Abstract
Objective
This study aims to assess the clinical efficacy and safety of CHF-II in combination with RG for treating AKI on CKD (A on C), and to explore potential therapeutic mechanisms through lipidomics analysis.
Methods
98 patients were enrolled and randomly assigned to the RG or RG + CHF groups. Both groups received RG therapy, with RG + CHF group additionally receiving CHF-II treatment over a duration of two weeks. Evaluation endpoints included changes in renal function, blood lipid profiles, urinary AKI biomarkers, and TCM symptoms before and after treatment. Serum samples were collected for lipid metabolite analysis.
Results
The total clinical effective rate in RG + CHF group was 73.5%, and that of RG group was 40.8%. TCM syndrome scores in RG + CHF group showed a more pronounced decrease (p < 0.05). Scr, BUN, and UA levels decreased while eGFR levels increased in both groups (p < 0.05), with a greater magnitude of change observed in the RG + CHF group. Urinary AKI biomarkers decreased more in RG + CHF group (p < 0.05). No serious adverse events occurred during the trial. 58 different lipid metabolites and 48 lipid biomarkers were identified. According to the KEGG database, the possible metabolic pathways involved triglyceride metabolic pathway and fat digestion and absorption metabolic pathways.
Conclusion
CHF-II effectively alleviated kidney injury and improved TCM syndrome scores in patients with A on C. Lipid differential metabolites could serve as diagnostic indicators for AKI in patients with CKD. The possible metabolic pathways might be implicated in therapeutic action of CHF-II in the prevention and treatment of patients with A on C.
1. Introduction
Acute kidney injury (AKI) is characterized by a sudden deterioration in kidney function that can lead to severe morbidity and mortality both in the short and long term [Citation1]. Chronic kidney disease (CKD) encompasses a range of chronic conditions caused by abnormalities in various indicators, particularly inflammation, oxidative stress, and metabolic abnormalities [Citation2]. Studies have shown a close relationship between AKI and CKD. Additionally, CKD is a non-negligible pathogenic factor in the progression of AKI, rendering the treatment of AKI on CKD (A on C) extremely challenging [Citation3].
Historically, in the absence of renal replacement therapy (RRT), traditional Chinese medicine (TCM) has been employed to treat kidney disorders in ancient times. Chuan Huang Fang (CHF) is an empirical prescription for the treatment of A on C formulated by Professor Gong Xuezhong at the Shanghai Municipal Hospital of Traditional Chinese Medicine [Citation4,Citation5]. Our previous single-center clinical studies [Citation6–8] have suggested that CHF may confer renal protective benefits in patients with A on C by reducing oxidative damage and inflammatory responses. To further improve clinical efficacy, CHF was optimized to form CHF-II, and the current small sample clinical efficacy observation was conducted to evaluate the actual effect of CHF-II on A on C patients. Reduced glutathione (RG) helps to control local inflammation, reduce the accumulation of reactive oxygen species and inflammatory markers, and mitigate oxidative stress in tissues and organs [Citation9–11]. Recent studies [Citation12,Citation13] have demonstrated RG’s potential to enhance the effectiveness of high-throughput hemodialysis in patients with severe AKI. Therefore, we have optimized the original protocol and adopted CHF-II combined with RG as a new drug therapy protocol for patients with A on C.
RRT is primarily used to treat severe AKI (grade 3), but there is currently no recognized drug treatment strategy for grade 1-2 AKI. In accordance with KDIGO recommendations [Citation14], the primary treatment method for AKI is to regulate the body’s internal environment, and most therapies focus on supportive rather than curative measures. During the early stages of the disease, it is critical to determine the underlying cause of AKI, maintain hemodynamic stability, and manage serious consequences. The maintenance of hemodynamic stability needs to be widely considered due to impaired auto-regulation mechanisms in patients with AKI. As mentioned before, RG has the potential to attenuate detrimental peroxide metabolites, reduce oxidative stress, and perform specific metabolic functions in the production of inflammatory components.
Dyslipidemia is a marker of CKD, while dyslipidemia metabolism is closely related to CKD stage and mortality rates [Citation15,Citation16]. However, due to the immense structural diversity of lipids, a comprehensive understanding of how lipids are dysregulated in patients with CKD is challenging. Modern lipidomics techniques aim to systematically identify and quantify lipid species from biological systems [Citation17]. The rapid advancement of mass spectrometry-based analysis platforms has enabled the precise and in-depth identification of complex lipids. Early identification of differential lipid metabolites and distinctive lipid signatures holds promise for timely diagnosis and treatment of A on C. Investigating the effect of CHF-II on the lipid metabolism pathways associated with A on C will further elucidate the precise mechanistic underpinnings of this treatment approach.
This study is a multicenter, randomized, controlled, post-hoc analysis of clinical data trial. Based on previous research results, it can be considered that CHF-II could effectively alleviate kidney injury and improve the clinical TCM symptoms in A on C patients. On this basis, we will further evaluate the efficacy and safety of CHF-II combined with RG in patients with A on C, and explore its mechanism of action from the perspective of lipidomics. The smooth development of this study will collect more relevant data and help to solve the clinical dilemma.
2. Materials and methods
2.1. Participants
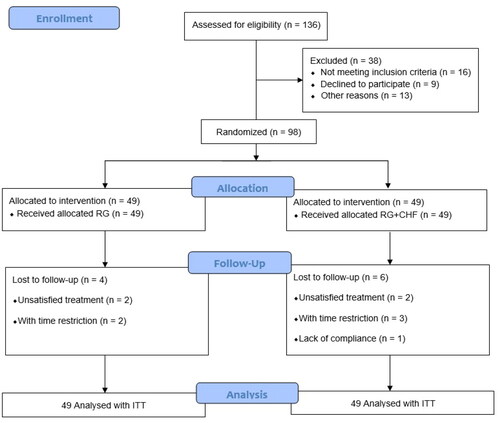
From September 2021 to December 2022, 98 patients with stage 2- 4 CKD complicated with grade 1- 2 AKI were enrolled (). The study subjects were AKI inpatients from the Department of Nephrology of Shanghai Municipal Hospital of Traditional Chinese Medicine, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and Minhang Branch of Yueyang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. Participants were enrolled if they satisfy all the following criteria: (1) diagnostic criteria for CKD stages 2–4 and AKI grades 1–2; (2) diagnostic criteria of TCM syndrome differentiation; (3) 24 h U-pro ≤ 2.5 g; (4) between 18 and 70 years old; and (5) voluntary to participate in the clinical trial and sign informed consents. To reduce enrollment bias that might affect the clinical outcomes, strict blind design is adopted. The three hospitals finally enrolled 38, 34 and 26 cases. This clinical trial was approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (No.2020SHL-KYYS-60). When each patient was enrolled, researchers introduced the purpose, procedure, and possible risks of the research trial, and patients should sign the informed consent.
2.2. Study interventions
Conventional treatment: All patients received conventional treatments to correct water, electrolyte, acid-base balance disorders, control blood pressure, improve anemia, correct renal bone diseases, etc.
Control group: RG group, on the basis of conventional treatment, 1.8g of RG (Chongqing Yaoyou Pharmaceutical Co., LTD., 0.6g/branch) for intravenous injection (IV) was added to 0.9% normal saline or 5% glucose injection 250 ml, once a day for 2 weeks. Normal saline enema was given once a day, and after 5 enemas, rest for 2 days, a total of 10 times.
Treatment group: RG + CHF group, IV of RG plus CHF-II decoction orally twice a day, once in the morning and once in the evening, a total of 2 weeks; At the same time, the concentrated liquid of CHF-II was given once a day enema for 5 days, and rest 2 days, a total of 10 times. CHF-II pharmaceutical composition mainly included prepared rhubarb (Zhida Huang), Ligusticum wallichii (Chuan Xiong), Codonopsis pilosula (Dangshen), Coptidis rhizome (Huanglian), and Smilacis glabrae (Tufuling), etc. The CHF-II administered was produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd.; hence, the quantity and quality of medicine were guaranteed.
2.3. Specimen collection method
On the day of sample collection, an 8 mL fasting blood sample was collected through a vein. The collected blood was left at 4 °C for 30 min, centrifuged (3500 r/min, 4 °C, 10 min, centrifugation radius 10 cm), and the upper serum layer was collected and stored at −80 °C. The serum samples were slowly thawed before analysis, and 10 μL of serum was drawn using a pipette and transferred into a 1.5 mL centrifuge tube. Subsequently, 150 μL internal standard methanol solution, 500 μL methyl tert-butyl ether solution, and 125 μL ultra-pure water were added successively. The mixture was swirled for 30 s, centrifuged (13,200 r/min, 4 °C, 10 min, centrifuge radius 8 cm, the same below), and the supernatant was extracted for subsequent use. Under the positive ion detection mode, 100 μL of the prepared sample was transferred to a 1.5 mL centrifuge tube. After nitrogen drying, acetonitrile-isopropyl alcohol-water (65:30:5, the same as below) was added to 200 μL of complex solution, vortexed for 30 s, and centrifuged. Subsequently, 150 μL of the supernatant was transferred to the sample vial for measurement. Under the negative ion detection mode, 300 μL of the prepared sample was used, followed by nitrogen drying and the addition of a 100 μL compound solution. The mixture was swirled for 30 s and centrifuged, and 50 μL of the supernatant was used for measurement.
2.4. Statistical analysis for clinical outcomes
Full Analysis Set (FAS) was the primary analysis to be performed. Patients accomplishing the whole research were defined as per-protocol samples. According to Intend-to-Treat (ITT) principle, all participants receiving medical treatments were included in a safety analysis.
Continuous variables were presented as mean ± standard deviation or by the median. Categorical variables were displayed in the form of numbers and percentages. Student’s t-tests or one-way ANOVA were applied for continuous data exhibiting normal distribution; in contrast, Pearson’s chi-square test (or Fisher’s exact test for cell count < 5 in any cell) was adopted for comparing categorical variables. All statistical analyses were conducted using a two-sided design. p < 0.05 was considered statistical significance. The analyses of statistical data were carried out by an independent biostatistician using the SPSS (version 21.0) software package. There were no further analyses or interim analyses conducted in this study.
2.5. Lipidomics test methods
2.5.1. Chromatographic conditions:
Chromatography was performed using an ACQUITY UPLC BEH C8 column (2.1 mm × 100 mm, 1.7 μm) with acetonitrile-aqueous solution (6:4, A) in mobile phase containing 5 mmol/L ammonium formate and acetonitrile-isopropyl alcohol solution (1:9, 1) in mobile phase containing 5 mmol/L ammonium formate. A gradient elution scheme was applied as follows: 0-1.0 min, 100%A; 1.0 ∼ 2.0 min, 100%∼70%A; 2.0-12.0 min, 70%-30% A; 12.0 ∼ 12.5 min, 30%∼5%A; 12.5 ∼ 13.0 min, 5%∼0%A; 13.0 ∼ 14.0 min, 0%A. The column temperature was maintained at 55 °C, with a flow rate of 0.26 mL/min. Sample injection volumes were 1 μL for positive and 2 μL for negative ion modes.
2.5.2. Mass spectrum conditions:
Signal acquisition was conducted using an electrospray ion source in multi-reaction monitoring mode. The parameters were set as follows: capillary voltage 1 kV, cone hole voltage 20 V, ion source temperature 150 °C, solvent gas temperature 400 °C, 800 L/h, and cone hole gas flow 150 L/h. The mass spectrum scanning range was m/z 50 ∼ 1 200 Da.
2.6. Lipidomics analysis method
2.6.1. Multidimensional statistical analysis
2.6.1.1. Principal component analysis (PCA)
The lipid data matrix from 98 samples was subjected to PCA analysis using standardized lipid data. In Simca14.1 software, the data matrix was transformed by log10, standardized using UV (Auto scaling), and automatically fitted using 7-fold cross-validation. The PCA results revealed a total of 10 principal components. The first principal component fitted 35.9% of the variance. The RG + CHF group was more concentrated than the RG group, indicating discernible differences between the groups, with the RG + CHF group displaying higher consistency. The PCA score chart is shown in the figure below ().
Figure 2. (A) PCA scores of serum samples in the two groups (t[Citation1] represents the first principal component and t[Citation2] represents the second principal component); (B) Scores of OPLS-DA model in serum samples of two groups; (C) VIP scores of serum samples in the two groups (Red and yellow are the parts of VIP ≥ 1. In order to show that bar does not contain 0 area, the chart with yellow bar is displayed.); (D) Results of 999 permutation and combination experiments.
![Figure 2. (A) PCA scores of serum samples in the two groups (t[Citation1] represents the first principal component and t[Citation2] represents the second principal component); (B) Scores of OPLS-DA model in serum samples of two groups; (C) VIP scores of serum samples in the two groups (Red and yellow are the parts of VIP ≥ 1. In order to show that bar does not contain 0 area, the chart with yellow bar is displayed.); (D) Results of 999 permutation and combination experiments.](/cms/asset/615481d2-a632-405c-957a-4beab5aae40a/irnf_a_2356021_f0002_c.jpg)
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
The OPLS-DA score map exhibited significant separation distance between groups, indicating substantial differences between the groups. The variable importance plot (VIP) of OPLS-DA was computed to measure the impact of lipid expression patterns on sample classification and interpretation in each group. A VIP value typically greater than 1.0, with zero not included, served as the screening criterion for marker lipids. The data matrix was transformed using -log10, standardized with Par (Pareto scaling), and automatically fitted by 7-fold cross-validation to establish the OPLS-DA model for each comparison group. Model evaluation parameters are summarized in the table below, accompanied by the model score chart, replacement test chart, and VIP score chart presented in the figures below ( and ).
The following figure shows the model validation of OPLS-DA with 999 substitution tests. The quality of the multivariate statistical analysis model depends on the replacement test results. When the Q2 regression line intercept is less than 0, or the Q2 score of 999 replacement tests is lower than the Q2 score of the original model, the model is very reliable and indicates that the model does not overfit.
There is still no universally accepted standard for evaluating the quality of multivariate statistical analysis models (R2 intercept <0.4, Q2 intercept <0). However, based on experience, it is recommended to make reference when the slope of the Q2 regression line is greater than 1; when the Q2 slope is negative, it is recommended not to use multivariate statistical analysis results. Multivariate statistical analysis is not a mandatory statistical approach for metabolomics data analysis. When the model quality is notably poor, the results of multivariate statistical analysis can be discarded, and reliance on the T-test combined with Fold Change Analysis or only the T-test results is suggested ().
2.6.2. Analysis of differential lipid expression
2.6.2.1. Venn analysis of significantly difference lipids
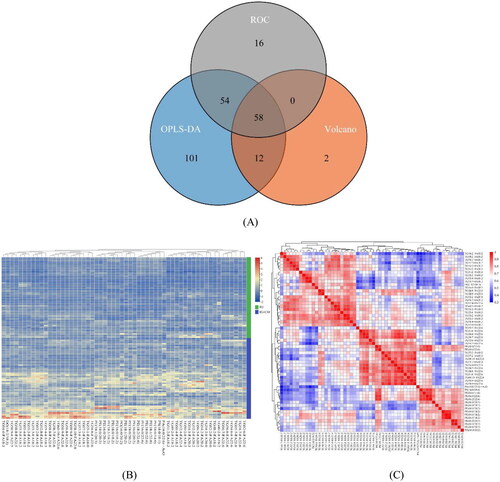
Using VIP values obtained from the OPLS-DA model, the differential lipids with clinical significance were measured and mined. VIP > 1 was used as the screening threshold to distinguish lipids between the two groups. Lipids with multidimensional statistical analysis VIP ≥ 1, univariate statistical analysis p ≤ 0.05, |log2(FC)|≥1, and AUC ≥ 0.85 were selected as lipids exhibiting statistically significant differences. Venn analysis was used to demonstrate common and specific differential lipids based on different screening criteria. Each circle in the figure represents a model (ROC, OPLS-DA, Volcano), the number represents the amount of differential lipids, and the overlapping and non-overlapping parts represent the amount of common and unique differential lipids, respectively ().
2.6.2.2. Differential lipid cluster heat map analysis
To visually represent the relative content of differential lipids among inter-group samples and the consistency within intra-group samples, cluster heat maps were used for visualization. In Figure, colors denote the relative content of the substance in the sample, with red and blue representing higher and lower content, respectively ().
2.6.2.3. Differential lipid correlation analysis
Spearman correlation analysis was used to calculate correlation coefficients among significantly different lipids, which are displayed in the form of a matrix heat map (Figure). The correlation coefficient (R) between the differential lipids ranged between −1 and +1, where R > 0 indicates a positive correlation (shown in red) and R < 0 signifies a negative correlation (shown in blue). The correlation significance was indicated by asterisks (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). It is important to note that high correlation does not imply causation ().
3. Results
3.1. Clinical results
3.1.1. Participants baseline data
98 participants participated in this clinical study. There were 31 males and 18 females in the RG group, the mean age was (59.8 ± 11.4) years, and the duration of CKD was (48.6 ± 13.2) months. There were 32 males and 17 females in the RG + CHF group. The mean age was (57.6 ± 11.1) years and the duration of CKD was (50.3 ± 12.6) months. The concomitant diseases of the patients were also analyzed. According to the statistical results, there were no significant differences in gender, age, CKD course and associated diseases between the two groups (p > 0.05), and the two basic characteristics were relatively balanced between the groups, and the clinical study effect was good and comparable ().
Table 1. Participants baseline data.
3.1.2. Analysis of underlying CKD diseases and A on C inducements
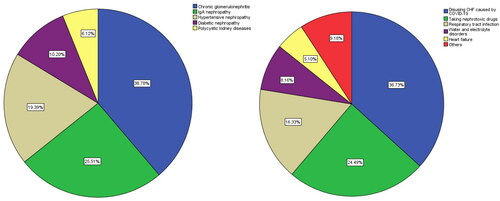
The main underlying diseases of CKD patients were chronic glomerulonephritis in 38 cases (38.78%), IgA nephropathy in 25 cases (25.51%), hypertensive nephropathy in 19 cases (19.39%), diabetic nephropathy in 10 cases (10.20%), polycystic kidney diseases in 6 cases (6.12%). According to the etiological analysis of patients at the time of enrollment, the main factors inducing AKI are: Disusing CHF caused by COVID-19 were 36 cases (36.73%), 24 cases (24.49%) of taking nephrotoxic drugs, 16 cases (16.33%) of respiratory tract infection, 8 cases (8.16%) of water and electrolyte disorders, 5 cases (5.10%) of heart failure, and 9 cases (9.18%) of others (insufficient effective blood volume, stress, etc.) ().
3.1.3. Clinical outcomes
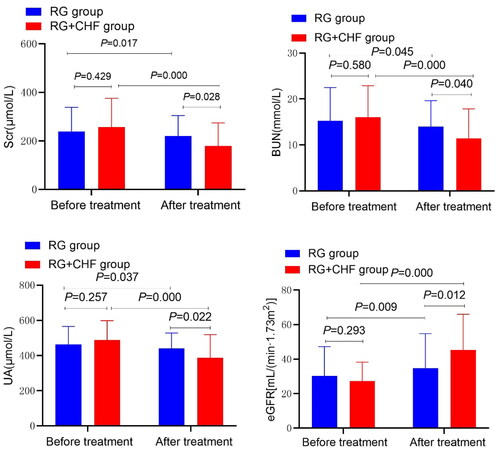
The statistical results showed that the mean ± standard deviation of Scr after treatment in RG group and RG + CHF group were 219.6 ± 84.9 and 178.9 ± 95.4, respectively. After treatment, Scr in both groups was significantly decreased compared with that before treatment (p < 0.01). Scr decreased more significantly in RG + CHF group than in RG group, and the difference was statistically significant (p < 0.05). Compared with RG group, BUN and UA decreased more significantly in RG + CHF group, and the difference was statistically significant (p < 0.01). eGFR was calculated by the Modification of Diet in Renal Disease equation (eGFRMDRD) with eGFR improved, and the difference was statistically significant (p < 0.01) ( and ).
Table 2. Comparison of clinical outcome between two groups.
The statistical results showed that the mean ± standard deviation of urinary TG after treatment in RG group and RG + CHF group were 1.63 ± 0.28 and 1.46 ± 0.24, respectively. Compared with RG group, TG in RG + CHF group decreased more significantly, and the difference was statistically significant (p < 0.05). HDL-C increased significantly in RG + CHF group, the difference was statistically significant (p < 0.01). TC and LDL-C showed no statistically significant (p > 0.05).
The statistical results showed that the mean ± standard deviation of urinary NGAL after treatment in RG group and RG + CHF group were 146.7 ± 66.9 and 105.7 ± 41.0, respectively. Compared with RG group, urinary NGAL in RG + CHF group decreased more significantly, and the difference was statistically significant (p < 0.05). IL-18 decreased significantly in RG + CHF group, the difference was statistically significant (p < 0.01). TIMP2 and IGFBP7 decreased significantly in RG + CHF group, and the difference was statistically significant (p < 0.05).
The statistical results showed that the mean ± standard deviation of TCM syndrome score in RG group and RG + CHF group after treatment were 37.0 ± 11.4 and 32.0 ± 8.3, respectively. The score of RG + CHF group was lower than that of RG group, and the difference was statistically significant (p < 0.05).
3.1.4. Comparison of effective rate between two groups
As shown in the following table, in the RG group, 13 cases (26.5%) were significant and 7 cases (14.3%) were effective. In RG + CHF group, there were 22 (44.9%) obvious effects and 14 (28.6%) effective cases. The total effective rate of RG + CHF group (73.5%) was significantly higher than that of RG group (40.8%), and the difference was statistically significant (p < 0.01) ().
Table 3. Comparison of effective rate between two groups.
3.1.5. Safety evaluation
In both the RG and RG + CHF groups, no serious adverse events requiring discontinuation were reported throughout the treatment period.
3.2. Lipidomics results
3.2.1. Identification and screening results of differential metabolism
We identified 98 clinical samples based on Spearman correlation in this data analysis process. Subsequently, we utilized PCA, OPLS-DA, Volcano, and ROC models to analyze the differences in lipids between groups. Based on the clinical data from the 98 cases, we established an OPLS-DA model with R2(cum)=0.932 and Q2(cum)=0.822, indicating that the model had excellent explanatory and predictive ability. To ascertain the model’s stability and reliability, 999 arrangement and combination experiments were performed, revealing the intercepts of R2 and Q2 as 0.688 and −0.486, respectively. With OPLS-DA model VIP ≥ 1, bar excluding 0, P-value ≤ 0.05, |log2(FC)|≥1, AUC ≥ 0.8 as screening criteria, we screened significantly different lipids and constructed a Wayne diagram. Differences between groups and fluctuations within groups can be observed based on differential lipid clustering heat maps. Additionally, differential lipid correlation analysis provided indications of potential associations between substances to some extent, but this internal association needs to be verified by further correlation experiments.
We finally obtained 58 lipid differential metabolites using the aggregation method (). These were identified by comparing the experimental spectra with the metabolic spectra libraries, including triglyceride (TG), phosphatidylserine (PS), phosphatidyl glycerol (PG), phosphatidyl ethanolamine (PE), phosphatidic acid (PA), and diacylglycerol (DG).
Table 4. VIP, P and FDR values of 58 different metabolites.
3.2.2. Identification and screening results of potential lipid markers
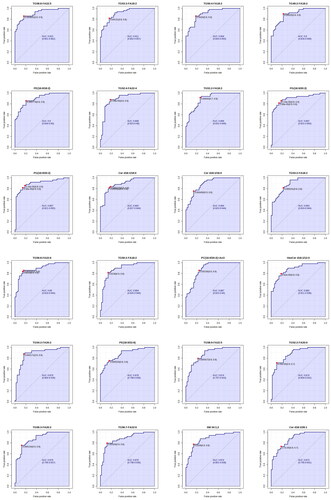
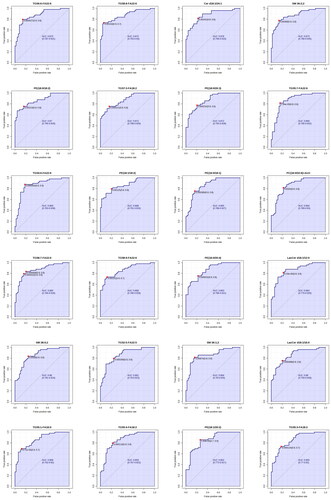
The ROC analysis can better evaluate the accuracy and discriminant effect of the results by combining sensitivity and specificity. In metabolomics, ROC analysis of lipids could indicate the ability of biomarkers to distinguish between experimental and control groups. The area under the curve (AUC) falls between 1.0 and 0.5, with values closer to 1, indicating superior diagnostic efficacy. An AUC > 0.7 indicates that the model has a certain degree of accuracy.
According to the detection results, AUC value > 0.85 was selected, and a total of 48 biomarkers were identified (). They are: TG58:8-FA22:5, TG53:3-FA18:2, TG50:4-FA18:2, TG49:2-FA18:2, PE(16:0/18:2), TG52:4-FA22:4, TG51:2-FA18:2, PG(16:0/20:2), PE(16:0/20:2), Cer d18:1/18:0, Cer d18:1/16:0, TG53:2-FA18:2, TG56:8-FA22:6, TG50:2-FA18:2, PC(16:0/20:2)+AcO, HexCer d18:1/12:0, TG54:2-FA20:2, PE(18:0/22:6), TG58:9-FA22:5, TG52:2-FA20:0, TG56:3-FA20:2, TG56:7-FA22:6, SM 34:1;2, Cer d18:1/26:1, TG56:6-FA22:6, TG58:8-FA22:6, Cer d18:1/24:1, SM 36:2;2, PE(18:0/18:2), TG57:3-FA18:2, PE(18:0/20:3), TG55:7-FA22:6, TG54:6-FA22:6, PE(18:1/18:2), PE(16:0/18:1), PC(16:0/22:6)+AcO, TG58:7-FA22:6, TG58:9-FA22:6, PE(16:0/20:4), LacCer d18:1/12:0, SM 38:0;2, TG52:5-FA22:5, SM 36:1;2, LacCer d18:1/16:0, TG55:1-FA16:0, TG55:4-FA18:2, PE(18:1/20:2), TG55:3-FA18:2. It can be summarized into four categories such as glycerides, glycerol phospholipids, sphingolipids, and cholesterol lipids. Together, these results suggest that these classes of lipid small molecules could serve as potential lipid markers.
Figure 6. ROC profile of lipid markers in serum samples of the two groups.
3.2.3. Potential lipid biomarkers pathway analysis
The analysis reveals that potential lipid biomarkers predominantly fall into five major categories: glyceride, glycerol phospholipid, sphingolipid, cholesterol ester, and amide. These categories were cross-referenced with the KEGG database (https://www.genome.jp/kegg/pathway.html), combined with relevant clinical and laboratory lipid data to deduce potential metabolic pathways, as listed below.
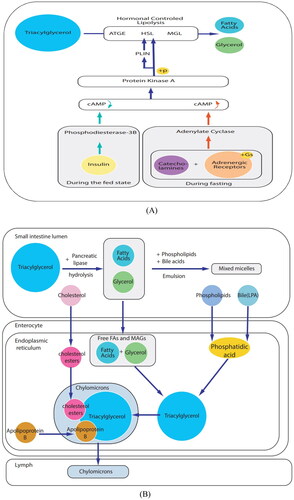
3.2.3.1. Triglyceride metabolic pathway
Within cells, lipolysis involves the hydrolysis of triacylglycerol to release fatty acids and glycerol for use as an energy substrate under the regulation of hormones. During fasting, catecholamines activate adenylate cyclase, elevating cAMP levels and activating protein kinase A (PKA) by binding to GS-coupled adrenergic receptors (-AR). PKA phosphorylates target proteins, such as hormone-sensitive lipase and periilipin 1 (PLIN). PLIN phosphorylation is a pivotal event in the sequential activation of TAG hydrolysis of fatty triglyceride lipase, HSL, and monotriglyceride lipase. During feeding, insulin inhibits catecholamine-induced lipolysis by activating phosphodiesterase 3b, which degrades cAMP ().
3.2.3.2. Metabolic pathways of fat digestion and absorption
Fat is an important source of energy in food, with over 95% comprising long-chain triacylglycerols and the remainder consisting of phospholipids and sterols. In the small intestinal lumen, pancreatic lipase hydrolyzes dietary TAG into fatty acids (FA) and monoacylglycerol. These products are then emulsified with the help of phospholipids and bile acids to form micelles. Intestinal cells absorb free FAs and monoacylglycerol, rapidly resynthesizing them in the endoplasmic reticulum to form TAG. Phospholipids from food and bile are also absorbed by intestinal epithelial cells and acylated to form phosphatidic acid, which is subsequently converted into TAG. Moreover, cholesterol (CL) acyl is absorbed to form cholesterol ester (CE). In the endoplasmic reticulum, TAG combines with CE and apolipoprotein B to form chylomicrons, which enter circulation through the lymphatic system ().
4. Discussion
Studies have shown that AKI and CKD are interrelated and influence each other, thus characterizing A on C as a complex clinical syndrome [Citation3]. Related research [Citation18,Citation19] has uncovered that the most common cause of A on C is CKD itself or concomitant disease and is often accompanied by other AKI-inducing factors. CKD is an important risk factor for AKI, and when AKI occurs in patients with CKD, it is typically more severe and challenging to recover from. Reasons why the cause of CKD are not different from elsewhere in the world might as follows: The clinical trial was conducted during the COVID-19 pandemic period when many patients, especially older patients, couldn’t have access to effective medical treatments. According to published data, numerous studies [Citation20–22] have reported COVID-19 infection is associated with a high incidence of AKI or A on C; In addition, the limited number of patients (98 cases) included in this clinical trial also had a certain impact on the results; Besides, the influence of regional reasons also has a certain relationship. The presence of CKD poses a threat to kidney function and delays the recovery of kidney function in patients with AKI. The relationship between AKI and CKD is bidirectional, wherein patients with CKD are more likely to develop AKI than the general population. Meanwhile, patients with AKI are at a heightened risk of exacerbating CKD, potentially progressing to end-stage renal disease (ESRD) [Citation23]. In clinical practice, prompt identification and effective correction of factors contributing to the acute aggravation of patients with A on C can significantly enhance disease recovery and prognosis. On the contrary, neglecting these factors may accelerate disease progression, possibly culminating in an irreversible outcome.
AKI biomarkers typically reflect specific components of pathophysiology, including tubule injury, cell cycle arrest, systemic inflammatory pathways, and glomerular filtration. These biomarkers are instrumental in predicting clinical outcomes, guiding clinical decision-making, and evaluating therapeutic responses [Citation24]. Whether employed individually or in combination, these novel biomarkers exhibit robust associations with short-term outcomes such as in-hospital mortality, the need for RRT, and length of hospitalization. Urinary biomarkers such as TIMP-2/IGFBP7, IL-18, and KIM-1 have been shown to be associated with the prediction and diagnosis of AKI, serving as a urinary stress biomarker with potential roles in risk assessment and AKI prediction [Citation25–27]. Proteomic studies have identified urinary NGAL and IL-18 as important biomarkers of AKI clinical conditions such as renal ischemia or xenobiological exposure [Citation28,Citation29]. NGAL is filtered by the glomeruli and subsequently reabsorbed by the proximal tubules. Decreased renal tubule reabsorption during AKI leads to increased urinary NGAL levels. Due to its rapid expression during renal cell stress or injury, NGAL is used at the emergency room admission to predict the risk of mortality, need for dialysis, and AKI after cardiac surgery in adult patients [Citation30]. Compared to other biomarkers of single-mechanism damage, TIMP-2/IGFBP7 may serve as novel biomarkers that are highly sensitive to kidney damage [Citation31]. Recently, Adler et al. [Citation32] hypothesized that the ratio of TIMP-2 to IGFBP7 levels could serve as a biomarker to predict AKI in patients with non-traumatic shock.
Serum total cholesterol (TC) can be used to assess the nutritional status of the body and is recognized as one of the acute phase markers. Research shows that hypocholesterolemia observed in critically ill surgical patients is associated with reduced synthesis of plasma and liver-related proteins [Citation33–35]. Additionally, a surge in inflammatory factors such as TNF-α, IL-1β, and IL-6, accompanied by a significant reduction in lipoprotein synthesis, is a pivotal contributing factor to hypocholesterolemia. Triglyceride (TG) levels in patients with high TG are prone to deposition in various tissues, including cardiovascular, liver, pancreas, muscle, and kidneys, potentially leading to chronic renal function injury [Citation36]. High-density lipoprotein cholesterol (HDL-C) exhibits reverse cholesterol transport, anti-inflammatory responses, and antioxidant effects [Citation37,Citation38]. A clinical trial conducted by Smith et al. revealed that higher pre-surgery HDL-C concentrations were associated with lower postoperative Scr [Citation39], with the strongest association observed between elevated HDL-C levels and reduced postoperative AKI incidence. In addition, a single-center cohort study conducted by Zhou et al. [Citation40] found that among 74,284 surgical procedures, the incidence of postoperative 7d-AKI was 4.4%. After adjusting for various confounders using propensity score matching analysis, the preoperative HDL-C < 1.03 mmol/L was closely associated with an increased risk of postoperative AKI. Low-density lipoprotein cholesterol (LDL-C) is routinely measured in patients with cardiovascular disease and aids in evaluating various disease conditions. Elevated LDL-C levels have been identified as a risk factor for CI-AKI after emergency treatment. The underlying mechanism might be attributed to LDL-C stimulated oxygen free radical production, NO degradation, and impairment of NOS synthesis and NO activity [Citation41]. As a vasodilator, NO plays an important role in maintaining the blood oxygen supply in the medulla of renal tubules. Dysfunction of vascular endothelial cells and decreased NO activity are crucial factors in CI-AKI onset [Citation42].
Dyslipidemia serves as a hallmark of CKD, with the severity of dyslipidemia closely correlated with CKD stage and mortality [Citation43]. However, understanding the genesis and progression of lipid dysregulation is challenging due to the diversity of lipid structures. In recent years, the rapid development of various analytical platforms based on mass spectrometry (MS) has enabled the precise and in-depth identification of complex lipids [Citation17,Citation44]. AKI is a prevalent complication in critically ill patients, and early identification of high-risk patients is critical [Citation27]. Emerging evidence implicates early lipid alterations in AKI pathogenesis [Citation45,Citation46], with lipidomics analysis providing biomarkers for early identification and diagnosis [Citation47]. CKD and its associated complications lead to significant multi-organ metabolic disorders, with dyslipidemia being considered a contributory factor in disease development. In CKD, dyslipidemia characterized by lipid oxidation, lipid uptake, and lipogenesis aberrations is considered to be the cause of disease progression and, in some instances, may even contribute to patient mortality [Citation48–50].
According to the partial clinical results, (1) After treatment, the total clinical effective rate of RG + CHF group was 73.5%, and that of RG group was 40.8%, and the efficacy of the former was significantly better than that of the latter; (2) TCM syndrome score: After treatment, the total TCM syndrome score of both groups was significantly lower than that before treatment (p < 0.01), and the total score of RG + CHF patients was lower than that of RG (p < 0.05); (3) Renal function: Compared with before treatment, Scr, BUN and UA levels in 2 groups were decreased (p < 0.05), eGFR levels were increased (p < 0.05), and Scr, BUN and UA levels in RG + CHF patients were decreased and eGFR levels were increased more than RG; (4) Blood Lipids: Compared with RG group, TG decreased and HDL-C increased more significantly in RG + CHF group, and the difference was statistically significant (p < 0.05). TC and LDL-C showed no statistically significant (p > 0.05). (5) Urinary AKI markers: After treatment, the levels of urinary NGAL, IL-18, TIMP2 and IGFBP7 were decreased to varying degrees (p < 0.05), and the reduction range in RG + CHF group was greater than that in RG group (p < 0.05). (6) Adverse reactions: No serious adverse events occurred in both groups.
Results of lipidomics: (1) Differential lipid analysis: PCA, OPLS-DA, Volcano and ROC models were used to analyze the different lipids between the groups, and 58 different lipid metabolites were obtained by aggregation method. They are: triglyceride (TG), phosphatidylserine (PS), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylacid (PA), diglycerol (DG), etc. (2) Lipid marker screening: According to the test results, AUC value > 0.85 was selected, and a total of 48 biomarkers were identified, which could be summarized into four categories, including triglycerides, glycerolipids, sphingolipids, and cholesterol lipids. These results indicated that small molecule substances of these categories of lipids could be used as potential lipid markers. (3) Biomarker pathway analysis: According to the selected potential lipid markers, the KEGG database was searched, and the possible metabolic pathways were found to include triglyceride metabolic pathway and fat digestion and absorption metabolic pathway.
Based on the clinical treatment experience, this trial adopted the combination of CHF-II oral and enema to improve the clinical efficacy of patients and provide a more comprehensive assessment of CHF-II’s impact. Notably, both treatment groups exhibited improvements in renal function was improved in both groups after treatment compared with before treatment. However, the RG + CHF group demonstrated more pronounced reductions in Scr, BUN, and UA, accompanied by a significant increase in eGFR. Additionally, the level of urinary AKI biomarkers and TCM syndrome exhibited greater reductions in the RG + CHF group, indicative of superior renal protection compared to the RG subgroup. This enhanced effect is postulated to be associated with CHF-II’s influence on regulating lipid metabolism in the body. Our previous single-center clinical studies [Citation6–8] have shown that CHF has a renal protective effect on patients with A on C. Furthermore, an article on the treatment of AKI by CHF in animal and cell models was also published, which delved into the clinical dose inhibition effect of arsenic trivalent and the mechanism of renal toxicity [Citation51]. The work revealed CHF’s ability to effectively inhibit renal toxicity induced by clinical doses of arsenic trivalent, with the underlying molecular mechanism attributed to the inhibition of caspase 3-induced apoptosis of renal tubular epithelial cells. We also studied the mechanism of apoptosis of renal tubular epithelial cells in rats with contrast-induced acute kidney injury (CI-AKI) and found that the activation of the p38-MAPK pathway plays an important role in the pathogenesis of CI-AKI [Citation52]. Notably, the combination of Zhidahuang (rhubarb) and Chuanxiong may alleviate the kidney damage of CI-AKI rats by inhibiting the activation of the p38-MAPK pathway. Based on these results, we further uncovered the involvement of the Nrf2/HO-1 pathway [Citation53], which is activated and contributes to renal protection. Notably, Zhidahuang (rhubarb)-Chuanxiong drug pair inhibits this pathway, ultimately exerting renal protective effects. Tetramethylpyrazine (TMP), an effective ingredient in Ligustrum chuanxium, can prevent AKI by improving oxidative stress damage, inhibiting inflammatory response, inhibiting renal cell apoptosis, and regulating autophagy [Citation54,Citation55]. Besides, CHF was shown to exert renal protective effects and inhibit fibrosis by reducing TGF-β1 and CTGF [Citation5]. Our recently published data [Citation56] on Rhizoma Chuanxiong and Radix et Rhizoma Rhei (rhubarb) against AKI and renal fibrosis based on network pharmacology and experimental validation demonstrated that this herbal pair might inhibit tubular epithelial cell apoptosis and improve AKI and renal fibrosis by inhibiting the p38-MAPK/p53 signaling. The triglyceride metabolic pathway and fat digestion and absorption metabolic pathway discovered in this study might become new targets for CHF-II to treat A on C, and specific research strategies will be established for the next study. Our research group will continue to conduct relevant studies and further verify them in subsequent studies.
AKI, regardless of their severity or cause, can have profound effects on the kidneys and throughout the body. It is important to emphasize not only the early detection and timely management of AKI, but also the need for long-term follow-up of AKI patients. Current research in the field of AKI has several potential limitations:AKI is a multifactorial disease that is difficult to represent in a single animal model. Diagnostic inaccuracy and lack of reliable early biomarkers to define AKI and CKD. In low-income countries, AKI is associated with neglected diseases affecting their epidemiology. The problem is exacerbated by a lack of resources in science and technology due to neglect. The main reasons for drug trial failure include heterogeneity of AKI and insufficient research. Although there have been unique research and molecular findings on AKI, the mortality and morbidity of patients with this clinical disease remains high. New management and technologies are urgently needed to improve AKI care, as well as important health strategies that focus on prevention. In the early detection of AKI, new biomarkers are emerging to prevent disease progression and increased health care costs. However, more reliable clinical trials with greater statistical power are needed. Improved surgical conditioning techniques or drug disease management are feasible in humans to avoid loss of kidney function or loss of transplanted organs.
5. Conclusion
In conclusion, the renal protection effect of RG + CHF group was better than that of RG subgroup. CHF-II could effectively alleviate kidney injury in patients with A on C and improved TCM syndrome scores. The 58 lipid differential metabolites and 48 lipid markers screened could be used as lipid markers for the diagnosis of AKI in CKD patients. According to the selected potential lipid markers, KEGG database was searched, and the triglyceride metabolic pathway, fat digestion and absorption metabolic pathway might be the lipid metabolic pathway for CHF-II to prevent and treat patients with A on C.
6. Limitations
Due to the limitation of the decoction form, the double-blind method could not be adopted in this research, which may have certain psychological implications or pharmacal efficacy differences for patients in this clinical protocol. During the COVID-19 epidemic period, combined with our previous researches, the sample size was determined as 98 cases. Thus, the results might have certain limitations, and samples scale would be expanded in the later clinical studies. Another limitation of the current study is the lack of common and proven methods for validating lipid analytes. Compared with samples from cells, mice and other sources, clinical samples often have large individual differences, so it is difficult to control the high consistency of metabolites. In addition, the results produced by single omics are often one-sided, and are not comprehensive enough to reveal the potential mechanism of biological activities. To improve the reliability of biomarkers, large-scale targeted metabolomics studies would be conducted based on the screened biomarkers.
Ethical approval
The studies were reviewed and approved by the Institutional Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (reference number2020SHL-KYYS-60). Written informed consents were necessary before participants were enrolled to participate in this study.
Authors’ contributions
XZG was the chief investigator in charge of this trial program. He designed this study, and coordinated various personnel and material resources to ensure the smooth implementation of the program. In addition, he participated in, instructed, and supervised the whole study. LC understood the task of data collection, analysis, and interpretation. Besides, LC mainly drafted the manuscript and assisted the implement of subsequent trial program. QW, TLL, and LJL assisted to consult relevant literatures, collect clinical samples and they contributed many useful advices to this manuscript. CW and BX were responsible for the enrollment of participants in other two hospitals, and they coordinated related affairs with XZG. The manuscript is critically revised by XZG and finally approved by all authors for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data from this study will not be disclosed to the public because the experimental data contain sensitive data and patients’ privacy. All authors confirm the authenticity and availability of data upon request. If original data and materials are needed, request should be sent to the corresponding author, Xuezhong Gong ([email protected]).
Additional information
Funding
7. References
- Laing C. Database research in acute kidney injury: time to take stock?. Am J Kidney Dis. 2022;79(4):1–18. doi: 10.1053/j.ajkd.2021.09.028.
- August P. Chronic kidney Disease - Another step forward. N Engl J Med. 2023;388(2):179–180. doi: 10.1056/NEJMe2215286.
- He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms Kidney Int. 2017;92(5):1071–1083. doi: 10.1016/j.kint.2017.06.030.
- Chen L, Gong X. Efficacy and safety of chuan huang fang combining reduced glutathione in treating acute kidney injury (grades 1-2) on chronic kidney disease (stages 2-4): study protocol for a multicenter randomized controlled clinical trial. Evid Based Complement Alternat Med. 2022;2022:1099642. doi: 10.1155/2022/1099642.
- Chen L, Ye Z, Wang D, et al. Chuan huang fang combining reduced glutathione in treating acute kidney injury (grades 1-2) on chronic kidney disease (stages 2-4): a multicenter randomized controlled clinical trial. Front Pharmacol. 2022;13:969107. doi: 10.3389/fphar.2022.969107.
- Gong X, Tang X, Wang Q, et al. Observe the clinical efficacy of chuanhuang decoction combined with lipo PGE1 in treating acute kidney injury (AKI) on phase 2 ∼ 4 chronic kidney disease (CKD) patients. Chin J IntegrTrad West Med Nephrol. 2014;15(9):784–787.
- Gong X, Duan Y, Wang Y, et al. Effects of chuanhuang decoction on renal function and oxidative stress in patients of chronic kidney disease at stage 2-4 complicated with acute kidney injury. J Shanghai Univ Trad Chin Med Sci. 2020;34(1):11–16. doi: 10.16306/j.1008-861x.2020.01.002.
- Gong X, Ye Z, Xu X, et al. Effects of chuanhuang formula combined with prostaglandin E1 in treating patients of chronic kidney disease complicated with acute kidney injury and its influence on NLRP3 J Shanghai Univ Trad Chin Med Sci. 2021;35(06):12–16. doi: 10.16306/j.1008-861x.2021.06.002.
- Wang WM, Wu Y. Clinical effect of reduced glutathione combined with jinshuibao in the treatment of acute renal injury. Guide China Med, 2021, 19 (24), 7–9.
- Zhang L, Bai L. Therapeutic effect of reduced glutathione on acute kidney injury in patients with sepsis[J]. Chin Mode Med. 2019;26(9):54–60.
- Kim DH, Choi HI, Park JS, et al. Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes. Redox Biol. 2022;54:102382. doi: 10.1016/j.redox.2022.102382.
- Yang F, Tong J, Li H, et al. Effects of reduced glutathione combined with high-flux hemodialysis on serum CysC, KIM-1 and scr in patients with acute kidney injury. . Med J West China. 2020;32(6):863–867.
- Hu DJ. Reduced glutathione with high flux hemodialysis therapy of acute renal injury in clinical study. . J Clin Nephrol. 2015;15(1):39–41.
- Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 2020;98(2):294–309. doi: 10.1016/j.kint.2020.04.020.
- Kochan Z, Szupryczynska N, Malgorzewicz S, et al. Dietary lipids and dyslipidemia in chronic kidney disease. Nutrients. 2021;13(9):3138. doi: 10.3390/nu13093138.
- Theofilis P, Vordoni A, Koukoulaki M, et al. Dyslipidemia in chronic kidney disease: contemporary concepts and future therapeutic perspectives. Am J Nephrol. 2021;52(9):693–701. doi: 10.1159/000518456.
- Cao Y, Li H, Sun Y, et al. Integration of multi-omics in investigations on the mechanisms of action of Chinese herbal medicine interventions in metabolic diseases. Tradit Med Res. 2022;7(4):31. doi: 10.53388/TMR20220117001.
- Acosta-Ochoa I, Bustamante-Munguira J, Mendiluce-Herrero A, et al. Impact on outcomes across KDIGO-2012 AKI criteria according to baseline renal function. J Clin Med. 2019;8(9):1323. doi: 10.3390/jcm8091323.
- Soliman KM, Campbell RC, Fülöp T, et al. Acute kidney injury in subjects with chronic kidney disease undergoing total joint arthroplasty. Am J Med Sci. 2019;358(1):45–50. doi: 10.1016/j.amjms.2019.04.002.
- Aklilu AM, Kumar S, Nugent J, et al. COVID-19-associated acute kidney injury and longitudinal kidney outcomes. JAMA Intern Med. 2024;184(4):414–423. doi: 10.1001/jamainternmed.2023.8225.
- Ng PY, Ip A, Ng AK, et al. Risk of acute kidney injury in critically-ill patients with COVID-19 compared with seasonal influenza: a retrospective cohort study. EClinl Med. 2024;70:102535. doi: 10.1016/j.eclinm.2024.102535.
- Li Y, Gong Y, Xu G. New insights into kidney disease after COVID-19 infection and vaccination: histopathological and clinical findings. QJM. 2023:1–21. doi: 10.1093/qjmed/hcad159.
- Zarbock A, Forni LG, Ostermann M, et al. Designing acute kidney injury clinical trials. Nat Rev Nephrol. 2024;20(2):137–146. doi: 10.1038/s41581-023-00758-1.
- Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018;44(3):323–336. doi: 10.1007/s00134-018-5126-8.
- Molinari L, Del Rio-Pertuz G, Smith A, et al. Utility of biomarkers for sepsis-associated acute kidney injury staging. JAMA Netw Open. 2022;5(5):e2212709. doi: 10.1001/jamanetworkopen.2022.12709.
- Ix JH, Shlipak MG. The promise of tubule biomarkers in kidney disease: a review. Am J Kidney Dis. 2021;78(5):719–727. doi: 10.1053/j.ajkd.2021.03.026.
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/s0140-6736(19)32563-2.
- Merchant ML, Brier ME, Slaughter MS, et al. Biomarker enhanced risk prediction for development of AKI after cardiac surgery. BMC Nephrol. 2018;19(1):102. doi: 10.1186/s12882-018-0902-9.
- Wen Y, Parikh CR. Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci. 2021;58(5):354–368. doi: 10.1080/10408363.2021.1879000.
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66(6):993–1005. doi: 10.1053/j.ajkd.2015.06.018.
- Endre ZH. Using biomarkers for acute kidney injury: barriers and solutions. Nephron Clin Pract. 2014;127(1–4):180–184. doi: 10.1159/000363555.
- Adler C, Heller T, Schregel F, et al. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors[J]. Crit Care. 2018;22(1):126. doi: 10.1186/s13054-018-2042-9.
- Sailon AM, Wasserburg JR, Kling RR, et al. Influence of large-Volume liposuction on metabolic and cardiovascular health: a systematic review. Ann Plast Surg. 2017;79(6):623–630. doi: 10.1097/sap.0000000000001195.
- Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, et al. Empagliflozin attenuates non-Alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 2021;22(2):818. doi: 10.3390/ijms22020818.
- Wang G, Wu B, Zhang L, et al. The protective effects of trelagliptin on high-fat diet-induced nonalcoholic fatty liver disease in mice. J Biochem Mol Toxicol. 2021;35(4):e22696. doi: 10.1002/jbt.22696.
- Wang J, Zhang F, Hu L, et al. Correlation of acute kidney injury with serum uric acid and lipid profiles levels. . Chin J Health Lab Tec. 2017;27(03):377–379.
- Smith LE. High-Density lipoproteins and acute kidney injury. Semin Nephrol. 2020;40(2):232–242. doi: 10.1016/j.semnephrol.2020.01.013.
- Wei M, Zhang Z, Ma Z, et al. Ratios of neutrophil, lymphocyte, and monocyte to high-density lipoprotein cholesterol in acute pancreatitis complicated with acute kidney injury. J Clin Nephrol. 2021;21(1):1–9. doi: 10.3969/j.issn.1671-2390.m20-147.
- Smith LE, Smith DK, Yancey PG, et al. Perioperative high density lipoproteins, oxidative stress, and kidney injury after cardiac surgery. J Lipid Res. 2021;62:100024. doi: 10.1016/j.jlr.2021.100024.
- Zhou Y, Yang HY, Zhang HL, et al. High-density lipoprotein cholesterol concentration and acute kidney injury after noncardiac surgery. BMC Nephrol. 2020;21(1):149. doi: 10.1186/s12882-020-01808-7.
- Yang K, Chen J, Liu L, et al. Analysis of the incidence and influencing factors of acute contrast induced renal injury after emergency PCI. J Clin Emer. 2021;22(06):390–394. doi: 10.13201/j.issn.1009-5918.2021.06.005.
- Hao Y, Pan Y, Gao H, et al. Comparison of the value of preoperative serum LP(a) and LDL-C levels in predicting short-term adverse prognosis in patients with acute coronary syndrome after PCI. J Clin Cardio. 2020;36(12):1115–1119. doi: 10.13201/j.issn.1001-1439.2020.12.010.
- Baek J, He C, Afshinnia F, et al. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol. 2022;18(1):38–55. doi: 10.1038/s41581-021-00488-2.
- Cui H, Yang L, Li Y, et al. Omics technology: an important tool in mechanism studies of Chinese herbal formulas. Tradit Med Res. 2021;6(1):2. doi: 10.53388/TMR20200920199.
- Rao S, Walters KB, Wilson L, et al. Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. Am J Physiol Renal Physiol. 2016;310(10):F1136–47. doi: 10.1152/ajprenal.00100.2016.
- Stasi A, Intini A, Divella C, et al. Emerging role of lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2017;32(1):24–31. doi: 10.1093/ndt/gfw250.
- Wang S, Xiao C, Liu C, et al. Identification of biomarkers of sepsis-associated acute kidney injury in pediatric patients based on UPLC-QTOF/MS. Inflammation. 2020;43(2):629–640. doi: 10.1007/s10753-019-01144-5.
- Wang YN, Hu HH, Zhang DD, et al. The dysregulation of eicosanoids and bile acids correlates with impaired kidney function and renal fibrosis in chronic renal failure. Metabolites. 2021;11(2):127. doi: 10.3390/metabo11020127.
- Ren JL, Dong H, Han Y, et al. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan baiyao formulation. Phytomedicine. 2020;77:153266. doi: 10.1016/j.phymed.2020.153266.
- Dou F, Miao H, Wang JW, et al. An integrated lipidomics and phenotype study reveals protective effect and biochemical mechanism of traditionally used alisma orientale juzepzuk in chronic kidney disease. Front Pharmacol. 2018;9:53. doi: 10.3389/fphar.2018.00053.
- Gong X, Zheng J, Duan Y, et al. Effect of Chuanhuang fang on apoptosis of renal tubular epithelial cells in rats with trivalent arsenic nephrotoxicity. Beijing Med J. 2019;41:1089–1093. doi: 10.15932/j.0253-9713.2019.12.009.
- Gong X, Wang Q, Fu D, et al. Research on radix et Rhizoma Rhei-Rhizoma Ligustici Chuanxiong restraining renal tubular epithelial cell apoptosis in contrast-induced nephropathy rats. . Shanghai J Trad Chin Med. 2013;47(03):69–71. doi: 10.16305/j.1007-1334.2013.03.023.
- Gong X, Qiu A, Duan Y, et al. Effects of couplet medicines of prepared radix et rhizoma Rhei-Rhizoma ligustici chuanxiong on Nrf2/HO-1 signaling pathway in renal tissue of contrast induced nephropathy rats. J Shanghai Univ Trad Chin Med Sci. 2017;31(06):58–61. doi: 10.16306/j.1008-861x.2017.06.014.
- Chen L, Gong X. Drug-induced acute kidney injury: epidemiology, mechanisms, risk factors, and preventive treatment of traditional Chinese medicine. Integr Med Nephrol Androl. 2022;9(1):5. doi: 10.4103/2773-0387.345767.
- Li J, Gong X. Tetramethylpyrazine: an active ingredient of Chinese herbal medicine with therapeutic potential in acute kidney injury and renal fibrosis. Front Pharmacol. 2022;13:820071. doi: 10.3389/fphar.2022.820071.
- Li J, Gong X. Bibliometric and visualization analysis of kidney repair associated with acute kidney injury from 2002 to 2022. Front Pharmacol. 2023;14:1101036. doi: 10.3389/fphar.2023.1101036.