Abstract
Objectives
Peritoneal dialysis (PD) serves as a vital renal replacement therapy for patients with end-stage kidney disease (ESKD). γ-Gamma-glutamyl transferase (γ-GGT) is a recognized predictor of oxidative stress and mortality. This study aimed to assess the prognostic significance of γ-GGT in predicting all-cause and cardiovascular mortality among PD patients.
Methods
A retrospective study was conducted, enrolling 640 PD patients from a single center. The one-year, three-year, and five-year mortality rates for all causes and cardiovascular causes were evaluated. Kaplan–Meier survival analysis and multivariate Cox regression analysis were performed.
Results
Within five years of initiating PD, the observed all-cause mortality rates at one, three, and five years were 11.72%, 16.09%, and 23.44%, while cardiovascular mortality rates were 2.97%, 7.34%, and 11.09%, respectively. Lower γ-GGT levels were associated with decreased all-cause mortality during one-, three-, and five-year follow-ups, along with reduced cardiovascular mortality in the first and third years, as indicated by Kaplan–Meier analysis on median γ-GGT groupings. Multivariate Cox regression analysis showed significantly decreased hazard ratios (HRs) for one- to five-year all-cause mortality and cardiovascular mortality in the lower γ-GGT group compared to higher groups. However, when sex differences were eliminated using separate tertile groupings for males and females, only the one- and three-year all-cause mortality rates demonstrated significantly reduced hazard ratios (HRs) in the lower γ-GGT groups.
Conclusion
This retrospective study suggests that γ-GGT levels have prognostic significance in predicting one- and three-year all-cause mortality among PD patients when accounting for sex differences.
Introduction
Peritoneal dialysis (PD), an initial renal replacement treatment for end-stage kidney disease (ESKD), was administered to 114,000 patients in China in 2020 [Citation1]. In recent years, accumulating clinical studies have demonstrated that ESKD patients are at higher risk for cardiovascular (CV) disease, which takes the leading position of mortality in ESKD patients [Citation2]. Moreover, patients receiving dialysis suffer a 10 to 20 times higher CV mortality risk than general populations [Citation2]. Conventional perspective have pointed out that the high CV mortality and all cause mortality in patients receiving dialysis are mainly associated with some important risk factors such as age, hypertension, diabetes mellitus, dyslipidemia and so on [Citation3,Citation4]. In recent years, oxidative stress(OS) had been considered as a novel risk factor contributing to CV morality, especially in chronic kidney disease patients [Citation5]. Studies have revealed that the level of OS is significantly elevated remarkably in ESKD and further exaggerated in patients undergoing PD [Citation6].
γ-Gamma-glutamyl transferase (γ-GGT) is a recognized circulating indicator of alcohol-related liver disease [Citation7]. Interestingly, its prognostic value applies well beyond hepatic disease. Augmented γ-GGT activity is an indicator of antioxidant deficiency and causes oxidative stress due to its role in enabling the metabolism of glutathione and glutathionylated xenobiotics and resisting oxidative stress [Citation8]. Growing evidence has revealed that a higher γ-GGT level is associated with an increased risk of CVD, diabetes, metabolic syndrome, and all-cause mortality, regardless of liquor consumption or hepatic disease [Citation9,Citation10]. Few studies have focused on the prognostic value of γ-GGT in patients undergoing PD. A study including 820 PD patients found that high γ-GGT levels were positively associated with the risk of all-cause mortality in these patients [Citation11]. However, the conclusion is uncertain because the aforementioned study observed significant sex differences between γ-GGT subgroups, but did not investigate whether there were sex differences in the predictive effect of GGT on mortality. In addition, there is currently a lack of literature specifically reporting on the relationship between γ-GGT and cardiovascular mortality in patients undergoing PD. Hence, the objective of this study was to explore the association between γ-GGT levels and both cardiovascular and all-cause mortality among individuals undergoing peritoneal dialysis.
Methods
Study population
As shown in , all the participants in this retrospective study were inpatients who began receiving PD treatment between November 2005 and May 2016 at the First Affiliated Hospital of Nanchang University in China. The exclusion criteria were as follows: (1) age < 18 years at PD initiation; (2) cessation of PD within 90 days of initial treatment; (3) shift from renal transplantation failure or maintenance hemodialysis; (4) incomplete data (e.g., laboratory data, such as baseline γ-GGT, and mortality status); (5) with malignant tumor; (6) with alcohol abuse; (7) with hepatic diseases; (8) alanine aminotransferase (ALT) >100 u/L; and (9) aspartate aminotransferase (AST) >80 u/L; The flowchart is provided in . Finally, 640 patients were included in the analysis. This study adhered to the ethical principles of the Helsinki Declaration and was approved by the Human Ethics Committee of Nanchang University (Application ID: [2021]5-074). As all data were collected from the electronic medical record system, this study did not involve personal privacy and commercial interests, and the risk to participants was minimal, it had been approved by the Ethics Committee for exemption from informed consent.
Data collection
All participants were followed up for five years, unless cessation of PD treatment or death occurred. At the start of PD therapy, baseline demographic characteristics, such as sex, age, diabetic or hypertensive status, and history of CVD, as well as clinical and laboratory data, such as blood pressure, body mass index (BMI), white blood cell (WBC), hemoglobin (HB), serum albumin (sALB), ALT, AST, γ-GGT, alkaline phosphatase (AKP), free blood glucose (FBG), blood chlorine, and creatinine (sCr) were obtained from the Electronic Medical Record System of the First Affiliated Hospital of Nanchang University. All variables were assessed in the central laboratory of the First Affiliated Hospital of Nanchang University. Data on medication usage was retrieved from the patients’ electronic medical records. Diabetes was diagnosed according to the diagnostic criteria of the American Diabetes Association (ADA) in 2023, which are as follows: fasting plasma glucose (FPG) of ≥7.0 mmol/L (126 mg/dl); 2-h plasma glucose (2hPG) of ≥11.1 mmol/L (200 mg/dl) after an oral glucose tolerance test (OGTT); hemoglobin A1c (HbA1c) of ≥6.5%;In an individual with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (≥11.1 mmol/L). The diagnosis of hypertension is based on the general definition of hypertension, which includes a systolic blood pressure (SBP) of ≥140 mmHg or a diastolic blood pressure (DBP) of ≥90 mmHg, persistently elevated blood pressure on repeated measurements, or the use of antihypertensive drugs. BMI was calculated as weight (kg) divided by height (m) squared. The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.
Statistical analysis
We divided the patients into two groups according to the medium of γ-GGT; thereafter, the Kaplan–Meier method and log-rank test were used to construct cumulative survival curves for one-year, three-year, and five-year cardiovascular and all-cause mortality. To address the issue of sex differences, we stratified the γ-GGT levels based on the trimean of γ-GGT among male and female patients separately. By doing so, we were able to compare mortality risks across similar ranges of γ-GGT in both sexes. Categorical variables were represented using frequencies and percentages, while continuous variables were represented using measures such as means and standard deviations (SDs) or medians accompanied by quartile ranges, depending on the underlying distribution. Comparisons between the two γ-GGT groups were made using the chi-squared or Kruskal–Wallis test to determine the differences among categorical or continuous variables.
The main endpoints were one-year, three-year, and five-year cardiovascular mortality. The Kaplan–Meier method and log-rank test were used to construct cumulative survival curves for one-year, three-year, and five-year cardiovascular and all-cause mortality. The hazard ratio (HR) for cardiovascular and all-cause mortality, along with its 95% confidence interval (CI) was evaluated using the Cox proportional hazards regression model. Potential confounding factors, including age, sex, diabetic and hypertensive status, CVD history, body mass index (BMI), systolic and diastolic blood pressure, free Blood glucose (FBG), estimated glomerular filtration rate (eGFR), sALB, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), high-density lipoprotein (HDL), cholesterol (CHOL), triglyceride (TG), low density lipoprotein-cholesterol (LDL-C), white blood cells (WBC) and Neutrophils (NEU), Calcium (Ca), intact parathyroid hormone (iPTH), daily dialysis dose/100, residual urine volume/100, and daily ultrafiltration volume/100 were adjusted for in the analysis. A subgroup analysis was conducted to evaluate the potential influence of altering the association between γ-GGT and cardiovascular as well as all-cause mortality across various confounding factors such as sex, age, hypoalbuminemia status, hypertension, diabetes mellitus, and history of cardiovascular disease. We used Cox proportional hazard models to estimate HRs (95% CI) for mortality and to present the above results. Statistical significance was set at p < .05. All statistical analyses were conducted using SPSS software version 19.0 (SPSS Inc., Chicago, IL).
Results
Baseline characteristics of participants
A total of 640 participants (289 men, 45.2%) undergoing incident PD were finally included in the present study, and their mean age was 49.66 ± 14.67 (mean ± SD) years. The medium of γ-GGT is 19 and all the participants were evenly divided into two groups according to the medium of γ-GGT. A comparison of the baseline characteristics of the participants is presented in . Distinct differences were observed in sex, AST, ALT, AKP, sALB, iPTH and HDL between the two γ-GGT groups. Specifically, patients with lower γ-GGT levels had a higher proportion of female patients, and lower level of ALT, AST and AKP. They also exhibited higher level of sALB, iPTH and HDL. Additionally, there is a tendency for differences in the number of WBC and neutrophils between the two γ-GGT groups. No significant differences were found in age, BMI, diabetes and hypertension status, CVD history, hemoglobin, platelet count, blood urea nitrogen (BUN), uric acid (UA), eGFR, FBG, CHOL,TG, LDL-C, calcium, daily dialysis dose, residual urine volume, and daily ultrafiltration volume between the two groups.
Table 1. Characteristics of subjects stratified by γ-GGT medium.
In order to reduce the influence of sex disparities, we categorized the γ-GGT levels by dividing them into groups based on the trimean of γ-GGT among male and female patients individually. A comparison of the baseline characteristics of these participants was showed in . Significant differences were observed in AST, ALT, AKP, and sALB among the three γ-GGT groups.
Table 2. Characteristics of subjects stratified by trimean of γ-GGT among male and female patients individually.
Association between the γ-GGT and one-year, three-year, and five-year all-cause and cardiovascular mortality
As showed in , in this study, the all-cause mortality rates at one, three and five years after starting peritoneal dialysis were 11.72%, 16.09%, and 23.44%, respectively, while the corresponding cardiovascular mortality rates were 2.97%, 7.34%, and 11.09%, respectively. Compared to the higher γ-GGT group, all-cause and cardiovascular mortality rates at one and three years were significantly lower in the higher γ-GGT group (p = .002 for one-year and three-year all-cause mortality, p = .010 for one-year cardiovascular mortality and p = .022 for three-year cardiovascular mortality, respectively). All-cause mortality (p = .022), but not cardiovascular mortality(p = .158), was significantly higher in the higher γ-GGT group at five years.
Table 3. All-cause and cardiovascular mortality rate stratified by γ-GGT medium.
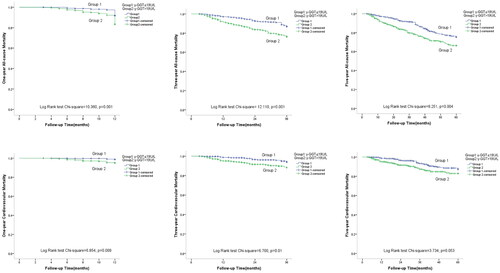
Kaplan–Meier estimates of one-year, three-year, and five-year all-cause and cardiovascular mortality for participants from both γ-GGT level groups are shown in . Patients in the higher γ-GGT group had significantly higher all-cause and cardiovascular mortality at one year (log-rank 10.360, p = .001 for all-cause mortality; log-rank 6.854, p = .009 for cardiovascular mortality, respectively) and three years (log-rank 12.110, p = .001 for all-cause mortality; log-rank 6.854, p = .009 cardiovascular mortality, respectively) compared with those in the lower γ-GGT group. The patients with higher γ-GGT had a significantly higher five-year all-cause mortality (log-rank 8.251, p = .004), while the five-year cardiovascular mortality showed a higher trend (log-rank 3.734, p = .053).
Figure 2. Crude analyses of cardiovascular and all-cause mortality by γ-GGT with Kaplan–Meier estimates. Abbreviation: γ-GGT: γ-gamma-glutamyl transferase.
Cox proportional hazards models were used to evaluate the prognostic value of the γ-GGT for one-year, three-year, and five-year all-cause and cardiovascular mortality, respectively (). Model 1 was adjusted for age, sex, diabetic status, hypertensive status, cardiovascular history, body mass index(BMI), systolic and diastolic blood pressure. Model 2 was further adjusted for eGFR, sALB, ALT, AST, AKP, FBG, HDL, CHOL, TG, LDL-C, WBC, and NEU based on model 1. Model 3 surpassed model 2 by incorporating adjustments for daily dialysis dose/100, residual urine volume/100, daily ultrafiltration volume/100, Calcium, and iPTH. When compared to individuals in the higher γ-GGT group, those in the lower γ-GGT group demonstrated a reduced hazard ratio (HR) for one-year all-cause mortality across multiple models: model 1 (HR = 0.454, 95% CI 0.277–0.741, p = .002), model 2 (HR = 0.501, 95% CI 0.303–0.828, p = .007), and model 3 (HR = 0.491, 95% CI 0.297–0.809, p = .005). Similarly, the HR for three-year all-cause mortality was reduced in the lower γ-GGT group: model 1 (HR = 0.494, 95% CI 0.327–0.745, p = .001), model 2 (HR = 0.551, 95% CI 0.363–0.836, p = .005), and model 3 (HR = 0.516, 95% CI 0.340–0.783, p = .002). Furthermore, the HR for five-year all-cause mortality in the lower γ-GGT group was statistically significant in model 1 (HR = 0.636, 95% CI 0.458–0.883, p = .007), model 2 (HR = 0.667, 95% CI 0.476–0.934, p = .018), and model 3 (HR = 0.607, 95% CI 0.431–0.854, p = .004) (showed in ).
Table 4. The associations of γ-GGT with all-cause and cardiovascular mortality.
Additionally, upon examining cardiovascular mortality, the hazard ratio (HR) with a 95% confidence interval (CI) for one-year mortality in the lower γ-GGT group was significant in all three models. Specifically, it was 0.258 (0.083–0.799) in model 1 (p = .019), 0.266 (0.085–0.838) in model 2 (p = .024), and 0.266 (0.085–0.838) in model 3 (p = .004). For three-year cardiovascular mortality, the HR (95% CI) remained significant across the models: 0.445 (0.240–0.827) in model 1 (p = .010), 0.478 (0.256–0.893) in model 2 (p = .021), and 0.478 (0.256–0.893) in model 3 (p = .021). However, when considering five-year cardiovascular mortality, the HR (95% CI) was only significant in model 3, with an HR of 0.558 (95% CI0.343–0.907, p = .019) (showed in ).
Subgroup analyses of confounding factors on the predictive role of γ-GGT on mortality
A subgroup analysis was performed to assess the impact of various confounding factors, including sex, age categorization based on 60 years old, hypoalbuminemia status, hypertension, diabetes mellitus, and history of cardiovascular disease, on the association between γ-GGT and both cardiovascular and all-cause mortality. The aim was to gain a deeper understanding of how these variables influence the relationship between γ-GGT levels and mortality outcomes.
Our findings demonstrated a statistically significant association between γ-GGT levels and all-cause mortality at one-year and three-year follow-ups for both males and females. This association was particularly pronounced in female subjects in terms of five-year all-cause mortality. However, there was no significant association between γ-GGT and cardiovascular mortality in either sex.
Patients over 60 years old, as well as those with hypertension, exhibited a strong association between γ-GGT and all-cause mortality at one-year, three-year, and five-year follow-ups. Conversely, in patients without hypertension, only a strong association with three-year all-cause mortality was observed. Regarding cardiovascular mortality, a strong association with γ-GGT was seen at one-year and five-year follow-ups among hypertensive patients, while in non-hypertensive patients, the association was significant at three-year follow-up.
Furthermore, patients without diabetes and without hypoalbuminemia displayed a strong association between γ-GGT and three-year all-cause mortality. Patients with a history of cardiovascular disease demonstrated a strong association between γ-GGT and five-year cardiovascular mortality.
Moreover, age significantly influenced the association between γ-GGT levels and all-cause and cardiovascular mortality within five years. Hypertension had a significant impact on the association between γ-GGT levels and all-cause mortality during five-year follow-ups, as well as three-year cardiovascular mortality. A history of cardiovascular disease altered the association between γ-GGT and one-year all-cause mortality, as well as one-year and five-year cardiovascular mortality. The presence of hypoalbuminemia significantly affected the relationship between γ-GGT and all-cause mortality within five years of follow-up, as well as one-year cardiovascular mortality. It is noteworthy that sex did not significantly affect the association between γ-GGT levels and both all-cause mortality and cardiovascular mortality (showed in and ).
Table 5. Subgroup analyses of the association between γ-GGT binary classification and all-cause mortality among patients on peritoneal dialysis.
Table 6. Subgroup analyses of the association between γ-GGT binary classification and cardiovascular mortality among patients on peritoneal dialysis.
Table 7. Subgroup analyses of the association between γ-GGT tertiles derived separately for both sexes and all-cause mortality among patients on peritoneal dialysis.
The confounding effect of sex on the predictive role of γ-GGT on mortality
In addition, to further minimize the impact of sex differences, we replaced the median γ-GGT groupings of all enrolled patients with tertile groupings derived separately for both sexes mentioned above in model 3 to form Cox proportional hazard model 4. Compared to the third tertile (T3) of γ-GGT, the HR (95% CI) for one-year all-cause mortality was 0.522 (0.294–0.928)(p = .027) in the first tertile (T1) group of γ-GGT, and 0.494 (0.276–0.884)(p = .018) in the second tertile (T2) group. For three-year all-cause mortality, the HR (95% CI) was 0.585 (0.359–0.953) in T1 group with a p value of .031 and .585 (0.362–0.947) in T2 group with a p value of .029. Finally, for five-year all-cause mortality, the HR (95% CI) was 0.737 (0.498–1.090) in T1 group with a p value of .127 and .565 (0.375–0.862) in T2 group with a p value of .006, underscore the significant impact of the third γ-GGT tertiles on all-cause mortality over time. However, there was no significant difference in the HR (95% CI) for one-year, three-year, and five-year cardiovascular mortality among the three tertiles (showed in ). Similarly, when γ-GGT levels were grouped into tertiles separately for each sex, the association between these levels and both all-cause mortality and cardiovascular mortality remained unaffected by sex (showed in ).
Discussion
The retrospective cohort study revealed two noteworthy findings, including: (1) Even after adjusting for sex confounding and interaction, γ-GGT remains a powerful predictor of all-cause mortality during the first and third years following the initiation of peritoneal dialysis; (2) However, after adjusting for sex confounding and interaction, γ-GGT fails to serve as a predictor of cardiovascular mortality within five years of peritoneal dialysis initiation. To our understanding, this study represents one of the initial attempts to examine the interaction between sex and γ-GGT in order to determine the prognostic impact of γ-GGT on mortality among incident PD patients.
γ-GGT, a crucial enzyme residing on the cell membrane, plays a fundamental role in glutathione metabolism, a vital antioxidant pathway that shields cells from oxidative stress triggered by its interaction with iron [Citation12,Citation13]. Increased γ-GGT activity indicates an insufficient antioxidant capacity and induces oxidative stress. Given its association with oxidative stress, γ-GGT has been recognized as a sensitive and reliable marker of cellular damage and stress response [Citation14,Citation15].Beyond its role in oxidative stress, γ-GGT has also been implicated in inflammatory processes, endothelial dysfunction, and atherosclerosis [Citation6,Citation16,Citation17]. This association is particularly relevant in the context of PD, where chronic inflammation and oxidative stress are key factors contributing to the pathogenesis of associated comorbidities [Citation2]. Therefore, we hypothesize that γ-GGT levels may serve as a bridge between these pathophysiological processes and the increased mortality risk associated with PD.
Patients undergoing PD experience high mortality, predominantly attributed to CVD [Citation18,Citation19]. Consistent with several previous studies, 23.44% of the 640 PD patients included in this study suffered all-cause mortality within five years follow-up, with nearly half attributed to cardiovascular death [Citation2,Citation18,Citation19].
Cumulative evidence has revealed a positive relationship between γ-GGT level and CVD or all-cause mortality [Citation13,Citation20,Citation21]. However, there are conflicting evidences on the predictive significance of γ-GGT in patients with CKD, with some prospective studies finding a high association [Citation22–24] and others finding none [Citation25,Citation26]. Nonetheless, it is yet unclear how γ-GGT affects prognosis in the dialysis population. Increased γ-GGT levels have been linked to CV risk, infection-related and all-cause mortality, as well as CV hospitalization in hemodialysis patients, according to a prior study [Citation17]. In this retrospective study, we analyzed 640 PD patients from a single center and observed that in the two categories of γ-GGT median values across both sexes, those with lower levels of γ-GGT exhibited significantly lower all-cause and cardiovascular mortality rates at one, three, and five years.
The level of γ-GGT exhibits a significant sex difference, with female levels generally being lower than those of males [Citation27,Citation28]. To further minimize sex differences, we replaced the median γ-GGT groupings of all enrolled patients with tertile groupings derived separately for both sexes in the Cox proportional hazards model adjusting several confounding factors. Our findings suggest that γ-GGT remains a significant predictor of all-cause mortality during the initial and subsequent three years following the initiation of peritoneal dialysis, despite accounting for conventional CVD risks such as CVD history, diabetes and hypertension status, markers of malnutrition/inflammation including sALB, WBC, and neutrophils, renal failure-specific factors such as calcium and iPTH, PD-specific risk factors such as daily dialysis dose, residual urine volume, and daily ultrafiltration volume, as well as sex-related interaction effects. While the ultimate conclusion of our study aligns with previous research conducted in South Korea [Citation11], our approach stands apart in its rigorous consideration of sex-specific factors, ultimately resulting in a more dependable conclusion.
Nevertheless, our comprehensive analysis revealed no significant difference in the risk of cardiovascular mortality across tertile groupings for both sexes or in the subgroup analysis adjusting for sex-specific associations between γ-GGT and all-cause mortality. The findings hint that non-CVD factors may be the primary contributors to all-cause mortality; however, further in-depth research is warranted to identify the specific influencing factors.
Additionally, our subgroup analysis uncovered a significant association between γ-GGT and all-cause mortality among older patients (≥60 years) and those with hypertension during a five-year follow-up period. Age and hypertension emerged as key modifiers of this relationship, consistent with previous studies [Citation29,Citation30].
To mitigate the potential interference of medication use, we conducted a thorough analysis examining the impact of various medications, including ACEIs, ARBs, beta-blockers, statins, antiplatelet agents, and iron agents, as confounding factors on the relationship between γ-GGT and mortality. In our study, medication usage data was available for 446 patients. For these patients, we conducted a Cox regression analysis using an enhanced Model 2 that incorporated ACEIs, ARBs, beta-blockers, statins, antiplatelet agents, and iron agents, building upon Model 3.Our results indicate that γ-GGT remains a predictor of all-cause mortality at one, three, and five years, while antiplatelet agents, ACEIs, ARBs, beta-blockers, and statins were not associated with mortality (presented in Supplementary Tables 13–18).
Interestingly, despite the theoretical dependence of γ-GGT on iron to alleviate oxidative stress and studies linking iron overload to mortality in patients with ESKD, our analysis failed to reveal a statistically significant correlation between iron therapy and death rates (presented in Supplementary Tables 13–18) [Citation11]. This finding is consistent with clinical studies involving iron therapy, which have also failed to unequivocally demonstrate a direct and significant relationship between iron therapy and mortality among patients undergoing dialysis [Citation31–33].
The current investigation was subject to specific constraints. The present study, being a retrospective observational study conducted at a single center, may potentially exhibit some bias. Furthermore, a considerable number of patients were initially diagnosed with ESKD without a clear identification of the underlying primary kidney disease. One limitation of the study is that the collection of γ-GGT data was exclusive to the baseline and not continuous, potentially resulting in an oversight of the impact of its changing trend on mortality over the follow-up period. Ultimately, while the majority of pertinent confounding variables were incorporated into the Cox regression analysis, certain confounding factors previously identified, namely ferritin, peritonitis episodes, smoking and vitamin D, were not obtained or evaluated within the scope of this investigation. Thus, it is imperative to conduct a more extensive multicenter and prospective investigation to assess the precision of these findings.
However, the current research possesses various advantages. This study represents one of the first survey to utilize the γ-GGT as a prognosticator of all-cause and cardiovascular mortality hazard among patients with PD. This study constitutes an early effort to investigate the interaction between sex and γ-GGT, with the aim of ascertaining the prognostic implications of γ-GGT on mortality in PD patients. The study’s sample size was considerable for a single PD center, and the Cox regression analysis included a relatively comprehensive set of confounding factors.
Conclusion
In summary, the present study demonstrated strong and independent associations between γ-GGT and all-cause mortality rates at one-year and three-year intervals in patients who underwent peritoneal dialysis, with no significant influence of sex on the prognostic value of γ-GGT for mortality at these time points.
Author contributions
Yan-Bing Chen, Xiao-Jiang Zhan, Jun-Xiao, and Heng-Mei Zhu contributed to Conceptualization, Methodology, Formal analysis, Data Curation, Writing-original draft and Writing- review & editing; and Heng-Mei Zhu contributed to Project Administration and Funding acquisition.
Supplemental Material
Download MS Word (53.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data sharing statement
The datasets used in this work may be requested from the corresponding author upon reasonable request.
Additional information
Funding
References
- Project Group of “White Paper on the Status of Peritoneal Dialysis Management in China.” White paper on the current status of peritoneal dialysis management in China. Chin J Nephrol. 2022;38(12):1–13. doi: 10.3760/cma.j.cn441217-20220418-00158.
- Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18(12):779–793. doi: 10.1038/s41581-022-00623-7.
- Jegatheesan D, Cho Y, Johnson DW. Clinical studies of interventions to mitigate cardiovascular risk in peritoneal dialysis patients. Semin Nephrol. 2018;38(3):277–290. doi: 10.1016/j.semnephrol.2018.02.007.
- Zhang J, Lu X, Li H, et al. Risk factors for mortality in patients undergoing peritoneal dialysis: a systematic review and meta-analysis. Ren Fail. 2021;43(1):743–753. doi: 10.1080/0886022x.2021.1918558.
- Piko N, Bevc S, Hojs R, et al. The role of oxidative stress in kidney injury. Antioxidants. 2023;12(9):1772. doi: 10.3390/antiox12091772.
- Roumeliotis S, Eleftheriadis T, Liakopoulos V. Is oxidative stress an issue in peritoneal dialysis? Semin Dial. 2019;32(5):463–466. doi: 10.1111/sdi.12818.
- Hernandez-Tejero M, Clemente-Sanchez A, Bataller R. Spectrum, screening, and diagnosis of alcohol-related liver disease. J Clin Exp Hepatol. 2023;13(1):75–87. doi: 10.1016/j.jceh.2022.10.002.
- Corti A, Belcastro E, Dominici S, et al. The dark side of gamma-glutamyltransferase (GGT): pathogenic effects of an ‘antioxidant’ enzyme. Free Radic Biol Med. 2020;160:807–819. doi: 10.1016/j.freeradbiomed.2020.09.005.
- Yi SW, Lee SH, Hwang HJ, et al. Gamma-glutamyltransferase and cardiovascular mortality in Korean adults: a cohort study. Atherosclerosis. 2017;265:102–109. doi: 10.1016/j.atherosclerosis.2017.08.028.
- Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026.
- Park W-Y, Kim S-H, Kim YO, et al. Serum gamma-glutamyltransferase levels predict mortality in patients with peritoneal dialysis. Medicine. 2015;94(31):e1249. doi: 10.1097/md.0000000000001249.
- Torino C, Mattace-Raso F, van Saase JLCM, et al. Oxidative stress as estimated by gamma-glutamyl transferase levels amplifies the alkaline phosphatase-dependent risk for mortality in ESKD patients on dialysis. Oxid Med Cell Longev. 2016;2016:8490643. doi: 10.1155/2016/8490643.
- Ndrepepa G, Holdenrieder S, Cassese S, et al. A comparison of gamma-glutamyl transferase and alkaline phosphatase as prognostic markers in patients with coronary heart disease. Nutr Metab Cardiovasc Dis. 2018;28(1):64–70. doi: 10.1016/j.numecd.2017.09.005.
- Mitrić A, Castellano I. Targeting gamma-glutamyl transpeptidase: a pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic Biol Med. 2023;208:672–683. doi: 10.1016/j.freeradbiomed.2023.09.020.
- Liu D, Zhou L, Yang M, et al. Oxidative stress mediates the association between dietary fat intake and cognition in US older adults. Am J Geriatr Psychiatry. 2022;30(7):761–773. doi: 10.1016/j.jagp.2022.01.001.
- Alkaabi J, Sharma C, Yasin J, et al. Relationship between lipid profile, inflammatory and endothelial dysfunction biomarkers, and type 1 diabetes mellitus: a case-control study. Am J Transl Res. 2022;14(7):4838–4847.
- Wang H, Zhang X, Li P, et al. Prediction of early atherosclerotic plaques using a sequence-activated fluorescence probe for the simultaneous detection of γ-glutamyl transpeptidase and hypobromous acid. Angew Chem Int Ed Engl. 2024;63(1):e202315861. doi: 10.1002/anie.202315861.
- Zhan X, Pan D, Wei X, et al. Monocyte to high-density lipoprotein ratio and cardiovascular events in patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2020;30(7):1130–1136. doi: 10.1016/j.numecd.2020.03.011.
- Bartosova M, Schaefer B, Bermejo JL, et al. Complement activation in peritoneal dialysis-Induced arteriolopathy. J Am Soc Nephrol. 2018;29(1):268–282. doi: 10.1681/asn.2017040436.
- Cho EJ, Jeong S-M, Chung GE, et al. Gamma-glutamyl transferase and risk of all-cause and disease-specific mortality: a nationwide cohort study. Sci Rep. 2023;13(1):1751. doi: 10.1038/s41598-022-25970-0.
- Ke P, Zhong L, Peng W, et al. Association of the serum transaminase with mortality among the US elderly population. J Gastroenterol Hepatol. 2022;37(5):946–953. doi: 10.1111/jgh.15815.
- Caravaca-Fontán F, Azevedo L, Bayo M, et al. High levels of both serum gamma-glutamyl transferase and alkaline phosphatase are independent predictors of mortality in patients with stage 4-5 chronic kidney disease. Nefrologia. 2017;37(3):267–275. doi: 10.1016/j.nefro.2016.11.010.
- Chen T, Ren Y, Gao Y, et al. Serum gamma-glutamyl transferase and ferritin synergistically associated with the rate of chronic kidney disease. Dis Markers. 2017;2017:9765259–9765257. doi: 10.1155/2017/9765259.
- Lee DY, Han K, Yu JH, et al. Gamma-glutamyl transferase variability can predict the development of end-stage of renal disease: a nationwide population-based study. Sci Rep. 2020;10(1):11668. doi: 10.1038/s41598-020-68603-0.
- Navise NH, Mokwatsi GG, Gafane-Matemane LF, et al. Kidney dysfunction: prevalence and associated risk factors in a community-based study from the North West province of South Africa. BMC Nephrol. 2023;24(1):23. doi: 10.1186/s12882-023-03068-7.
- Fan Y, Jin X, Man C, et al. Association of serum gamma-glutamyltransferase with chronic kidney disease risk: a meta-analysis. Free Radic Res. 2018;52(8):819–825. doi: 10.1080/10715762.2018.1492120.
- Dai H, Zhu L, Pan B, et al. The relationship between serum γ-glutamyltransferase (GGT) and diabetic nephropathy in patients with type 2 diabetes mellitus: a cross-sectional study. Clin Exp Med. 2023;23(7):3619–3630. doi: 10.1007/s10238-023-00991-9.
- Jung DH, Park B, Ryu HE, et al. Sex-specific associations of γ-glutamyltransferase to HDL-cholesterol ratio and the incident risk of cardiovascular disease: three Korean longitudinal cohorts from different regions. Front Endocrinol. 2023;14:1231502. doi: 10.3389/fendo.2023.1231502.
- Liu Y-H, Chen S-C, Lee W-H, et al. Liver-function parameters are associated with incident hypertension in a large Taiwanese population follow-up study. J Hum Hypertens. 2023;37(6):496–501. doi: 10.1038/s41371-022-00694-w.
- Fard MT, Najafi F, Rezaeian S, et al. Association between serum liver enzymes and hypertension using propensity score matching analysis: evidence from a large Kurdish prospective cohort study. BMC Cardiovasc Disord. 2022;22(1):476. doi: 10.1186/s12872-022-02884-3.
- Macdougall IC, White C, Anker SD, et al; PIVOTAL Investigators and Committees. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):380–502. doi: 10.1056/NEJMx180044.
- Macdougall IC, Bhandari S, White C, et al. Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL trial. J Am Soc Nephrol. 2020;31(5):1118–1127. doi: 10.1681/asn.2019090972.
- Hougen I, Collister D, Bourrier M, et al. Safety of intravenous iron in dialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2018;13(3):457–467. doi: 10.2215/cjn.05390517.