Abstract
Research has shown that patients undergoing hemodialysis experience seasonal variations in their serum potassium levels. There was inconsistent seasonal fluctuation in serum potassium levels among the hemodialysis population across different locations. In the form of narrative review for the first time, the article discusses the seasonal changes of serum potassium in this population and its potential reasons, this article demonstrates that it is primarily attributable to seasonal dietary potassium intake. However, existing studies have not quantified seasonal dietary potassium intake, so the results are still speculative. Furthermore, future research ought to further expound upon the clinical implications of seasonal variations in serum potassium levels among dialysis patients, as well as other influencing mechanisms such as the pathophysiological causes of these seasonal changes, particularly those pertaining to dietary, geographical, and regional factors. These findings contribute to a more thorough interpretation of laboratory results in hemodialysis patients and provide important guidance for their individualized dietary management.
Introduction
Potassium plays a crucial role in maintaining normal cell function. Approximately 90% of excess potassium intake is excreted in the urine [Citation1], whereas the remainder is excreted through the gastrointestinal tract [Citation2]. The kidney can maintain potassium homeostasis despite high dietary intake by regulating urinary potassium excretion. In the general population, a U.K. study evaluating the effect of ambient temperature on serum potassium levels showed that serum potassium values followed seasonal patterns; peaks in winter and troughs in summer [Citation3]. A Swiss study showed the lowest point in October and the highest point in April [Citation4]. Several studies also have reported seasonal variations in serum potassium levels in Maintenance hemodialysis (MHD) patients [Citation5–7]. Seasonal variations in dietary patterns have been reported as a specific cause of serum potassium changes in hemodialysis patients [Citation4,Citation8]. Potassium intake follows a seasonal pattern in a Swiss study, peaking in April and lowest in October [Citation9]. Studies have revealed seasonal variations in the increase of fruit and vegetable consumption [Citation10]. Due to the influence of geographical and climatic factors, various potassium-rich seasonal vegetables and fruits vary between regions, and the potassium intake of hemodialysis patients may fluctuate seasonally. Because impaired renal excretion alters potassium homeostasis in hemodialysis patients, potassium is excreted primarily through dialysis and the colon [Citation11–14], hemodialysis patients are more susceptible to hyperkalemia, which increases the risk of malignant arrhythmia and sudden death [Citation15–18]. Although the mechanism underlying this phenomenon is unknown, recognizing these changes may help explain laboratory results and clinical management of these patients. This article is the first narrative review of seasonal variations in serum potassium in hemodialysis patients. The basic characteristics and results of the clinical researches included in our study are shown in .
Table 1. Characteristics and seasonal variation in serum potassium levels of seven clinical researches in hemodialysis patients.
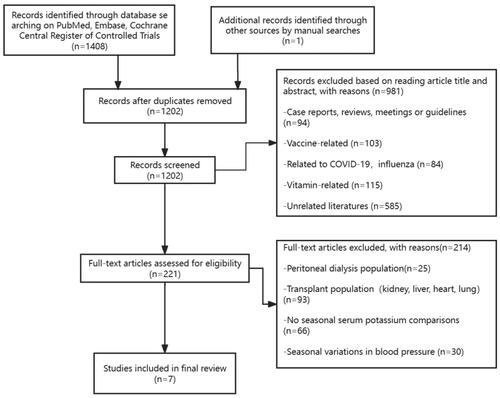
From inception to March 29, 2024, we searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials using the terms “Seasons, Season or Seasonal Variation and dialysis, renal disease, or kidney failure.” Two reviewers (Z.S. and Z.B.) independently screened the title and abstract of each search result, and studies on seasonal serum potassium differences in hemodialysis patients were included by reading the abstracts and full texts of the articles. Any disagreements were resolved through discussions with the third independent reviewer (H.Y.) to avoid bias or oversight. Finally, we included seven studies to analyze the seasonal variation of serum potassium in hemodialysis population (; ).
Table 2. Causes of seasonal variations in serum potassium and conclusions of seven clinical researches in hemodialysis patients.
Serum potassium metabolism in hemodialysis patients
In people with normal renal function, the kidneys are the main organ for maintaining potassium homeostasis, and the amount of potassium excreted through the gastrointestinal tract only accounts for 5–10% of the potassium intake [Citation19,Citation20]. Insulin, catecholamines, and aldosterone are key factors in maintaining potassium balance in the body. Under normal circumstances, when serum potassium increases, especially after a meal, insulin release can not only regulate serum glucose concentration but also promote the transfer of potassium into liver and muscle cells by enhancing the activity of Na+/K + ATPase, until the kidneys excrete the potassium load, thereby reestablishing normal systemic potassium levels [Citation20,Citation21]. Elevated serum potassium also causes the release of catecholamines, which increase the activity of Na+/K + ATPase throughβ2-Adrenergic receptors to transfer potassium into cells [Citation20,Citation21]. In addition, when sodium intake decreases and potassium intake increases, aldosterone secretion is stimulated and urinary potassium excretion is increased to regulate serum potassium level [Citation20,Citation21]. Typically, serum potassium disturbances caused by cell transfer are transient, whereas persistent hyperkalemia results from impaired renal excretory function [Citation20]. Therefore, patients with chronic kidney disease (CKD) and end-stage renal disease (ESKD) receiving hemodialysis are more likely to develop hyperkalemia [Citation22].
For dialysis patients, in addition to being affected by the potassium load of dietary intake and the removal of potassium through hemodialysis, the use of drugs such as the renin-angiotensin-aldosterone system (RAAS) blockers and mineralocorticoid receptor antagonist (MRA) to inhibit aldosterone secretion, insulin deficiency, hyperglycemia, and metabolic acidosis lead to decreased cellular potassium uptake, reduced distal tubular sodium and water transport due to decompensated heart failure and hypovolemia, and altered catecholamine signaling, all of the above factors will affect serum potassium metabolism [Citation19,Citation23–25].
Additionally, with aspect to the mode of potassium load elimination in dialysis patients, aldosterone also enhances potassium excretion in the distal colon when serum potassium concentrations are elevated by dietary potassium intake [Citation26,Citation27]. Studies compared the fecal potassium excretion of healthy people and ESKD patients and found that the fecal potassium excretion of ESKD patients was three times higher than that of the control group [Citation28–30]. It has also been reported that human gastrointestinal-renal kaliuretic signaling axis regulates potassium excretion independently of variations in aldosterone and serum potassium concentration [Citation31].
Literatures on seasonal variation of serum potassium in dialysis patients
In a multicenter study of hemodialysis (HEMO) sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases [Citation7], 1445 patients were examined for seasonal changes in 21 clinical and laboratory variables, and 13 indicators showed statistically significant seasonal variation (p < .01). Serum potassium peaked in February, which had a significant seasonal effect (p = .007). The study also concluded that seasonal variation in pre-dialysis urea nitrogen levels was significant, presumably due to dietary protein intake changes. In addition, it is emphasized that the study’s various indicators only demonstrate association, not causation, and seasonal changes of these indicators may lead to deviations in the interpretation of clinical research results.
Yanai et al. [Citation5] assessed the annual rhythm of routine laboratory parameters in Japanese hemodialysis patients, including 150 hemodialysis patients, and collected 38 laboratory parameters over a period of 2 years. The study found that serum potassium was highest in December and lowest in June, with seasonal variations [(5.0 ± 0.60 vs. 4.8 ± 0.60) mmol/L, p < .001], but serum potassium did not show a significant sinusoidal regression trend over time (p = .320). The study also examined the seasonal variations of additional indicators. The peaks of urea nitrogen and phosphate occur in February and August, respectively. Summertime in Tokyo, Japan, due to the high temperature and humidity, people tend to lose their appetite and prefer low-fat, light foods. A recent retrospective study examining the correlation between variability in serum potassium levels and mortality among hemodialysis patients in Japan also identified seasonal variation in potassium levels, revealing increases during the winter season and reduced levels during the summer season. Considering the study was conducted in Nagasaki Prefecture, a renowned orange producer, there is a likelihood that patients undergoing dialysis have ample opportunities to consume oranges throughout the winter months, thereby potentially augmenting their serum potassium levels [Citation32]. Furthermore, it is consistent with Shahar et al. [Citation33], who reported that total energy, total fat, and other nutrient intake increased significantly in winter when investigating seasonal changes in dietary intake, indicating that the seasonal differences in urea nitrogen and phosphate (winter high and low in summer) could be attributable to changes in dietary intake, particularly protein intake. Interannual rhythms in laboratory-measured parameters may be attributable to seasonal variations in food intake, and since this study did not monitor dietary intake, the observed results are speculative, and these seasonal variations should be taken into account when interpreting laboratory results.
Usvyat et al. [Citation6] conducted a 5-year cohort study to assess seasonal variations in mortality, clinical and laboratory parameters in hemodialysis patients involving 15 nephrology institutes from six different climate states in the United States of 15,056 hemodialysis patients, which is also the largest cohort study to date. The study found significantly higher all-cause and cardiovascular mortality in winter compared with other seasons (14.2 deaths per 100 patient-years in winter, 13.1 in spring, 12.3 in autumn, and 11.9 in summer). The ratio of neutrophils/lymphocytes, serum potassium and platelets varied seasonally, being highest in winter and lowest in summer; serum calcium was highest in autumn and lowest in spring. Consistent with the findings of Yanai et al. this study discovered that serum potassium levels varied seasonally, peaking in the winter and possibly reflecting higher dietary intake during the winter [Citation34]. In contrast to the findings of Yanai et al. there was no significant seasonal variation in phosphate levels. Consistent across climate regions, the study found notable seasonal differences in overall and cardiovascular mortality among a large number of dialysis patients. There were also seasonal differences in other physiological and laboratory parameters, and seasonality in physiological and laboratory parameters was associated with mortality differences. When designing and interpreting longitudinal studies of dialysis patients, seasonal variation should be taken into account.
In a recent multicenter cohort study investigating seasonal changes in serum potassium levels in hemodialysis patients [Citation35], 279 hemodialysis patients were included in the analysis, and it was found that the serum potassium of the patients in the moderate group (K+,4.7 ± 0.4 mmol/L) reached its peak in January (4.83 ± 0.74 mmol/L) and the lowest in April (4.46 ± 0.59 mmol/L); the serum potassium of the patients in the high group (K+, 5.6 ± 0.4 mmol/L) reached its peak in August (5.71 ± 0.70 mmol/L) and the lowest in April (5.43 ± 0.74 mmol/L). In the high potassium group, potassium levels were substantially higher in summer than in autumn (p < .001) and spring (p = .007) [Citation35]. The analysis of this study showed that serum potassium levels peaked in winter in the moderate potassium group, which is consistent with several previous reports [Citation5–7]. However, new findings were discovered in the high potassium group, which had higher mean potassium levels throughout the year than the moderate group, and in serum potassium levels that peaked in summer, suggesting that cases of fatal hyperkalemia may occur more frequently in summer [Citation35]. Patients in the high potassium group, on the other hand, were younger and had been on hemodialysis for a longer period of time than those in the moderate serum potassium group [Citation35]. Younger age was reported to be the most important demographic correlate of nonadherence, including skipping dialysis treatments or not adhering a restricted diet [Citation36,Citation37]; longer hemodialysis duration was also associated with lower residual renal function [Citation38], which is also a risk factor for developing hyperkalemia. This study therefore recommends caution and close monitoring for hyperkalemia in younger patients who have been on longer dialysis and have relatively high year-round potassium levels, especially in summer [Citation35]. This study also noted that mean levels of serum albumin and phosphorus were higher in the high potassium group than in the moderate group, a possible explanation being that higher mean potassium levels may indicate not only poorer dietary adherence but also better nutritional status [Citation39]. The study concluded that serum potassium showed distinct seasonal variation patterns in the middle and high potassium groups, the high potassium group was significantly increased in summer, and the medium potassium group was significantly increased in winter. These findings may provide important evidence for individualized management of patients [Citation35].
However, two studies located in the Mediterranean region showed different conclusions. Tsiagka et al. [Citation40] investigated the prevalence, recurrence and seasonality of hyperkalemia in 149 maintenance hemodialysis patients located in Thessaloniki, Greece. This study discovered substantial differences in the occurrence of hyperkalemia 5.1, 5.5, and 6.0 mmol/L among the four seasons, with hyperkalemic episodes occurring more frequently in summer (p < .001 for all three comparisons). Another prospective multicenter study [Citation41] of 14 chronic hemodialysis centers in the Lorraine region of France also observed a significant seasonal trend in serum potassium >6 mmol/L, which was highest in summer and the incidence of hyperkalemia (>5.1 and 5.5 mmol/L) was also higher in summer.
Summarize the seasonal variations in serum potassium described in the preceding studies. Cheung et al. [Citation7] reported that potassium levels peaked in February in hemodialysis patients in various regions of the United States; in the largest cohort study to date conducted by Usvyat et al. [Citation6], seasonal variations in potassium levels in hemodialysis patients across regions of the United States were also shown, with highest in winter and lowest in summer; Yanai et al. and Yamaguchi et al. [Citation5,Citation32] reported that potassium levels in Japanese hemodialysis patients peaked in winter and troughed in summer; a recent multicenter cohort study of seasonal variation in serum potassium levels in hemodialysis patients was conducted in Korea [Citation35] and found that patients in the moderate potassium group(K+, 4.7 ± 0.4 mmol/L) peaked in January and those in the high group(K+, 5.6 ± 0.4 mmol/L) peaked in August. Whereas two studies [Citation40,Citation41] conducted in the Mediterranean region consistently demonstrated that summer is the season when hyperkalemia is more prevalent and severe, contrary to the findings of Cheung, Usvyat, Yanai et al. [Citation5–7] in the United States and Japan.
Effect of diet on seasonal variation of serum potassium in hemodialysis patients
Existing studies indicate that the seasonal variation of serum potassium in hemodialysis patients is inconsistent. For instance, potassium levels in hemodialysis patients in various regions of Japan and the United States tend to be higher in winter, and studies have indicated that peak potassium levels in winter are associated with higher total dietary intake during this season [Citation5,Citation34]. Consider that, as a result of the summer’s high temperatures, individuals typically lose their appetite. As early as the previous animal study by Sakata et al. it was found that in the high temperature environment of 31 °C in rats, the synthesis of neuronal histamine in the hypothalamus increased, the satiety center was stimulated, and the food intake decreased [Citation42,Citation43]. Other studies have also shown that the majority of nutrients are obtained more in the winter than in the summer [Citation44,Citation45]. It may also be due to a high basal metabolic rate and a high food consumption during winter time as a result of the cold weather [Citation46–48]. In contrast, in two studies conducted in France and Greece in the Mediterranean region, the eating habits/patterns of the patients were quite distinct and consistently demonstrated that summer was the season in which hyperkalemia was more frequent and severe, possibly as a result of the Mediterranean diet, they regularly ingest a lot of potassium-rich vegetables, such as leafy greens, fruits, grains, nuts, and beans and legumes [Citation49]. This is a more likely explanation, as the foods rich in potassium vary by geographical location and climate, resulting in seasonal changes in dietary potassium intake, which may be the most important mediator of the heterogeneity of seasonal variation in hyperkalemia. However, the validity of this speculation has lately been called into question through several observational studies that found no correlation between dietary potassium intake and pre-dialysis serum potassium levels [Citation50–54]. Other studies have found that adhering to a “healthy” plant-based diet has little effect on potassium homeostasis and does not exacerbate the risk of hyperkalemia in hemodialysis patients [Citation50,Citation52,Citation54]. Studies have shown that the bioavailability of dietary potassium is affected by other nutrients in the food, such as vitamins, antioxidants, carbohydrates, and fiber [Citation19]. Typically, consumption of meats high in potassium results in net acid production, but consumption of high potassium fruits and vegetables results in net base production [Citation19]. Compared with high-potassium meat, high-potassium fruits and vegetables can contain high fiber content, which can promote bowel movements and increase stool volume by stimulating intestinal peristalsis, thereby increasing potassium excretion [Citation55]. These differing observations on the effect of dietary potassium intake on serum potassium are influenced by factors such as type of food, potassium content, content of other nutrients, dietary pattern, and adequacy of dialysis. However, seasonal dietary potassium intake in hemodialysis patients was not collected in the existing studies, so these are still hypotheses.
Other factors affecting the seasonal fluctuations in serum potassium levels and related clinical significance
Another likely cause is that in the summer, a substantial amount of potassium is lost through sweat, it has previously been shown that the potassium concentration of sweat from renal failure patients is much higher than that of healthy controls [Citation56]. Besides, a substantial negative correlation between daily temperature and mean serum potassium concentration has also been reported, and a high-temperature environment stimulates glucose metabolism and cellular uptake of K+ [Citation57–59]. Therefore, compared with summer, the incidence of hyperkalemia is relatively high in winter when the temperature is lower [Citation60]. Furthermore, the majority of dialysis patients are older and have a variety of complex chronic conditions, including heart failure, hypertension, left ventricular hypertrophy, lung disorder, etc., which are more susceptible to the above factors in winter. If metabolic acidosis caused by severe acid-base imbalance occurs, blood potassium will increase.
Usvyat et al. [Citation6] reported a significant increase in all-cause and cardiovascular mortality in hemodialysis patients during the winter, with seasonal changes in mortality associated with physiological and laboratory parameters. In addition, the aforementioned study by Kim et al. [Citation35] showed no difference in mortality between hemodialysis patients in the moderate and high groups, but changes in potassium levels were strongly related to mortality. In a survey from the International Monitoring of Dialysis Outcomes (MONDO) Consortium database, Guinsburg et al. [Citation61] explored seasonal variations in mortality and clinical indicators in a hemodialysis population. Among the 87,399 hemodialysis patients included, the northern temperate (n = 63,671); the northern tropical (n = 7,159); the southern temperate (n = 13,917); and the southern tropical (n = 2,652). The results showed that winter had the highest mortality rate globally. After stratification, mortality was significantly lower in spring and summer than in winter in temperate regions, but not in tropical regions. Higher interdialytic weight gain (IDWG), increased peripheral vasoconstriction, increased release of thrombotic factors [Citation62–64], and higher rates of infectious and subsequent cardiovascular complications could explain the observed seasonal mortality disparities [Citation65]. Other factors, such as alterations in sympathetic nervous system activity, may also contribute to the rise in arrhythmia [Citation66,Citation67]. Consequently, during seasons of high mortality, it is imperative to monitor the clinical and laboratory parameters of patients.
Seasonal fluctuations in serum potassium levels could facilitate a more comprehensive understanding of laboratory findings, enable personalized patient treatment, and, most significantly, contribute to the development of enhanced preventive strategies against hyperkalemia. Yamaguchi et al. [Citation32] demonstrated that serum potassium level fluctuation is associated with prognosis, even after correcting for confounding factors such as age and dialysis duration, and that changes in serum potassium levels are more detrimental than high serum potassium levels. The study found that the relative risk of death in the group with higher coefficient of variation of potassium level was higher than that in the group with lower coefficient of variation of potassium level (RR 1.98, 95% Cl 1.19–3.29, p = .01). Consequently, meticulous monitoring of the potassium level and its fluctuations is required.
Conclusion
In conclusion, the aforementioned studies have demonstrated seasonal variations in serum potassium in hemodialysis patients, which may be related to dietary potassium intake, as well as seasonal variations in mortality in hemodialysis patients, which may be associated to arrhythmia caused by hyperkalemia. Nevertheless, the current study lacks further investigation into the exact cause of death of patients as well as quantification of dietary potassium intake, therefore the conclusions are still hypothetical. And besides, this phenomenon and causes may be subject to publication bias. Future studies, we believe, should shed more light on the pathophysiological causes of these seasonal variations, particularly in relation to dietary, geographic, and regional factors, as well as the clinical significance of seasonal variations in dialysis patients. These findings can provide valuable guidance for the individualized dietary management of hemodialysis patients so as to make their serum potassium fluctuations in seasonal changes tend to remain stable and reduce the frequency of hyperkalemia.
Acknowledgments
We would like to appreciate all members participated in this work and the Department of Family Medicine of the University of Hong Kong-Shenzhen Hospital for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol. 1998;274(5):F817–33.
- Hayslett JP, Binder HJ. Mechanism of potassium adaptation. Am J Physiol. 1982;243(2):F103–12.
- Durose CL, Holdsworth M, Watson V, et al. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. J Am Diet Assoc. 2004;104(1):35–41. doi: 10.1016/j.jada.2003.10.016.
- Cox BD, Whichelow MJ, Prevost AT. Seasonal consumption of salad vegetables and fresh fruit in relation to the development of cardiovascular disease and cancer. Public Health Nutr. 2000;3(1):19–29. doi: 10.1017/s1368980000000045.
- Yanai M, Satomura A, Uehara Y, et al. Circannual rhythm of laboratory test parameters among chronic haemodialysis patients. Blood Purif. 2008;26(2):196–203. doi: 10.1159/000117310.
- Usvyat LA, Carter M, Thijssen S, et al. Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin J Am Soc Nephrol. 2012;7(1):108–115. doi: 10.2215/CJN.03880411.
- Cheung AK, Yan G, Greene T, et al. Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol. 2002;13(9):2345–2352. doi: 10.1097/01.ASN.0000026611.07106.A7.
- Kovacic V, Kovacic V. Seasonal variations of clinical and biochemical parameters in chronic haemodialysis. Ann Acad Med Singap. 2004;33(6):763–768. doi: 10.47102/annals-acadmedsg.Kovac.
- Marti-Soler H, Pommier C, Bochud M, et al. Seasonality of sodium and potassium consumption in Switzerland. Data from three cross-sectional, population-based studies. Nutr Metab Cardiovas Dis. 2017;27(9):792–798.
- Hussain A, Zulfiqar F, Saboor A. Changing food patterns across the seasons in rural Pakistan: analysis of food variety, dietary diversity and calorie intake. Ecol Food Nutr. 2014;53(2):119–141. doi: 10.1080/03670244.2013.792076.
- Rajendran VM, Sandle GI. Colonic potassium absorption and secretion in health and disease. Compr Physiol. 2018;8(4):1513–1536. doi: 10.1002/cphy.c170030.
- de Rooij ENM, Dekker FW, Le Cessie S, et al. Serum potassium and mortality risk in hemodialysis patients: a cohort study. Kidney Med. 2022;4(1):100379. doi: 10.1016/j.xkme.2021.08.013.
- Yamada S, Inaba M. Potassium metabolism and management in patients with CKD. Nutrients. 2021;13(6):1751. doi: 10.3390/nu13061751.
- Pani A, Floris M, Rosner MH, et al. Hyperkalemia in hemodialysis patients. Sem Dialysis. 2014;27(6):571–576. doi: 10.1111/sdi.12272.
- Yusuf AA, Hu Y, Singh B, et al. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44(3):179–186. doi: 10.1159/000448341.
- Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi: 10.1159/000479802.
- Kovesdy CP, Rowan CG, Conrad A, et al. Real-world evaluation of Patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019;4(2):301–309. doi: 10.1016/j.ekir.2018.10.020.
- Montford JR, Linas S. How dangerous is hyperkalemia? JASN. 2017;28(11):3155–3165. doi: 10.1681/ASN.2016121344.
- Clase CM, Carrero J-J, Ellison DH, et al. Potassium homeostasis and management of Dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020;97(1):42–61. doi: 10.1016/j.kint.2019.09.018.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480–490. doi: 10.1152/advan.00121.2016.
- Borrelli S, Matarazzo I, Lembo E, et al. Chronic hyperkaliemia in chronic kidney disease: an old concern with new answers. Int J Mol Sci. 2022;23(12):6378. doi: 10.3390/ijms23126378.
- D'Alessandro C, Cumetti A, Pardini E, et al. Prevalence and correlates of hyperkalemia in a renal nutrition clinic. Intern Emerg Med. 2021;16(1):125–132. doi: 10.1007/s11739-020-02353-9.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10(6):1050–1060. doi: 10.2215/CJN.08580813.
- Bilbrey GL, Carter NW, White MG, et al. Potassium deficiency in chronic renal failure. Kidney Int. 1973;4(6):423–430. doi: 10.1038/ki.1973.138.
- Seliger SL. Hyperkalemia in patients with chronic renal failure. Nephrol Dial Transplant. 2019;34(Suppl 3):iii12–iii18. doi: 10.1093/ndt/gfz231.
- Giebisch G, Krapf R, Wagner C. Renal and extrarenal regulation of potassium. Kidney Int. 2007;72(4):397–410. doi: 10.1038/sj.ki.5002288.
- Epstein M, Lifschitz MD. The unappreciated role of extrarenal and gut sensors in modulating renal potassium handling: implications for diagnosis of Dyskalemias and interpreting clinical trials. Kidney Int Rep. 2016;1(1):43–56. doi: 10.1016/j.ekir.2016.03.001.
- Hayes CP, Jr., McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216.
- Mathialahan T, Maclennan KA, Sandle LN, et al. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206(1):46–51. doi: 10.1002/path.1750.
- Bastl C, Hayslett JP, Binder HJ. Increased large intestinal secretion of potassium in renal insufficiency. Kidney Int. 1977;12(1):9–16. doi: 10.1038/ki.1977.73.
- Preston RA, Afshartous D, Rodco R, et al. Evidence for a gastrointestinal-renal kaliuretic signaling axis in humans. Kidney Int. 2015;88(6):1383–1391. doi: 10.1038/ki.2015.243.
- Yamaguchi K, Kitamura M, Otsuka E, et al. Association between annual variability of potassium levels and prognosis in patients undergoing hemodialysis. Clin Exp Nephrol. 2023;27(10):873–881. doi: 10.1007/s10157-023-02368-4.
- Shahar DR, Yerushalmi N, Lubin F, et al. Seasonal variations in dietary intake affect the consistency of dietary assessment. Eur J Epidemiol. 2001;17(2):129–133. doi: 10.1023/a:1017542928978.
- Shephard RJ, Aoyagi Y. Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol. 2009;107(3):251–271. doi: 10.1007/s00421-009-1127-1.
- Kim Y, Yun SH, Koo H, et al. Different seasonal variations of potassium in hemodialysis patients with high longitudinal potassium levels: a multicenter cohort study using DialysisNet. Yonsei Med J. 2021;62(4):315–324. doi: 10.3349/ymj.2021.62.4.315.
- Leggat JE, Orzol SM, Hulbert-Shearon TE, et al. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis. 1998;32(1):139–145. doi: 10.1053/ajkd.1998.v32.pm9669435.
- Kugler C, Vlaminck H, Haverich A, et al. Nonadherence with diet and fluid restrictions among adults having hemodialysis. J Nurs Scholarsh. 2005;37(1):25–29. doi: 10.1111/j.1547-5069.2005.00009.x.
- Shemin D, Bostom AG, Laliberty P, et al. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38(1):85–90. doi: 10.1053/ajkd.2001.25198.
- Lee JE, Kim H-J, Lee MJ, et al. Comparison of dietary intake patterns in hemodialysis patients by nutritional status: a cross-sectional analysis. Kidney Res Clin Pract. 2020;39(2):202–212. doi: 10.23876/j.krcp.20.037.
- Tsiagka D, Georgianos PI, Pikilidou MI, et al. Prevalence, recurrence and seasonal variation of hyperkalemia among patients on hemodialysis. Int Urol Nephrol. 2022;54(9):2327–2334. doi: 10.1007/s11255-022-03142-3.
- Rossignol P, Lamiral Z, Frimat L, et al. Hyperkalaemia prevalence, recurrence and management in chronic haemodialysis: a prospective multicentre French regional registry 2-year survey. Nephrol Dial Transplant. 2017;32(12):2112–2118. doi: 10.1093/ndt/gfx053.
- Sakata T, Yoshimatsu H, Kurokawa M. Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism. Nutrition. 1997;13(5):403–411. doi: 10.1016/s0899-9007(97)91277-6.
- Fujimoto K, Sakata T, Ookuma K, et al. Hypothalamic histamine modulates adaptive behavior of rats at high environmental temperature. Experientia. 1990;46(3):283–285. doi: 10.1007/BF01951767.
- Westerterp KR, Plasqui G, Goris AH. Water loss as a function of energy intake, physical activity and season. Br J Nutr. 2005;93(2):199–203. doi: 10.1079/bjn20041310.
- Capita R, Alonso-Calleja C. Differences in reported winter and summer dietary intakes in young adults in Spain. Int J Food Sci Nutr. 2005;56(6):431–443. doi: 10.1080/09637480500407875.
- Osiba S. The seasonal variation of basal metabolism and activity of thyroid gland in man. Jpn J Physiol. 1957;7(4):355–365. doi: 10.2170/jjphysiol.7.355.
- Leonard WR, Levy SB, Tarskaia LA, et al. Seasonal variation in basal metabolic rates among the Yakut (Sakha) of northeastern Siberia. Am J Hum Biol. 2014;26(4):437–445. doi: 10.1002/ajhb.22524.
- Henry CJ, Ponnalagu S, Bi X, et al. Does basal metabolic rate drive eating rate? Physiol Behav. 2018;189:74–77. doi: 10.1016/j.physbeh.2018.03.013.
- Davis C, Bryan J, Hodgson J, et al. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139–9153. doi: 10.3390/nu7115459.
- Bernier-Jean A, Wong G, Saglimbene V, et al. Dietary potassium intake and all-cause mortality in adults treated with hemodialysis. Clin J Am Soc Nephrol. 2021;16(12):1851–1861. doi: 10.2215/CJN.08360621.
- Ramos CI, González-Ortiz A, Espinosa-Cuevas A, et al. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2021;36(11):2049–2057. doi: 10.1093/ndt/gfaa232.
- Garagarza C, Valente A, Caetano C, et al. Potassium intake-(Un)expected non-predictor of higher serum potassium levels in hemodialysis DASH diet consumers. Nutrients. 2022;14(10):2071. doi: 10.3390/nu14102071.
- Narasaki Y, Okuda Y, Kalantar SS, et al. Dietary potassium intake and mortality in a prospective hemodialysis cohort. J Ren Nutr. 2021;31(4):411–420. doi: 10.1053/j.jrn.2020.05.008.
- González-Ortiz A, Xu H, Ramos-Acevedo S, et al. Nutritional status, hyperkalaemia and attainment of energy/protein intake targets in haemodialysis patients following plant-based diets: a longitudinal cohort study. Nephrol Dial Transplant. 2021;36(4):681–688. doi: 10.1093/ndt/gfaa194.
- St-Jules DE, Goldfarb DS, Sevick MA. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26(5):282–287. doi: 10.1053/j.jrn.2016.02.005.
- Yosipovitch G, Reis J, Tur E, et al. Sweat electrolytes in patients with advanced renal failure. J Lab Clin Med. 1994;124(6):808–812.
- Masters PW, Lawson N, Marenah CB, et al. High ambient temperature: a spurious cause of hypokalaemia. BMJ. 1996;312(7047):1652–1653. doi: 10.1136/bmj.312.7047.1652.
- Ulahannan TJ, McVittie J, Keenan J. Ambient temperatures and potassium concentrations. Lancet. 1998;352(9141):1680–1681. doi: 10.1016/S0140-6736(05)61452-3.
- Sinclair D, Briston P, Young R, et al. Seasonal pseudohyperkalaemia. J Clin Pathol. 2003;56(5):385–388. doi: 10.1136/jcp.56.5.385.
- Sodi R, Davison AS, Holmes E, et al. The phenomenon of seasonal pseudohypokalemia: effects of ambient temperature, plasma glucose and role for sodium-potassium-exchanging-ATPase. Clin Biochem. 2009;42(9):813–818. doi: 10.1016/j.clinbiochem.2009.01.024.
- Guinsburg AM, Usvyat LA, Etter M, et al. Seasonal variations in mortality and clinical indicators in international hemodialysis populations from the MONDO registry. BMC Nephrol. 2015;16(1):139. doi: 10.1186/s12882-015-0129-y.
- Keatinge WR, Donaldson GC. The impact of global warming on health and mortality. South Med J. 2004;97(11):1093–1099. doi: 10.1097/01.SMJ.0000144635.07975.66.
- Crawford VL, McCann M, Stout RW. Changes in seasonal deaths from myocardial infarction. QJM. 2003;96(1):45–52. doi: 10.1093/qjmed/hcg005.
- Mercer JB. Cold–an underrated risk factor for health. Environ Res. 2003;92(1):8–13. doi: 10.1016/S0013-9351(02)00009-9.
- Dalrymple LS, Mohammed SM, Mu Y, et al. Risk of cardiovascular events after infection-related hospitalizations in older patients on dialysis. Clin J Am Soc Nephrol. 2011;6(7):1708–1713. doi: 10.2215/CJN.10151110.
- Fries RP, Heisel AG, Jung JK, et al. Circannual variation of malignant ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators and either coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79(9):1194–1197. doi: 10.1016/s0002-9149(97)00081-7.
- Anand K, Aryana A, Cloutier D, et al. Circadian, daily, and seasonal distributions of ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Am J Cardiol. 2007;100(7):1134–1138. doi: 10.1016/j.amjcard.2007.04.063.