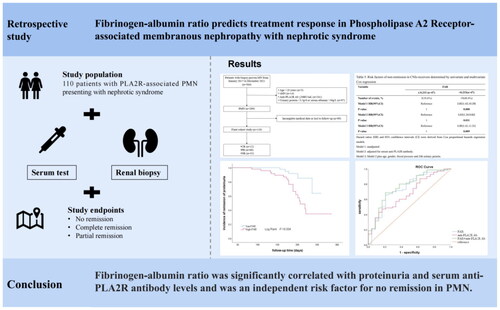
Abstract
Background
The M-type phospholipase A2 receptor (PLA2R)-associated primary membranous nephropathy (PMN) is an immune-related disease in adults with increasing morbidity and variable treatment response, in which inflammation may contribute to the multifactorial immunopathogenesis. The relationship between fibrinogen-albumin ratio (FAR), serving as a novel inflammatory biomarker, and PMN is still unclear. Therefore, this study aims to clarify the association between FAR and disease activity and therapy response of PMN.
Methods
110 biopsy-proven phospholipase A2 receptor (PLA2R) -associated PMN participants with nephrotic syndrome from January 2017 to December 2021 were recruited in the First Affiliated Hospital of Nanjing Medical University. The independent risk factors of non-remission (NR) and the predictive ability of FAR were explored by Cox regression and receiver-operating characteristic (ROC) curve analysis. According to the optimal cutoff value, study patients were categorized into the low-FAR group (≤the cutoff value) and the high-FAR group (>the cutoff value). Spearman’s correlations were used to examine the associations between FAR and baseline clinicopathological characteristics. Kaplan-Meier method was used to assess the effects of FAR on remission.
Results
In the entire study cohort, 78 (70.9%) patients reached complete or partial remission (CR or PR). The optimal cutoff value of FAR for predicting the remission outcome (CR + PR) was 0.233. The Kaplan-Meier survival analysis demonstrated that the high-FAR group (>0.233) had a significantly lower probability to achieve CR or PR compared to the low-FAR group (≤0.233) (Log Rank test, p = 0.021). Higher levels of FAR were identified as an independent risk factor for NR, and the high-FAR group was associated with a 2.27 times higher likelihood of NR than the low-FAR group (HR 2.27, 95% CI 1.01, 5.13, p = 0.048). These relationships remained robust with further analysis among calcineurin inhibitors (CNIs)-receivers. In the multivariate Cox regression model, the incidence of NR was 4.00 times higher in the high-FAR group than in the low-FAR group (HR 4.00, 95% CI 1.41, 11.31, p = 0.009). Moreover, ROC analysis revealed the predictive value of FAR for CR or PR with a 0.738 area under curve (AUC), and the AUC of anti-PLA2R Ab was 0.675. When combining FAR and anti-PLA2R Ab, the AUC was boosted to 0.766.
Conclusions
FAR was significantly correlated with proteinuria and anti-PLA2R Ab in PMN. As an independent risk factor for NR, FAR might serve as a potential inflammation-based prognostic tool for identifying cases with poor treatment response, and the best predictive cutoff value for outcomes was 0.233.
Introduction
Primary membranous nephropathy (PMN) is well-known as an immune-related kidney disease, with an increasing incidence rate in recent years [Citation1]. It is characterized by immune complexes deposited in subepithelial, thickened basement membrane, and podocytes injury. Phospholipase A2 receptor (PLA2R), a signature antigen for PMN, expresses on podocytes in a normal population, and circulating anti-PLA2R antibody (anti-PLA2R Ab) increases under pathologic conditions in 70-80% MN patients [Citation2]. Serum anti-PLA2R Ab has been used to assist in diagnosis, monitor treatment, and evaluate prognosis in clinical practice. Numerous studies have suggested that anti-PLA2R Ab titer was associated with immunological remission, and epitope spreading of antibodies was negatively correlated with clinical remission [Citation3,Citation4]. Although recent years have witnessed increasing attention in the application of anti-PLA2R Ab for MN diagnosis, disease monitoring, the judgment of treatment efficacy, and prognostic evaluation, the clinical characteristics and prognosis of PMN still vary considerably, with an urgent need to identify the risk factors for the renal outcome of PLA2R-associated PMN and therefore to evaluate the most appropriate treatment in each case [Citation5].
Serum fibrinogen-albumin ratio (FAR), a novel inflammatory biomarker, has important prognostic value in many malignant solid tumors, hematologic malignancies, coronary heart disease (CHD), some autoimmune diseases, and the assessment of end-stage kidney disease (ESKD) [Citation6–9]. And urinary fibrinogen has been suggested as an independent risk factor for progression of CKD including PMN to ESKD, the underlying mechanism of which may be associated with the glomerular injury mediated by the Toll-like receptor 4 signaling pathway [Citation10]. In addition, the activation of inflammatory pathways was considered one of the vital mechanisms in the pathogenesis of PMN [Citation11]. However, whether the inflammatory index FAR from a routine blood testing in clinical practice has a role in the PLA2R-associated PMN has yet to be proven.
The current study aimed to evaluate FAR, calculated by dividing the plasma fibrinogen level by the serum albumin level, in combination with coagulation and nutritional status to investigate its association with baseline clinicopathological parameters and predictive value of treatment response and prognosis of biopsy-proven PLA2R-associated PMN patients with nephrotic syndrome.
Methods
Participants selection
Patients proven with PMN by renal biopsy in the First Affiliated Hospital of Nanjing Medical University from January 2017 to December 2021 were retrospectively reviewed. Inclusion criteria were as follows: (1) age ≥ 18 years; (2) newly diagnosis of MN confirmed by renal biopsy and positive serum anti-PLA2R Ab titer at diagnosis (>20RU/mL); (3) patients with nephrotic syndrome (urinary protein ≥ 3.5g/d and serum albumin ≤ 30g/L); (4) treatment-naïve at the time of renal biopsy, i.e.without a drug history of immunosuppressive therapy or corticosteroids; (5) compliance with follow-up. Exclusion criteria: (1) secondary to other diseases, including Hepatitis B virus (HBV) and other infections, tumors, and autoimmune diseases; (2) patients with eGFR ≤ 15 mL/min/1.73 m2 or received renal replacement therapy at baseline; (3) patients with incomplete clinical examination, such as FAR, and anti-PLA2R Ab titer, were also excluded from this study.
Finally, a total of 110 patients with PLA2R-associated PMN concurrent with nephrotic syndrome were enrolled in this single-center retrospective study (). Our study complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist: cross-sectional studies (see Supplementary Table 1).
Data collection
All demographics data (sex, age, body mass index (BMI)) and clinical data, including systolic (SBP) and diastolic blood pressure (DBP), anti-PLA2R Ab, 24h urinary protein, blood coagulation function testing encompassing Prothrombin time (PT), International Normalized Ratio (INR), Activated Partial Thromboplastin Time (APTT), serum fibrinogen, Thrombin Time (TT) and D-Dimer (D-D), Total Cholesterol (TC), Triglyceride (TG), High-Density Lipoprotein Cholesterol (HLD-C), Low Density Lipoprotein Cholesterol (LDL-C), serum albumin, serum creatinine, estimated Glomerular Filtration Rate (eGFR), serum Uric Acid (UA), serum Retinol Binding Protein (RBP), C-reactive protein (CRP), serum neutrophil cell count, serum lymphocyte cell count, serum platelet count, Hemoglobin (Hb), Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin M (IgM), Complement 3 (C3), Complement 4 (C4) and the history of hypertension, diabetes mellitus (DM) were collected from medical records at the time of renal biopsy. Clinical response to treatment, progression to eGFR reduction of 30% from baseline or ESKD, whatever came first, were also recorded for each patient. These patients were followed up for at least about six months. For patients without outcomes, the end date was defined as the time of the latest hospitalization or the latest clinic visit. The follow-up time for each patient was calculated as the number of days between the origin and end date. 24h urinary protein, eGFR, and the therapeutic regimen (glucocorticoids, immunosuppressive agents, and RAAS blockades) during follow-up were recorded. FAR was the serum fibrinogen to serum albumin ratio. NLR was the serum neutrophil cell to serum lymphocyte cell ratio, and PAR was the serum platelet to serum albumin ratio. eGFR was calculated according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation12]. BMI was calculated as the ratio of weight in kilograms divided by the square of the height in meters.
Kidney biopsies
All patients underwent a renal biopsy before treatment. Each biopsy specimen was evaluated by light microscopy, direct immunofluorescence, and electron microscopy. Renal biopsy specimens were fixed in Hollande’s fixative, embedded in paraffin wax, and processed routinely. Frozen sections containing 4 μm of the samples were used for direct immunofluorescence to detect IgG, fibrinogen, C3 and PLA2R. The fluorescence intensity was determined using a semi-quantitative scale of 0–3: 0, negative; 1, weak; 2, moderate; 3, strong staining. Glomerular MN lesions were classified into four stages according to Ehrenreich and Churg’s criteria [Citation13]. The chronic tubulointerstitial injury was defined as tubular atrophy and interstitial fibrosis, grading semi-quantitatively from 0 to 4: 0, 0–5%; 1, 6–25% of tubulointerstitial tissue involved; 2, 26–50% of tubulointerstitial tissue involved; 3, >50% of tubulointerstitial tissue involved. Additionally, the acute tubulointerstitial injury included the loss of tubular brush border and interstitial mononuclear cell infiltration, which was graded from 0 to 1, for none and existence, respectively.
Treatment and follow-up
The use of corticosteroids and immunosuppressive agents and the definitions of remission were in compliance with the KDIGO (Kidney Disease: Improving Global Outcomes) guideline for glomerulonephritis [Citation14]. CR was defined as urinary protein excretion < 0.3g/d with a stable GFR. PR was defined as urinary protein excretion < 3.5g/d and a reduction of at least 50% from baseline values with a stable GFR. Otherwise, the outcome was defined as non-remission or non-response (NR). ESKD was defined as the initiation of maintenance dialysis or kidney transplantation.
Statistical analysis
Mean and standard deviation (SD), median with interquartile range (IQR) or frequencies with percentages were employed separately to describe data distribution as appropriate. Correlations between FAR and clinicopathological parameters were performed by Spearman’s correlation. Comparisons between groups were performed using one-way analysis of variance (ANOVA), Kruskal–Wallis test or χ2 test as appropriate. Receiver-operating characteristic (ROC) curve analysis was used to explore the predictive optimal cutoff point of the FAR level to remission rate. Kaplan–Meier analysis and the log-rank test were used to compare the remission rate between the high-FAR group (>cutoff value) and the low-FAR group (≤cutoff value). The predicting ability of FAR, anti-PLA2R Ab, and FAR + anti-PLA2R Ab was depicted in ROC Curve with the relevant Area Under Curve (AUC) and pairwise comparison of ROC curves was adopted by software medcalc. Univariate and multivariate Cox proportional hazards models were employed to investigate the risk factors of NR, model 1 was unadjusted, model 2 was adjusted for serum anti-PLA2R antibody and model 3 was defined by the addition of indicators, including age, gender, blood pressure and 24h urinary protein. All values were analyzed by IBM SPSS Statistics 26, and a two-tailed P value of <0.05 was considered significant. In addition, we used PASS 2021 software to calculate the sample size with α = 0.05 and tolerance error = 0.1.
Results
Comparisons of baseline parameters between groups with different outcomes
110 patients with biopsy-proven PMN concurrent with nephrotic syndrome (NS) were recruited. During the follow-up, 78 patients reached remission, including 12 (10.9%) with complete remission (CR) and 66 (60.0%) with partial remission (PR). Accordingly, patients were divided into two groups: remission group (CR + PR) (n = 78) and no remission group (NR) (n = 32). As shown in , levels of serum anti-phospholipase A2 receptor (PLA2R) antibody (Ab), serum fibrinogen, CRP, D-dimer and fibrinogen-albumin ratio (FAR) were significantly lower, while levels of albumin and retinol binding protein (RBP) were higher in remission group compared to NR group (p < 0.05). Meanwhile, a higher percentage of infection was observed in patients with NR compared to those who reached remission (p < 0.05). However, there was no difference in demographics and other clinical parameters between the remission and NR group, such as age, sex, body mass index (BMI), systolic and diastolic blood pressure (SBP, DBP), 24h urinary protein, and estimated glomerular filtration rate (eGFR) (p > 0.05).
Table 1. Characteristics of patients with different outcomes.
Comparison of clinical and pathological characteristics according to different levels of FAR
Based on the results that FAR was significantly discriminated between remission and NR groups, further receiver-operating characteristic (ROC) curves to determine the predictive value of FAR on remission rate of PMN were performed. And other established inflammatory biomarkers including CRP, NLR, PAR in predicting the treatment response for PLA2R-MN was performed in Supplementary Figure 1A. The optimal cutoff value of FAR for predicting the remission outcome (CR or PR) was 0.233 with 68.7% specificity and 60.3% sensitivity, as calculated by obtaining the best Youden index (AUC = 0.669) (). Next, we analyzed the clinical and pathological characteristics in all enrolled patients stratified by the cutoff value of FAR (). Of the entire cohort, the majority were male (n = 78, 70.9%). The median age was 53.00 years (IQR 42.75, 60.25) with a 25.72 kg/m2 (IQR 23.37, 27.77) body mass index (BMI). At baseline, the median urinary protein was 7.62 g/d (IQR 5.57, 10.68) and eGFR was 98.36 mL/min/1.73m2 (IQR 79.58, 108.90). Enrolled patients had a median serum anti-PLA2R Ab titer of 108.91 (IQR 55.00, 242.30) RU/mL, and 89.1% of patients had positive PLA2R deposits. In addition, hypertension and diabetes mellitus were observed in 58 (52.7%) patients and 13 (11.8%) patients, respectively, and 26 (23.6%) patients suffered a 30% decline from baseline eGFR or ESKD during follow-up.
Figure 1. Flowchart of study participants. PMN, Primary membranous nephropathy; sMN, secondary membranous nephropathy; anti-PLA2R Ab, anti-phospholipase A2 receptor antibody; CR, complete remission; PR, partial remission; NR, non-remission.
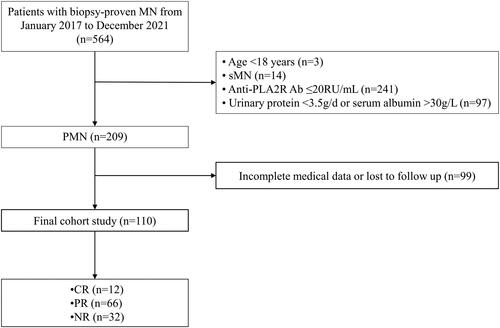
Figure 2. Association of FAR and treatment response in all enrolled patients. A. Predictive performances of FAR for remission outcomes (CR or PR) evaluated by ROC curves. B. Incidence of CR and PR between groups stratified for FAR levels by Kaplan-Meier survival curve. Anti-PLA2R Ab positive PMN patients with FAR ≤ the cutoff value at the time of renal biopsy had significantly higher probability to achieve CR or PR compared with the patients with FAR above the cutoff (Log Rank test, p = 0.021). AUC, the area under the ROC curve; ROC, receiver operating characteristic.
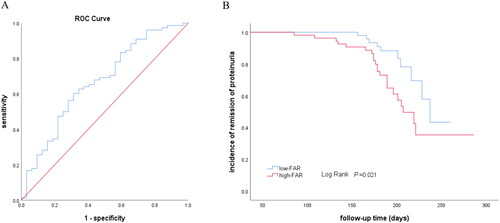
Table 2. Clinical and pathological features of patients with PMN according to different levels of FAR.
Compared with the low-FAR group (≤0.233), patients with a high level of FAR (>0.233) had higher levels of SBP, 24h urinary protein, thromboplastin time, fibrinogen, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), complement 3 (C3), complement 4 (C4), and were susceptible to hypertension and more frequent use of renin-angiotensin-aldosterone system (RAAS) inhibitors (p < 0.05). Meanwhile, patients in the low-FAR group had higher levels of albumin, uric acid, and IgG. Furthermore, the high-FAR group had a higher NR rate. However, there were no significant differences between the two groups in the use of glucocorticoids or immunosuppressive agents, serum creatinine, serum anti-PLA2R Ab, progression to 30% eGFR decline or ESKD, as well as pathological features in terms of fibrinogen, PLA2R, immunoglobulin or complement deposits, global sclerosis, chronic or acute tubulointerstitial injury, etc.
Correlations between FAR and clinical and pathological parameters
displayed the correlations between FAR and baseline clinicopathological parameters. A positive correlation between FAR and SBP (r = 0.263, p = 0.006), serum anti-PLA2R Ab (r = 0.238, p = 0.012), 24h urinary protein (r = 0.363, p < 0.001), TC (r = 0.363, p < 0.001), LDL-C (r = 0.334, p < 0.001), serum C4 (r = 0.237, p = 0.013), and IgG deposition (r = 0.149, p = 0.036). Meanwhile, FAR was negatively correlated with serum IgG (r=-0.314, p = 0.001). With respect to demographics and other clinical parameters, such as sex, age, BMI, DBP, TG, HDL-C, eGFR, serum IgA, IgM, C3 and pathological parameters (such as fibrinogen, PLA2R, immunoglobulin or complement deposits, global sclerosis, chronic or acute tubulointerstitial injury, etc.), no significant value was achieved.
Table 3. Correlations between FAR and clinical and pathologic parameters.
Association of FAR and remission rate in all enrolled PMN patients
As shown in , the Kaplan-Meier survival analysis demonstrated that the high-FAR group had a higher possibility of NR than the low-FAR group (Log Rank test, p = 0.021). Moreover, the association between the FAR and risks for NR was determined by Cox proportional hazards model (). In unadjusted models, the higher level of FAR was associated with an increased risk for NR (HR 2.35, 95% CI 1.11, 4.96, p = 0.026, model 1 in ). The results remained statistical significance in multivariate analysis after adjusting for serum anti-PLA2R antibody (HR 2.24, 95% CI 1.05, 4.77, p = 0.037, model 2 in ). In a fully adjusted model including serum anti-PLA2R antibody, age, gender blood pressure and 24h urinary protein, the high-FAR group was associated with 2.27 times higher likelihood of NR compared to the low-FAR group, with an HR of 2.27 (95% CI 1.01, 5.13, p = 0.048, model 3 in ).
Table 4. Risk factors of non-remission in PMN determined by univariate and multivariate cox regression.
Association of FAR and remission rate in calcineurin inhibitors (CNIs)-receivers
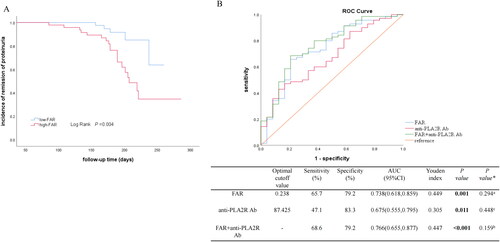
A total of 110 participants were included in this study, among which 94 patients received glucocorticoids with calcineurin inhibitors (CNIs). To minimize the effect of different therapeutic strategies on treatment response, patients who received CNIs treatment were selected further to analyze the prediction value of FAR on remission rate. The clinicopathological characteristics in CNIs-receivers stratified by the cutoff value of FAR were summarized in Supplementary Table 1. During the median follow-up period of 187 days, 12 cases received CR, and 58 received PR. Kaplan–Meier survival analysis revealed that the low-FAR group (≤0.233) also had a higher possibility of CR or PR (Log Rank test, p = 0.004, ). Further ROC curves confirmed the predictive ability of FAR on the remission outcomes (CR or PR), with a sensitivity of 65.7% and a specificity of 79.2% (AUC = 0.738). Meanwhile, the predictive ability of anti-PLA2R Ab were observed a relatively low sensitivity (47.1%) but a high specificity (83.3%) (AUC = 0.675). When combining FAR and anti-PLA2R Ab, the sensitivity was boosted to 68.6%, and the specificity was as high as 79.2% (AUC = 0.766) (). Furthermore, pairwise comparison of ROC curves showed that, FAR had similar performance with anti-PLA2R Ab or the combination of FAR and anti-PLA2R Ab in discriminating different treatment responses (FAR v.s anti-PLA2R Ab, p = 0.448; FAR v.s FAR + anti-PLA2R Ab, p = 0.294; anti-PLA2R Ab v.s FAR + anti-PLA2R Ab, p = 0.159). Additionally, other established inflammatory biomarkers including CRP, NLR, PAR in predicting the treatment response for CNIs-receivers was performed in Supplementary Figure 1B.
Figure 3. Association of FAR and treatment response in CNIs-receivers (A) Incidence of CR and PR between groups stratified for FAR levels by Kaplan-Meier survival curve in CNIs-receivers. (B) Predictive performances of variables for remission evaluated by ROC curves. ROC curve of FAR, anti-PLA2R Ab, and combination of FAR and anti-PLA2R Ab for remission outcomes. The AUC and the cutoff value of variables were presented in a separate table under the figure. *: Pairwise comparison; aFAR vs FAR + anti-PLA2R Ab; banti-PLA2R Ab vs FAR + anti-PLA2R Ab; cFAR vs anti-PLA2R Ab. FAR, fibrinogen-albumin ratio; CNIs, calcineurin Inhibitors; CR, complete remission; PR, partial remission; ROC, receiver operating characteristic. AUC, the area under the ROC curve.
The univariate Cox regression analysis identified that FAR was still an independent risk factor for NR in CNIs-receivers (HR 3.83, 95%CI 1.43,10.29, p = 0.008, model 1 in ). After adjustment for serum anti-PLA2R antibody, we also observed a significantly higher risk for NR in the higher level of FAR (>0.233) (HR 3.63, 95%CI 1.34, 9.82, p = 0.011, model 2 in ). This increased risk remained in the high-FAR, even after extensive adjustment for sex, age, blood pressure and 24h urinary protein (HR 4.00, 95%CI 1.41,11.31, p = 0.009, model 3 in ).
Table 5. Risk factors of non-remission in CNIs-receivers determined by univariate and multivariate cox regression.
Discussion
The results of our study supplied evidence concerning the associations between the FAR and disease activity and treatment response in PLA2R-associated PMN. First, a high level of FAR was demonstrated as an independent risk factor for NR in PLA2R-associated PMN by unadjusted and multivariate-adjusted Cox regression analyses. ROC curves confirmed the predictive ability of FAR on the remission rate, with a cutoff value of 0.233 with 60.3% sensitivity and 68.7% specificity. Moreover, Kaplan-Meier survival analysis demonstrated that PMN patients with the low FAR (<0.233) level had a higher possibility of remission. Secondly, FAR was positively correlated with proteinuria and anti-PLA2R Ab. These relationships remained robust with further analysis among CNIs-receivers who account for most enrolled patients. Additionally, ROC analysis revealed that FAR combined with anti-PLA2R Ab boosted the predictive strength of anti-PLA2R Ab on remission rate (AUC = 0.766). Our study provides an efficient and convenient blood-based index for the prediction of disease progression and treatment response in PLA2R-associated PMN.
Our findings offered a deeper insight into the interaction between inflammation and the pathogenesis of PMN. Serum albumin is critical in maintaining plasma osmotic pressure, reflecting nutrition conditions, and decreases in inflammatory state [Citation15]. Fibrinogen is well known for participating in coagulation cascade reactions and acts as an inflammatory index due to its effects on the synthesis of IL-6, TNF-α, and IL-1β [Citation16]. Moreover, it is one of the ligands of Toll-like receptor 4, which is expressed in podocytes and inflammatory cells [Citation17,Citation18]. Consequently, FAR, the combination of albumin and fibrinogen, was obtained from a facile and reliable calculation of a routine and extensively-used blood testing and was recognized as a novel inflammatory marker. It has recently been reported as a noninvasive predictor in breast cancer, chronic lymphocytic leukemia (CLL), epithelial ovarian cancer, non-small-cell lung cancer (NSCLC), or the prognosis of patients after coronary artery bypass grafting or percutaneous coronary intervention (PCI), and the assessment of ESKD [Citation6,Citation7,Citation9,Citation19–22]. The primary finding of the present study is that a higher level of FAR was an independent risk factor for NR in PLA2R-associated PMN. The optimal cutoff value of FAR for predicting remission was 0.233 with 68.7% specificity and 60.3% sensitivity. In addition, patients with the low-FAR level had a higher possibility of remission. The findings helped to supplement the multifactorial immunopathogenesis of PMN. As PLA2R expressed on podocytes was discovered as the primary target antigen, PMN has been widely recognized as immune-related organ-specific kidney disease [Citation2]. Apart from immune reaction and complement pathways activation, the effect of inflammation on the pathogenesis of PMN has also been stressed in recent years. One study proposed by Xie et al. revealed two inflammation-associated risk loci, nuclear factorκB1 (NFκB1) and interferon regulated factor 4 (IRF4) in a genome-wide association study of PMN [Citation23]. Another study claimed that Th-17-mediated inflammation reaction might increase the incidence of thrombosis events and relapse in PMN patients [Citation24]. Moreover, environmental factors played an essential role in PMN and PM2.5 might mediate inflammation via influencing the expression of IL-6 and IL-1β [Citation1,Citation25]. The potential association between inflammation and PLA2R in PMN has been suggested that PLA2R in an inflammatory environment may be more easily recognized by the immune system, thus triggering an autoimmune response [Citation26,Citation27]. Therefore, FAR considered as a novel indicator of systemic inflammatory status, was strongly suggested to be associated with the therapeutic response in PMN.
Another important observation was that FAR was positively correlated with serum anti-PLA2R Ab titer, which was recommended as a well-standardized and established biomarker for risk stratification and treatment strategy adjustment in clinical practice [Citation14]. Previous studies have suggested that anti-PLA2R Ab were closely related to the severity, treatment efficacy, and prognosis of PMN [Citation2]. Liu et al. have explained the potential mechanism of the close relationship between inflammatory status and anti-PLA2R Ab that oxidative stress associated with inflammation might induce the PLA2R-expressing cells such as airway epithelial cells in the lungs, alveolar macrophages, and neutrophils, to a conformation which contains pathogenic epitopes contributing to the formation of autoantibodies [Citation26]. On the other hand, PLA2R may be released into the inflammatory space and then be bounded by antigen-presenting cells, triggering the humoral immune response and producing anti-PLA2R Abs [Citation26]. Our study integrated novel inflammation status biomarkers and anti-PLA2R Ab titers at the same time and compared the predictive performance in unique CNIs-receivers. From ROC curves, both FAR and serum anti-PLA2R Ab titer presented intermediate accuracy in predicting remission. With the high specificity (79.2%) and fair sensitivity (68.6%), the combination of FAR and serum anti-PLA2R Ab would help boost the predictive strength as a potential tool for identifying cases with a high risk of NR.
Furthermore, FAR was positively correlated with levels of urinary protein and patients with FAR > 0.233 had more severe proteinuria even though all the enrolled patients suffered from nephrotic proteinuria. It might be involved in the underlying mechanism of an association between higher FAR level and increased risk of NR in PLA2R-associated PMN. Consistently, one previous study showed that the systemic immune-inflammation index, an inflammatory marker, was positively correlated with proteinuria [Citation28]. From a clinical point of view, the natural course of PMN is variable. Around one-third of PMN patients will have spontaneous remission, while about 60% of untreated patients will develop progressive loss of renal function [Citation2,Citation5]. In addition, 30-40% of cases progressed to ESKD within 5-15 years [Citation5]. Proteinuria is still an irreplaceable criterion for the evaluation of disease progression, treatment response, and prognostic assessment recommended by the 2021 KDIGO guideline [Citation14]. Numerous clinical trials and retrospective studies have demonstrated that CNIs therapy is a useful therapeutic option for nephrotic patients with well-preserved kidney function. Most patients experience remission with a significant reduction in the risk of deteriorating kidney function [Citation29]. Cyclosporine and tacrolimus are effective in MN, which induce CR or PR in more than 70-80% of cases [Citation5]. As such, based on that FAR > 0.233 at baseline indicated a high probability of more severe proteinuria and poor treatment response, those patients may be more likely to benefit from initial immunosuppressive treatment with cyclophosphamide associated with steroids, Rituximab (RTX), or CNIs with RTX which were recommended to high-risk cases by 2021 KDIGO guidelines [Citation14]. Further large, randomized controlled, well-designed studies are required to determine the ideal immunomodulatory approaches with risk-based and response-based decision tools, including FAR.
Several limitations to our study should be mentioned. Firstly, study limitations included being a single-center study, which lacks external validation of an independent patient cohort. And the primary endpoint was set during a relatively short follow-up period of 6 months. Secondly, this was a retrospective observational study, and the majority of enrolled patients received CNIs treatment, which might produce selection bias and limit the comparison of clinical response in different therapy regimens. The study’s design and analysis may be prone to suffer from various confounders and biases. Research with a prospective study design in the future is warranted. Thirdly, dynamic monitoring changes of anti-PLA2R Ab and FAR levels were missing. Lastly, the association of FAR and endpoints of progression to ESKD or changes in eGFR would be a subject of future studies. Multi-center, prospective studies with sufficient sample size, a long-term follow-up and standardized treatment and monitoring protocols would be required to ascertain the predictive value of FAR.
In conclusion, our study demonstrated that FAR was associated with proteinuria and anti-PLA2R Ab in PMN. Moreover, a high level of FAR was an independent risk factor for NR, and the best predictive cutoff value for NR was 0.233.
Author contributions
Suyan Duan and Yanggang Yuan designed the research. Si Chen contributed to the writing. Si Chen, Ying Pan, and Yifei Lu conducted the research. Chen Chen revised the manuscript. Si Chen and Fang Lu analyzed the data and performed the statistical analysis. Qing Li, Simeng Liu, Bo Zhang, and Huijuan Mao reviewed the manuscript. Changying Xing conceived and coordinated the study and was responsible for its final content. Yanggang Yuan is the guarantor of this work, who have complete access to all the data in the study and takes ultimate responsibility for the study design and integrity of data analysis. All authors have read the final paper and approved the submission.
Compliance with ethical standards
All experiments and methods were performed in accordance with relevant guidelines and regulations. This study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2020-SR-458). Informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download PDF (369.1 KB)Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Xu X, Wang G, Chen N, et al. Long-Term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27(12):1–11.
- Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7(1):69. doi:10.1038/s41572-021-00303-z.
- Seitz-Polski B, Dahan K, Debiec H, et al. High-Dose rituximab and early remission in PLA2R1-Related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14(8):1173–1182.
- Reinhard L, Zahner G, Menzel S, et al. Clinical relevance of domain-specific phospholipase a receptor 1 antibody levels in patients with membranous nephropathy. J Am Soc Nephrol. 2020;31(1):197–207.
- Nieto-Gañán I, Iturrieta-Zuazo I, Rita C, et al. Revisiting immunological and clinical aspects of membranous nephropathy. Clin Immunol. 2022;237:108976.
- Wang Y, Xu W, Wang Y. Prognostic role of preoperative fibrinogen to albumin ratio in breast cancer. Clin Chim Acta. 2020;510:360–362. doi:10.1016/j.cca.2020.07.055.
- Zou Y-X, Qiao J, Zhu H-Y, et al. Albumin-to-fibrinogen ratio as an independent prognostic parameter in untreated chronic lymphocytic leukemia: a retrospective study of 191 cases. Cancer Res Treat. 2019;51(2):664–671. doi:10.4143/crt.2018.358.
- Lee LE, Pyo JY, Ahn SS, et al. Fibrinogen to albumin ratio reflects the activity of antineutrophil cytoplasmic antibody-associated vasculitis. J Clin Lab Anal. 2021;35(4):e23731.
- Zou Y, Zhu Z, Zhou J, et al. Fibrinogen/albumin ratio: a more powerful prognostic index for patients with end-stage renal disease. Eur J Clin Invest. 2020; 50(8):e13266.
- Wang H, Zheng C, Lu Y, et al. Urinary fibrinogen as a predictor of progression of CKD. Clin J Am Soc Nephrol. 2017;12(12):1922–1929. doi:10.2215/CJN.01360217.
- Hoxha E, Reinhard L, Stahl RAK. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. 2022;18(7):466–478. doi:10.1038/s41581-022-00564-1.
- Levey AS, Stevens LA, Schmid CH, 3rd, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006.
- Ehrenreich T, Porush JG, Churg J, et al. Treatment of idiopathic membranous nephropathy. N Engl J Med. 1976;295(14):741–746. doi:10.1056/NEJM197609302951401.
- Rovin BH, Adler SG, Barratt J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753–779.
- Sheinenzon A, Shehadeh M, Michelis R, et al. Serum albumin levels and inflammation. Int J Biol Macromol. 2021;184:857–862.
- Jensen T, Kierulf P, Sandset PM, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(5):822–829. doi:10.1160/th07-01-0039.
- Anders H-J, Banas B, Schlöndorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi:10.1097/01.asn.0000121781.89599.16.
- Chen K, Huang J, Gong W, et al. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7(10):1271–1285. doi:10.1016/j.intimp.2007.05.016.
- Yu W, Ye Z, Fang X, et al. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. 2019;12(1):88. doi:10.1186/s13048-019-0563-8.
- Ying J, Zhou D, Gu T, et al. Pretreatment albumin/fibrinogen ratio as a promising predictor for the survival of advanced non small-cell lung cancer patients undergoing first-line platinum-based chemotherapy. BMC Cancer. 2019;19(1):288. doi:10.1186/s12885-019-5490-y.
- Wang P, Yuan D, Zhang C, et al. High fibrinogen-to-albumin ratio with type 2 diabetes mellitus is associated with poor prognosis in patients undergoing percutaneous coronary intervention: 5-year findings from a large cohort. Cardiovasc Diabetol. 2022;21(1):46. doi:10.1186/s12933-022-01477-w.
- Park S, Nam K, Kim TK. Association between preoperative fibrinogen-to-albumin ratio and all-Cause mortality after off-Pump coronary artery bypass grafting: a retrospective observational study. Anesth Analg. 2022;134(5):1021–1027. doi:10.1213/ANE.0000000000005948.
- Xie J, Liu L, Mladkova N, et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat Commun. 2020;11(1):1600. doi:10.1038/s41467-020-15383-w.
- Cremoni M, Brglez V, Perez S, et al. Th17-Immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol. 2020;11:574997. doi:10.3389/fimmu.2020.574997.
- Chen R, Li H, Cai J, et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect. 2018;126(1):017007. doi:10.1289/EHP1447.
- Liu W, Gao C, Dai H, et al. Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front Immunol. 2019;10:1809. doi:10.3389/fimmu.2019.01809.
- van de Logt AE, Fresquet M, Wetzels JF, et al. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96(6):1292–1302.
- Qin Z, Li H, Wang L, et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: a population-based study. Front Immunol. 2022;13:863640. doi:10.3389/fimmu.2022.863640.
- Scolari F, Alberici F, Mescia F, et al. Therapies for membranous nephropathy: a tale from the old and new millennia. Front Immunol. 2022;13:789713. doi:10.3389/fimmu.2022.789713.