Abstract
Objective
To determine the efficacy and safety of Astragalus combined with renin-angiotensin-aldosterone system (RAAS) blockers in treating stage III diabetic nephropathy (DN) by meta-analysis.
Methods
PubMed, Embase, Cochrane Library, Wiley, and Web of Science databases were searched for articles published between August 2007 and August 2022. Clinical studies on Astragalus combined with RAAS blockers for the treatment of stage III DN were included. Meta-analysis was performed by RevMan 5.1 and Stata 14.3 software.
Results
A total of 32 papers were included in this meta-analysis, containing 2462 patients from randomized controlled trials, with 1244 receiving the combination treatment and 1218 solely receiving RAAS blockers. Astragalus combined with RAAS blockers yielded a significantly higher total effective rate (TER) (mean difference [MD] 3.63, 95% confidence interval [CI] 2.59–5.09) and significantly reduced urinary protein excretion rate (UPER), serum creatinine (Scr), blood urine nitrogen (BUN) and glycosylated hemoglobin (HbAlc) levels. In subgroup analysis, combining astragalus and angiotensin receptor blocker significantly lowered fasting plasma glucose (FPG) and 24 h urinary protein (24hUTP) levels, compared with the combined astragalus and angiotensin-converting enzyme inhibitor treatment. Meanwhile, the latter significantly decreased the urinary microprotein (β2-MG). Importantly, the sensitivity analysis confirmed the study’s stability, and publication bias was not detected for UPER, BUN, HbAlc, FPG, or β2-MG. However, the TER, SCr, and 24hUTP results suggested possible publication bias.
Conclusions
The astragalus-RAAS blocker combination treatment is safe and improves outcomes; however, rigorous randomized, large-scale, multi-center, double-blind trials are needed to evaluate its efficacy and safety in stage III DN.
KEY SUMMARY POINTS
Renin-angiotensin-aldosterone system (RAAS) inhibitors are commonly used to treat diabetic neuropathy (DN) and Astragalus membranaceus components are known to improve DN symptoms.
We aimed to establish the efficacy and safety of using Astragalus combined with RAAS inhibitors.
Astragalus combined with RAAS inhibitors enhances the total effective rate of diabetic neuropathy response to treatment and reduces urinary protein excretion rate, serum creatinine, blood urea nitrogen and HbAlc.
Sensitivity analysis affirms study stability, while publication bias was detected for total effective rate, serum creatinine, and 24 h urinary protein levels.
1. Introduction
Diabetic nephropathy (DN) is a common microvascular complication caused by metabolic abnormalities of diabetes mellitus (DM) and is the second leading cause of end-stage renal disease (ESRD) [Citation1]. In clinical practice, renin-angiotensin-aldosterone system (RAAS) blockers are commonly administered to treat DN. Particularly, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) effectively block different portions of the RAAS, aiding in the reduction of urinary protein levels and controlling blood pressure. Although the clinical application of RAAS blockers can slow down the progression of DN, effectively reduce proteinuria in patients with severe proteinuria [Citation2] or reverse the pathological progression of DN has been challenging [Citation3]. If Western medicine is combined with traditional medical treatment, it has a better effect on DN [Citation4].
Recently, traditional Chinese medicine has accumulated broad success in treating DN. Traditional Chinese medicine has summarized diabetic nephropathy as “Xiaoke,” “edema,” and “turbiduria” [Citation5], and its main pathological characteristics as qi and yin deficiency. Associated methods treat DN primarily by tonifying the kidneys and qi [Citation6]. Evidently, combinatorial astragalus treatment with RAAS blockers can considerably alleviate the clinical symptoms and reduce the relevant outcomes of patients with stage III DN. Many studies have investigated the mechanisms of the active ingredients in Astragalus in treating DN [Citation7–10]. Oxidative stress and inflammatory response play an important role in the occurrence and development of DN [Citation11]. The core active ingredients quercetin, kawanferol, and isorhamnetin extracted from Astragalus membranaceus can reduce the oxidative stress response, inhibit the expression of inflammatory factors, and prevent renal interstitial fibrosis by regulating the therapeutic target of DN, thus delaying DN progression [Citation12,Citation13]. Hence, Astragalus has a clear role in improving DN. Accordingly, a meta-analysis of the efficacy and safety of Astragalus combined with RAAS blockers is necessary.
This meta-analysis aims to systematically evaluates the efficacy and safety of Astragalus combined with RAAS blockers in treating stage III DN to provide more reliable evidence for the clinical application of Astragalus.
2. Material and methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [Citation14]. The review was also registered in the PROSPERO registry for systematic reviews and meta-analyses (CRD42023453297).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
2.1. Search strategy
We searched electronic databases, including PubMed, Embase, Cochrane Library, Wiley, and Web of Science, for articles published between August 2007 and August 2022. We also screened the reference lists of included studies for additional eligible studies not retrieved by our search. Moreover, the search was rerun before the final analysis. Unpublished studies were also screened.
For English databases, the search terms we used were: “diabetic nephropathy” OR “diabetic kidney disease” OR “diabetic proteinuria” AND “Astragalus” OR “Astragaloside” AND “RAAS blockers” OR “Angiotensin-converting enzyme inhibitors” OR “Angiotensin receptor blockers” AND “randomized control”. For Chinese databases, the search terms we used were “Tang Niao Bing Shen Bing” (which means diabetic nephropathy in Chinese), AND “Huang Qi” OR “Huang Qi Jia Gan” (which is the alternative name of Astragalus or Astragaloside in Chinese and TCM), AND “RAAS Zu Zhi Ji” OR “Xue Guan Jin Zhang Su Zhuan Huan Mei Yi Zhi Ji” (which is the alternative name of RAAS blockers or Angiotensin-converting enzyme inhibitors in Chinese), AND “sui ji”. The titles and the abstracts were screened first, then the full-text version for inclusion and exclusion criteria were checked. The searches were carried out by two reviewers who evaluated the quality of the trials and extracted the data independently.
2.2. Inclusion criteria
Population: All patients included in the study were diagnosed with stage III DN, and were staged according to the Mogensen DN staging system [Citation15].
Interventions and comparison: The included articles primarily compared the efficacy and safety of combined astragalus-RAAS blocker and RAAS blockers (ACEI/ARB) regardless of dose, type, or treatment duration. The basic therapies were similar between the combined treatment group and RAAS blocker group.
Outcomes: The primary outcomes were the total effective rate (TER), urinary protein excretion rate (UPER), urinary microprotein (β2-MG), and 24-h urinary protein levels (24 h UTP). Secondary outcomes comprised serum creatinine (Scr), blood urine nitrogen (BUN), fasting plasma glucose (FPG), and glycosylated hemoglobin (HbAlc).
Studies: This study included randomized, controlled trials (RCTs) or quasi-RCTs, with no restrictions on blinding or concealing group assignments. We did not consider published literature types, language, or population characteristics.
2.3. Exclusion criteria
Articles were excluded if they contained many evaluation variables, were based on animal models or in vitro, comprised a literature review or theoretical article, were published repeatedly, or contained incomplete data.
2.4. Data extraction
Two researchers independently extracted the eligible literature data. A third researcher participated in the discussion to reach a consensus in case of disagreement. The following data were extracted from each study: author (years), sample size, stage of disease, age of patients, intervention(s), comparators, treatment period, outcome measures, and main results. Three investigators reviewed all literature that met the inclusion and exclusion criteria.
The heterogeneity of the included studies was evaluated to assess whether the data of each study could be analyzed. Data for characteristics and clinical report outcomes of the included studies were summarized and expressed in table format.
2.5. Quality assessment
We used the Cochrane Collaboration methodology to evaluate the study bias risk [Citation16]. The criteria included sequence generation of allocation (evaluated whether the allocation sequence was adequately generated using methods such as computer-generated random numbers or random number tables); allocation concealment (determined whether intervention allocations were concealed from both researchers and participants until assignment); masking of participants, staff, and outcome assessors (assessed whether the study subjects and investigators were blinded to the group assignments and outcome assessors were blinded to the intervention provided); completeness of outcome data (evaluated the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis); selectiveness of outcome reporting (checked if the study reported all of the study’s pre-specified outcomes in the pre-specified way); and other sources of bias (considered other potential sources of bias not covered in the other domains, such as the funding source or vested interests by authors).
2.6. Statistical analysis
All data were analyzed using RevMan 5.1 and Stata 14.3. Success rates with 95% CI per study and pooled analysis are shown as forest plots. The I2 index evaluated the degree of heterogeneity among the studies. The weighting of the studies was performed according to the DerSimonian and Laird stochastic random model and presented as a forest plot. Funnel plots represented the analysis of publication bias, and the magnitude of publication bias was further quantified by Egger’s and Begg’s tests. A subgroup analysis was performed to determine the intervention (ACEI or ARB) that was associated with outcome changes in participants. The sensitivity analysis determined the stability of the results included in the meta-analysis. Finally, the overall quality of each report outcome was assessed using the GRADE evaluation of evidence quality [Citation17]. The quality of evidence for the main findings was graded as GRADE working Group grades of evidence, ‘High quality’: Further research is very unlikely to change our confidence in the estimate of effect; ‘Moderate quality’: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; ‘Low quality’: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; ‘Very low quality’: We are very uncertain about the estimate.
3. Results
3.1. Literature search results
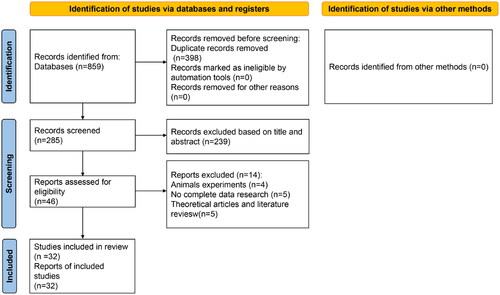
The database search provided 859 references. After removing duplicates, 398 abstracts were screened for eligibility, and 46 full-text articles were assessed. Overall, 32 references were included in the present review [Citation18–49]. All RCTs included herein proceeded in China. shows the literature search process for this study and study selection. The meta-analysis included 2462 patients, among whom 1244 received combined therapy, and 1218 received only a RAAS blocker (control). The treatment period was 3–48 weeks. shows the main characteristics of the included studies.
Table 1. Characteristics of included studies.
3.2. Quality of included articles
Randomization was mentioned in all 32 reports. However, only 13 trials indicated that the random grouping method applied was the random number table [Citation18,Citation22–24,Citation32–35,Citation37,Citation38,Citation40,Citation46,Citation49]. Nine trials did not mention the use of allocation concealment [Citation21,Citation29–31,Citation33,Citation38,Citation41,Citation45,Citation49]. Ten trials did not provide detailed information about participant blinding or personnel assessment [Citation18,Citation24,Citation27–31,Citation34,Citation42,Citation46]. Additional trial details are provided in Supplementary Figures 1 and 2.
3.2. Report outcomes
3.2.1. Total effective rate (TER)
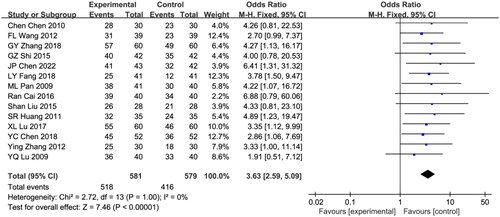
Fourteen eligible studies were included in this analysis, with a total sample size of 1160 patients, including 581 patients in the combination treatment group and 579 in the control group [Citation11,Citation13,Citation15–24,Citation29,Citation30,Citation32,Citation35–37,Citation41,Citation50,Citation51]. Due to a lack of heterogeneity among studies, the fixed effects model was analyzed (I2 = 0%; p = 1.0). During the treatment period, the total effective rate of the astragalus-RAAS blocker combination group was markedly higher than that of the RAAS blocker group (odds ratio [OR] = 3.63, 95% CI [2.59, 5.09], p < 0.00001; ).
3.2.2. Urinary protein excretion rate (UPER)
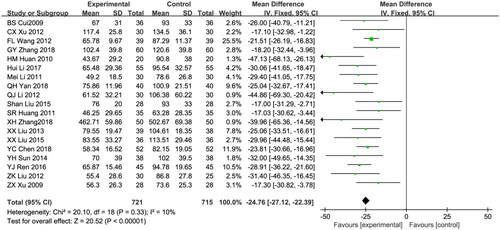
Nineteen studies reported UPER, a total of 1436 patients were included: 721 patients in the combination treatment group and 735 patients in the control group [Citation19,Citation21–23,Citation25,Citation26,Citation31,Citation33,Citation34,Citation36–42,Citation44,Citation46]. Due to the low heterogeneity among the studies (I2 = 10%; p = 0.33), the fixed effects model was used for data analysis. Compared with the control group, the astragalus-RAAS blocker combination group showed significantly lower UPER (weighted mean difference [WMD]: −24.76,95% CI [−27.12, −22.39], p < 0.00001; ).
3.2.3. Serum creatinine (Scr)
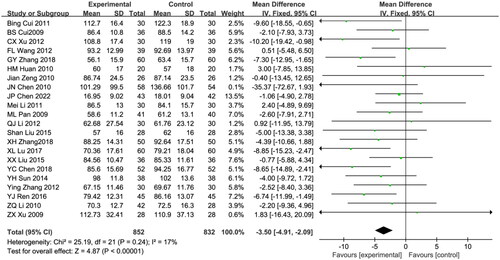
Twenty-two trials reported Scr, comprising 1685 patients (852 patients in the combination treatment group and 832 patients in the control group) [Citation18,Citation19,Citation21–23,Citation25,Citation27–29,Citation31,Citation32,Citation34,Citation36,Citation38,Citation40–44,Citation51]. The meta-analysis results showed low heterogeneity among the studies; hence, the fixed effect model was used for data analysis. (I2 = 17%; p = 0.24). Compared with the control group, the levels of Scr in the astragalus-RAAS blocker combination group was significantly decreased (WMD: −3.50, 95% CI [-4.91, −2.09], p < 0.00001; ).
3.2.4. Blood urine nitrogen (BUN)
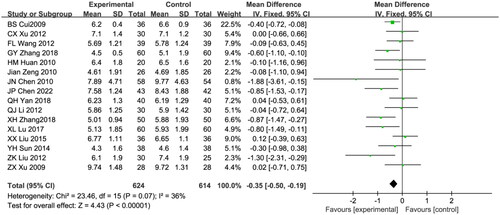
BUN was reported in sixteen eligible studies, including 1238 patients (624 in the treatment group and 614 in the control group) [Citation19,Citation21–23,Citation25,Citation27–29,Citation33,Citation34,Citation38,Citation40,Citation42,Citation46,Citation48,Citation49]. Due to the low heterogeneity of the test results, the fixed effects model was used for data analysis (I2 = 36%; p = 0.07). The astragalus-RAAS blocker combination group had lower levels of BUN than that of the control group (WMD: −0.35, 95% CI [−0.50, −0.19], p < 0.00001; ).
3.2.5. Glycosylated hemoglobin (HbAlc)
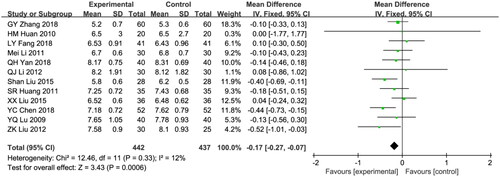
Twelve studies reported HbAlc and included 879 patients (442 in the treatment group and 437 in the control group [Citation23,Citation25,Citation30,Citation31,Citation33,Citation34,Citation36,Citation37,Citation40,Citation41,Citation45,Citation46]. A fixed effects model was employed due to the low heterogeneity (I2 = 12%; p = 0.33). The control group showed significantly higher HbAlc levels compared to the combination group (WMD: −0.17, 95% CI [−0.27, −0.07], p = 0.0006; ).
3.2.6. Subgroup analysis
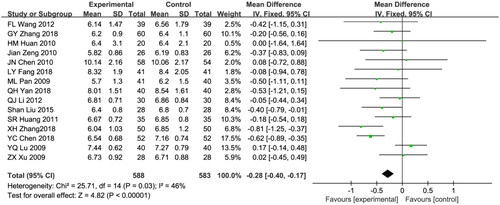
Fifteen studies included FPG in the results [Citation22,Citation23,Citation25,Citation27,Citation28,Citation30,Citation32–34,Citation36–38,Citation41,Citation45,Citation48,Citation51]. A total of 1171 patients were included in the studies, with 588 in the combined treatment group and 583 in the control group. A fixed effects model was used for data analysis due to the moderate heterogeneity among the studies (I2 = 46%; p = 0.03). The Astragalus combined RAAS blocker therapy showed a significantly larger decrease in FPG compared with the control group (WMD: −0.28, 95% CI [−0.40, −0.17], p < 0.00001; ).
Figure 7. Meta-analysis of fasting plasma glucose.
Mean difference, −0.28; 95% CI, −0.40, −0.17; p < 0.00001. CI: confidence interval; IV: inverse variance; SD: standard deviation.
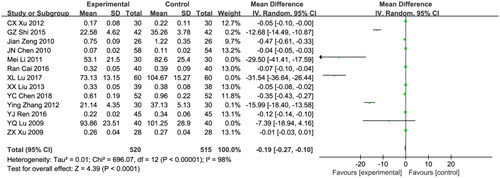
Thirteen studies included urinary microprotein (β2-MG) in the results [Citation21,Citation24,Citation27,Citation28,Citation31,Citation35,Citation39,Citation41,Citation43–45,Citation48,Citation49], comprising 1035 patients (520 in the treatment group and 515 in the control group). Due to the significant heterogeneity among the studies (I2 = 98%; p < 0.00001) a random effects model was employed. No significant difference was observed in efficacy between the astragalus-RAAS blocker combination group and control group (WMD:-0.19, 95% CI [−0.27, −0.10], p < 0.00001; ).
Figure 8. Meta-analysis of β2-MG.
Mean difference, −0.19; 95% CI, −0.27, −0.10; p < 0.0001. CI: confidence interval; IV: inverse variance; SD: standard deviation.
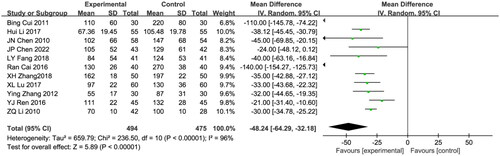
Eleven studies included 24hUTP in the results [Citation18,Citation26,Citation28–30,Citation35,Citation38,Citation43,Citation44,Citation47,Citation49]. comprising 969 patients (494 in the treatment group and 475 in the control group). As there was no significant heterogeneity among the studies (I2 = 96%; p < 0.00001), a random effects model was applied. The astragalus-RAAS blocker combination group showed a significantly lower 24hUTP than that of the control group (WMD: −48.24, 95% CI [−64.29, −32.18], p < 0.00001; ).
Figure 9. Meta-analysis of 24 h urinary protein level.
Mean difference, −48.24; 95% CI, −64.29, −32.18; p < 0.00001. CI: confidence interval; IV: inverse variance; SD: standard deviation.
This study aimed to identify the source of heterogeneity in the included studies using subgroup analysis. From the treatment perspective, the interventions differed, which may have caused heterogeneity. According to the type of RAAS blocker (ACEI/ARB) used in combination with Astragalus, the heterogeneity factors affecting FPG, β2-MG, and 24hUTP were analyzed by subgroup (). Astragalus combined with ARB had a significantly better effect on FPG (WMD: −0.37, 95% CI [−0.51, −0.24], p < 0.00001) and 24hUTP (WMD: −32.24, 95% CI [−36.04, −28.43], p < 0.00001) than Astragalus combined with ACEI. However, the effect of Astragalus combined with ACEI in reducing β2-MG (WMD:-0.06, 95% CI [−0.08, −0.04], p < 0.00001) was superior to that of Astragalus with ARB.
Table 2. Subgroup analysis of FPG, β2-MG, and 24-h UTP.
3.2.7. Publication bias and sensitivity analyses
The number of literatures included in the outcome indicators for publication bias analysis was more than 10. The funnel plots were largely symmetric (), suggesting no publication biases in the meta-analyses of UPER, BUN, HbAlc, FPG, and β2-MG. Egger’s and Begg’s tests were then used to quantitatively assess publication bias. The associated results for UPER, BUN, HbAlc, FPG, and β2-MG were not significant (p > 0.05, ). However, the funnel plots for TER, SCr, and 24hUTP were asymmetrical, suggesting the possible presence of a publication bias, which was supported by Egger’s test results (p < 0.05, ). It’s worth noting that 14 literatures were included in TER and 11 literatures were included in 24hUTP. The small sample size of the included studies would also affect the results of publication bias analysis.
Figure 10. Funnel plots for UPER, BUN, HbAlc, FPG, and β2-MG.
(A) UPER; (B) BUN; (C) HbAlc; (D) FPG; (E) β2-MG. β2-MG: urinary microprotein; BUN: blood urine nitrogen; FPG: fasting plasma glucose; HbAlc: glycosylated hemoglobin; MD: mean difference; SEM: standard error of the mean; UPER: urinary protein excretion rate.
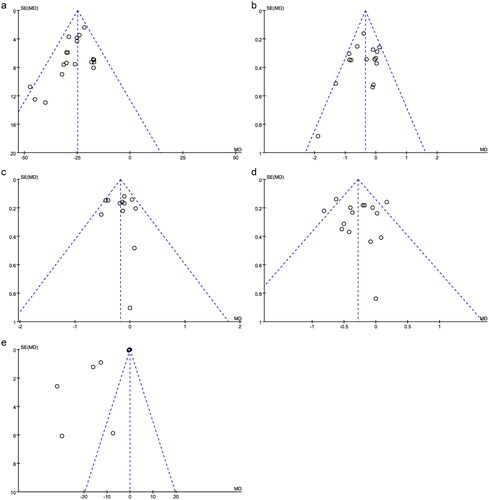
Table 3. Publication bias.
The sensitivity analysis was used to evaluate the stability of the study results. The random effects model and fixed effects model were respectively used for comparative analysis of the report outcomes included in the studies. The combined MD and 95% CI results of the two models were similar (), demonstrating the stability of our study.
Table 4. Sensitivity analysis.
3.2.8. GRADE evaluation of evidence quality
The GRADE evaluation system classifies the quality of evidence as high, moderate, low, and very low. According to the GRADE assessment results (), the quality of the evidence presented for UPER was high level, while that for BUN, HbAlc, FPG, and β2-MG was moderate, and that for TER, SCr, and 24hUTP was low.
Table 5. GRADE evaluation of evidence quality.
4. Discussion
DN is considered the most common microvascular complication of DM, which is the main cause of ESRD and renal replacement therapy. Currently, the first-line drugs for DN treatment include RAAS system blockers [Citation1]. However, western medicine alone has a limited effect on DN. In the current meta-analysis, 32 eligible RCTs were included to evaluate the clinical efficacy and safety of combined astragalus-RAAS blockers in treating stage III DN. A total of 2462 patients were included in the study, comprising 1244 in the combination therapy group and 1218 in the control monotherapy group.
Our results revealed significantly better clinical efficacy when RAAS blockers were combined with, than without astragalus (OR, 3.63; 95% CI, 2.59, 5.09; p < 0.05). Moreover, combined treatment decreased the UPER (WMD, −24.76; 95% CI, −27.12, −22.39), SCr (WMD, −3.50; 95% CI, −4.91, −2.09), BUN (WMD, −0.35; 95% CI, −0.50, −0.19), and HbAlc (WMD, −0.17; 95% CI, −0.27, −0.07; p < 0.05). More specifically, the subgroup analysis revealed significantly better effects on FPG (WMD, −0.37, 95% CI, −0.51, −0.24) and 24-h UTP (WMD, −32.24; 95% CI, −36.04, −28.43) when Astragalus was combined with an ARB than with an ACEI. Moreover, Astragalus combined with ACEI did not confer any obvious therapeutic advantages in terms of reducing FPG indicators (WMD, −0.06; 95% CI, −0.27, 0.15, p = 0.56). However, the capacity to reduce β2-MG was stronger when Astragalus was combined with an ACEI than an ARB (WMD, −0.06; 95% CI; −0.08, −0.04; p < 0.05). Currently, the treatment of proteinuria is mainly limited to ACEIs or ARBs [Citation51], whereas the comprehensive analysis of this study confirmed that astragalus-RAAS blockers inhibited proteinuria significantly better than ACEIs or ARBs alone. We did not perform subgroup analyses based on patient characteristics or disease severity due to incomplete descriptions of the included literature, but these potential effect modifiers may also influence treatment outcomes. This needs to be explored in further designed trials.
Our analysis further demonstrated that the β2-MG outcomes of the Astragalus combined with ARB and the 24 h UTP outcomes of the Astragalus combined with ACEI had significant heterogeneity. This may have resulted from different clinical baseline characteristics and intervention protocols among the studies. The included studies used different doses and types of Astragalus and RAAS blockers, which could lead to heterogeneity in the results.
Additionally, the sensitivity analysis demonstrated the stability of this study. According to the GRADE evaluation of the evidence quality, the UPER data was high quality, while all other variables ranged from low to moderate. The overall quality of evidence for these findings was poor, which may have a large impact on the quality of this meta-analysis.
This meta-analysis has several limitations that need to be considered. (1) Among the 32 studies included, 15 only mentioned using a “random” method for random assignment, but lacked specific descriptions. In addition, four studies did not explicitly mention using double-blind methods. These factors can lead to bias in selecting and implementing studies, resulting in false-positive results. (2) The 32 articles included were all single-center studies with a small sample size, which may cause publication bias, which could lead to an overestimation of the treatment effect. (3) Differences in routine interventions exist across studies, with varying dosages and methods of administering Astragalus, combined with multi-drug treatments, and a lack of placebo control groups. (4) The treatment periods in the included studies ranged from 3 to 48 weeks, which may not be long enough to fully assess the long-term efficacy and safety of the combination therapy. Therefore, future trials should employ a placebo-controlled randomized design and conduct comprehensive cost-effectiveness analyses (such as using Markov models or decision tree analyses). Additionally, considering the evolving landscape of DN treatment, it is pertinent to extend the exploration of Astragalus beyond RAAS blockers. Future research should include combinations with GLP-1 receptor agonists and SGLT2 inhibitors, which have shown promising results in recent studies [Citation52,Citation53]. Moreover, to advance our understanding of the therapeutic mechanisms, in vivo and in vitro experiments need to be designed for exploring mechanisms such as how astragalus active compounds modulate RAAS and their effects on DN inflammatory pathways, oxidative stress and tissue fibrosis. This will provide a more reliable framework for evaluating the therapeutic value and practicality of Astragalus in the combined treatment of DN.
In conclusion, the present results indicated that Astragalus combined with RAAS blockers is as safe as ACEI/ARB monotherapy. The combination conferred a greater advantage in improving TER, and reducing UPER, Scr, and HbAlc. However, our meta-analysis was limited, and more rigorous randomized double-blind trials of larger samples at several centers are needed to verify the efficacy and safety of Astragalus combined with RAAS blockers in the treatment of stage III DN. And a comprehensive analysis of adverse events associated with the combination therapy is further needed.
Ethical approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Authors’ contributions
All authors made a significant contribution to the work reported. Yuqiong Lin and Yuanrong Deng coordinated the review and organized the team. Yuanrong Deng and Feng Yu contributed to developing the protocol. Feng Yu and Huijun Chen assessed the studies and extracted data. Ying Xu, Xin Zheng, and Jingwen Zhang contributed to the data analysis and interpretation. Yuqiong Lin and Junfeng Liu drafted the review. Ying Xu and Xin Zheng edited and formatted the review text. Yuanrong Deng and Jin Lin reviewed the review and made important intellectual contributions.
Supplemental Material
Download Zip (1.4 MB)Acknowledgments
The authors would like to thank the Project of the university-level scientific research of Fujian Health College for their support in publishing this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Nomura H, Kuruppu S, Rajapakse NW. Stimulation of angiotensin converting enzyme 2: a novel treatment strategy for diabetic nephropathy. Front Physiol. 2021;12:1. doi: 10.3389/fphys.2021.813012.
- Leoncini G, Viazzi F, De Cosmo S, et al. Blood pressure reduction and RAAS inhibition in diabetic kidney disease: therapeutic potentials and limitations. J Nephrol. 2020;33(5):949–13. doi: 10.1007/s40620-020-00803-3.
- Lam AYR, Zheng HF, Teo KK, et al. Determining diabetic kidney disease severity using traditional Chinese medicine syndrome classification. Singapore Med J. 2021. Epub ahead of print. doi: 10.11622/smedj.2021179.
- Wen Y, Yan M, Zhang B, et al. Chinese medicine for diabetic kidney disease in China. Nephrology. 2017;22 Suppl 4(S4):50–55. doi: 10.1111/nep.13149.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Qi C, Mao X, Zhang Z, et al. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. 2017;2017:8637138–8637137. doi: 10.1155/2017/8637138.
- Weeks SM. The cochrane collaboration. West J Nurs Res. 2013;35(10):1249–1250. doi: 10.1177/0193945913491839.
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. 2004;4(1):38. doi: 10.1186/1472-6963-4-38.
- Bs C. Clinical observation of astragalus injection combined with captopril in the treatment of early diabetic nephropathy. J Clin Exp Med. 2009;8(11):2.
- Cui B, Wang HT. Clinical observation of lotensin combined with astragalus injection in the treatment of early diabetic nephropathy. Chin J Integr Trad West Nephrol. 2011;12(1):2.
- C C. Clinical observation of benazepril combined with astragalus in the treatment of diabetic nephropathy. Mod J Integr Trad Chin West Med. 2010;19(23):2.
- Xu CX. Effect of fosinopril combined with astragalus injection on early diabetic nephropathy. Chin J Rural Med. 2012;19(2):2.
- Wang FL, et al. Clinical observation of Yiqi Yangyin Xiaozhi Tongluo prescription in treating early diabetic nephropathy. Chin J Integr Trad West Med. 2012;1:4.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160.
- Shi GZ, Lin SS. Effect of astragalus injection on the clinical symptoms and urinary microalbumin in patients with early diabetic nephropathy. Mil Joint Logist. 2015;2(2):4.
- Huan HM, et al. Efficacy of losartan potassium combined with Astragalus injection in the treatment of early diabetic nephropathy. Chin J Integr Trad West Nephrol. 2010;11(5):2.
- Li H, Xu YC. Effect of Huangqi injection in combined with irbesartan on the vascular endothelial function and CGRP in patients with early DN. J Hainan Med Univ. 2017;23(3):3.
- Zeng J,Liu DF, Wang JY. Effect of combining Huangqi injection with enalapril in early DN for treatment of early diabetic nephropathy. J Mod Clin Med. 2010;36(2):2.
- Chen JN, Li ZX. Curative effect of irbesartan combined with astragalus injection on 58 patients with early diabetic nephropathy. Chin Community Doctors. 2010;12(22):1.
- Chen JP. Effect of Huangqi decoction combined with irbesartan on type 2 diabetic nephropathy. Pract Clin J Integr Trad Chin West Med. 2022;22(15):4.
- Fang LY, et al. Clinical study on the effect of Jinqi Yuquan formula in the treatment of diabetic nephropathy of qi and yin deficiency combined with blood stasis type. J Nanjing Univ Trad Chin Med. 2018;34(1):4.
- Li M. Combined therapy of Huangqi injection and irbesartan for early period type 2 diabetic nephropathy patients. Medical Forum. 2011;32(9):3.
- Pan ML, Xue FM. Bservation of curative effect of Tangshen decoction on early diabetic nephropathy. Beijing J Trad Chin Med. 2009;28(4):2.
- Yan QH, et al. Effect of qikui granule on microalbuminuria and progress in patients with early diabetic nephropathy. Chin J Integr Trad West Med. 2018;38(4):5.
- Li QJ. Effect of astragalus injection on cellular inflammatory factors in early diabetic nephropathy. Chin J Clin Res. 2012;25(10):2.
- Cai R, Cui JH. Effect of astragalus injection on urinary albumin and cytokines in patients with early diabetic nephropathy. Mod J Integr Trad Chin West Med. 2016;25(28):3.
- Liu S, et al. Effect of irbesartan combined with astragalus injection on connective tissue growth factor in elderly patients with diabetic nephropathy. Chin J Gerontol. 2015;35(1):3.
- Huang SR. Clinical study of shenan decoction in treating early diabetic nephropathy. J Changchun Univ Trad Chin Med. 2011;27(3):2.
- Zhang XH, Guo ZJ, Lan CF. Clinical observation of Bushen Jianpi, Yiqi Tongluo prescription combined with valsartan in the treatment of diabetic nephropathy proteinuria. Chin J Integr Trad West Nephrol. 2018;19(2):4.
- Lu XL, Chen XM. Effect of Shenqi Qinerxian decoction on diabetic nephropathy (stage III) and its influence on renal function. Glob Trad Chin Med. 2017;10(11):3.
- Liu XX, Xiao M, Liu B. Effect of astragalus injection on urinary albumin and cytokines in patients with early diabetic nephropathy. Chin J Exp Trad Med Formulae. 2013;19(9):5.
- Liu XX, Meng X, Wei N. Effect of astragalus membranaceus on endothelin and calcitonin gene-related peptide in early diabetic nephropathy patients. Chin J Gerontol. 2015;000(008):3.
- Yc C, et al. Clinical trial of qishen tongluo granules in the treatment of diabetic nephropathy in stage III. Chin J Clin Pharmacol. 2018;34(07):3.
- Sun YH, Zhao FL, Ding XY. Clinical observation of telmisartan combined with astragalus injection in the treatment of early diabetic nephropathy. Chin J Integr Trad West Nephrol. 2014;15:2.
- Zhang Y. Effect of astragalus injection combined with valsartan in treating diabetic nephropathy. Med Pharm J Chin Peoples Liberation Army. 2012;024(008):3.
- Ren YJ. Effect of Huangqi injection in combined with irbesartan on the microcirculation and CTGF in patients with early DN. J Hainan Med Univ. 2016;22(22):4.
- Lu Y. Q., clinical effect of huangqi granule combined with lotensin on early diabetic nephropathy. Chin J Exp Trad Med Formulae. 2009;15(7):3.
- Liu ZK, Xie R. Observation on the curative effect of irbesartan, astragalus injection and insulin in treating early diabetic nephropathy. Clin Med Eng.. 2012;19(6):2.
- Xu ZX. Clinical observation of 28 patients with early diabetic nephropathy treated with valsartan combined with Astragalus injection. GUANGXI Med J. 2009;31(6):3.
- Sonmez A. Challenges in the prevention and management of diabetic kidney diseases. Front Clin Diabetes Healthc. 2021;2:728320. doi: 10.3389/fcdhc.2021.728320.
- Ai X, et al. A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomed Pharmacother. 2020;132:110852.
- Zhou H, Ni W-J, Meng X-M, et al. Corrigendum: microRNAs as regulators of immune and inflammatory responses: potential therapeutic targets in diabetic nephropathy. Front Cell Dev Biol. 2022;10:875280. doi: 10.3389/fcell.2022.875280.
- Tan B, et al. The effect of ibrutinib on radiosensitivity in pancreatic cancer cells by targeting EGFR/AKT/mTOR signaling pathway. Biomed Pharmacother. 2020;128:110133.
- Guo M, et al. Astragalus polysaccharide ameliorates renal inflammatory responses in a diabetic nephropathy by suppressing the TLR4/NF-kappaB pathway. Drug Des Devel Ther. 2023;17:2107–2118.
- Ren G, et al. Baicalin exerts a protective effect in diabetic nephropathy by repressing inflammation and oxidative stress through the SphK1/S1P/NF-kappaB signaling pathway. Diabetes Metab Syndr Obes. 2023;16:1193–1205.
- Vodošek Hojs N, Bevc S, Ekart R, et al. Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants. 2020;9(10):925. doi: 10.3390/antiox9100925.
- Lin Y, et al. Astragaloside IV ameliorates streptozotocin induced pancreatic beta-Cell apoptosis and dysfunction through SIRT1/P53 and akt/GSK3beta/Nrf2 signaling pathways. Diabetes Metab Syndr Obes. 2022;15:131–140.
- Chen JY, et al. Based on the network pharmacology, and molecular docking to explore the mechanism of action of diabetes, renal Ann soup treatment of diabetic nephropathy. Chin J Gerontol. 2022;42(23):7.
- Li ZQ. Curative effect of astragalus injection combined with telmisartan in the treatment of early diabetic nephropathy. Clin Med Res. 2010;27(3):3.
- Zhang GY, Liu YS, Sui HY. Clinical observation of Yiqi Ziyin Tongluo prescription combined with Western medicine in the treatment of early diabetic nephropathy. Chin J Trad Med Sci Technol. 2018;25(4):3.
- Tu X, Liu F, Jordan JB, et al. Huang Qi Elixir’ for proteinuria in patients with diabetic nephropathy: a study protocol for a randomized controlled pilot trial. Trials. 2013;14(1):223. doi: 10.1186/1745-6215-14-223.
- Krisanapan P, Sanpawithayakul K, Pattharanitima P, et al. Safety and efficacy of GLP-1 receptor agonists in type 2 diabetes mellitus with advanced and end-stage kidney disease: a systematic review and meta-analysis. Diseases. 2024;12(1):14. doi: 10.3390/diseases12010014.
- Kanduri SR, et al. SGLT2 inhibitors and kidney outcomes in patients with chronic kidney disease. J Clin Med. 2020;9(9):2723.