Abstract
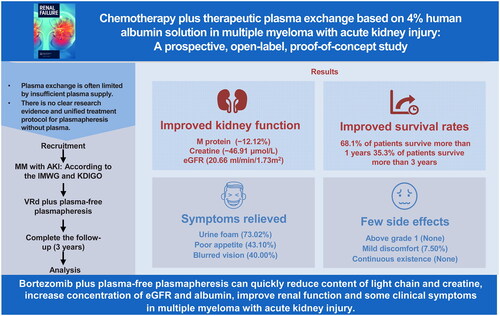
As no unified treatment protocol or evidence yet exists for plasmapheresis without plasma, this study explored the outcomes of using 4% human albumin (ALB) solution as a replacement solution in patients undergoing plasma exchange for multiple myeloma (MM) patients with acute kidney injury (AKI). This study was prospectively registered (ChiCTR2000030640 and NCT05251896). Bortezomib-based chemotherapy plus therapeutic plasmapheresis (TPP) with 4% human ALB solution was assessed for three years in patients with MM aged >18 years, with AKI according to the Kidney Disease Improving Global Outcomes criteria, and without previous renal impairment from other causes. The primary endpoints were changes in renal function over 18 weeks and survival outcomes at 36 months. The secondary endpoints were the incidence of adverse reactions and symptom improvement. Among the 119 patients included in the analysis, 108 experienced renal reactions. The M protein (absolute changes: median −12.12%, interquartile ranges (IQRs) −18.62 to −5.626) and creatine (median −46.91 μmol/L, IQR −64.70 to −29.12) levels decreased, whereas the estimated glomerular filtration rate (eGFR) increased (median 20.66 mL/(min·1.73 m2), IQR 16.03–25.29). Regarding patient survival, 68.1% and 35.3% of patients survived for >12 and >36 months, respectively. The three symptoms with the greatest relief were urine foam, poor appetite, and blurred vision. All 11 patients (7.6%) who experienced mild adverse reactions achieved remission. In conclusion, in MM patients with AKI, plasma-free plasmapheresis with 4% human ALB solution and bortezomib-based chemotherapy effectively alleviated light chain damage to kidney function while improving patient quality of life.
Introduction
Multiple myeloma (MM) generally occurs in older adults, of whom 88% are aged >50 years. MM begins with the monoclonal proliferation of B cells, resulting in the oversecretion of pathogenic immunoglobulins. Renal dysfunction occurs as a result of excessive light-chain (LC) secretion [Citation1]. Up to 50% of patients with MM have acute kidney injury (AKI), and approximately 10–15% require dialysis. Moreover, AKI is closely associated with a lower one-year survival rate and adverse quality of life [Citation2]. Early improvement of AKI is an important step in MM treatment. This is mentioned in every edition of the treatment guidelines published by the International Myeloma Working Group. Previous studies reported that plasmapheresis is an effective adjunct to chemotherapy for patients with MM with AKI [Citation3].
Therapeutic plasma exchange (TPE) plays a vital role in reducing progressive and permanent damage to the kidney by rapidly removing large amounts of LCs. The well-known immunomodulatory mechanisms of plasmapheresis include removing full-size pathological molecules, correcting blood flow disturbances, and ion homeostasis. Recent studies have shown that plasmapheresis also blocks Fc receptors, competitively inhibiting the release of immunoglobulins and specific pathogenic substances and the feedback response, thus preventing the production of pathological antibodies [Citation4,Citation5]. Thus, TPE can simultaneously correct several dysregulated biological pathways to restore homeostasis [Citation6–8].
Despite the current clinical experience regarding the efficacy of chemotherapy plus plasmapheresis, most studies were conducted before new drugs, such as bortezomib, and were almost inseparable from using fresh frozen plasma [Citation9–11]. Plasma, as a nonrenewable resource, can only be obtained from volunteer blood donors [Citation12]. In addition, massive plasma transfusions are associated with a high risk of adverse immune side effects, such as transfusion-related immune modulation and latent viral infections, further endangering the antitumor function and health of patients with MM [Citation13,Citation14]. Therefore, the large demand for plasma in TPE limits the development of treatments, and developing new methods that do not require plasma is challenging.
Owing to cancer and the use of drugs, patients often experience an albumin (ALB) deficiency and a decreased anion gap [Citation15]. Albumin solution is a tailored plasma derivative with excellent biocompatibility [Citation16]. There was no significant difference in the complication rate associated with the transfusion of ALB, plasma, or a combination of both (15.1%) [Citation17]. Currently, 4–5% ALB solutions are widely used in patients with liver cirrhosis and those requiring intensive support [Citation18]. Based on our past treatment experience, substituting 4% human ALB solution for plasma can correct hypoalbuminemia in patients with AKI while maintaining osmotic pressure. In addition, the massive proliferation of tumors creates an acidic and hypoxic microenvironment [Citation19]. This accumulation of acidic substances is not conducive to rapid chemotherapy. Thus, identifying a buffering molecule that can improve the plasma environment while protecting the proliferation of normal cells under hypoxic conditions is particularly important. We found that Ringer’s solution met these criteria and is commonly used for aggressive resuscitation. Therefore, we propose a new solution proportioning method for therapeutic plasmapheresis (TPP) that uses equal amounts of 4% ALB solution, Ringer’s solution, and normal saline. Herein, we examined the outcomes of using 4% human ALB solution (plasma-free plasmapheresis, PFP) as a replacement solution in patients undergoing plasma exchange for MM with AKI.
Materials and methods
Patients and treatment
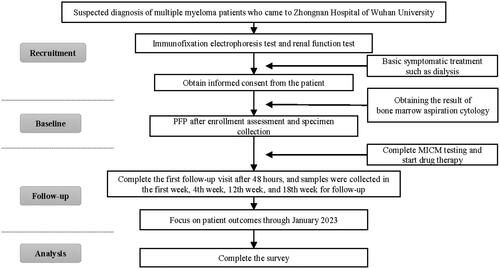
This prospective, open-label interventional trial was conducted at Zhongnan Hospital of Wuhan University from 2020 to 2022. Patients were screened within one day before enrollment. The inclusion criteria were male and female patients with MM accompanied by AKI with Durie–Salmon (DS) stage I–III, aged >18 years, without New York Heart Association (NYHA) D cardiac insufficiency, without psychiatric illness leading to hospitalization, without other hematological tumors, without previous renal impairment from other causes (such as hypertension and type 2 diabetes), who did not undergo other forms of TPP, who did not take part in other clinical trials, and who had no plans to use drugs other than chemotherapy drugs. The following laboratory tests were performed: bone marrow biopsy, chromosome analysis, genetic testing, flow cytometry, routine blood tests, coagulation function tests, liver and kidney function tests, electrolyte panels, myocardial protein determination, brain natriuretic peptide tests, electrocardiography, echocardiography, and plasma M protein content determination. Based on the renal function impairment standards defined by the International Myeloma Working Group, Kidney Disease Improving Global Outcomes criteria, and previous studies, AKI was defined as a serum creatinine >177 µmol/L or estimated glomerular filtration rate (eGFR) <50 [Citation20–22]. PFP was scheduled in conjunction with a standard chemotherapy regimen of bortezomib plus lenalidomide and dexamethasone (VRd). Patients with cast nephropathy were either confirmed by biopsy (20%) or exhibited a high likelihood (free LC assay >2 g/L). Patients with transient renal failure resulting from hydration or hypercalcemia were excluded because of the necessity for TPP.
Both the patients and primary guardians understood the specific details of the treatment. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2020037). Informed consent was obtained from all participants. If a patient or legal representative disagreed with the research, they were informed of their right to object to using the research data obtained from that patient. This study was initially registered in the Chinese Clinical Trial Registry (ChiCTR2000030640) and later in www.clinicaltrials.gov (NCT05251896). The study flowchart is illustrated in .
Measurements of physiological indicators and assessments
The patients then started bortezomib-based chemotherapy immediately until the 18th week or until the occurrence of the outcome event, for which they discussed the possibility of transplantation. Preoperative data were based on indicators at the time of initial enrollment. The PFP data were based on those obtained on the third day after treatment to exclude the interference of transient fluctuations. The following self-reported items were obtained from medical records using Wenjuanxing online questionnaire survey software. Telephone-based assessments were permitted when scheduling conflicts occurred. Sex, age, race, and ethnicity were self-reported. The researcher also reviewed other medical information from the patients’ medical records. The follow-up time was defined as the period from the day of surgery until the next day. All measurements were performed independently in a clinical biochemical laboratory. The funders had no role in the study’s design, analysis, or reporting.
The primary endpoints were survival outcomes in the 18th week and changes in renal function during those 18 weeks. The definition of renal response was given by the International Myasthenia Working Group (IMWG) [Citation23]. For a more comprehensive understanding of the long term, we extended our observation period to include the survival outcomes of patients up to the 36th month. The secondary endpoint was symptom improvement. Symptom improvement was defined as a reduction in the frequency of episodes (e.g., blurred vision), a decrease in the intensity of episodes (e.g., shortness of breath), or an increase in self-perceived well-being (e.g., improved appetite).
Equipment and replacement fluid
The development of plasmapheresis was based on the standard TPE program of the Fresenius Kabi blood cell separator (Com. Tec;) and a new replacement fluid formula. The displacement volume was 0.6 times the estimated plasma volume (EPV), calculated as Displacement volume (mL) = 39 × weight (kg) × (1–Hematocrit) [Citation24]. The operators ensured that the replacement fluid and the discarded plasma were equal volumes of approximately 1500 mL each. The replacement fluid was infused in the following order: 450 mL of normal saline, 500 mL of Ringer’s solution, 500 mL of 4% ALB, and 50 mL of normal saline. If the EPV was >1500 mL, the operators used more Ringer’s solution; otherwise, the amount of saline used in the first step was reduced [Citation25,Citation26]. Intravenous access was created on the left and right arms, plasma was centrifuged at 1500–2000 rpm, an anticoagulant (sodium citrate) was administered, and the blood flow rate was maintained at 30–55 mL/min (anticoagulant:whole blood = 1:10 to 1:12). The TPE process lasted for 1–2 h, during which time the patients underwent continuous electrocardiogram and oxygen saturation monitoring.
Statistical analyses
Both diagnosis and typing confirmation were completed within one week of the first visit or study enrollment. Quantile–quantile plots and the Shapiro–Wilk test were used to assess the normality of distributions of continuous variables. Normally distributed data are reported as the means ± standard deviations, whereas nonnormally distributed data are reported as medians (interquartile ranges, IQRs). Paired t-tests or Wilcoxon’s signed-rank tests for continuous variables and Chi-square tests for categorical variables were used to compare differences before and after treatment. The results were considered statistically significant at p < 0.05. Reductions in laboratory parameter values were defined as improvements; otherwise, they were described as ineffective. Treatment-related adverse events were assessed using the Common Terminology Criteria for Adverse Events v4.0. The proportion of missing values for a few variables was <10%; therefore, multiple imputation methods were applied using SPSS (SPSS Inc., Chicago, IL) [Citation27]. The final results were determined through discussion among three clinical experts. All analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY).
Results
Baseline characteristics
This study included 119 patients (54 women and 65 men) with confirmed MM accompanied by AKI who underwent VRd plus TPP with 4% human ALB solution. These patients received 145 fluid replacements, with 21.8% experiencing more than one replacement. The median number of operations was one (IQR 1–2). Only one 86-year-old woman underwent two rounds of TPP (four times in the first week and three times in the 10th week) due to repeated increases in LC levels. Because the interval between the two AKIs was long and was related to changes in LC levels, the two AKIs were considered independent events. Thirty percent of patients underwent autologous stem cell transplantation after 18 weeks. No patient underwent allogeneic stem cell transplantation. The subjective reports of each patient were based on nursing records and patient responses to questionnaires. The demographic data and clinical characteristics are presented in .
Table 1. Clinical characteristics of the primary analysis population.
Primary endpoints
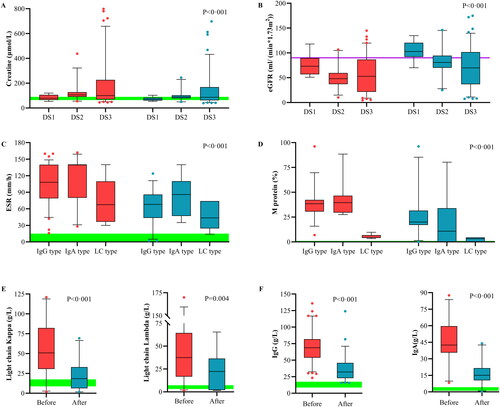
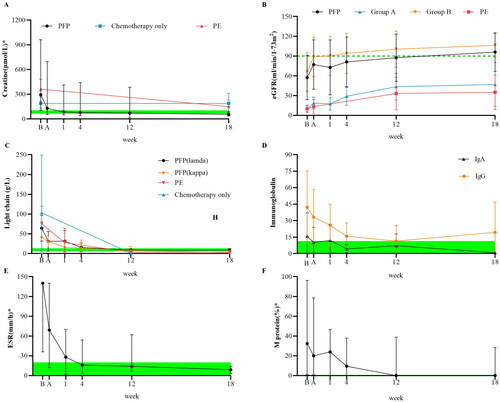
We assessed the ability of PFP to improve renal function in . A total of 108 (90.8%) patients experienced renal reactions. The beta-2 microglobulin (β2-MG) and creatine initial blood concentrations increased with increasing DS stage. PFP significantly reduced β2-MG (−107.7 mg/L, −7.6 to −227), creatine (−11.7 μmol/L, −5.6 to −31.3), and uric acid (UA, −103.8 μmol/L, −88.6 to −119.6) levels and significantly improved the eGFR (16.8 mL/(min·1.73 m2), 6.8 to 31.0). The eGFR in 80% of patients with DS1 was >90 mL/(min·1.73 m2). The absolute value and clearance rate were greater in patients with higher basal concentrations. Spearman’s rho analysis showed that a decrease in creatinine was negatively correlated with the International Scoring System (ISS) score (r = −0.205, p = 0.014) and positively correlated with the initial eGFR (r = 0.655, p < 0.001), whereas age was negatively correlated with an increase in the eGFR (r = −0.240, p = 0.006). Regarding eGFR, patients with the kappa type benefited more from treatment than those with the lambda type (median 17.97 vs. 16.79 mL/(min·1.73 m2)), and female patients benefited more from treatment than male patients (20.27 vs. 14.03 mL/(min·1.73 m2)). M protein levels were also significantly reduced (absolute changes: median −12.12%, −18.62 to −5.63). The initial erythrocyte sedimentation rate (ESR) for IgA type was much greater than that for the LC type (median 140.0 vs. 67.5 mm/h), but all types of ESR were effectively reduced (36.03 mm/h, −47.47 to −24.59). After PFP, the percentage of free LCs was reduced to 74.97% (range 33.10–89.54%), and the percentage of clearance in the 18th week was 92.29% (range 74.64–99.94%). IgG and IgA clearance rates of differed slightly (median −33.03 vs. −30.58 g/L). However, in LC typing, the clearance of LC kappa (LC κ, −23.39 g/L, −34.63 to −12.14) was better than that of LC lambda (LC λ, −27.26 g/L, −44.72 to −9.80).
Figure 2. Effects of PFP on the clearance of substances with different molecular weights. Box plots comparing the curative effects of renal biomarkers (A, B) and immunological biomarkers (C–F), demonstrating the ability of plasma-free plasmapheresis with 4% albumin (PFP) to scavenge small, medium, and large molecules. A paired t-test was performed with the values before (red) and after (blue) PFP. Green: normal reference range. The analysis results are shown as a box plot for the Durie–Salmon stage. Box: interquartile range (IQR) from the first to the third quartiles, with the median value in the middle. Whiskers: 5th to 95th percentiles. Circles: values outside this range.
shows the changes in renal function and pathological immune substances in patients according to treatment time. These values were then compared with those of chemotherapy plus TPE and chemotherapy only. The patient’s serum creatinine decreased rapidly after PFP and could be reduced to less than or equal to 150 µmol/L in most cases. Half of the patients’ creatinine returned to normal after one week. The renal function of patients at 18 weeks was significantly better than the initial renal function, and most patients recovered to age-matched renal function. Patients treated with PFP exhibited more rapid recovery within the first week and greater preservation of renal function at the 18th week. In patients with poor initial renal function (group A), PFP did not significantly differ from TPE. PFP and TPE did not differ significantly in the early removal of LCs but were significantly better than chemotherapy alone. Most patients with the IgG type had a greater initial pathological protein load, were more likely to recover quickly from the disease, and were more likely to have repeated increases in pathological proteins. Serum immunoglobulin levels in patients with the IgA type are more easily controlled by PFP. The initial ESR of most patients exceeded the detection limit of the instrument (140 mm/h). After 18 weeks, the patients’ median ESR decreased by more than or equal to 120 mm/h. After the first week of treatment, the median concentration of M protein in the serum decreased by 8.4%. By the end of the first month of treatment, the median serum M protein concentration had decreased by 23.0%.
Figure 3. Outcome measures at the 18-week follow-up. Follow-up testing of renal (A, B) and immunological biomarkers (C–F). The points on the polyline represent the median value. The whiskers represent the ranges of the maximum and minimum values. The green shading represents the normal reference range. Normally, the M protein is not visible. According to the initial renal function, the patients were divided into groups A and B according to a cutoff of 20 mL/(min·1.73 m2). The light chain (LC) and Ig types were determined using immunofixation electrophoresis at enrollment. The results of chemotherapy alone and therapeutic plasma exchange (TPE) were obtained from previous studies that included patients with the same baseline estimated glomerular filtration rate (eGFR) or chemotherapy plan.
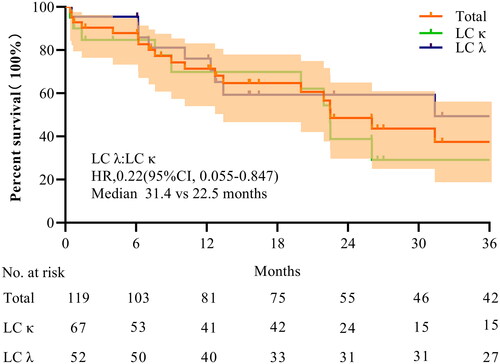
The follow-up results are shown in . The median survival time of patients with AKI was 26.03 months (IQR 14.6–37.44). At the 18th week, a total of 103 patients were alive. The overall survival rate was 86.86%, with survival rates of 83.58% for LC κ patients and 94.86% for LC λ patients. A total of 68.1% and 35.3% of patients survived for >12 and >36 months, respectively. At the 36th month, 42 patients were alive, with over half being LC λ patients. Furthermore, Kaplan–Meier’s survival curves of patients grouped according to free LC type showed that although the two curves did not differ significantly in the first year, they separated in the second year. LC lambda patients had longer median survival than did LC κ patients (median 31.4 vs. 22.5 months) but had more severe illness at the first visit (median 39.2 vs. 77.1 mL/(min·1.73 m2)).
Secondary endpoints
shows the results of secondary endpoints. To fully assess the status of the patients during the replacement process, we conducted a focused investigation of possible symptoms through bedside surveys of the patients’ subjective feelings. Approximately, 40.34% of the questionnaire-based surveys were conducted on site. Missing data were collected through telephone interviews (5.88%) or patient interviews conducted during the next hospital stay (14.20%). The survey results showed that the top three relief symptoms were urine foam (73.02%), poor appetite (43.10%), and blurred vision (40.00%).
Figure 5. Subjective feelings and the first hospitalization length of stay (FLOS). The statistical results for symptom improvement (A) are based on questionnaires and nursing records. Red: significant improvement, orange: mild improvement, blue: partial improvement, and grey: no relevant symptoms (NRS) at the time of onset. The violin plot (B) shows the FLOS of multiple myeloma hospitalizations for patients with different admission statuses. According to the estimated glomerular filtration rate (eGFR) at enrollment, group A included patients with an eGFR ≤20 mL/(min·1.73 m2), whereas group B included the remaining patients. Trends in overall survival and different subgroups are shown. Patients with severe infection, anuria, systemic failure, or rheumatic disease were considered critically ill, whereas the remaining patients were considered mildly ill. The horizontal black line in the middle of the violin represents the median value. The horizontal white lines below and above represent the IQRs from the first to the third quartiles.
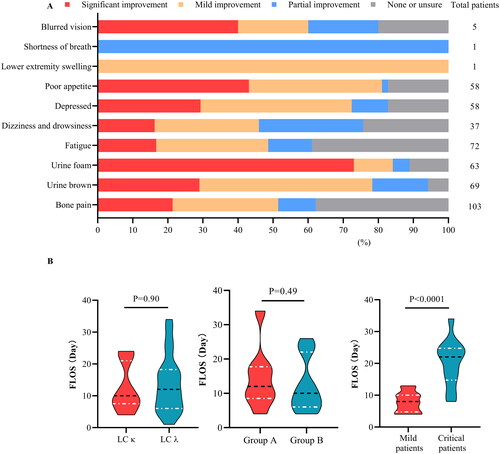
Among survivors, the LC immunophenotype had no significant effect on the length of hospital stay between the LC λ group and the LC κ group (median 12.0 vs. 10.0 days). However, patients with poor initial renal function had a significantly longer hospital stay (median 12.0 vs. 10.0 days) and were more likely to have hospitalizations >30 days. Furthermore, hospital stay was associated with initial comorbidities in all patients. The first hospitalizations of patients with severe infection, rheumatic immune disease, and severe organ failure were significantly prolonged (median 22.0 vs. 8.0 days).
Safety analysis
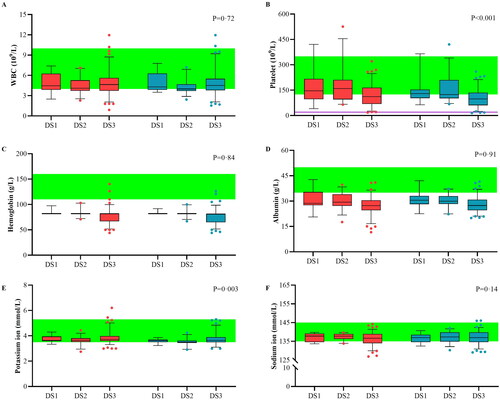
shows the fluctuations in intrinsic blood composition. The white blood cell (WBC, median 4.42 vs. 4.30 × 109/L) count and hemoglobin (median 76.00 vs. 76.00 g/L) levels showed negligible decreases. The platelet count tended to decrease (median −21.34 × 109/L, IQR −30.51 to −12.18) during the replacement process but was still >20 × 109/L. Most patients had mild to moderate anemia. PFP did not cause the destruction of red blood cells or worsen anemia. Albumin (mean 21.54 vs. 31.86 g/L) and sodium ion (mean 134.84 vs. 137.05 mmol/L) levels increased and play important roles in volume maintenance. Before treatment, seven patients developed mild hypokalemia, and two patients with stage 3 disease developed mild hyperkalemia. After PFP treatment, all patients with hyperkalemia were relieved without additional treatment. A total of 57.1% of patients with hypokalemia achieved complete remission. The remaining patients experienced symptom relief, which resolved with additional oral potassium supplementation. The change in potassium ion concentration (median −0.13 mmol/L, IQR −0.21 to −0.04) was statistically significant. After PFP treatment, the serum sodium ion concentration returned to normal in 75% of the patients. In patients with stage 3 DS and anuria, edema can be partially reduced through PFP, but hemodialysis is still required to remove small molecule waste.
Figure 6. Variability of critical blood components. The variability of major blood components was analyzed for plasma-free plasmapheresis with 4% albumin (PFP) using box plots for blood cells (A–C), colloid components (D), and crystal components (E, F). Red: values before PFP; blue: values after PFP; green: reference values. Analysis was performed using a paired t-test, and the data are presented as a box plot for the Durie–Salmon stage. Box: interquartile range (IQR) from the first to third quartiles, with the median value in the middle. Whiskers: 5th to 95th percentiles. Circles: values outside this range.
The results of adverse reactions are presented in . None of the patients developed adverse reactions above grade 1 or required treatment interruption during the treatment period, whereas 11 (7.59%) patients developed mild adverse reactions. However, these reactions did not affect the treatment course and eventually resolved on their own after nursing. The patient who reported mild dyspnea visited the hospital in the winter and reported a history of cold urticaria many times. After being provided with heat and oxygen inhalation, the patient’s symptoms disappeared after 20 min.
Table 2. Reported adverse events.
Discussion
This prospective study investigated the outcomes of using this novel plasma-free procedure in 119 patients who underwent MM with AKI. The standardized replacement volume and operating procedures eliminated the influence of confounding factors such as plasma and replacement fluids. This study spanned three years, with a median follow-up of 28.2 months, comparable to and possibly exceeding the latest staging standard R2-ISS stage 2 median progression-free survival [Citation28]. The median progression-free survival reached 26.03 months (95% confidence interval (CI) 13.1–53.6), which was comparable to and possibly exceeded the 24 months (95% CI 19–23) in Asian populations reported by the International Myeloma Working Group [Citation29]. Moreover, 68.1% of patients in our study survived >1 year, and 35.3% survived >3 years. The effect of LC type on long-term survival cannot be ignored (HR = 0.22). This study fills some gaps in clinical research on myeloma complicated by AKI since the advent of bortezomib, in the context of new staging systems, and after the launch of LC typing testing.
We advocate the art of balancing treatment efficacy and complications, which means that the composition of the replacement fluid deserves more attention [Citation30]. To correct hypoalbuminemia and exert the ultrafiltration effect of ALB, most fluid resuscitation methods use a 5% ALB solution. However, ALB does not work as well as it should in theory. In addition, high ALB levels may induce or worsen AKI in patients with sepsis or shock [Citation31]. Moreover, the use of expensive ALB also significantly increases medical expenses. In addition to normal saline, which can replenish the circulating blood volume, Ringer’s solution is also frequently used for aggressive resuscitation [Citation32]. It provides sodium lactate, a beneficial molecule in various illnesses and that humans can metabolize with lower oxygen consumption under ischemic conditions [Citation33]. Similarly, Ringer’s solution does not cause hyperchloremic acidosis, unlike normal saline, when infused in large volumes [Citation34].
Although TPP is a lifesaving method, replacement induces several stressors. This can induce stress in the internal environment, such as operation intensity, immunogenicity of solute fluxes, and electrolytic shifts. Renal excretion is an important part of the potassium cycle, and potassium imbalance is associated with adverse renal outcomes [Citation35]. Potassium ion levels were significantly correlated (p = 0.012, Pearson’s r = −0.22) with patient renal function and improvement in relevance (p = 0.003, Pearson’s r = −0.26) after controlling for age and sex. As patients with AKI have poor renal excretion capacity, it is more beneficial to adjust the ion concentration in the blood to a low-normal value simultaneously with TPP. Therefore, using 4% ALB solution followed by Ringer’s solution and normal saline is one advantage of PFP.
The deposition of pathogenic monoclonal ingredients causes varying degrees of kidney damage [Citation36]. Immediate interventions to rapidly reduce the level of nephrotoxic secretions by >50% are a prerequisite for renal recovery [Citation37]. Regardless of the type of TPP, the LC concentration quickly decreased in the first week; however, the PFP concentration decreased by 54.80–59.40% within 48 h after replacement, whereas the LC κ and IgG concentrations nearly did not rebound at the end of the first week. β2-MG, a potential biomarker for the early detection of glomerular disease, is correlated with progression-free survival and recurrence time in patients with MM [Citation38,Citation39]. Ongoing monitoring revealed a median decrease in β2-MG of 59.6% at one month as treatment progressed. However, ordinary TPE has no effect [Citation3]. In comparable studies, Burnette et al. conducted TPE with fresh frozen plasma in which patients showed the same baseline eGFR as those in group A [Citation40]. In contrast, the patients in the study by Costa et al. were only treated with VCd chemotherapy (bortezomib, cyclophosphamide, and dexamethasone) and showed initial renal function closer to that in group B [Citation41]. For chemotherapy alone, the median eGFR increased by 34.5 mL/(min·1.73 m2), and 71.43% of patients had a renal response. Moreover, the addition of TPE led to an 80% renal response rate, along with a 90 μmol/L reduction in UA and a 60 μmol/L reduction in creatine [Citation42,Citation43]. In our study, 90.8% of patients’ kidneys demonstrated a positive response to PFP, with a median eGFR increase to 56.15 mL/(min·1.73 m2), a 103.8 μmol/L reduction in UA, and a 164.0 μmol/L reduction in creatine. In particular, 80% of patients in DS1 had eGFR values >90 mL/(min·1.73 m2). For patients with severe AKI with an eGFR <20 mL/(min·1.73 m2), PFP also achieved a better eGFR than did TPE in the third month (mean 43.5 vs. 33.2 mL/(min·1.73 m2)). Therefore, the feasibility of PFP in patients with MM accompanied by AKI was confirmed.
Furthermore, we provided new observational data, such as patient symptoms. Cancer-related symptoms strongly affect the quality of life and progression-free survival of patients with MM [Citation44,Citation45]. The association between cancer-related depression and symptoms of light chain disease refers to the persistent and severe emotional downturn that may occur during cancer treatment or in the context of having light chain disease. The results of the questionnaire survey showed that the three most common symptoms were bone pain, fatigue, and urine abnormalities, whereas the three most common symptoms were urine foam, poor appetite, and blurred vision. Patients may experience feelings of emotional distress, helplessness, and a loss of interest in daily activities. Blurred vision and poor appetite may be related to tissue edema caused by increased local blood viscosity. These symptoms may be related to the deposition of LC in the kidney, brain, intestines, spleen, and other organs and the direct infiltration and invasion of myeloma cells [Citation46–49]. This depressive state may be triggered by cancer itself, light chain disease, or their respective treatments. The initial ESR of most patients exceeded 140 mm/h. The median ESR after PFP was only 63 mm/h. This finding appears to be consistent with patient-reported symptom relief. PFP can rapidly reduce the concentration of metabolites and blood viscosity, which may explain the improvement in fatigue symptoms to varying degrees. A significant improvement was observed in 90.2% of the patients with depressive symptoms. Thus, TPP may help improve patient quality of life.
The first day of hospitalization was related to the remission of the patient’s condition and the economic expenses of hospitalization. The median FLOS in most patients was 10 days (IQR 6.8–17.6 days), which is consistent with Dhakal et al.’s reported hospital stay of 11.9 days (IQR 7.2–17.6 days) under TPE treatment [Citation50]. The median FLOS of patients with severe infection, rheumatic immune disease, severe organ failure, etc., was significantly prolonged (22.0 days). This finding also implies possible delays in initiating chemotherapy, multiple rescue operations, and the occurrence of adverse outcomes. Even in the era of diverse targeted drug options, considering plasmapheresis as an adjuvant treatment is logical [Citation51]. However, improvement was closely related to the state before fluid replacement (Spearman’s rho r = 0.655). Complications such as LC λ, initial eGFR <20 mL/(min·1.73 m2), and coinfection can lead to extremely long hospitalizations. Moreover, significant renal function damage cannot be completely reversed, which can also affect the occurrence of extremely long hospitalizations (>25 days).
We also acknowledge the debate regarding the effectiveness of TPP in MM patients with AKI. Zucchelli et al. contend that this therapy significantly improves patients’ renal function and extends survival [Citation52]. Johnson et al. reported a negative correlation between treatment outcomes and pretreatment renal function [Citation42]. Conversely, Clark et al. argued that this therapy is ineffective [Citation53]. Notably, Zucchelli and Johnson had observation periods exceeding 30 months, whereas Clark’s follow-up spanned only one year. Our study revealed that the median survival time for patients exceeded 26 months, with a decrease in the survival rate after 12 months of treatment. Moreover, Johnson’s results underscore a greater recovery rate in patients with LC κ than in those with LC λ (66.7% vs. 44.4%), aligning with the outcomes derived from our FLOS data. Thus, observing beyond 28 months becomes crucial for understanding the sustained effects of TPE in this context. This extended period allows for a more nuanced comprehension of the long-term impact on patients.
TPP has undergone exploration for several generations and is a remarkable and generally safe procedure, although occasional complications may arise. We observed that the inherent blood components were unaffected. The average WBC count and hemoglobin levels were stable. A platelet concentration less than 20 × 109/L is considered an indication for the transfusion of fresh frozen blood. No patient required blood transfusions due to PFP. Complications of TPE primarily include nausea and vomiting due to blood volume fluctuations at the onset of treatment (4%), hypocalcemia (3%), and pruritus (1.7%). Infrequently, it may lead to glucose abnormalities, thrombosis, and pulmonary infections, but symptomatic consequences related to thrombosis are extremely rare. Among these factors, the risks of blood volume fluctuations and hypocalcemia are more significant for patients with lower total circulating blood volume and electrolyte imbalances. However, these risks can be mitigated by controlling the exchange rate and adjusting the formulation of the replacement fluid. Therefore, in our protocol, the total exchange volume is correlated with the patient’s body weight and hematocrit. The amount of anticoagulant used (anticoagulant:whole blood) was also increased from the usual 1:9 ratio to 1:10 to 1:12. Hypoproteinemia and hyponatraemia were corrected, which reduced the risk of worsening AKI or the occurrence of adverse reactions [Citation54]. Moreover, only 2.06% of patients experienced hypotension during PFP replacement, and recovered spontaneously after reducing the flow rate or completing treatment. Five patients (4.2%) experienced mild adverse reactions, resolved with oxygen inhalation, rest, and other care. As an adjuvant treatment, PFP was associated with fewer adverse effects than fresh frozen plasma (7.6% vs. 20%) [Citation55]. This provides a new approach for clinical practice.
This study has several limitations and warrants further research. First, the study participants did not include ethnic minorities or other races, which may have resulted in slight and acceptable differences in the survival and tolerability of the substitution fluid. Second, owing to medical restrictions, we only evaluated sensitive indicators at the serological level without renal biopsy. However, in preliminary research and the standard from the International Myeloma Working Group on myeloma nephropathy treatment by TPE, we included patients with a high probability of cast nephropathy with free LC levels >5 g/L [Citation28,Citation40]. Based on the survival differences among immunotypes, subsequent studies should perform immunofluorescence pathological examinations. Third, the platelets were relatively light and were easily lost at a median of 21.34 × 109/L during the replacement process, and patients with coagulation disorders are at greater risk of bleeding. Therefore, these patients should be supplemented with coagulation factors or TPE.
In essence, our study not only provides novel insights into the treatment approach for MM accompanied by AKI but also provides valuable data and methodologies, particularly in the context of TPP with 4% human ALB solution. Simultaneously, we contend that an observation period exceeding 28 months and a clear delineation of patients’ free light chain types are imperative for resolving some of the controversial results observed in prior studies. Our research sets the stage for future investigations, suggesting a need for in-depth exploration into the long-term effects of TPP and the varied responses within different patient subgroups. Before extending this method to a broader patient population, larger-scale randomized controlled trials are still warranted to validate our findings further.
Conclusions
Bortezomib plus TPP with 4% human ALB solution can quickly reduce the light chain and creatine content, increase the eGFR and ALB concentration, and improve renal function. Some clinical symptoms in patients with MM accompanied by AKI. Standardized operating procedures and detailed replacement fluid formulas are provided for reference. Considering that the replacement fluid is easier to obtain, PFP may constitute a new replacement method for secretory myeloma.
Author contributions
Data analysis and design: FL Zhou, Tianzhi Wu, and Dandan Liu; administrative support: FL Zhou and Tianzhi Wu; provision of study materials or patients: FL Zhou, Tianzhi Wu, Shangqin Liu, Hui Xiao, Bei Xiong, Yi Zhou, Jiang Wu, and Minghui Liu; collection and assembly of data: FL Zhou, Tianzhi Wu, Dandan Liu, Yafen Xiong, Qin Cui, Hongli Liu, Yiming Li, Meixin Wang, Xueqin Bao, and Ye Li; manuscript writing: all authors; final approval of manuscript: all authors.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University.
Consent form
Written informed consent was obtained from all patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets and resources analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):1–13. doi: 10.1002/jca.21705.
- Lameire N, Vanholder R, Van Biesen W, et al. Acute kidney injury in critically ill cancer patients: an update. Crit Care. 2016;20(1):209. doi: 10.1186/s13054-016-1382-6.
- Premuzic V, Batinic J, Roncevic P, et al. Role of plasmapheresis in the management of acute kidney injury in patients with multiple myeloma: should we abandon it? Ther Apher Dial. 2018;22(1):79–86. doi: 10.1111/1744-9987.12606.
- Manook M, Flores WJ, Schmitz R, et al. Measuring the impact of targeting FcRn-mediated IgG recycling on donor-specific alloantibodies in a sensitized NHP model. Front Immunol. 2021;12:660900. doi: 10.3389/fimmu.2021.660900.
- Díaz F, Cores C, Atenas O, et al. Rationale of therapeutic plasma exchange as rescue immunomodulatory treatment for MIS-C with multiorgan failure. Pediatr Infect Dis J. 2021;40(7):e259–e262. doi: 10.1097/INF.0000000000003169.
- Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130(11):5967–5975. doi: 10.1172/JCI140970.
- Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26(11):1701–1707. doi: 10.1038/s41591-020-1054-6.
- Diorio C, McNerney KO, Lambert M, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4(23):6051–6063. doi: 10.1182/bloodadvances.2020003471.
- Sethi J, Ramachandran R, Malhotra P, et al. Plasma exchange in the management of new onset multiple myeloma with cast nephropathy treated with bortezomib based chemotherapy. Nephrology. 2017;22(12):1035–1036. doi: 10.1111/nep.12979.
- Leung N, Gertz MA, Zeldenrust SR, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73(11):1282–1288. doi: 10.1038/ki.2008.108.
- El-Achkar TM, Sharfuddin AA, Dominguez J. Approach to acute renal failure with multiple myeloma: role of plasmapheresis. Ther Apher Dial. 2005;9(5):417–422. doi: 10.1111/j.1744-9987.2005.00322.x.
- Stanworth SJ, New HV, Apelseth TO, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–e764. doi: 10.1016/S2352-3026(20)30186-1.
- Alcorn K, Ramsey G, Souers R, et al. Appropriateness of plasma transfusion: a College of American pathologists Q-Probes study of guidelines, waste, and serious adverse events. Arch Pathol Lab Med. 2017;141(3):396–401. doi: 10.5858/ARPA.2016-0047-CP.
- McCullagh J, Cardigan R, Brunskill SJ, et al. Assessing the risks of haemolysis as an adverse reaction following the transfusion of ABO incompatible plasma-containing components – a scoping review. Blood Rev. 2022;56:100989. doi: 10.1016/j.blre.2022.100989.
- Rumman A, Candia R, Sam JJ, et al. Public versus private drug insurance and outcomes of patients requiring biologic therapies for inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:7365937. doi: 10.1155/2017/7365937.
- Covington ML, Voma C, Stowell SR. Shortage of plasma-derived products: a looming crisis? Blood. 2022;139(21):3222–3225. doi: 10.1182/blood.2021015370.
- Bai Z, Chen Y, Dong L. Experience of therapeutic plasma exchange in rheumatic diseases: albumin may be a suitable substitute for plasma. Arch Rheumatol. 2021;36(3):398–408. doi: 10.46497/ArchRheumatol.2021.8447.
- Yu X, Gan L, Wang Z, et al. Chemotherapy with or without plasmapheresis in acute renal failure due to multiple myeloma: a meta-analysis. Int J Clin Pharmacol Ther. 2015;53(5):391–397. doi: 10.5414/CP202245.
- Hasan MN, Capuk O, Patel SM, et al. The role of metabolic plasticity of tumor-associated macrophages in shaping the tumor microenvironment immunity. Cancers. 2022;14(14):3331. doi: 10.3390/cancers14143331.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789.
- Joshua DE, Bryant C, Dix C, et al. Biology and therapy of multiple myeloma. Med J Aust. 2019;210(8):375–380. doi: 10.5694/mja2.50129.
- Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib–doxorubicin–dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28(30):4635–4641. doi: 10.1200/JCO.2010.28.1238.
- Dimopoulos MA, Merlini G, Bridoux F, et al. Management of multiple myeloma-related renal impairment: recommendations from the International Myeloma Working Group. Lancet Oncol. 2023;24(7):e293–e311. doi: 10.1016/S1470-2045(23)00223-1.
- Ahmed S, Kaplan A. Therapeutic plasma exchange using membrane plasma separation. Clin J Am Soc Nephrol. 2020;15(9):1364–1370. doi: 10.2215/CJN.12501019.
- Wald R, Kirkham B, daCosta BR, et al. Fluid balance and renal replacement therapy initiation strategy: a secondary analysis of the STARRT-AKI trial. Crit Care. 2022;26(1):360. doi: 10.1186/s13054-022-04229-0.
- Inkinen N, Pettilä V, Valkonen M, et al. Non-interventional follow-up versus fluid bolus in RESPONSE to oliguria in hemodynamically stable critically ill patients: a randomized controlled pilot trial. Crit Care. 2022;26(1):401. doi: 10.1186/s13054-022-04283-8.
- Little RJA, Rubin DB. Statistical analysis with missing data. 3rd ed. Hoboken (NJ): Wiley; 2020. xii, 449 pp.
- D’Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: a European Myeloma Network (EMN) report within the HARMONY project. J Clin Oncol. 2022;40(29):3406–3418. doi: 10.1200/JCO.21.02614.
- Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242.
- Canaud B, Stephens MP, Nikam M, et al. Multitargeted interventions to reduce dialysis-induced systemic stress. Clin Kidney J. 2021;14(Suppl. 4):i72–i84. doi: 10.1093/ckj/sfab192.
- Hryciw N, Joannidis M, Hiremath S, et al. Intravenous albumin for mitigating hypotension and augmenting ultrafiltration during kidney replacement therapy. Clin J Am Soc Nephrol. 2021;16(5):820–828. doi: 10.2215/CJN.09670620.
- Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer’s versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2021;67(8):4131–4139. doi: 10.1007/s10620-021-07269-8.
- Ichai C, Orban JC, Fontaine E. Sodium lactate for fluid resuscitation: the preferred solution for the coming decades? Crit Care. 2014;18(4):163. doi: 10.1186/cc13973.
- Singh S, Kerndt CC, Davis D. Ringer’s lactate. Treasure Island (FL): StatPearls; 2022.
- Rodan AR. Potassium: friend or foe? Pediatr Nephrol. 2017;32(7):1109–1121. doi: 10.1007/s00467-016-3411-8.
- Fermand J-P, Bridoux F, Dispenzieri A, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132(14):1478–1485. doi: 10.1182/blood-2018-04-839480.
- Bridoux F, Leung N, Belmouaz M, et al. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. 2021;99(3):570–580. doi: 10.1016/j.kint.2020.11.010.
- Liu S, Shang J, Lin Y, et al. Analysis of the clinical effects and outcome of patients with double-hit high-risk multiple myeloma. Zhonghua Zhong Liu Za Zhi. 2021;43(11):1209–1214. doi: 10.3760/cma.j.cn112152-20200109-00016.
- Li Q, Hu C-D, Zhang C-C, et al. Predictive value of newly diagnosed IgG level in patients with IgG-type multiple myeloma after initial treatment. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(6):1825–1830. doi: 10.19746/j.cnki.issn.1009-2137.2021.06.023.
- Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364(24):2365–2366. doi: 10.1056/NEJMc1101834.
- Costa LJ, Abbas J, Ortiz-Cruz KL, et al. Outcomes of patients with multiple myeloma and renal impairment treated with bortezomib, cyclophosphamide, and dexamethasone without plasma exchange. Eur J Haematol. 2012;89(5):432–434. PMID: 22971164. doi: 10.1111/ejh.12008.
- Johnson WJ, Kyle RA, Pineda AA, et al. Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med. 1990;150(4):863–869. doi: 10.1001/archinte.1990.00390160111022.
- Kalpakci Y, Hacibekiroglu T, Darcin T, et al. Efficacy and safety of plasmapheresis in symptomatic hyperviscosity and cast nephropathy: a multicenter experience in Turkey. Transfus Apher Sci. 2021;60(5):103244. doi: 10.1016/j.transci.2021.103244.
- Chen F, Leng Y, Ni J, et al. Symptom clusters and quality of life in ambulatory patients with multiple myeloma. Support Care Cancer. 2022;30(6):4961–4970. doi: 10.1007/s00520-022-06896-9.
- Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(10):1317–1330. doi: 10.1016/S1470-2045(20)30452-6.
- Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818–829. doi: 10.1002/ajh.26569.
- Sethi S, Rajkumar SV, D’Agati VD. The complexity and heterogeneity of monoclonal immunoglobulin-associated renal diseases. J Am Soc Nephrol. 2018;29(7):1810–1823. doi: 10.1681/ASN.2017121319.
- Sobue Y, Takemura G, Kawamura S, et al. Coexistence of amyloidosis and light chain deposition disease in the heart. Cardiovasc Pathol. 2021;51:107315. doi: 10.1016/j.carpath.2020.107315.
- Mercado JJ, Markert JM, Meador W, et al. Primary CNS nonamyloidogenic light chain deposition disease: case report and brief review. Int J Surg Pathol. 2017;25(8):755–760. doi: 10.1177/1066896917717338.
- Dhakal B, Miller S, Rein L, et al. Trends in the use of therapeutic plasma exchange in multiple myeloma. J Clin Apher. 2020;35(4):307–315. doi: 10.1002/jca.21798.
- Fabbrini P, Finkel K, Gallieni M, et al. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the Onconephrology Work Group of the Italian Society of Nephrology. J Nephrol. 2016;29(6):735–746. doi: 10.1007/s40620-016-0347-9.
- Zucchelli P, Pasquali S, Cagnoli L, et al. Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int. 1988;33(6):1175–1180. doi: 10.1038/ki.1988.127.
- Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143(11):777–784. doi: 10.7326/0003-4819-143-11-200512060-00005.
- Wiedermann CJ, Wiedermann W, Joannidis M. Correction to: hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2021;47(2):262. doi: 10.1007/s00134-020-06268-z.
- Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994;23(6):817–827. doi: 10.1016/s0272-6386(12)80135-1.