Abstract
Background
This study aims to investigate the incidence and prognosis of malignancy in individuals with thrombospondin type-1 domain-containing 7A (THSD7A)-associated membranous nephropathy (MN).
Methods
First, we performed a systematic literature review of prevalence of malignancy in THSD7A-associated MN. Then, we conducted a retrospective analysis of 454 patients diagnosed with MN through renal biopsy at our hospital between January 2016 and December 2020. We assessed the presence of serum anti-THSD7A antibodies and performed immunohistochemical staining of renal tissue for THSD7A. Subsequently, we followed patients with THSD7A-associated MN for a minimum of 3–5 years, collecting their clinical, pathological characteristics, and prognosis. Additionally, we conducted a literature review on patients with THSD7A-associated MN in conjunction with malignancy.
Results
We identified a total of nine articles containing comprehensive data on THSD7A-associated MN and malignancy. Among 235 patients with THSD7A-positive MN, 36 individuals had concurrent malignancies, resulting in a malignancy prevalence of 13.3% (95% CI: 8.9–17.7%). In our center, we followed up with 15 patients diagnosed with THSD7A-associated MN and observed three cases of concomitant tumors: two cases of lung adenocarcinoma and one case of small cell lung cancer with multiple metastases. The prevalence of malignancy in our cohort was 20%. Notably, we detected positive THSD7A staining in both renal and lung cancer tissues in one patient with small cell lung cancer.
Conclusions
Patients with THSD7A-associated MN should undergo vigilant follow-up assessments, with a particular focus on actively seeking potential tumorigenic lesions to prevent misdiagnosis or oversight.
Introduction
Membranous nephropathy (MN) is a prevalent cause of nephrotic syndrome (NS) among adults, accounting for 9.83–30% of primary glomerulonephritis [Citation1,Citation2]. It is characterized by the formation of immune complexes beneath epithelial cells and diffuse thickening of the glomerular basement membrane, as observed through light microscopy [Citation3]. While approximately 75% of MN cases are idiopathic, the remaining cases are associated with various factors such as infections, malignancies, autoimmune diseases, and drug toxicity. In 2009, Beck et al. [Citation4] initially identified the presence of M-type PLA2R antibodies in the blood circulation of patients with idiopathic membranous nephropathy (IMN), which were detected in around 70–80% of IMN patients and exhibited a strong association with disease activity and prognosis. Subsequently, thrombospondin type-1 domain-containing 7A (THSD7A) was identified as a second autoantigen in adult IMN [Citation5].
The prevalence of THSD7A-associated MN is relatively low, approximately 3–5% in patients with IMN, primarily manifesting in PLA2R-negative cases [Citation6–10]. A systematic review encompassing 4121 MN patients from 10 studies revealed a THSD7A positivity rate of 3% among all patients and 10% among PLA2R-negative patients [Citation8]. The prevalence of malignancy in THSD7A-positive MN patients ranged from 6% to 25% [Citation8]. Hoxha et al. [Citation9] assessed serum samples from 1276 MN patients across three cohorts and identified 40 patients (3.1%) with THSD7A-associated MN, eight of whom developed malignancies within a median follow-up period of 3 months from MN diagnosis. These findings suggest a potential association between THSD7A-associated MN and malignancy.
To gain a comprehensive understanding of THSD7A’s role in MN patients with malignancies, we conducted a systematic review to investigate the prevalence of malignancy in THSD7A-positive patients. Additionally, we present a summary of 15 patients with THSD7A-associated MN from our center, aiming to elucidate the characteristics of THSD7A-associated MN and the prevalence of malignancy in THSD7A-positive individuals.
Materials and methods
Systematic literature review
Data sources and searches
We conducted literature searches from Chinese and English databases, English literature databases including PubMed, Embase, Web of Science, Cochrane Library, and Chinese literature databases including CKNI, VIP, and WANGFANG from January 2014 to December 2023. The English search term was ‘Glomerulonephritis, Membranous [MeSH]’, ‘thrombospondin type-1 domain-containing 7A (THSD7A)’, and ‘Anti-THSD7A antibody’. PubMed search term was ((thrombospondin type-1 domain-containing 7A) OR (Anti-THSD7A antibody)) AND (MN or PMN or Glomerulonephritis, Membranous OR Glomerulonephritides, Membranous OR Membranous Glomerulonephritides OR Membranous Glomerulonephritis OR Nephropathy, Membranous OR Membranous Glomerulopathy OR Glomerulopathy, Membranous OR Membranous Nephropathy OR Extramembranous Glomerulopathy OR Glomerulopathy, Extramembranous OR Membranous Glomerulonephropathy OR Glomerulonephropathy, Membranous OR Heymann Nephritis OR Nephritis, Heymann OR Idiopathic Membranous Glomerulonephritis OR Glomerulonephritides, Idiopathic Membranous OR Glomerulonephritis, Idiopathic Membranous OR Idiopathic Membranous Glomerulonephritides OR Membranous Glomerulonephritides, Idiopathic OR Membranous Glomerulonephritis, Idiopathic OR Idiopathic Membranous Nephropathy OR Membranous Nephropathy, Idiopathic OR Nephropathy, Idiopathic Membranous).
Study selection
Two reviewers assessed eligible cross-sectional, prospective, and retrospective studies using a standardized approach. Any disagreement was adjudicated by a third reviewer. The inclusion criteria: (1) the types of studies included cross-sectional, prospective, and retrospective studies; (2) the purpose of the study was to explore the prevalence of malignancy in THSD7A-positive patients. Therefore, patients with THSD7A-associated MN in the articles included in the systematic review had to be described with concomitant tumors. (3) The study subjects were MN patients and the gold standard for diagnosing MN was pathological diagnosis; (4) the original data are complete. The exclusion criteria: (1) repeated reports, case reports, reviews, and abstracts; (2) animal and cellular experiments and studies.
Data extraction and quality assessment
Information on the author, publication year, type of study, race of subjects, sample size, the number of THSD7A positive cases, and the number of malignancies in THSD7A positive cases, patients’ age, and gender were extracted. Two investigators independently evaluated the quality of each study using the Newcastle-Ottawa Quality Assessment Scale. Any discrepancies in data extraction and quality assessment were resolved by the third reviewer.
Outcomes
Clinical outcomes included prevalence of malignancy in THSD7A-positive MN patients.
Clinical case study
Case selection
We conducted a comprehensive follow-up and analysis of 454 patients diagnosed with MN through renal biopsy at China-Japan Friendship Hospital, spanning from January 2016 to December 2020. Immunohistochemical staining for THSD7A was consistently performed on all kidney biopsy specimens. Additionally, we systematically assessed sera from all study participants for PLA2R1-Ab and THSD7A-Ab. We diligently tracked patient progress over time, recording data related to proteinuria, serum creatinine levels, and treatment regimens. Subsequently, we compiled and analyzed clinical and pathological data as part of our investigation.
This study was approved by the Ethics Committee of China-Japan Friendship Hospital (approval number: 2019-17-K12).
Diagnostic criteria
Inclusion criteria for patients with THSD7A-related MN were defined as follows: (1) confirmation of MN through renal biopsy [Citation5]; (2) presence of positive THSD7A staining in renal tissues or serum anti-THSD7A antibodies; (3) exclusion of secondary causes such as systemic lupus erythematosus, hepatitis B, hepatitis C infection, non-steroidal anti-inflammatory drugs, gold agents, penicillamine, and other drugs usage, as well as no history of exposure to organic solvents and mercury.
Clinical and biological data
We collected comprehensive clinical data for each patient, encompassing age, gender, urine red blood cell count, 24-h urine protein quantification, serum albumin levels, blood creatinine levels, estimated glomerular filtration rate (eGFR) calculated using the EPI formula [Citation11], and anti-PLA2R antibody status. Additionally, we recorded the primary clinical manifestations, etiology, and prognosis of THSD7A-associated MN. Information regarding the treatment of THSD7A-associated MN, remission following treatment, and other pertinent details were also documented.
Renal biopsy processing techniques
Percutaneous renal puncture was performed in all cases to obtain kidney tissue specimens, which were divided into three parts for examination using light microscopy, immunofluorescence, and electron microscopy. (1) Light microscopy: Specimens for light microscopy contained a minimum of 10 glomeruli, were paraffin-embedded, sectioned to a thickness of 2 μm, and subsequently stained with HE, PAS, PASM, and MASSON. (2) Immunofluorescence: Frozen sections were utilized to detect IgG, IgA, IgM, C3, C1q, FRA, and IgG subclasses (IgG1, IgG2, IgG3, and IgG4) through direct immunofluorescence. Immunofluorescence results were semiquantitatively scored from 0 to 3 (0, negative; 1, weak; 2, moderate; 3, strong). (3) Electron microscopy: All kidney biopsy specimens were sent to the electron microscopy laboratory at China-Japan Friendship Hospital for examination.
Immunohistochemistry staining for PLA2R, THSD7A, and other MN antigens
In the realm of immunohistochemistry, patients afflicted with THSD7A-associated MN underwent immunohistochemical staining of renal tissues for PLA2R, THSD7A, NELL1, SEMA3B, PCDH7, NCAM1, and EXT1. Kidney tissue was meticulously processed, fixed, paraffin-embedded, and sliced to a thickness of 3 μm. Subsequently, after dewaxing through a hydration procedure, renal tissue underwent antigen retrieval. For PLA2R, THSD7A, NELL1, EXT1, and SEMA3B, high-pressure thermal retrieval was employed for antigen retrieval. Afterward, endogenous peroxidase activity was quenched using hydrogen peroxide following the retrieval process. The sections were then washed with PBS and immersed in a goat serum working solution for 30 min. Subsequently, the sections were incubated overnight at 4 °C with the following antibodies (all from Abcam, Cambridge, UK): (1) PLA2R antibody (ab211573), (2) THSD7A antibody (ab242371) diluted to 1:400, (3) NELL1 antibody (ab23457) diluted to 1:400, (4) EXT1 antibody (ab126305) diluted to 1:100, (5) SEMA3B antibody (ab48197) diluted to 1:200, and (6) NCAM1 antibody (ab75813) diluted to 1:400. Following incubation, the sections were washed with PBS, and the reaction was visualized through horseradish peroxidase staining using diaminobenzidine. The sections were examined under a microscope (Nikon, Tokyo, Japan) at ×400 magnification, utilizing a Moticam 2506 instrument (Motic, Xiamen, China), and images were captured using a digital camera system (Nikon, Minato City, Japan) for blinded assessment. The presence of granular staining in glomerular capillary loops, equal to or exceeding trace levels, was considered positive. In cases with concurrent malignancy, paraffin sections of tumor tissues were also subjected to THSD7A immunostaining.
Serum testing for PLA2R and THSD7A antibodies
Patients’ serum samples, collected 1–3 days before renal biopsy, underwent immediate testing using a human anti-phospholipase A2 receptor antibody enzyme immunoassay kit (Shanghai Lianshuo Biological, Shanghai, China). Testing procedures strictly adhered to kit instructions, with a serum anti-PLA2R antibody level ≥20 IU/mL deemed positive.
Subsequently, serum samples were subjected to THSD7A-antibody analysis via an indirect immunofluorescence test as per the manufacturer’s protocol (EUROIMMUN, Lübeck, Germany). Patients’ sera were diluted in PBST at a 1:10 ratio and applied to a reagent tray’s reaction field. After covering with a BIOCHIP, incubation occurred at room temperature for 30 min, followed by slide removal and washing. For the detection of bound IgG antibodies, a FITC-conjugated anti-human IgG antibody was employed, and the fluorescence intensity of the encapsulated cells was assessed via fluorescence microscopy (Nikon, Minato City, Japan).
Statistical analysis
Continuous data are given as median and 1st–3rd quartile (Q1–Q3), and categorical data as counts and percentages (%). In the systematic literature review, statistical analysis was conducted using STATA 15.0 (StataCorp, College Station, TX). The prevalence of tumors in THSD7A-associated MN was calculated with a random-effects model using the metaprop method.
Results
Description of the included studies
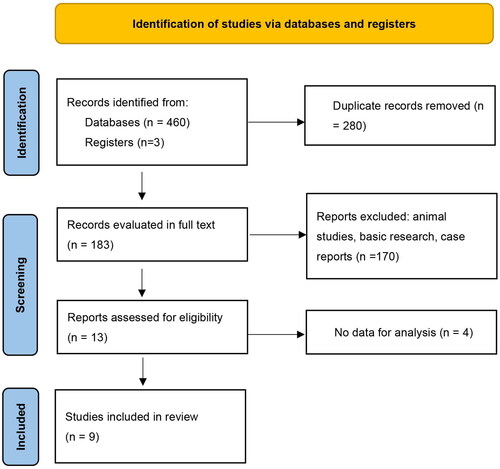
The search retrieved 463 citations for screening, from which nine articles with comprehensive data on THSD7A-associated MN complicating malignancy were finally included [Citation9,Citation10,Citation12–18] ().
Malignancy prevalence
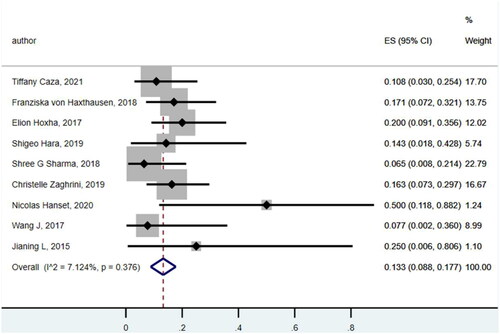
A total of 231 patients with THSD7A-associated MN, with a median mean age of 60–65 years, including 196 patients with primary MN and 35 patients with THSD7A-associated MN with malignancy were found.
The prevalence of malignancy was 0.133 (95% CI: 0.088, 0.177) () with a variety of tumor types observed, including nine cases of prostate cancer, three cases of breast cancer, four cases of colon cancer, four cases of gastric cancer, two cases of lung cancer, two cases of head and neck squamous cell carcinoma, one case of kidney cancer, and one case of the other types ().
Reporting bias
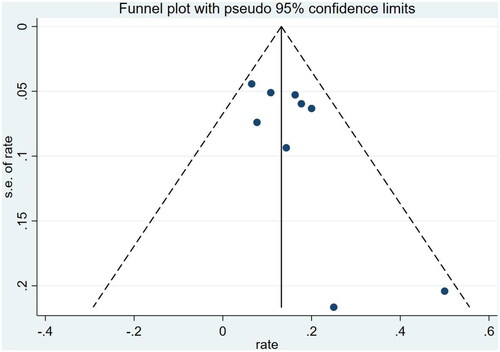
To describe the report bias of the studies included, a funnel plot was constructed. The funnel plot is symmetrical and the bias of the study is not significant ().
Clinical case reports of malignancy in THSD7A-associated membranous nephropathy in our center
In our center, a cohort of 454 patients, initially diagnosed with MN via renal biopsy, underwent follow-up and analysis. Among them, 204 exhibited positive serum anti-PLA2R antibodies, while 250 were negative. Fifteen patients demonstrated positive THSD7A staining in their renal tissue, with three of them also having positive serum anti-THSD7A antibodies. The prevalence of THSD7A-associated MN was 3.3% among all MN patients and 6.0% among those with negative serum anti-PLA2R antibodies.
As depicted in , out of the 15 patients with THSD7A-associated MN, three individuals were subsequently diagnosed with tumors during their follow-up. Specifically, one patient was diagnosed with small cell lung cancer featuring multiple metastases, while the other two were diagnosed with adenocarcinoma of the lung. All three patients achieved remission of MN following surgical or chemotherapy interventions. Among the remaining 12 patients, no secondary causes were identified. Three patients were in partial remission, while nine patients attained complete remission.
Patient no. 13, experiencing increased foamy urine, was found to have a right lower lung nodule via chest CT nine months after the MN diagnosis. Surgical treatment confirmed adenocarcinoma as the diagnosis. Patient no. 14 initially admitted for bilateral lower limb edema and MN diagnosis, presented with a cough and hemoptysis after 13 months. Chest CT revealed central lung cancer in the upper lobe of the left lung with pulmonary atelectasis. Puncture pathology and PET-CT results indicated small cell lung cancer with multiple metastases, rendering it inoperable, leading to chemotherapy treatment. Patient no. 15, admitted with bilateral lower extremity edema, also exhibited a ground glass nodule in the upper lobe of the right lung on chest CT. Surgical intervention five months later revealed adenocarcinoma as the diagnosis.
Patient no. 14, diagnosed with small cell lung cancer, showed positive THSD7A staining in both kidney and tumor tissues ().
Renal tissues of patients with malignancy-associated MN exhibited positive IgG1 and IgG2 staining. Among the remaining THSD7A-associated MN cases, IgG1 and IgG4 were the predominant subtypes, while IgG3 staining was largely negative ().
Among the 15 patients with THSD7A-associated MN, one case exhibited positive serum anti-PLA2R antibodies, three cases had positive serum anti-THSD7A antibodies, and four patients demonstrated positive staining for both PLA2R and THSD7A in renal tissue. Conversely, other antigens associated with MN, including Nell-1, SEMA3B, PCDH7, NCAM1, and EXT1/EXT2, were all negative ().
Discussion
In recent years, THSD7A has emerged as a novel autoantigen in IMN, representing 2–3% of adult MN cases, with a role analogous to PLA2R in this context [Citation5,Citation19]. THSD7A is recognized as a membrane-associated N-linked glycoprotein, and its soluble form is produced by endothelial and neurogenic cells, promoting angiogenesis by enhancing endothelial cell migration, tube formation, and sprouting [Citation20]. Approximately, 10% of PLA2R-negative patients exhibit detectable THSD7A, and THSD7A-associated MN is linked to malignancy development. The prevalence of malignancy in THSD7A-associated MN ranges from 15% to 20%, signifying a heightened malignancy risk in these patients [Citation8]. Messenger RNA of THSD7A has been identified in certain neoplastic tissues, such as gallbladder cancer, and THSD7A protein has been detected in dendritic cells within tumor-infiltrated lymph node growth centers [Citation21,Citation22]. Anti-THSD7A antibodies have the potential to directly disrupt podocyte integrity, resulting in podocyte damage and proteinuria [Citation8]. THSD7A exhibits differential expression across various solid tumors, with frequent and/or intense expression observed in breast cancer, kidney cancer, and colorectal cancer [Citation23]. Therefore, THSD7A detection may offer valuable insights into the differentiation of malignancy-associated nephropathy.
We identified eight articles containing comprehensive data on THSD7A-associated MN and malignancy, revealing an overall malignancy prevalence of 13.3%. Our center’s statistical analysis demonstrated a higher malignancy prevalence of 20% in THSD7A-associated MN. In our follow-up of 15 patients with THSD7A-associated MN, three were subsequently diagnosed with tumors, including one case of small cell lung cancer with multiple metastases and two cases of lung adenocarcinoma. All three patients achieved MN remission through surgical or chemotherapy interventions. Notably, one patient with small cell lung cancer exhibited positive THSD7A staining in both renal and lung cancer tissue.
THSD7A-associated MN exhibits distinct characteristics from PLA2R-associated MN, namely, a female predominance, unexplained, and a strong association with malignancy [Citation5,Citation9]. The link between MN and malignancy has long been debated. A study revealed that only 9% of MN patients with anti-PLA2R antibodies eventually developed malignancy, often occurring later, suggesting a coincidental process [Citation24]. PLA2R has been proposed to promote senescence and function as a tumor suppressor, with loss of PLA2R associated with breast cancer [Citation25,Citation26]. In contrast, patients with THSD7A-associated MN face a significantly elevated malignancy risk. Hoxha et al. [Citation9] and Hanset et al. [Citation17] reported that 20% and 50% of patients with THSD7A-associated MN were diagnosed with malignancy during follow-up, respectively. In two illustrative cases, one with endometrial carcinoma metastasis and another with gallbladder tumor, THSD7A was detected in follicular dendritic cells within lymph nodes featuring metastatic infiltration. Analysis of cancerous tissues revealed heightened THSD7A mRNA levels and increased THSD7A protein expression, suggesting active synthesis by cancer cells [Citation27,Citation28]. Notably, after tumor remission, kidney disease can ameliorate, even without immunosuppressive agents. Stahl et al. [Citation23] reported that THSD7A, as a novel tumor antigen, plays a potential role in human cancer. Differential THSD7A expression across various cancers, based on clinical stage and differentiation degree, indicates its involvement in vascular invasion, cancer progression, metastasis, and angiogenesis mechanisms supporting cancer growth [Citation29,Citation30]. Approximately, 20% of patients with THSD7A-associated MN develop malignancies within a median time of 3 months after MN diagnosis, compared to a much lower incidence of malignancy in the general MN population (10%) [Citation31]. Furthermore, THSD7A is detectable in primary tumor tissue and corresponding metastatic lymph nodes, reinforcing a causal link between THSD7A expression as a tumor antigen and MN pathogenesis in certain cases [Citation32]. Our cases illustrate that THSD7A-associated MN can be triggered by lung cancer, with one small cell lung cancer patient exhibiting THSD7A expression in both kidney and lung cancer tissues, underscoring THSD7A’s involvement in the pathogenesis of lung cancer-associated MN.
Our study is a single-center investigation, and the incidence of THSD7A-related MN is relatively low, resulting in a smaller sample size. At this stage, initiating large-scale multicenter collaborations across different regions is challenging to achieve. Should the conditions permit in the future, we will consider undertaking more extensive multicenter collaborations to include a larger patient cohort, thereby validating our research findings with greater rigor.
Conclusions
In summary, THSD7A-associated MN is relatively rare, accounting for 3–5% of IMN cases, and is linked to malignancies, with a 13.3% malignancy prevalence based on the literature review. Consequently, vigilant follow-up of MN patients with THSD7A positivity is essential for monitoring tumor development. Further research is required to elucidate the pathogenesis of malignancy-associated MN.
Author contributions
Qianqian Xu: conceptualization; formal analysis; methodology; writing – original draft. Jiayi Li: data curation; methodology; writing – original draft. Yue Yang: data curation; methodology. Li Zhuo: data curation; methodology. Hongmei Gao: methodology. Shimin Jiang: investigation; methodology, validation; Wenge Li: conceptualization; data curation; investigation; project administration; validation. Writing – review and editing: all authors. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Ethics statement
Approval of the research protocol by an Institutional Reviewer Board: The studies involving human participants were reviewed and approved by Ethics Committee of China-Japan Friendship Hospital (approval number: 2019-17-K12).
Acknowledgements
We would like to thank all study participants for their valuable contributions. We would also like to thank the Department of Nephrology, China-Japan Friendship Hospital for identifying potential participants for our study and providing the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Wu YQ, Wang Z, Xu HF, et al. Frequency of primary glomerular disease in northeastern China. Braz J Med Biol Res. 2011;44(8):1–11. doi: 10.1590/S0100-879X2011007500089.
- Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–923.
- Beck LH, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int. 2010;77(9):765–770. doi: 10.1038/ki.2010.34.
- Beck LHJr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457.
- Tomas NM, Beck LHJr., Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354.
- Iwakura T, Ohashi N, Kato A, et al. Prevalence of enhanced granular expression of thrombospondin type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLOS One. 2015;10(9):e0138841. doi: 10.1371/journal.pone.0138841.
- Larsen CP, Cossey LN, Beck LH. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol. 2016;29(4):421–426. doi: 10.1038/modpathol.2016.32.
- Ren S, Wu C, Zhang Y, et al. An update on clinical significance of use of THSD7A in diagnosing idiopathic membranous nephropathy: a systematic review and meta-analysis of THSD7A in IMN. Ren Fail. 2018;40(1):306–313.
- Hoxha E, Beck LHJr., Wiech T, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28(2):520–531. doi: 10.1681/ASN.2016010050.
- Wang J, Cui Z, Lu J, et al. Circulating antibodies against thrombospondin type-I domain-containing 7A in Chinese patients with idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(10):1642–1651.
- Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462.
- Caza TN, Hassen SI, Dvanajscak Z, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99(4):967–976. doi: 10.1016/j.kint.2020.07.039.
- von Haxthausen F, Reinhard L, Pinnschmidt HO, et al. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front Immunol. 2018;9:3035. doi: 10.3389/fimmu.2018.03035.
- Hara S, Tsuji T, Fukasawa Y, et al. Clinicopathological characteristics of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. Virchows Arch. 2019;474(6):735–743. doi: 10.1007/s00428-019-02558-0.
- Sharma SG, Larsen CP. Tissue staining for THSD7A in glomeruli correlates with serum antibodies in primary membranous nephropathy: a clinicopathological study. Mod Pathol. 2018;31(4):616–622. doi: 10.1038/modpathol.2017.163.
- Zaghrini C, Seitz-Polski B, Justino J, et al. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int. 2019;95(3):666–679. doi: 10.1016/j.kint.2018.10.024.
- Hanset N, Aydin S, Demoulin N, et al. Podocyte antigen staining to identify distinct phenotypes and outcomes in membranous nephropathy: a retrospective multicenter cohort study. Am J Kidney Dis. 2020;76(5):624–635. doi: 10.1053/j.ajkd.2020.04.013.
- Jianing L. Diagnostic study of serum anti-M type phospholipase A2 receptor (PLA2R) antibody negative idiopathic membranous nephropathy [dissertation]. Peking Union Medical College; 2015.
- De Vriese AS, Glassock RJ, Nath KA, et al. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28(2):421–430. doi: 10.1681/ASN.2016070776.
- Kuo MW, Wang CH, Wu HC, et al. Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLOS One. 2011;6(12):e29000. doi: 10.1371/journal.pone.0029000.
- Hoxha E, Wiech T, Stahl PR, et al. A mechanism for cancer associated membranous nephropathy. N Engl J Med. 2016;374(20):1995–1996. doi: 10.1056/NEJMc1511702.
- Zhang C, Zhang M, Chen D, et al. Features of phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in malignancy-associated membranous nephropathy. J Clin Pathol. 2019;72(10):705–711. doi: 10.1136/jclinpath-2019-205852.
- Stahl PR, Hoxha E, Wiech T, et al. THSD7A expression in human cancer. Genes Chromosomes Cancer. 2017;56(4):314–327. doi: 10.1002/gcc.22440.
- Timmermans SA, Ayalon R, van Paassen P, et al. Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am J Kidney Dis. 2013;62(6):1223–1225. doi: 10.1053/j.ajkd.2013.07.019.
- Augert A, Payré C, de Launoit Y, et al. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10(3):271–277. doi: 10.1038/embor.2008.255.
- Bernard D, Vindrieux D. PLA2R1: expression and function in cancer. Biochim Biophys Acta. 2014;1846(1):40–44. doi: 10.1016/j.bbcan.2014.03.003.
- Lin F, Zhang D, Chang J, et al. THSD7A-associated membranous nephropathy in a patient with neurofibromatosis type 1. Eur J Med Genet. 2018;61(2):84–88.
- Zhang Z, Gong T, Rennke HG, et al. Duodenal schwannoma as a rare association with membranous nephropathy: a case report. Am J Kidney Dis. 2019;73(2):278–280.
- Wang CH, Chen IH, Kuo MW, et al. Zebrafish THSD7A is a neural protein required for angiogenic patterning during development. Dev Dyn. 2011;240(6):1412–1421.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220.
- Leeaphorn N, Kue-A-Pai P, Thamcharoen N, et al. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol. 2014;40(1):29–35. doi: 10.1159/000364782.
- Xian L, Dong D, Luo J, et al. Expression of THSD7A in neoplasm tissues and its relationship with proteinuria. BMC Nephrol. 2019;20(1):332. doi: 10.1186/s12882-019-1489-5.
Appendix
Figure 4. Representative images of glomerular and tumor staining for THSD7A. (A) Patient no. 13 with positive glomerular THSD7A staining. (B) Patient no. 14 with positive glomerular THSD7A staining. (C) Patient no. 15 with positive glomerular THSD7A staining. (D) Patient no. 14 with positive THSD7A staining of small cell lung cancer tissue (immunohistochemistry staining, magnification ×400).
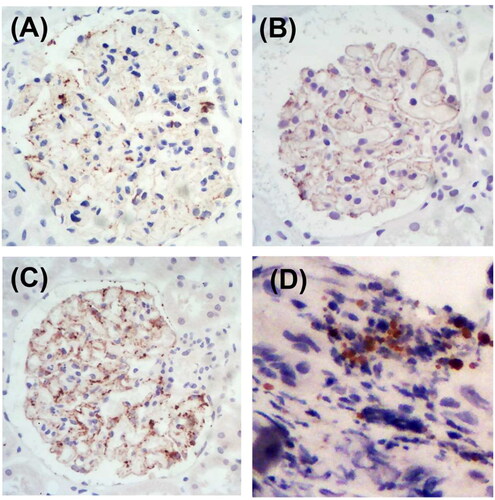
Table A1. Quality evaluation of each study using the Newcastle-Ottawa Quality Assessment Scale.
Table 1. Literature review of malignancy in THSD7A-associated MN.
Table 2. Clinical manifestations and prognosis of 15 patients with THSD7A-associated MN in our center.
Table 3. Immunofluorescence deposition of renal tissue in 15 patients with THSD7A-associated MN.
Table 4. Serum antibodies and antigen staining of renal tissues in 15 cases of THSD7A-associated MN.