Abstract
Background: Recent studies have shown that the baseline values of absolute aortic root diameter (ARD) and indexed diameter are associated with all-cause mortality and cardiovascular events in the general population, even in the absence of aneurysmal aortic disease. However, there is limited available data on the association between ARD and prognosis in end-stage renal disease (ESRD) patients receiving maintenance hemodialysis (MHD). Accordingly, the purpose of this study is to investigate the predictive value of ARD for all-cause mortality and cardiovascular events in this specific population.Methods: ARD was measured by echocardiography at the level of the sinuses of Valsalva at end diastole and indexed to body surface area (BSA). The primary endpoint was all-cause mortality. The secondary endpoint was major adverse cardiovascular events (MACE), including cardiovascular mortality, myocardial infarction and stroke. Cox proportional hazards models were conducted to evaluate the association between baseline ARD/BSA and clinical outcomes.Results: A total of 391 patients were included in this study. The primary endpoint occurred in 95 (24.3%) patients while the secondary endpoint occurred in 71 (18.2%) patients. Multivariate Cox regression analysis showed that ARD/BSA was an independent prognostic factor for all-cause mortality (HR, per 1-SD increase, 1.403; 95% CI, 1.118–1.761; p = 0.003) as well as MACE (HR, per 1-SD increase, 1.356; 95% CI, 1.037–1.772; p = 0.026).Conclusions: Our results show that ARD/BSA is predictive of all-cause mortality and MACE in MHD patients with ESRD and support the view that assessment of ARD/BSA may refine risk stratification and preventive strategies in this population.
Introduction
The global prevalence of patients suffering from end-stage renal disease (ESRD) is steadily increasing [Citation1]. Hemodialysis, the most common form of renal replacement therapy, accounts for approximately 69% of all renal replacement therapies and 89% of dialysis cases [Citation2]. It has been shown to effectively prolong survival times and reduce the risk of mortality [Citation3]. However, despite these benefits, ESRD patients undergoing maintenance hemodialysis (MHD) remain to have a mortality rate 6.5–7.9 times higher than the general population [Citation1]. And cardiovascular disease (CVD) is the leading cause of death in these patients. Cardiovascular mortality risk in ESRD patients undergoing MHD is observed to be 20 times that of the general population [Citation4]. In addition, CVD is also the most common complication in ESRD patients. The prevalence of CVD among ESRD patients aged 22 to 44 years is approximately 50% and increases substantially to over 75% among ESRD patients over age 65 years [Citation5]. However, Traditional risk factors such as age, hypertension, diabetes mellitus and anemia fail to fully explain the disproportionately elevated mortality and incidence of CVD in this population [Citation5,Citation6]. Therefore, there is a growing need for emerging prognostic factors to improve the risk stratification and therapy for ESRD patients.
Recent studies have shown that the baseline values of absolute aortic root diameter (ARD) and indexed diameter served as independent predictors of cardiovascular events and all-cause mortality in the general population, even in the absence of aneurysmal aortic disease [Citation7–9]. ESRD patients on MHD have increased arterial stiffness caused by calcification of elastic lamellae, elastinolysis, accumulated collagen content, collagen cross-linking, apoptosis of vascular smooth muscle cells, and chronic inflammation, resulting in premature vascular aging and aortic dilatation [Citation10]. In addition, Mulé et al. demonstrated an inverse correlation between glomerular filtration rate (GFR) and ARD in hypertensive patients with a high prevalence of chronic kidney disease (CKD) [Citation11]. Consequently, it is conceivable that aortic root dilatation is common among patients with ESRD receiving MHD.
However, there is limited available data on the association between ARD and prognosis in ESRD patients receiving MHD. Accordingly, the purpose of this study is to investigate the predictive value of ARD for cardiovascular events and all-cause mortality events in this specific population.
Materials and methods
Study design and population
We conducted a retrospective single-center cohort study. The study population consisted of 391 patients aged > 18 years with ESRD receiving MHD for at least 3 months from the Fifth Affiliated Hospital of Sun Yat-sen University. Study subjects were recruited between 01 October 2017 and 31 December 2021. Exclusion criteria were as follows: (1) connective tissue diseases (i.e. Marfan’s syndrome and Ehlers-Danlos syndrome) and aortic aneurysm. (2) Aortic stenosis or moderate-severe aortic regurgitation. (3) Diagnosis with malignancies or liver failure. (4) Unsatisfactory echocardiographic image. (5) Incomplete clinical data.
This study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (IRB approval number K185-1). The requirement for patient informed consent was waived due to the retrospective design of the study.
Echocardiography
The baseline time was defined as the time at which the patients underwent their first echocardiography at our institution after enrollment in the study. All echocardiographic measurements were obtained using a commercially ultrasound imaging system (Vivid 7; GE Health Medical, Milwaukee, WI, USA) with a multifrequency transducer (M3S 1.7/3.4 MHz). Echocardiographic parameters, including aortic root diameter (ARD), left atrial diameter (LAD), left ventricular end-diastolic diameter (LVDd), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT) and left ventricular ejection fraction (LVEF), were measured by 4 sonographers who had specialized in cardiac ultrasound for more than 5 years, strictly following the protocol based on the American Society of Echocardiography (ASE) guidelines [Citation12]. LVDdi was calculated by dividing LVDd by the body surface area (BSA). ARD was measured in the parasternal long-axis view at sinuses of Valsalva in end-diastole, using the leading-edge to leading-edge method and then normalized to BSA (ARD/BSA). Left ventricular mass was calculated using the Cube formula (0.8 × 1.04 × [(IVST + LVDd + LVPWT)3 – LVDd3] + 0.6) (g) and normalized to BSA (left ventricular mass index, LVMi). Left ventricular hypertrophy was defined as LVMi >115 g/m2 in men and >95 g/m2 in women.
Clinical covariates
Data for other covariates were collected within the week before and after baseline echocardiography from electronic medical records. Demographic characteristics including age, gender, height and weight were collected. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Body surface area (BSA) was calculated using the Dubois formula, which was 0.20247 × height (m)0.725×weight (kg)0.425. Of note, both BMI and BSA were calculated on the basis of dry weight at the time of enrollment. Comorbidities were assessed using the Charlson Comorbidity Index (CCI), which has previously been validated for mortality prediction in hemodialysis patients [Citation13]. Dialysis-related variables included dialysis duration and Kt/V. Routine laboratory parameters included serum creatinine, estimated glomerular filtration rate, hemoglobin, serum albumin, lipid (triglyceride, total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol), uric acid, calcium, phosphorus, etc. All laboratory values were measured using automated and standardized methods at a centralized laboratory.
In addition, we used weekly averaged blood pressure (WAB) and pulse WAB (pWAB) to represent blood pressure (BP) levels of MHD patients in this study. WAB and pWAB were calculated referring to the method reported by Io et al. before [Citation14]. Briefly, we recorded 9 BP measurements over a one-week period for each patient at baseline. BP was measured just before HD on each dialysis day (day 1, 3, and 5) (pre-HDBP) and again after each dialysis (post-HDBP). Home BP (HBP) was measured in the morning on dialysis days 1 and 5 of each week. Finally, BP was measured in the morning on the non-dialysis day (day 4 of each week). If the patient’s baseline echocardiography was performed during hospitalization, then the 3 home BPs would be replaced by the morning BPs on the corresponding days during that hospitalization. The WAB was defined as the average of these 9 BP measurements. The mean pulse pressure in one week, named pulse weekly averaged blood pressure (pWAB), was equal to systolic WAB minus diastolic WAB.
Follow-up and outcome
The primary endpoint of this study was defined as all-cause mortality. For those who died in hospital, the date of death and attributed cause of death were obtained from the clinical records. For patients who died outside the hospital, family members were interviewed by telephone to determine a detailed cause of death. The secondary endpoint was major adverse cardiovascular events (MACE), including cardiovascular mortality, acute myocardial infarction and stroke. And the follow-up time for MACE was censored at the date of first MACE. All patients were followed-up until the occurrence of endpoint or September 18, 2022. Follow-up was censored for patients who received a kidney transplant, switched to peritoneal dialysis, or transferred to another renal unit.
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD) or median (interquartile range [IQR]), whereas categorical variables were expressed as the frequency and percentage. Conformity to normal distribution was evaluated for continuous variables using both the Kolmogorov–Smirnov and Shapiro–Wilk tests. When comparisons were made in terms of clinical features and echocardiographic parameters among groups, one-way ANOVA test or non–parametric Kruskal-Wallis test was used to make comparisons for continuous variables, whereas the χ2-test or Fisher’s exact test were applied for categorical variables. A multiple linear regression model with stepwise selection was used to identify variables independently correlated with the ARD/BSA. Variables were selected by stepwise selection (α = 0.05); Kaplan-Meier analysis combined with log rank test was used to compare the differences between ARD/BSA tertile groups. Cox proportional hazard models were used to evaluate the association between ARD/BSA and clinical outcomes. The proportional hazards assumptions were tested through Schoenfeld residuals. Variables with P-values < 0.1 in the univariate Cox regression analysis and known prognostic factors were included in the multivariate Cox regression analysis. We reported HRs with 95% CIs per 1-unit greater SD of ARD/BSA. Statistical analyses were performed in SPSS software (version 25.0, SPSS, Chicago, IL) and R language (R version 4.1.0). A two-tailed p-value < 0.05 was considered as statistically significant for all analysis executed.
Results
Clinical features of the enrolled population
In this study, a total of 391 patients were enrolled based on the inclusion and exclusion criteria (), of whom 246 (62.9%) were male. The mean age of the study population was 55.9 ± 14.6 years. The mean ARD/BSA of the enrolled population was 2.05 ± 0.25 cm/m2. 6 (1.5%) patients received kidney transplant during follow-up. Patients were categorized into three categories based on the tertiles of ARD/BSA. The baseline characteristics among the groups are shown in . The lower and upper tertiles for ARD/BSA were determined as 1.94 cm/m2 and 2.15 cm/m2, respectively. No significant differences were observed in terms of systolic WAB, diastolic WAB and dialysis duration. However, an increase in ARD/BSA was associated with a linear trend toward lower pWAB and hemoglobin level. Age, HDL-C levels, and the proportion of female increase progressively with an increase in ARD/BSA. Furthermore, patients with greater ARD/BSA displayed a lower prevalence of diabetes mellitus. In terms of echocardiographic parameters, there was a positive correlation between ARD/BSA and LVMi as well as LVDdi.
Table 1. Clinical features in end-stage renal disease patients grouped by ARD/BSA tertiles.
Correlation analyses
To investigate potential variables correlated to ARD/BSA, multiple linear regression analysis was carried out. The results revealed positive correlations between ARD/BSA and age, HDL-C and LVDdi, whereas an inverse correlation was observed between ARD/BSA and diabetes, hemoglobin, pWAB ().
Table 2. Multiple linear regression analysis for variables correlated to aortic root diameter (ARD)/body surface area (BSA).
Association of ARD/BSA with all-cause mortality
During a median follow-up of 32.7 months, all-cause death occurred in 95 (24.3%) patients, of which 41 (43.2%) were due to cardiovascular events. Patients who experienced an all-cause mortality event during follow-up had higher ARD/BSA (ARD/BSA = 2.14 ± 0.28 cm/m2) compared to their counterparts without events (ARD/BSA = 2.02 ± 0.23 cm/m2, p < 0.001).
The Kaplan–Meier analysis revealed a significantly higher all-cause mortality in the third tertile group (log-rank test, p = 0.041; ). shows relative HRs for the risk of all-cause mortality associated with 1-SD increase in ARD/BSA. After adjustment for age, sex, dialysis duration, Kt/V, CCI, serum albumin, hemoglobin, LAD and LVEF < 50%, each 0.25 cm/m2 increase in ARD/BSA was associated with a 40.3% higher risk of all-cause mortality (HR, per 1-SD increase, 1.403; 95% CI, 1.118–1.761; p = 0.003). In addition, in the multivariable Cox proportional hazards model, we also found that older age (HR, 1.050; 95% CI, 1.031–1.070; p < 0.001), lower hemoglobin (HR, 0.985; 95% CI, 0.97–50.996; p = 0.005) and higher CCI (HR, 1.155; 95% CI, 1.022–1.306; p = 0.021) were associated with a higher risk of all-cause mortality in ESRD patients on MHD.
Figure 2. Kaplan-Meier analyses of clinical outcomes categorized by ARD/BSA. (A), All-cause mortality. (B), Major adverse cardiovascular events (MACE).
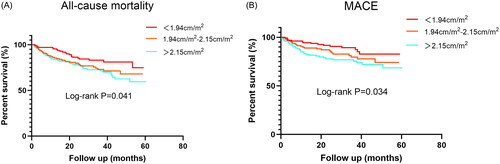
Table 3. Univariate and multivariate cox regression analysis for all-cause mortality.
Association of ARD/BSA with MACE
The association between ARD/BSA and MACE was also explored in our study. During a median follow-up of 31.6 months, MACE occurred in 71 (18.2%) patients, which included 38 cardiovascular deaths, 22 strokes and 11 acute myocardial infarctions. Patients who experienced MACE during follow-up had higher ARD/BSA (ARD/BSA = 2.15 ± 0.29 cm/m2) compared to their counterparts without MACE (ARD/BSA = 2.03 ± 0.23 cm/m2, p < 0.001).
The Kaplan–Meier analysis revealed a significantly higher rate of MACE in the third tertile group (log-rank test, p = 0.034; ). shows relative HRs for the risk of MACE associated with 1-SD increase in ARD/BSA. After adjustment for age, sex, dialysis duration, CCI, low-density lipoprotein cholesterol, serum albumin, ferritin, left ventricular hypertrophy and LVEF < 50%, each 0.25 cm/m2 increase in ARD/BSA was associated with a 35.6% higher risk of MACE (HR, per 1-SD increase, 1.356; 95% CI, 1.037–1.772; p = 0.026).
Table 4. Univariate and multivariate cox regression analysis for MACE.
Discussion
The main finding of this study was that baseline ARD/BSA has an important prognostic value for all-cause mortality and cardiovascular events in ESRD patients on MHD. After adjusting for relevant risk factors, ARD/BSA emerged as a significant predictor of all-cause mortality and MACE in this population. Several previous studies had described the relationship between ARD/BSA and poor prognosis in the general population. In the Jackson Heart Study, Kamimura et al. found that larger ARD/BSA was associated with increased risk of cardiovascular events and all-cause mortality [Citation7]. Similarly, in the Chin-Shan Community Cardiovascular Cohort, Lai et al. demonstrated a significant association between ARD/BSA and all-cause death in adults aged less than 65 years in an ethnic Chinese population [Citation8]. Our study results produce comparable results, extending the finding to a population with ESRD undergoing MHD. In addition, other studies had confirmed that absolute ARD and ARD/height are also associated with all-cause mortality and CVD [Citation7,Citation9]. It is worth noting that the association of absolute ARD and ARD/height with all-cause mortality was also examined in our patients during the study, but no significant association was found (Table S1–2). In addition, we also explored the association of absolute ARD and ARD/height with MACE in our study. And we found that only ARD/height was associated with MACE (Table S3). However, ARD indexed to BSA had been recommended in the current guidelines [Citation12]. And compared with ARD/height, ARD/BSA was more associated with poor prognosis in our patients. Therefore, BSA was regarded as more appropriate to normalize ARD in ESRD patients receiving MHD.
Several pathophysiological mechanisms may be involved in the relationship between aortic dilation and poor prognosis in this population. Our study revealed a positive correlation between ARD/BSA and age. Age-associated enlargement of ARD is thought to be associated with tissue remodeling of the aortic wall that includes reduced elastin fiber content, increased collagen deposition, and enhanced calcification [Citation15]. These structural alterations are associated with increased aortic stiffness, leading to elevated pulse wave velocity (PWV). Previous study has proven that PWV is a strong independent predictor of all-cause mortality and cardiovascular events in ESRD patients on MHD [Citation16,Citation17]. Thus, we speculate that the functional changes of the aorta might contribute to the relationship between ARD/BSA and poor prognosis observed in this study.
However, our cross-sectional analysis revealed a negative association between ARD/BSA and pWAB. This finding suggests that although the increase in ARD/BSA may reflect aortic wall remodeling, the expanded diameter itself does not directly contribute to increased aortic stiffness. Previous studies have already reported the inverse association between pulse pressure and proximal aortic diameter [Citation18,Citation19]. Several reports have suggested that the enlargement of the arterial lumen diameter might compensate for age-related increases in arterial stiffness and help preserve the buffering capacity of the central artery. However, this compensatory mechanism could be maladaptive, ultimately leading to a poorer prognosis [Citation7,Citation20,Citation21]. This concept could potentially explain the discrepancy between the negative correlation of ARD/BSA with pWAB and the positive association of ARD/BSA with all-cause mortality and MACE observed in our study.
Several other aspects of our research require attention. We found that ARD/BSA was independently associated with age, diabetes, HDL-C and LVDdi, thus confirming and extending previous studies [Citation22,Citation23]. The positive correlation between ARD/BSA and HDL-C in our study seemed to go against the conventional wisdom. However, it was in line with the result previously reported in the peritoneal dialysis population [Citation22]. In addition, Chen et al. also found that HDL-C had a significantly positive effect on the diameter of ascending and abdominal aorta [Citation24]. The underlying mechanism is currently unclear and need further investigation. Most previous investigations have primarily focused on the relationship between diabetes and abdominal aortic aneurysm (AAA) or thoracic aortic aneurysm (TAA) [Citation25,Citation26]. However, only a limited number of studies have investigated the correlation between ARD and diabetes, and similar studies involving ESRD patients receiving MHD are lacking [Citation22,Citation23].
In our study, the diabetic patients were older, which could have theoretically result in larger ARD/BSA values. However, multivariate analyses revealed an inverse correlation between diabetes and ARD/BSA in this population, even after adjusting for age, sex, and other potential confounders. The reason behind the smaller ARD in diabetic patients remains unclear. Several mechanisms influencing the relationship between diabetes and aortic aneurysm may explain the lower ARD in diabetic patients. Aneurysm formation is believed to result from altered TGF-β signaling, enhanced inflammation and activation of matrix metalloproteinases (MMPs), which increases the degradation of extracellular matrix (ECM) and weakens the vessel wall [Citation27]. Previous studies have shown that stimulation of the TGF-β signaling can prevent AAA, whereas its blockade can accelerate AAA formation [Citation28,Citation29]. Li et al. demonstrated that Cell Division Autoantigen 1 (CDA1) is upregulated in diabetic patients, which plays a role in reducing susceptibility to aneurysm formation by enhancing TGF-β signaling [Citation30]. Furthermore, hyperglycemia has been shown to increase the expression of plasminogen activator inhibitor-1 and reduce plasma levels of MMP-9 in mice, which may attenuate aortic aneurysm diameter [Citation31]. In addition, we observed that LVDdi was positively correlated with ARD/BSA in this population, which suggested that systemic and cardiac growth factors involved in cardiac remodeling may be also involved in aortic root dilatation, thereby contributing to corresponding increases in cardiac and aortic dimensions [Citation32].
Even though arterial hypertension is commonly recognized as a predisposing condition for the development of thoracic aortic aneurysms, the role of BP as a determinant of aortic root enlargement is disputed. Additionally, in ESRD patients receiving MHD, the relationship between ARD/BSA and BP remains unclear.
Blood pressure fluctuations due to fluid removal and renin-angiotensin-aldosterone system (RAAS) activation during each hemodialysis session are very common [Citation33]. It is therefore important not to limit BP assessment to a single measurement, such as before or after dialysis, but to take multiple BP measurements and then average them. Io et al. have previously reported that the weekly averaged BP (WABP) is a useful method for estimating the BP of MHD patients [Citation14]. In this study, we measured the WABP to estimate the BP of MHD patients and examined its relationship with ARD/BSA. In the cross-sectional analysis, we found that ARD/BSA was negatively associated with pWAB, which is consistent with the results of previous studies in non-hemodialysis population. Aortic remodeling, as previously indicated, could act as a compensatory mechanism to counteract the rise in stress and impedance resulting from aortic wall stiffening. This compensatory vessel enlargement may prevent excessive impedance during ventricular ejection, and thereby mitigate the increase in pulse pressure [Citation34,Citation35].
Moreover, we found an inverse correlation between ARD/BSA and hemoglobin in this population, which has not been reported in previous studies. Although the underlying mechanism is currently unclear, it suggests that we need to be proactive in improving anemia in ESRD patients undergoing MHD to improve their prognosis.
Limitations
Some limitations of our study need to be acknowledged. First, it is a retrospective single-center cohort study with a small sample size. Further multicenter studies are necessary to validate these findings in larger populations. Second, our study may exist selection bias because we excluded some patients with unsatisfactory echocardiographic images or incomplete clinical data. Third, the observational design of the present study precludes investigation into the longitudinal changes of ARD/BSA. Consequently, it remains unclear whether and how changes in ARD/BSA over time may have affected outcomes in the study cohort. Last, since ARD measurement were conducted at a single level, we cannot exclude the possibility that measurements at multiple levels (i.e. annulus, supra-aortic ridge, and ascending aorta) may have yielded different findings in this population. Besides, ARD was measured by 4 sonographers in this study and inter-observer variability might have an impact on our results. However, all these 4 sonographers had specialized in cardiac ultrasound for more than 5 years and ARD measurements were performed strictly according to the protocol based on the ASE guidelines.
Conclusions
In summary, our study provides the initial evidence that ARD indexed to BSA can be used as a predictor of all-cause mortality and MACE in ESRD patients undergoing MHD.
Supplemental Material
Download MS Word (55.1 KB)Acknowledgments
We sincerely thank Professor Man Li for her generous help in statistical analysis of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request due to privacy/ethical restrictions.
Additional information
Funding
References
- Johansen KL, Chertow GM, Gilbertson DT, et al. US renal data system 2021 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2022;79(4 Suppl 1):1–9. doi: 10.1053/j.ajkd.2022.02.001.
- Thurlow JS, Joshi M, Yan G, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52(2):98–107. doi: 10.1159/000514550.
- Wang F, Yang C, Long J, et al. Executive summary for the 2015 annual data report of the China kidney disease network (CK-NET). Kidney Int. 2019;95(3):501–505. doi: 10.1016/j.kint.2018.11.011.
- Cozzolino M, Mangano M, Stucchi A, et al. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl_3):iii28–iii34. doi: 10.1093/ndt/gfy174.
- Lai AC, Bienstock SW, Sharma R, et al. A personalized approach to chronic kidney disease and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2021;77(11):1470–1479. doi: 10.1016/j.jacc.2021.01.028.
- Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388(10041):276–284. doi: 10.1016/S0140-6736(16)30508-6.
- Kamimura D, Suzuki T, Musani SK, et al. Increased proximal aortic diameter is associated with risk of cardiovascular events and all-cause mortality in blacks the Jackson heart study. J Am Heart Assoc. 2017;6(6):e005005. Published 2017 Jun 21. doi: 10.1161/JAHA.116.005005.
- Lai CL, Chien KL, Hsu HC, et al. Aortic root dimension as an independent predictor for all-cause death in adults <65 years of age (from the Chin-Shan community cardiovascular cohort study). Echocardiography. 2010;27(5):487–495. doi: 10.1111/j.1540-8175.2009.01072.x.
- Gardin JM, Arnold AM, Polak J, et al. Usefulness of aortic root dimension in persons > or = 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the cardiovascular health study). Am J Cardiol. 2006;97(2):270–275. doi: 10.1016/j.amjcard.2005.08.039.
- London GM. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018;45(1-3):154–158. doi: 10.1159/000485146.
- Mulé G, Nardi E, Morreale M, et al. Relationship between aortic root size and glomerular filtration rate in hypertensive patients. J Hypertens. 2016;34(3):495–504; discussion 505. doi: 10.1097/HJH.0000000000000819.
- Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. doi: 10.1016/j.echo.2018.06.004.
- Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77(2):141–151. doi: 10.1038/ki.2009.413.
- Io H, Nakata J, Inoshita H, et al. Relationship among left ventricular hypertrophy, cardiovascular events, and preferred blood pressure measurement timing in hemodialysis patients. J Clin Med. 2020;9(11):3512. Published 2020 Oct 30. doi: 10.3390/jcm9113512.
- Kim SH, Monticone RE, McGraw KR, et al. Age-associated proinflammatory elastic fiber remodeling in large arteries. Mech Ageing Dev. 2021;196:111490. doi: 10.1016/j.mad.2021.111490.
- Matschkal J, Mayer CC, Sarafidis PA, et al. Comparison of 24-hour and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am J Nephrol. 2019;49(4):317–327. doi: 10.1159/000499532.
- Korjian S, Daaboul Y, El-Ghoul B, et al. Change in pulse wave velocity and short-term development of cardiovascular events in the hemodialysis population. J Clin Hypertens . 2016;18(9):857–863. doi: 10.1111/jch.12843.
- Tosello F, Guala A, D’ascenzo F, et al. Central pulse pressure is inversely associated with proximal aortic remodelling. J Hypertens. 2021;39(5):919–925. doi: 10.1097/HJH.0000000000002730.
- Kamimura D, Uchino K, Ogawa H, et al. Small proximal aortic diameter is associated with higher Central pulse pressure and poor outcome in patients with congestive heart failure. Hypertens Res. 2014;37(1):57–63. doi: 10.1038/hr.2013.111.
- Vasan RS, Song RJ, Xanthakis V, et al. Aortic root diameter and arterial stiffness: conjoint relations to the incidence of cardiovascular disease in the Framingham heart study. Hypertension. 2021;78(5):1278–1286. doi: 10.1161/HYPERTENSIONAHA.121.17702.
- Sugawara J, Otsuki T, Maeda S, et al. Effect of arterial lumen enlargement on carotid arterial compliance in normotensive postmenopausal women. Hypertens Res. 2005;28(4):323–329. doi: 10.1291/hypres.28.323.
- Ye M, Zhang J, Li J, et al. Diabetes attenuated age-related aortic root dilatation in end-stage renal disease patients receiving peritoneal dialysis. J Diabetes Investig. 2019;10(6):1550–1557. doi: 10.1111/jdi.13055.
- Nardi E, Mulè G, Nardi C, et al. Inverse association between type 2 diabetes and aortic root dimension in hypertensive patients. Int J Cardiol. 2017;228:233–237. doi: 10.1016/j.ijcard.2016.11.163.
- Chen T, Yang X, Fang X, et al. Potential influencing factors of aortic diameter at specific segments in population with cardiovascular risk. BMC Cardiovasc Disord. 2022;22(1):32. Published 2022 Feb 5. doi: 10.1186/s12872-022-02479-y.
- Nordness MJ, Baxter BT, Matsumura J, et al. The effect of diabetes on abdominal aortic aneurysm growth over 2 years. J Vasc Surg. 2022;75(4):1211–1222.e1. doi: 10.1016/j.jvs.2021.10.019.
- D’cruz RT, Wee IJY, Syn NL, et al. The association between diabetes and thoracic aortic aneurysms. J Vasc Surg. 2019;69(1):263–268.e1. doi: 10.1016/j.jvs.2018.07.031.
- Gao J, Cao H, Hu G, et al. The mechanism and therapy of aortic aneurysms. Signal Transduct Target Ther. 2023;8(1):55. Published 2023 Feb 3. doi: 10.1038/s41392-023-01325-7.
- Wang Y, Ait-Oufella H, Herbin O, et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120(2):422–432. doi: 10.1172/JCI38136.
- Chen X, Rateri DL, Howatt DA, et al. TGF-β neutralization enhances AngII-Induced aortic rupture and aneurysm in both thoracic and abdominal regions. PLOS One. 2016;11(4):e0153811. Published 2016 Apr 22. doi: 10.1371/journal.pone.0153811.
- Li J, Huynh P, Dai A, et al. Diabetes reduces severity of aortic aneurysms depending on the presence of cell division autoantigen 1 (CDA1). Diabetes. 2018;67(4):755–768. doi: 10.2337/db17-0134.
- Dua MM, Miyama N, Azuma J, et al. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery. 2010;148(2):429–435. doi: 10.1016/j.surg.2010.05.014.
- Cipolli JA, Souza FA, Ferreira-Sae MC, et al. Sex-specific hemodynamic and non-hemodynamic determinants of aortic root size in hypertensive subjects with left ventricular hypertrophy. Hypertens Res. 2009;32(11):956–961. doi: 10.1038/hr.2009.134.
- Bansal N, Artinian NT, Bakris G, et al. Hypertension in patients treated with in-center maintenance hemodialysis: current evidence and future opportunities: a scientific statement from the American Heart association. Hypertension. 2023;80(6):e112–e122. doi: 10.1161/HYP.0000000000000230.
- Guala A, Rodriguez-Palomares J, Dux-Santoy L, et al. Influence of aortic dilation on the regional aortic stiffness of bicuspid aortic valve assessed by 4-Dimensional flow cardiac magnetic resonance: comparison with Marfan syndrome and degenerative aortic aneurysm. JACC Cardiovasc Imaging. 2019;12(6):1020–1029. doi: 10.1016/j.jcmg.2018.03.017.
- Guala A, Camporeale C, Ridolfi L. Compensatory effect between aortic stiffening and remodelling during ageing. PLOS One. 2015;10(10):e0139211. Published 2015 Oct1. doi: 10.1371/journal.pone.0139211.