Abstract
Background and objectives
Acute kidney injury (AKI) is one of the most common and severe clinical syndromes of diffuse proliferative lupus nephritis (DPLN), of which poor prognosis is indicated by aggravated renal function deterioration. However, the specific therapy and mechanisms of AKI in DPLN remain to be explored.
Methods
The correlation between AKI and clinical pathological changes in DPLN patients was analyzed. Expression of STAT3 signaling was detected in MRL/lpr mice with DPLN using immunohistochemical staining and immunoblotting. Inhibition of STAT3 activation by combination therapy was assessed in MRL/lpr mice.
Results
Correlation analysis revealed only the interstitial leukocytes were significantly related to AKI in endocapillary DPLN patients. MRL/lpr mice treated with vehicle, which can recapitulate renal damages of DPLN patients, showed upregulation of STAT3, pSTAT3 and caspase-1 in renal cortex. FLLL32 combined with methylprednisolone therapy significantly inhibited the STAT3 activation, improved acute kidney damage, reduced the interstitial infiltration of inflammatory cells and decreased the AKI incidence in MRL/lpr mice.
Conclusion
STAT3 activation may play an important role in the pathogenesis of DPLN and the development of AKI. Hence, STAT3 inhibition based on the combination of FLLL32 with methylprednisolone may represent a new strategy for treatment of DPLN with AKI.
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease. Lupus nephritis (LN) is one of the most common manifestations of SLE, affecting approximately 40–60% of patients with lupus. It is a major risk factor for morbidity and mortality, and 10% of LN patients will develop end-stage renal disease (ESRD) [Citation1,Citation2]. LN therapy has remained largely unchanged, with a probability of achieving complete or partial remission not exceeding 60–70% [Citation3–7]. Acute kidney injury (AKI) is common in lupus nephritis and is an independent risk factor for ESRD [Citation8–10]. The molecular mechanisms have been implicated in the pathogenesis of lupus and lupus nephritis [Citation11–25]. The pathogenesis of AKI in LN is incompletely understood and current therapies largely relying on the use of high-dose corticosteroids with cytotoxic agents have limited efficacy and carry significant risks of toxicity. A rational approach for therapeutic design requires a detailed understanding of AKI pathogenesis in LN.
Emerging studies have shown that signal transducer and activator of transcription-3(STAT3) signaling participate in the pathogenesis of LN, which is also strongly implicated in establishing a lupus inflammatory microenvironment [Citation20–22]. These studies found that T cell specific STAT3 deficiency or STAT3 inhibition prevents the development of glomerular nephritis in MRL/lpr mice. However, the mechanisms leading to tubulo-interstitial injury and AKI in LN have received relatively less attention to date. Although immune complexes are predominantly deposited in the glomerulus, approximately 70% of LN patients also have demonstrated tubulo-interstitial inflammation and injury [Citation26–29]. Our previous work had found the important role of STAT3 activation on AKI [Citation30]. We hypothesized that STAT3 signaling may also play critical role on the molecular pathogenesis of AKI and tubulo-interstitial inflammation in LN. FLLL32 is a diketone analog of curcumin, specifically as an inhibitor of the inflammatory signaling molecule Jak2/STAT3. The use of FLLL32 in lupus nephritis has not been explored. To develop mechanistic hypotheses that AKI in LN is associated with interstitial inflammation, we analyzed the relationship between AKI and renal pathological changes from diffuse proliferative lupus nephritis (DPLN) patients. The effects of combinative therapy of FLLL32 with methylprednisolone (MP) on the renal function and interstitial inflammation in MRL/lpr murine model would be tested.
Materials and methods
Study population
The study cohort came from DPLN patients reported in our previous study [Citation10]. Patients with renal pathological changes of subepithelial immune deposits, crescent, fibrinoid necrosis, hyaline deposits or chronic change (such as glomerular sclerosis, tubular atrophy or interstitial fibrosis) were excluded. 48 endocapillary DPLN patients without chronic changes were enrolled and divided into two groups: AKI and noAKI group. The protocol was approved by Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Animal models
MRL/lpr mice and five female C57BL/6 mice were obtained from the Sibeifu Laboratory Animal Co. Ltd (Peking, China) and maintained under specific pathogen-free conditions. Animal studies were approved by the Wenzhou Medical University Institutional Animal Care Committee. Fifteen 14-week-old female MRL/lpr mice with urine albumin over creatinine ratio (UACR) more than 1.0 g/g were selected and randomized into three groups with five animals each. Vehicle group was treated with DMSO intraperitoneally daily. MP group was treated with MP (0.4 mg/kg) orally daily. FLLL32 with MP group was treated with FLLL32 (50 mg/kg) intraperitoneally combined with MP (0.4 mg/kg) orally daily. At an age of 18 weeks, MRL/lpr mice and C57 mice were killed for determination of the extent of renal injury. Plasma and urine samples were collected at the terminal time point for biochemical measurements. Kidney specimens were harvested and fixed in 4% paraformaldehyde prior to histological examination and kidney cortices extracted for immunoblotting analysis.
Diagnosis of AKI
AKI was diagnosed according to the kidney disease: Improving Global Outcomes (KDIGO) AKI criteria [Citation31]. Diagnosis of patients AKI was increase in Scr by X26.5 μmol/l within 48 h or increase in Scr to X1.5 times baseline within the prior 7 days. Diagnosis of murine AKI was increase in Scr to X1.5 times baseline within the prior 7 days. The mean Scr level of C57 mice was used as baseline level.
Plasma and urine assay
The urine albumin-to-creatinine ratio (UACR) was determined using Albuwell M and the Creatinine Companion (Exocell, Phil., US). Scr were quantified by sarcosine oxidase enzymatic (SOE) assays and blood urea nitrogen (BUN) levels were determined by urease-UV fixed rate (enzymatic method). The testing of Scr, BUN and UACR was performed by qualified technicians in the department of Clinical Laboratory, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China. All biochemical tests were performed and analyzed by blinded experimenters.
Histopathological examination
Periodic Acid-Schiff (PAS) or Hematoxylin and eosin (H&E) staining of renal tissues were performed at Kidney Pathology Center of the First Affiliated Hospital of Wenzhou Medical University utilizing standard procedures. Histologic changes of renal tissue were scored based on the proposed modified NIH lupus nephritis activity scoring system [Citation32]. The severity of renal pathological lesions was scored according to the following criteria: Endocapillary hypercellularity in <25% (1 point), 25%–50% (2 points), or >50% (3 points) of glomeruli; Neutrophils and/or karyorrhexis in <25% (1 point), 25%–50% (2 points), or >50% (3 points) of glomeruli; Interstitial leukocytes in <25% (1 point), 25%–50% (2 points), or >50% (3 points) in the cortex.
Western blot analysis
Renal cortical tissues were lysed in Radio Immunoprecipitation Assay (RIPA) buffer containing protease inhibitor cocktail (Thermo Scientific, Shanghai.China). Protein samples were loaded on 4%-12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, MA, USA), probed with specific antibodies, and visualized with the Luminescent Imaging Workstation (Tanon, China). The band intensities were quantified using Image lab. Detection was performed using antibodies: STAT3, phospho-STAT3 (pSTAT3), Caspase-1 from Cell Signaling Technology (CST-US subsidiary in China. Pudong Shanghai). Mouse anti-β-actin antibody (CST-US subsidiary, China) was used as loading control.
Immunohistochemistry staining
For immunohistochemistry, formalin-fixed and paraffin-embedded 5 μm sections of the renal tissue specimens were stained using a Ventana Benchmark Immunostainer (VMS, Inc.,AZ, USA). Renal tissue sections were dewaxed and endogenous peroxidase activity was blocked using the Ventana’s universal DAB inhibitor. Primary antibodies against pSTAT3 (CST-US subsidiary in China) were diluted according to the manufacturer’s instruction and incubated with the sections. After washing, the tissue sections were incubated with biotinylated secondary antibodies, followed by incubation with an avidin-biotin-peroxidase complex for DAB substrate development using the ABC kit at room temperature, and they were mounted using Aqua PolyMount (Poly-sciences, Inc., PA, USA). A parallel paraffin-embedded section was prepared without the primary antibody as a negative control. Slides were scored by two reviewers independently according to the distribution of light staining in renal tissues.
RNA extraction and quantitative Real-Time PCR
RNA was extracted from snap-frozen renal tissues using TRIzol (Invitrogen. Thermo Fisher Scientific-CN, Pudong Shanghai, China). Reverse transcriptions were performed using the cDNA Reverse Transcription Kit (Invitrogen. Thermo Fisher Scientific, Pudong Shanghai, China) according to the manufacturer’s instruction. The Light Cycler and SYBR Green PCR Master Mix (Roche Life Science, China) were applied to detect mRNA expression with primer pair sequences. β-actin was used as an internal control. The 2−ΔΔct method was used to analyze the relative changes in mRNA expression. The sequences of primers used for qRT-PCR were listed as follows: β-actin, forward 5′AGGAGTACGATGAGTCCGGC-3′; reverse 5′AGGGTGTAAAACGCAGCTCAG-3′; STAT3 forward 5′CTGTGTGACACCATTCATTGATGC-3′; Reverse 5′- CGACTCAAACTGCCCTCCTG −3′; Caspase-1 forward 5′-TTT CCG CAA GGT TCG ATT TTC A −3′, reverse 5′-GGC ATC TGC GCT CTA CCA TC −3′;
Statistical analyses
Data were reported as means with standard deviation (SD). One-way ANOVA was used for analysis of three or more groups followed by Tukey’s test and unpaired t-test was used for analysis of two groups. For the correlation analysis, R2 was obtained and analyzed with Pearson correlation test for continuous variables and Spearman rank correlation test for categorical data. Significance was set at p < 0.05. p-value is indicated as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; p > 0.05, not significant (ns).
Results
AKI was associated with interstitial leukocytes infiltration in endocapillary DPLN patients
To investigate the main factors which contributed to the pathogenesis of AKI in endocapillary DPLN, the clinical and pathological factors of 48 endocapillary DPLN patients were analyzed. AKI patients had a significant increase in interstitial inflammation score compared with noAKI patients (1.6 ± 0.6 vs. 0.6 ± 0.5, p < 0.001). There were no significant differences in age, SBP, DBP, Hb, ALB, C3, dsDNA, UACR, eGFR, endocapillary hypercellularity in glomeruli, neutrophils in glomeruli between AKI and noAKI patients (). Representative histological pictures showed the monocyte infiltration around the glomeruli with interstitial edema in AKI patient () compared to noAKI patients (). Correlation analysis revealed only the interstitial leukocytes in the cortex was significantly related to AKI, R = 0.7, p < 0.001().
Figure 1. Representative pathological images (original magnification, ×200) and correlation analysis in DPLN patients. A, Representative images of noAKI patients showed endocapillary proliferation; B, Representative images of AKI patients showed endocapillary proliferation with interstitial leukocytes. C, Correlation analysis between AKI and clinical pathological characteristics revealed AKI was related to interstitial leukocytes in DPLN patients. AKI, acute kidney injury; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb: hemoglobin; ALB: serum albumin; C3, Serum complement3; dsDNA, anti-double stranded DNA; UACR, urine albumin to creatinine ratio; eGFR, evaluated glomerular filtration rate; Endo, endocapillary hypercellularity; Neutro, neutrophils; Intersti, interstitial leukocytes; Corr, correlation (R).
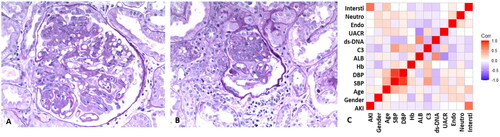
Table 1. Clinical characteristics of endocapillary DPLN patients.
18-week-old MRL/lpr mice recapitulated the AKI and renal pathological changes of DPLN patients
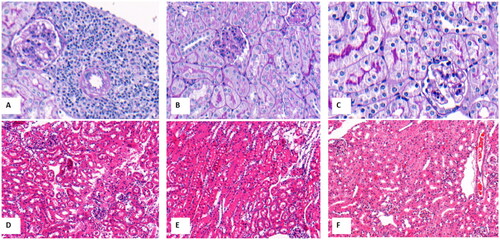
Vehicle group exhibited a significant increase in Scr compared with C57 group (0.13 ± 0.04 vs 0.08 ± 0.01 mg/dl, p < 0.001). Three of five mice developed AKI in vehicle group. AKI incidence in vehicle group was similar to that in DPLN patients (3/5 vs 20/48, p = 0.431). The incidence of overt UACR(≥0.3g/g) in vehicle group was higher significantly than that in C57 group (3/5 vs 0/5, p = 0.038). Vehicle group recapitulated endocapillary proliferation and interstitial inflammatory infiltration of the DPLN patients. AKI mice () had remarkable endocapillary proliferation and interstitial leukocytes infiltration compared to noAKI mice () in vehicle group and C57 group ().
Figure 2. The representative pathological changes of MRL/lpr mice and C57 mice. A, AKI mice with endocapillary proliferation and interstitial inflammation in vehicle group (PAS staining, ×200); B, noAKI mice with endocapillary proliferation in vehicle group(PAS staining, ×200); C, C57 mice with normal renal pathological changes(PAS staining, ×200); D, AKI mice with endocapillary proliferation and interstitial inflammation in vehicle group (H&E staining, ×100); E, noAKI mice with endocapillary proliferation in vehicle group (H&E staining, ×100); F, C57 mice with normal renal pathological changes (H&E staining, ×100).
FLLL32 with MP therapy inhibited STAT3 activation and caspase-1 expression
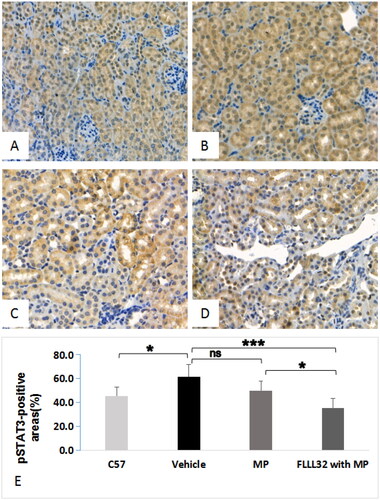
The expression of STAT3, pSTAT3, and caspase-1 in renal cortex was assessed by western blotting (). Compared with C57 group, vehicle group had remarkably higher expression of all these proteins (p < 0.001). Compared with vehicle group, FLLL32 with MP group had significantly lower expression of these proteins. However, in MP group, only the expression of caspase-1, but not STAT3 and pSTAT3, was significantly decreased, compared with that in vehicle group (). The mRNA expression of STAT3 was significantly increased in MRL/lpr mice compared to C57 mice, but not different among vehicle group, MP group and FLLL32 with MP group (). Compared with vehicle group, FLLL32 with MP group and MP group had remarkably lower expression of caspase-1 mRNA (). Immunohistochemistry in renal cortex showed a reduced expression of pSTAT3 in FLLL32 with MP group () compared to C57 group (), vehicle group () or MP group (). The percentage of pSTAT3-positive areas in renal cortex was 61.8 ± 10.2% and 35.6 ± 7.8% (p < 0.001) in FLLL32 with MP group and vehicle group ().
Figure 3. Expression of STAT3, pSTAT3 and Caspase-1. (A) Western blot analyses of STAT3, pSTAT3, Caspase -1 in renal cortex tissues. (B) Relative STAT3 protein expression. (C) Relative pSTAT3 protein expression. (D) Relative caspase-1 protein expression. (E) STAT3 mRNA expression. (F) Caspase-1 mRNA expression.
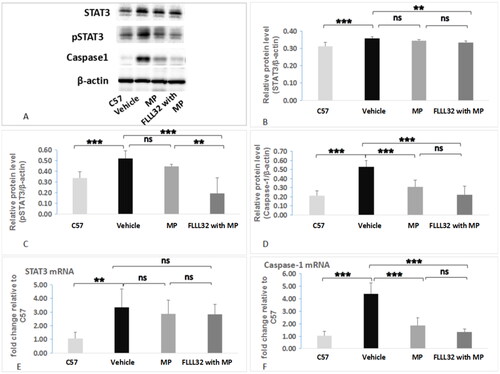
Figure 4. Representative images of pSTAT3 expression in renal tissue (immunochemistry staining, ×200) A. pSTAT3 expression in C57 mice; B, pSTAT3 expression in MRL/lpr mice treated with vehicle group; C, pSTAT3 expression of MRL/lpr mice treated with MP; D, pSTAT3 expression of MRL/lpr mice treated with FLLL32 with MP; E, Quantification analysis of pSTAT3-positive areas among four groups.
FLLL32 with MP therapy reduced AKI incidence and severity of interstitial inflammation in MRL/lpr mice
The AKI incidence decreased significantly in FLLL32 with MP group compared to vehicle group (0/5 vs 3/5, p = 0.038). The AKI incidence between MP group and vehicle group was not different (1/5 vs 3/5, p = 0.197). Scr levels in FLLL32 with MP group, but not in MP group, were significantly lower than that in vehicle group (). Interstitial infiltration of inflammatory cells was significantly more severe in vehicle group () than in MP group () and FLLL32 with MP group (). The mean score of interstitial inflammation in FLLL32 with MP group and MP group were lower significantly compared to vehicle group ().
Figure 5. Representative images (H&E staining, original magnification, ×50) and renal interstitial leukocytes. A, AKI mice in vehicle group showed remarkable interstitial infiltration of inflammatory cells; B, noAKI mice in MP group with less interstitial infiltration of inflammatory cells; C, noAKI mice in FLLL32 with MP group with less interstitial infiltration of inflammatory cells. D, comparison of interstitial inflammation.
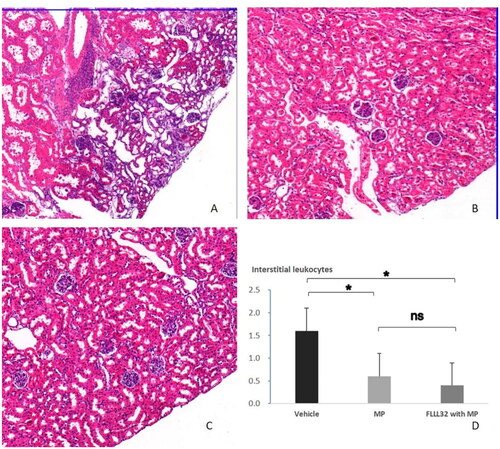
Table 2. Comparison of characteristics among four groups.
Discussion
Lupus nephritis displays a wide variety of glomerular histopathologic manifestations and clinical syndromes. There are several variations of histopathology involving those representatives of endocapillary proliferative, extracapillary proliferative and wire loop types in lupus nephritis type IV, which are supposed to be the causes of AKI in DPLN patients. Tubulointerstitial lesions have been found to determine the renal prognosis of patients with LN in previous studies [Citation33–36]. However, few studies focus on the pathophysiology of AKI in DPLN. In our study, 20 of 48 endocapillary DPLN patients developed AKI, which was associated with the severity of interstitial inflammations, but not with the severity of endocapillary proliferation. Previous studies also found that the degree of interstitial inflammation was positively correlated with the Scr level at the time of biopsy [Citation34,Citation35].
In order to understand the pathogenesis of AKI and interstitial inflammation in endocapillary DPLN, MRL/lpr mice was selected as the study model due to a good consistency with the process of human LN. Previous studies reported that glomerular proliferation and interstitial inflammation developed in 11–18 weeks old MRL/lpr mice, but the AKI incidence was unclear [Citation24,Citation37–39]. In our study, diffusive endocapillary proliferation and interstitial infiltration of inflammatory cells were observed in MRL/lpr mice. Moreover, vehicle group showed significant Scr elevation compared to C57 group and had the similar AKI incidence to DPLN patients. Thus, 18-week-old MRL/lpr mice in vehicle group can mimic the renal function and pathological changes of DPLN patients.
To investigate whether STAT3 signaling plays a role in LN, we analyzed expression of STAT3, pSTAT3 and caspase-1 in C57 group, vehicle group, MP group and FLLL32 with MP group. Previous studies found that increased STAT3 activation was observed in T cells from lupus patients and in the B cells from lupus prone mice [Citation40,Citation41]. Our findings of increased STAT3 and pSTAT3 expression in renal tissue of MRL/lpr mice in vehicle group are in agreement with data published in recent studies [Citation22]. The expression of STAT3, pSTAT3 and caspase-1 in FLLL32 with MP group was markedly decreased. The expression of STAT3 mRNA was not different among vehicle group, MP group and FLLL32 with MP group. However, the expression of caspase-1 mRNA was deceased significantly in FLLL32 with MP group and MP group compared to vehicle group. pSTAT3 can promote the activation of downstream Caspase-1 and down-regulation of pSTAT3 can repress caspase-1 activation [Citation42,Citation43]. Reduction of caspase-1 expression in FLLL32 with MP mice may be explained by inhibition of STAT3 activation in our study. Recent studies reported that combination therapy with mycophenolate mofetil, tacrolimus and steroids prevented interstitial immune cells infiltration through inhibition of caspase-1/GSDMD-mediated pyroptosis in MRL/lpr mice [Citation24]. Sato S. reported that STAT3 was significantly lower in the kidney of MRL/lpr Fli-1+/−, but leukocyte infiltration to inflamed tubulointerstitial lesions was not inhibited [Citation43], so reduction of interstitial infiltration in FLLL32 with MP group in our study was associated with the inhibition of STAT3 activation but not the lower expression of STAT3.
In our study, AKI diagnosis in MRL/lpr mice defined as increase in Scr to X1.5 times baseline, which derived from KDIGO AKI diagnosis, can adequately mimic AKI in DPLN patients. The baseline Scr level from 18-week-old C57 mice was similar to that from 17-week-old MRL/lpr mice reported by Kerstin Renner [Citation44], which suggested AKI diagnosis in our study was relatively reasonable. AKI incidence in FLLL32 with MP mice, but not in MP mice, decreased significantly compared to vehicle mice, suggesting that FLLL32 treatment has additive protective effects on renal function in MRL/lpr AKI mice.
This study has several limitations. First, the interpretation might be biased owing to small-size mice per group. Second, chemoattractant of inflammatory cells and downstream genes of STAT3 pathway weren’t examined completely to clarify the mechanism of renal interstitial lesion and AKI. Third, the dosage of FLLL32 in our study was based on previously tumor models and optimal dosage in DLPN was not explored.
Together, the AKI of DPLN is associated with renal interstitial leukocytes infiltration and STAT3 activation, which is a previously unknown pathological and molecular mechanism. The combination therapy of FLLL32 with MP, which inhibits STAT3 activation and prevents interstitial inflammation, offers a new and promising way to treat AKI in DPLN. In vitro and in vivo experiments to minimize undesirable interferences were needed to confirm the role of pSTAT3 in DPLN with AKI.
Ethical approval
The DPLN clinical study was approved by Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University with a waiver of the need for an informed consent. Animal studies were approved by the Wenzhou Medical University Institutional Animal Care Committee.
Author contributions
TXC conceived study. JFZ collected animal renal tissue and blood sample. TXC, JFZ, YJC and YLC wrote the manuscript. TXC analyzed experimental data and interpreted results. YQL reviewed pathological image. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Zhouqing Huang and Tingting Wang, Fanfan Li, Mo Shen, Xinxin Zhou, Keqing Shi and Jinrong Tian for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Additional information
Funding
References
- Morales E, Galindo M, Trujillo H, et al. Update on lupus nephritis: looking for a new vision. Nephron. 2021;145(1):1–13. doi:10.1159/000511268.
- Parikh SV, Almaani S, Brodsky S, et al. Update on lupus nephritis: core curriculum 2020. Am J Kidney Dis. 2020;76(2):265–281. doi:10.1053/j.ajkd.2019.10.017.
- Mok CC, Ying KY, Yim CW, et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann Rheum Dis. 2016;75(1):30–36. doi:10.1136/annrheumdis-2014-206456.
- Mok CC, Ho LY, Ying SKY, et al. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann Rheum Dis. 2020;79(8):1070–1076. doi:10.1136/annrheumdis-2020-217178.
- Prasad N, Kurian J, Agarwal V, et al. Long-term outcomes of lupus nephritis treated with regimens based on cyclophosphamide and mycophenolate mofetil. Lupus. 2020;29(8):845–853. doi:10.1177/0961203320926256.
- Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi:10.1016/S0140-6736(10)61354-2.
- Cheng H, Zhang X-Y, Yang H-D, et al. Efficacy and safety of belimumab/low-dose cyclophosphamide therapy in moderate-to-severe systemic lupus erythematosus. Front Immunol. 2022;13:911730. doi:10.3389/fimmu.2022.911730.
- Chen T, Ding X, Chen B. Value of the RIFLE classification for acute kidney injury in diffuse proliferative lupus nephritis. Nephrol Dial Transplant. 2009;24(10):3115–3120. doi:10.1093/ndt/gfp235.
- Zhu D, Qu Z, Tan Y, et al. Acute kidney injury in chinese patients with lupus nephritis: a large cohort study from a single center. Lupus. 2011;20(14):1557–1565. doi:10.1177/0961203311417035.
- Chen T, Zhou Y, Zhang J, et al. Long-term predictive value of acute kidney injury classification in diffuse proliferative lupus nephritis with acute kidney injury. BMC Nephrol. 2020;21(1):13. doi:10.1186/s12882-019-1676-4.
- Chen SY, Liu MF, Kuo PY, et al. Upregulated expression of STAT3/IL-17 in patients with systemic lupus erythematosus. Clin Rheumatol. 2019;38(5):1361–1366. doi:10.1007/s10067-019-04467-8.
- Wang X, Blanco LP, Carmona-Rivera C, et al. Effects of gasdermin D in modulating murine lupus and its associated organ damage. Arthritis Rheumatol. 2020;72(12):2118–2129.
- Rasmussen TK, Andersen T, Bak RO, et al. Overexpression of microRNA-155 increases IL-21 mediated STAT3 signaling and IL-21 production in systemic lupus erythematosus. Arthritis Res Ther. 2015;17(1):154. doi:10.1186/s13075-015-0660-z.
- Goropevšek A, Holcar M, Avčin T. The role of STAT signaling pathways in the pathogenesis of systemic lupus erythematosus. Clin Rev Allergy Immunol. 2017;52(2):164–181. doi:10.1007/s12016-016-8550-y.
- Yiu G, Rasmussen TK, Tsai BL, et al. High interferon signature leads to increased STAT1/3/5 phosphorylation in PBMCs from SLE patients by single cell mass cytometry. Front Immunol. 2022;13:833636. doi:10.3389/fimmu.2022.833636.
- Talaat RM, Mohamed SF, Bassyouni IH, et al. Th1/Th2/Th17/treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine. 2015;72(2):146–153. doi:10.1016/j.cyto.2014.12.027.
- Jeruc J, Vizjak A, Rozman B, et al. Immunohistochemical expression of activated caspase-3 as a marker of apoptosis in glomeruli of human lupus nephritis. Am J Kidney Dis. 2006;48(3):410–418. doi:10.1053/j.ajkd.2006.05.019.
- Arazi A, Rao DA, Berthier CC, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. 2019;20(7):902–914. doi:10.1038/s41590-019-0398-x.
- Collison J. Lupus nephritis: targeting bcl-2 prevents nephritis in mice. Nat Rev Rheumatol. 2016;12(7):376–376. doi:10.1038/nrrheum.2016.90.
- Yoshida N, He F, Kyttaris VC. T cell-specific STAT3 deficiency abrogates lupus nephritis. Lupus. 2019;28(12):1468–1472. doi:10.1177/0961203319877242.
- Edwards LJ, Mizui M, Kyttaris V. Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice. Clin Immunol. 2015;158(2):221–230. doi:10.1016/j.clim.2015.04.004.
- Li Y, Ding T, Chen J, et al. The protective capability of hedyotis diffusa willd on lupus nephritis by attenuating the IL-17 expression in MRL/lpr mice. Front Immunol. 2022;13:943827. doi:10.3389/fimmu.2022.943827.
- Paquissi FC, Abensur H. The Th17/IL-17 axis and kidney diseases, with focus on lupus nephritis. Front Med (Lausanne). 2021;8:654912. doi:10.3389/fmed.2021.654912.
- Oliveira CB, Lima CAD, Vajgel G, et al. The role of NLRP3 inflammasome in lupus nephritis. Int J Mol Sci. 2021;22(22):12476. doi:10.3390/ijms222212476.
- Yung S, Chan TM. Molecular and immunological basis of tubulo-interstitial injury in lupus nephritis: a comprehensive review. Clin Rev Allergy Immunol. 2017;52(2):149–163. doi:10.1007/s12016-016-8533-z.
- Yung S, Tsang RC, Sun Y, et al. Effect of human anti-DNA antibodies on proximal renal tubular epithelial cell cytokine expression: implications on tubulointerstitial inflammation in lupus nephritis. J Am Soc Nephrol. 2005;16(11):3281–3294. doi:10.1681/ASN.2004110917.
- Giannakakis K, Faraggiana T. Histopathology of lupus nephritis. Clin Rev Allergy Immunol. 2011;40(3):170–180. doi:10.1007/s12016-010-8207-1.
- Yung S, Ng CY, Ho SK, et al. Anti-dsDNA antibody induces soluble fibronectin secretion by proximal renal tubular epithelial cells and downstream increase of TGF-beta1 and collagen synthesis. J Autoimmun. 2015;58:111–122.
- Chen T, Fang Z, Zhu J, et al. ACE2 promoted by STAT3 activation has a protective role in Early-Stage acute kidney injury of murine sepsis. Front Med (Lausanne). 2022;9:890782. doi:10.3389/fmed.2022.890782.
- Kidney disease: improving global outcomes (KDIGO)acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl 1):1–138.
- Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the international society of nephrology/renal pathology society classification for lupus nephritis: clarification of definitions, and modified national institutes of health activity and chronicity indices. Kidney Int. 2018;93(4):789–796.
- Park MH, D'Agati V, Appel GB, et al. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44(4):309–319. doi:10.1159/000184012.
- Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus. 2013;22(14):1446–1454. doi:10.1177/0961203313507986.
- Londoño Jimenez A, Mowrey WB, Putterman C, et al. Brief report: tubulointerstitial damage in lupus nephritis: a comparison of the factors associated with tubulointerstitial inflammation and renal scarring. Arthritis Rheumatol. 2018;70(11):1801–1806. doi:10.1002/art.40575.
- Chang A, Clark MR, Ko K. Cellular aspects of the pathogenesis of lupus nephritis. Curr Opin Rheumatol. 2021;33(2):197–204. doi:10.1097/BOR.0000000000000777.
- Nakatani K, Fujii H, Hasegawa H, et al. Endothelial adhesion molecules in glomerular lesions: association with their severity and diversity in lupus models. Kidney Int. 2004;65(4):1290–1300. doi:10.1111/j.1523-1755.2004.00537.x.
- Zhang L, Chen S, Liu Y, et al. P-selectin blockade ameliorates lupus nephritis in MRL/lpr mice through improving renal hypoxia and evaluation using BOLD-MRI. J Transl Med. 2020;18(1):116. doi:10.1186/s12967-020-02284-1.
- Allam R, Sayyed SG, Kulkarni OP, et al. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22(11):2016–2027. doi:10.1681/ASN.2011010045.
- Harada T, Kyttaris V, Li Y, et al. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40(1):1–8. doi:10.1080/08916930601095148.
- Wu T, Qin X, Kurepa Z, et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J Clin Invest. 2007;117(8):2186–2196. doi:10.1172/JCI30398.
- Feng Y, Li W, Wang Z, et al. The p-STAT3/ANXA2 axis promotes caspase-1-mediated hepatocyte pyroptosis in non-alcoholic steatohepatitis. J Transl Med. 2022;20(1):497. doi:10.1186/s12967-022-03692-1.
- Li J, Zhou Y, Liu Y, et al. Sorafenib inhibits caspase-1 expression through suppressing TLR4/stat3/SUMO1 pathway in hepatocellular carcinoma. Cancer Biol Ther. 2018;19(11):1057–1064.
- Sato S, Zhang XK, Temmoku J, et al. Ets family transcription factor fli-1 promotes leukocyte recruitment and production of IL-17A in the MRL/lpr mouse model of lupus nephritis. Cells. 2020;9(3):714. doi:10.3390/cells9030714.
- Renner K, Hermann FJ, Schmidbauer K, et al. IL-3 contributes to development of lupus nephritis in MRL/lpr mice. Kidney Int. 2015;88(5):1088–1098.
