG-treated GECs can activate GMCs, thereby promoting DKD renal fibrosis. The communication between renal tubular epithelial cells and fibroblasts in the renal tubulointerstitium promotes renal interstitial fibrosis [Citation63]. Tsai et al. [Citation64] reported that miR-92a-1-5p in EVs derived from proximal RTECs in a high-glucose environment induce endoplasmic reticulum stress and myofibroblast transdifferentiation in GMCs by targeting reticulocalbin-3 (RCN3), thereby accelerating renal fibrosis. Therefore, urinary miR-92a-1-5p can predict renal injury in patients with type 2 diabetes. Bai [Citation65] reported that the content of circ-DLGAP4 was increased in both DKD patients and DKD rat models and that circ-DLGAP promoted the proliferation and fibrosis of GMCs by adsorbing miR-143 to regulate the ERBB3/NF-κB/MMP-2 axis. Liu et al. [Citation66] found that HNRNPA1-mediated EVs can transport miR-483-5p in RTECs to the urine, thereby reducing the inhibitory effect of intracellular miR-483-5p on MAPK1 and TIMP2 mRNA, causing excessive accumulation of ECM and promoting DKD renal interstitial fibers.
4.4. EVs and intercellular communication
EVs are involved in intercellular communication by transporting goods, including microRNAs, mRNAs and proteins, and play a role in the biological functions of cells [Citation67]. There is evidence that EVs transfer their cargo between renal cells and are involved in the progression of diabetic nephropathy [Citation68]. At present, the pathogenesis of DKD is not yet clear, but studies have shown that the structure or function of the glomerular filtration barrier is abnormal, which has an important impact on the final progression to proteinuria and renal failure [Citation69]. Podocytes are terminally differentiated glomerular visceral epithelial cells that are the final barrier for glomerular filtration of plasma and are closely related to maintaining the integrity of the barrier and preventing proteinuria [Citation70]. Many studies have shown that podocyte injury plays an important role in the occurrence and development of DKD [Citation71–73]. The communication between podocytes and glomerular endothelial cells (GECs) and between podocytes and glomerular mesangial cells (GMCs) affects the progression of DKD.
Recent research has shown that abnormal crosstalk between GECs and podocytes plays a key role in DKD [Citation74]. Wu et al. [Citation75] showed that HG-treated GECs secrete more EVs than normal glucose (NG)-treated GECs. Moreover, these TGF-β1-containing EVs can mediate podocyte injury and proteinuria through the typical Wnt/β-catenin signaling pathway. In vivo studies have shown that EVs secreted by high glucose-stimulated GECs can activate GMCs, leading to mesangial cell proliferation and extracellular matrix deposition in the mouse mesangial area and promoting renal fibrosis [Citation76]. In the diabetic state, GMCs are involved in a variety of pathophysiological injury pathways. In vitro experiments by Wang et al. [Citation77] revealed that high glucose-induced GMC-derived EVs can activate the PI3K-AKT signaling pathway in podocytes through TGF-β1 to mediate communication between mesangial cells and podocytes, thereby inducing podocyte apoptosis and inhibiting podocyte adhesion.
4.5. EVs and insulin resistance
Insulin resistance refers to the fact that the target cells and target tissues of insulin cannot normally process blood glucose, inhibit the generation and cleavage of endogenous glucose, and stimulate the synthesis of high-concentration glycogen in plasma, which is an important feature of T2DM [Citation78]. The main cause of insulin resistance is the obstruction of insulin signal transduction, which is due to the disordered phosphorylation of tyrosine residues in insulin receptor substrate 1 (IRS-1) and the inactivation of protein kinase B (PKB) [Citation79]. Insulin receptors are expressed in renal tubular cells and podocytes, and insulin signaling plays an important role in podocyte viability and renal tubular function [Citation80]. Insulin resistance is associated with the clinical manifestations of DKD, such as glomerular hyperfiltration, proteinuria, and renal failure, and the structural characteristics of DKD are already obvious in the insulin resistance stage [Citation81].
Previous studies have shown that inflammation plays an important role in insulin resistance in obesity-related T2DM, and it is believed that proinflammatory cytokines secreted from tissue macrophages, such as TNF-α, can directly inhibit insulin sensitivity [Citation82]. MicroRNAs (miRNAs) are a class of small noncoding RNAs that act as regulators of mRNA expression and translation efficiency in most cell types and are involved in processes such as insulin production and secretion and insulin resistance in tissues [Citation83]. Adipose tissue macrophages (ATMs) secrete EVs containing miRNA cargoes, which can be transferred to insulin target cell types through paracrine or endocrine regulatory mechanisms and have a strong effect on cellular insulin action, insulin sensitivity in vivo, and overall glucose homeostasis [Citation84]. MiR-155 is one of the differentially expressed miRNAs in obese ATM-Exos. miR-155 can inhibit insulin signal transduction and glucose tolerance by directly inhibiting its target gene PPARg.
5. Therapeutic perspectives of EVs in DKD
DKD is associated with high morbidity and mortality and seriously affects the quality of life of patients. At present, there is no effective drug for the treatment of diabetic neuropathy [Citation85]. Therefore, novel and effective strategies for improving diabetic neuropathy at an early stage should be identified. The development of safer and more effective treatments for DKD is urgently needed. Many experimental therapies are currently being studied, including stem cell therapy and drugs targeting the inflammatory, oxidative or profibrotic pathways activated during the progression of diabetic neuropathy. The therapeutic potential of stem cells depends in part on their secretory capacity. EVs are gradually being discovered and applied in the treatment of DKD due to their unique properties and functions (). One study reported that exosomal miR-22-3p derived from mesenchymal stem cells (HUC-MSCs) can reduce the expression of IL-6, IL-1β, IL-18 and TNF-α in the podocytes of DKD mice by inhibiting the activation of the NLRP3 signaling pathway, thus exerting anti-inflammatory effects [Citation86]. Another report demonstrated that adipose-derived stem cell (ADSC) EVs regulate the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and kelch-like ECH-associated protein 1 (Keap1) by targeting FAM129B, thereby improving oxidative stress and inflammation in the podocytes and kidney tissues of DKD mice [Citation87]. Similarly, in bone marrow mesenchymal stem cell (BMSC)-derived EVs, miR-30e-5p can reduce the expression of pyroptosis-related factors such as IL-1β, IL-18, caspase-1, and NLRP3 in high glucose–induced renal tubular HK-2 cells [Citation88]. Moreover, exosomal miR-424-5 derived from mesenchymal stem cells (MSCs) may reverse apoptosis and the epithelial–mesenchymal transition (EMT) to reduce renal fibrosis in DKD patients [Citation89]. Chen et al. [Citation90] cultured EVs derived from HUC-MSCs with islet β cells under hypoxic conditions and found that miR-21 contained in EVs could inhibit β cell apoptosis by reducing hypoxia-mediated endoplasmic reticulum stress, thereby improving blood glucose. A more recent report revealed a promising therapeutic approach using satellite cell-derived EVs to construct a platform for the targeted delivery of miR-23a/27a/26a clusters, which can enhance fibrosis resistance and effectively improve renal tubulointerstitial fibrosis in DKD patients [Citation91]. Zhuang et al. [Citation92] reported that G-aminobutyric acid (GABA) can regulate the Tnpo1/ATXN3 signaling pathway by affecting the expression of exosomal miR-21a-5p/miR-25-3 secreted by macrophages under HG conditions, thereby promoting podocyte proliferation and inhibiting podocyte apoptosis. Furthermore, Li et al. [Citation93] reported that HUC-MSCs activate autophagy through the AMPK/mTOR pathway and improve the expression of ageing-related genes (p16, p53 and p21) and autophagy-related genes (beclin-1, p62 and LC3), thereby reducing the autophagy of podocytes induced by high glucose in rats. Wang [Citation94] reported that under high glucose conditions, the secretion of miR-93-5p in M2 macrophage-derived EVs is increased, and EV-derived miR-93-5p can target Toll-like receptor 4 (TLR4) to reduce lipopolysaccharide-induced podocyte apoptosis. Therefore, EVs can improve podocyte autophagy and podocyte apoptosis and delay DKD renal injury.
Table 2. Potential therapeutic roles of EVs in DKD.
Therefore, EVs have potential therapeutic effects in terms of inhibiting renal inflammation, oxidative stress, and fibrosis and improving podocyte injury and insulin resistance.
6. Prospects
In summary, the diagnosis, mechanism and potential therapeutic effect of EVs in DKD is discussed in this paper. As a new biomarker, EVs may play an important role in the early diagnosis, disease monitoring and therapeutic effect evaluation of DKD. Urinary EVs have unique advantages as noninvasive biomarkers of DKD, among which miRNAs and proteins are the most studied molecules. However, there are few studies on serum EVs as biomarkers for the diagnosis of DKD, and further research and analysis are still needed. EVs are mainly involved in the inflammatory response, oxidative stress, renal fibrosis, podocyte injury and insulin resistance through the substances they carry. Many studies have shown that EVs participate in the treatment of DKD in a multidimensional, multichannel and multiform manner, and the curative effect is remarkable; thus, EVs are expected to become an important means of clinical DKD treatment. However, the exact molecular mechanism by which EVs treat DKD has not been fully elucidated and still needs to be further verified in basic and clinical trials. Extracellular vesicles are stable and specific, and their potential as drugs or nanocarriers for drug delivery deserves further exploration. Therefore, EVs are currently the main candidate for the development of new therapeutic methods for targeted drug carriers for DKD treatment. At present, most studies have shown that EVs administered systematically are easily delivered to glomerular podocytes and renal tubular epithelial cells. It is believed that with the continuous development of related technologies, the molecular mechanism underlying the effects of EVs will be further studied to determine their potential in the treatment of DKD.
Authors’ contributions
ZM conceptualized the ideas. JJ wrote the first draft of the manuscript. JJ prepared the figures. ZM revised the manuscript. All the authors approved the final version of the manuscript.
Disclosure statement
The authors declare that there are no conflicts of interest related to this work.
Additional information
Funding
References
- Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1–11. Published 2021 Jul 8. doi:10.1155/2021/1497449.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi:10.2215/CJN.11491116.
- Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102(2):248–260. doi:10.1016/j.kint.2022.05.012.
- Zoja C, Xinaris C, Macconi D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Front Pharmacol. 2020;11:586892. Published 2020 Dec 21. doi:10.3389/fphar.2020.586892.
- Liu H, Feng J, Tang L. Early renal structural changes and potential biomarkers in diabetic nephropathy. Front Physiol. 2022;13:1020443. Published 2022 Nov 8. doi:10.3389/fphys.2022.1020443.
- Tiwary M, Milder TY, Stocker SL, et al. Sodium-glucose co-transporter 2 inhibitor therapy: use in chronic kidney disease and adjunctive sodium restriction. Intern Med J. 2022;52(10):1666–1676. doi:10.1111/imj.15727.
- Choi JG, Winn AN, Skandari MR, et al. First-line therapy for type 2 diabetes with sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a cost-effectiveness study. Ann Intern Med. 2022;175(10):1392–1400. doi:10.7326/M21-2941.
- Lu Y, Liu D, Feng Q, et al. Diabetic nephropathy: perspective on extracellular vesicles. Front Immunol. 2020;11:943. Published 2020 Jun 3. doi:10.3389/fimmu.2020.00943.
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727.
- Sykaras AG, Christofidis K, Politi E, et al. Exosomes on endometrial cancer: a biomarkers treasure trove? Cancers (Basel). 2022;14(7):1733. doi:10.3390/cancers14071733.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977.
- Toh WS, Lai RC, Zhang B, et al. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843–853. doi:10.1042/BST20180079.
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi:10.1016/j.devcel.2011.05.015.
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326.
- Zhang Y, Bi J, Huang J, et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. Published 2020 Sep 22. doi:10.2147/IJN.S264498.
- Migliano SM, Teis D. ESCRT and membrane protein ubiquitination. Prog Mol Subcell Biol. 2018;57:107–135.
- Tran PHL, Xiang D, Tran TTD, et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 2020;32(18):e1904040.
- Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 2019;20(19):4684. Published 2019 Sep 21. doi:10.3390/ijms20194684.
- Zhou LI, Lv T, Zhang Q, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett. 2017;407:84–92. doi:10.1016/j.canlet.2017.08.003.
- Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi:10.1016/j.plipres.2017.03.001.
- Chopra N, Dutt Arya B, Jain N, et al. Biophysical characterization and drug delivery potential of exosomes from human wharton’s jelly‑derived mesenchymal stem cells. ACS Omega. 2019;4(8):13143–13152. doi:10.1021/acsomega.9b01180.
- Zhuoyang Y, Yan T, Jing Y, et al. The role of exosomes in adult neurogenesis: implications for neurodegenerative diseases. Neural Regen Res. 2024;19(2):282–288. doi:10.4103/1673-5374.379036.
- Jia L, Huang X, Peng H, et al. Pregnancy-specific beta-1-glycoprotein 1-enriched exosomes are involved in the regulation of vascular endothelial cell function during pregnancy. Placenta. 2023;139:138–147. doi:10.1016/j.placenta.2023.06.014.
- Hong Y, Heo J, Kang S, et al. Exosome-mediated delivery of gga-miR-20a-5p regulates immune response of chicken macrophages by targeting IFNGR2, MAPK1, MAP3K5, and MAP3K14. Anim Biosci. 2023;36(6):851–860. doi:10.5713/ab.22.0373.
- Wang KH, Ding DC. The role and applications of exosomes in gynecological cancer: a review. Cell Transplant. 2023;32:9636897231195240. doi:10.1177/09636897231195240.
- Chen C, Wang C, Hu C, et al. Normoalbuminuric diabetic kidney disease. Front Med. 2017;11(3):310–318. doi:10.1007/s11684-017-0542-7.
- Mulder S, Hamidi H, Kretzler M, et al. An integrative systems biology approach for precision medicine in diabetic kidney disease. Diabetes Obes Metab. 2018;20(Suppl 3):6–13. doi:10.1111/dom.13416.
- Gudehithlu KP, Garcia-Gomez I, Vernik J, et al. In diabetic kidney disease urinary exosomes better represent kidney specific protein alterations than whole urine. Am J Nephrol. 2015;42(6):418–424. doi:10.1159/000443539.
- Wang G, Szeto CC. Quantification of gene expression in urinary sediment for the study of renal diseases. Nephrology (Carlton). 2007;12(5):494–499. doi:10.1111/j.1440-1797.2007.00836.x.
- Sinha N, Kumar V, Puri V, et al. Urinary exosomes: potential biomarkers for diabetic nephropathy. Nephrology (Carlton). 2020;25(12):881–887. doi:10.1111/nep.13720.
- Lv LL, Cao YH, Pan MM, et al. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin Chim Acta. 2014;428:26–31. doi:10.1016/j.cca.2013.10.003.
- Zhao Y, Shen A, Guo F, et al. Urinary exosomal MiRNA-4534 as a novel diagnostic biomarker for diabetic kidney disease. Front Endocrinol (Lausanne). 2020;11:590. Published 2020 Aug 28. doi:10.3389/fendo.2020.00590.
- Feng Y, Zhong X, Ni HF, et al. Urinary small extracellular vesicles derived CCL21 mRNA as biomarker linked with pathogenesis for diabetic nephropathy. J Transl Med. 2021;19(1):355. Published 2021 Aug 17. doi:10.1186/s12967-021-03030-x.
- Wang J, Tao Y, Zhao F, et al. Expression of urinary exosomal miRNA-615-3p and miRNA-3147 in diabetic kidney disease and their association with inflammation and fibrosis. Ren Fail. 2023;45(1):2121929.
- Han LL, Wang SH, Yao MY, et al. Urinary exosomal microRNA-145-5p and microRNA-27a-3p act as noninvasive diagnostic biomarkers for diabetic kidney disease. World J Diabetes. 2024;15(1):92–104. doi:10.4239/wjd.v15.i1.92.
- Su J, Li SJ, Chen ZH, et al. Evaluation of podocyte lesion in patients with diabetic nephropathy: wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract. 2010;87(2):167–175. doi:10.1016/j.diabres.2009.10.022.
- Kalani A, Mohan A, Godbole MM, et al. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One. 2013;8(3):e60177. doi:10.1371/journal.pone.0060177.
- Guerrero-Hernandez A, Verkhratsky A. Calcium signalling in diabetes. Cell Calcium. 2014;56(5):297–301. doi:10.1016/j.ceca.2014.08.009.
- Li T, Ci Liu T, Liu N, et al. Changes in urinary exosomal protein CALM1 may serve as an early noninvasive biomarker for diagnosing diabetic kidney disease. Clin Chim Acta. 2023;547:117466. doi:10.1016/j.cca.2023.117466.
- Regmi A, Liu G, Zhong X, et al. Evaluation of serum microRNAs in patients with diabetic kidney disease: a nested case-controlled study and bioinformatics analysis. Med Sci Monit. 2019;25:1699–1708. Published 2019 Mar 5. doi:10.12659/MSM.913265.
- Prattichizzo F, De Nigris V, Sabbatinelli J, et al. CD31+ extracellular vesicles from patients with type 2 diabetes shuttle a miRNA signature associated with cardiovascular complications. Diabetes. 2021;70(1):240–254. doi:10.2337/db20-0199.
- Prattichizzo F, Matacchione G, Giuliani A, et al. Extracellular vesicle-shuttled miRNAs: a critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics. 2021;11(3):1031–1045. doi:10.7150/thno.51605.
- Olivieri F, Prattichizzo F, Giuliani A, et al. miR-21 and miR-146a: the microRNAs of inflammaging and age-related diseases. Ageing Res Rev. 2021;70:101374. doi:10.1016/j.arr.2021.101374.
- Kangas R, Pöllänen E, Rippo MR, et al. Circulating miR-21, miR-146a and fas ligand respond to postmenopausal estrogen-based hormone replacement therapy–a study with monozygotic twin pairs. Mech Ageing Dev. 2014;143-144:1–8. doi:10.1016/j.mad.2014.11.001.
- Kidney disease: improving global outcomes (KDIGO) diabetes work group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115.
- da Silva Novaes A, Borges FT, Maquigussa E, et al. Influence of high glucose on mesangial cell-derived exosome composition, secretion and cell communication [published correction appears in sci rep. 2020 jan 29;10(1):1730]. Sci Rep. 2019;9(1):6270. Published 2019 Apr 18. doi:10.1038/s41598-019-42746-1.
- Donate-Correa J, Luis-Rodríguez D, Martín-Núñez E, et al. Inflammatory targets in diabetic nephropathy. J Clin Med. 2020;9(2):458. Published 2020 Feb 7. doi:10.3390/jcm9020458.
- Tian S, Chen SY. Macrophage polarization in kidney diseases. Macrophage (Houst). 2015;2(1):e679.
- Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. doi:10.1016/j.intimp.2021.107823.
- Liu Y, Li X, Zhao M, et al. Macrophage-derived exosomes promote activation of NLRP3 inflammasome and autophagy deficiency of mesangial cells in diabetic nephropathy. Life Sci. 2023;330:121991. doi:10.1016/j.lfs.2023.121991.
- Zhao J, Chen J, Zhu W, et al. Exosomal miR-7002-5p derived from highglucose-induced macrophages suppresses autophagy in tubular epithelial cells by targeting Atg9b. FASEB J. 2022;36(9):e22501.
- Zhang Y, Le X, Zheng S, et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res Ther. 2022;13(1):171. Published 2022 Apr 27. doi:10.1186/s13287-022-02855-7.
- Li Y, Liu J, Liao G, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41(5):2629–2639. doi:10.3892/ijmm.2018.3501.
- Ma L, Wu F, Shao Q, et al. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther. 2021;15:3207–3221. Published 2021 Jul 21. doi:10.2147/DDDT.S319260.
- Jha JC, Banal C, Chow BS, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–684. doi:10.1089/ars.2016.6664.
- Hasegawa K, Wakino S, Yoshioka K, et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372(1):51–56. doi:10.1016/j.bbrc.2008.04.176.
- Zeng S, Wu X, Chen X, et al. Hypermethylated in cancer 1 (HIC1) mediates high glucose induced ROS accumulation in renal tubular epithelial cells by epigenetically repressing SIRT1 transcription. Biochim Biophys Acta Gene Regul Mech. 2018;1861(10):917–927. doi:10.1016/j.bbagrm.2018.08.002.
- Gao C, Wang B, Chen Q, et al. Serum exosomes from diabetic kidney disease patients promote pyroptosis and oxidative stress through the miR-4449/HIC1 pathway. Nutr Diabetes. 2021;11(1):33. Published 2021 Nov 3. doi:10.1038/s41387-021-00175-y.
- Zeng LF, Xiao Y, Sun L. A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Adv Exp Med Biol. 2019;1165:49–79.
- Mora C, Navarro JF. Inflammation and pathogenesis of diabetic nephropathy. Metabolism. 2004;53(2):265–266. doi:10.1016/j.metabol.2003.11.005.
- Ma TT, Meng XM. TGF-β/smad and renal fibrosis. Adv Exp Med Biol. 2019;1165:347–364.
- Wu XM, Gao YB, Cui FQ, et al. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5(4):484–491. doi:10.1242/bio.015990.
- Zhang Y, Qin X, Yang Y, et al. Ginkgo biloba extract attenuates cisplatin-induced renal interstitial fibrosis by inhibiting the activation of renal fibroblasts through down-regulating the HIF-1α/STAT3/IL-6 pathway in renal tubular epithelial cells. Phytomedicine. 2023;115:154809. doi:10.1016/j.phymed.2023.154809.
- Tsai YC, Kuo MC, Hung WW, et al. Proximal tubule-derived exosomes contribute to mesangial cell injury in diabetic nephropathy via miR-92a-1-5p transfer. Cell Commun Signal. 2023;21(1):10. Published 2023 Jan 13. doi:10.1186/s12964-022-00997-y.
- Bai S, Xiong X, Tang B, et al. Exosomal circ_DLGAP4 promotes diabetic kidney disease progression by sponging miR-143 and targeting ERBB3/NF-κB/MMP-2 axis. Cell Death Dis. 2020;11(11):1008. Published 2020 Nov 23. doi:10.1038/s41419-020-03169-3.
- Liu D, Liu F, Li Z, et al. HNRNPA1-mediated exosomal sorting of miR-483-5p out of renal tubular epithelial cells promotes the progression of diabetic nephropathy-induced renal interstitial fibrosis. Cell Death Dis. 2021;12(3):255. Published 2021 Mar 10. doi:10.1038/s41419-021-03460-x.
- Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for Next-Generation theranostic platforms. Adv Mater. 2019;31(2):e1802896.
- Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol. 2022;18(8):499–513. doi:10.1038/s41581-022-00586-9.
- Tung CW, Hsu YC, Shih YH, et al. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton). 2018;23 Suppl 4:32–37. doi:10.1111/nep.13451.
- Barutta F, Bellini S, Gruden G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin Sci (Lond). 2022;136(7):493–520. doi:10.1042/CS20210625.
- Lu Z, Liu H, Song N, et al. METTL14 aggravates podocyte injury and glomerulopathy progression through N6-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis. 2021;12(10):881. Published 2021 Sep 27. doi:10.1038/s41419-021-04156-y.
- Li XZ, Jiang H, Xu L, et al. Sarsasapogenin restores podocyte autophagy in diabetic nephropathy by targeting GSK3β signaling pathway. Biochem Pharmacol. 2021;192:114675. doi:10.1016/j.bcp.2021.114675.
- Zhang H, Yan Y, Hu Q, et al. LncRNA MALAT1/microRNA let-7f/KLF5 axis regulates podocyte injury in diabetic nephropathy [retracted in: life sci. 2023 apr 1;318:121420]. Life Sci. 2021;266:118794. doi:10.1016/j.lfs.2020.118794.
- Guo F, Song Y, Wu L, et al. SUMO specific peptidase 6 regulates the crosstalk between podocytes and glomerular endothelial cells in diabetic kidney disease. Biochim Biophys Acta Mol Basis Dis. 2023;1869(5):166685. doi:10.1016/j.bbadis.2023.166685.
- Wu X, Gao Y, Xu L, et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7(1):9371. Published 2017 Aug 24. doi:10.1038/s41598-017-09907-6.
- Ling L, Tan Z, Zhang C, et al. CircRNAs in exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells. Am J Transl Res. 2019;11(8):4667–4682.
- Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFβ1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185–192. doi:10.1016/j.ejphar.2018.01.034.
- Marušić M, Paić M, Knobloch M, et al. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827–6613829. doi:10.1155/2021/6613827.
- Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–S148. doi:10.1055/s-2001-18576.
- Artunc F, Schleicher E, Weigert C, et al. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–737. doi:10.1038/nrneph.2016.145.
- Adeva-Andany MM, Fernández-Fernández C, Funcasta-Calderón R, et al. Insulin resistance is associated with clinical manifestations of diabetic kidney disease (glomerular hyperfiltration, albuminuria, and kidney function decline). Curr Diabetes Rev. 2022;18(7):e171121197998.
- De Taeye BM, Novitskaya T, McGuinness OP, et al. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007;293(3):E713–E725. doi:10.1152/ajpendo.00194.2007.
- Mafi A, Aghadavod E, Mirhosseini N, et al. The effects of expression of different microRNAs on insulin secretion and diabetic nephropathy progression. J Cell Physiol. 2018;234(1):42–50. doi:10.1002/jcp.26895.
- Ying W, Riopel M, Bandyopadhyay G, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384.e12. doi:10.1016/j.cell.2017.08.035.
- Zhang L, Li K, Liu X, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22(23):3074–3086. doi:10.1089/scd.2013.0142.
- Wang Y, Liu J, Wang H, et al. Mesenchymal stem cell-derived exosomes ameliorate diabetic kidney disease through the NLRP3 signaling pathway. Stem Cells. 2023;41(4):368–383. doi:10.1093/stmcls/sxad010.
- Ren P, Qian F, Fu L, et al. Adipose-derived stem cell exosomes regulate Nrf2/Keap1 in diabetic nephropathy by targeting FAM129B. Diabetol Metab Syndr. 2023;15(1):149. Published 2023 Jul 4. doi:10.1186/s13098-023-01119-5.
- Lv J, Hao YN, Wang XP, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-30e-5p ameliorates high-glucose induced renal proximal tubular cell pyroptosis by inhibiting ELAVL1. Ren Fail. 2023;45(1):2177082.
- Cui C, Zang N, Song J, et al. Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J. 2022;36(10):e22517.
- Chen J, Chen J, Cheng Y, et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11(1):97. Published 2020 Mar 4. doi:10.1186/s13287-020-01610-0.
- Ji JL, Shi HM, Li ZL, et al. Satellite cell-derived exosome-mediated delivery of microRNA-23a/27a/26a cluster ameliorates the renal tubulointerstitial fibrosis in mouse diabetic nephropathy. Acta Pharmacol Sin. 2023;44(12):2455–2468. doi:10.1038/s41401-023-01140-4.
- Zhuang Y, Zheng H, Yang Y, et al. GABA alleviates high glucose-induced podocyte injury through dynamically altering the expression of macrophage M1/M2-derived exosomal miR-21a-5p/miR-25-3p. Biochem Biophys Res Commun. 2022;618:38–45. doi:10.1016/j.bbrc.2022.06.019.
- Li X, Guo L, Chen J, et al. Intravenous injection of human umbilical cord-derived mesenchymal stem cells ameliorates not only blood glucose but also nephrotic complication of diabetic rats through autophagy-mediated anti-senescent mechanism. Stem Cell Res Ther. 2023;14(1):146. Published 2023 May 29. doi:10.1186/s13287-023-03354-z.
- Wang Z, Sun W, Li R, et al. miRNA-93-5p in exosomes derived from M2 macrophages improves lipopolysaccharide-induced podocyte apoptosis by targeting toll-like receptor 4. Bioengineered. 2022;13(3):7683–7696. doi:10.1080/21655979.2021.2023794.