Abstract
Objectives
Most functional magnetic resonance research has primarily examined alterations in the affected kidney, often neglecting the contralateral kidney. Our study aims to investigate whether imaging parameters accurately depict changes in both the renal cortex and medulla in a unilateral ureteral obstruction rat model, thereby showcasing the utility of intravoxel incoherent motion (IVIM) in evaluating contralateral renal changes.
Methods
Six rats underwent MR scans and were subsequently sacrificed for baseline histological examination. Following the induction of left ureteral obstruction, 48 rats were scanned, and the histopathological examinations were conducted on days 3, 7, 10, 14, 21, 28, 35, and 42. The apparent diffusion coefficient (ADC), pure molecular diffusion (D), pseudodiffusion (D*), and perfusion fraction (f) values were measured using IVIM.
Results
On the 10th day of obstruction, both cortical and medullary ADC values differed significantly between the UUO10 group and the sham group (p < 0.01). The cortical D values showed statistically significant differences between UUO3 group and sham group (p < 0.01) but not among UUO groups at other time point. Additionally, the cortical and medullary f values were statistically significant between the UUO21 group and the sham group (p < 0.01). Especially, the cortical f values exhibited significant differences between the UUO21 group and the UUO groups with shorter obstruction time (at time point of 3, 7, 10, 14 day) (p < 0.01).
Conclusions
Significant hemodynamic alterations were observed in the contralateral kidney following renal obstruction. IVIM accurately captures changes in the unobstructed kidney. Particularly, the cortical f value exhibits the highest potential for assessing contralateral renal modifications.
Keywords:
Introduction
Urinary obstruction represents a significant etiology of obstructive nephropathy. Obstructive nephropathy results in renal interstitial fibrosis in the affected kidney [Citation1] and concomitant injury in the contralateral kidney. While numerous studies have elucidated the mechanisms of injury in the obstructed kidney, the molecular mechanism underlying injury in the contralateral kidney remains to be fully explored.
The unilateral ureteral obstruction (UUO) model offers several advantages, including the absence of both exogenous toxins and a uremic environment, controllable obstruction duration, and the opportunity to study recovery following recanalization. As a result, it serves as a classical animal model for investigating obstructive nephropathy and renal fibrosis [Citation2,Citation3]. Additionally, UUO model is widely used to study renal injury mechanisms such as apoptosis, inflammation, and fibrosis [Citation4].
Currently, renal dynamic imaging (RDI), a noninvasive modality, is commonly employed to evaluate the degree of hydronephrosis in the affected kidney [Citation5–7]. Furthermore, computer electron beam computerized tomography (EBCT) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) enable noninvasive evaluation of intrarenal hemodynamics and tubular dynamics in the single kidney in vivo [Citation8–10]. However, the use of contrast agents of RDI, EBCT, and DCE-MRI is generally associated with the risk of inducing or exacerbating renal failure [Citation11]. Conversely, certain functional magnetic resonance sequences (fMRI) sequences allow for noninvasively assessment of renal function, including oxygenation, diffusion, perfusion, and other aspects without the need for contrast agents. Intravoxel incoherent motion (IVIM) finds extensive utility in kidney diseases, including early detection of diabetic nephropathy or evaluation of renal fibrosis and hemodynamic alterations caused by renal artery stenosis [Citation12–14].
This study aimed to acquire IVIM-derived parameters of the contralateral kidney using UUO model, comprehensively analyze the alterations in IVIM-derived parameters of the renal cortex and medulla and explore the utility of IVIM in evaluating changes in contralateral renal microenvironment.
Materials and methods
Animal protocol
Following the experimental design and animal ethics guidelines, we utilized six rats per group for the study, totaling 54 rats. Fifty-four male Sprague–Dawley rats weighing 250 ± 20 g were sourced from the HUNAN SJA LABORATORY ANIMAL CO., LTD. (Animal license number: Xiang2019-0004). The ambient conditions were maintained at 22 ± 1 °C and a humidity level of 55 ± 5% with a 12-h light and 12-h dark cycles. Throughout the experiment, all animals had ad libitum access to water and standard rat feed. All researchers strictly adhered to ethical guidelines for animal welfare during all experimental procedures.
Prior to inducing left ureteral obstruction, random six rats underwent MR scan and were subsequently sacrificed for baseline histological examination. Left ureters were ligated in remaining 48 rats to establish animal models of obstructive nephropathy. All rats underwent MR scans on days 3, 7, 10, 14, 21, 28, 35, 42 after creating the obstruction. Following MRI scans at each time point, six rats were randomly selected and sacrificed for pathological analysis.
MRI
MR scans were performed using a 3.0 T MR scanner (MR 750, GE Healthcare, Waukesha, WI, USA) equipped with a 4-channel animal coil. Rats were anesthetized with 3% pentobarbital sodium injection (2 mL/kg) and positioned in a prone, head-forward position. The coronal plane of the right kidney was aligned using the axial and sagittal planes.
The IVIM-derived parameters were set as follows: replication time (TR)/echo time (TE) = 2000/80; slice thickness = 2.9 mm; slice spacing = 0 mm; field of view (FOV) = 80 × 64; voxels = 1.56 × 1.83 × 2.50; and 13 b values (0, 20, 30, 50, 80, 100, 150, 200, 400, 500, 600, 700, 800 s/mm2).
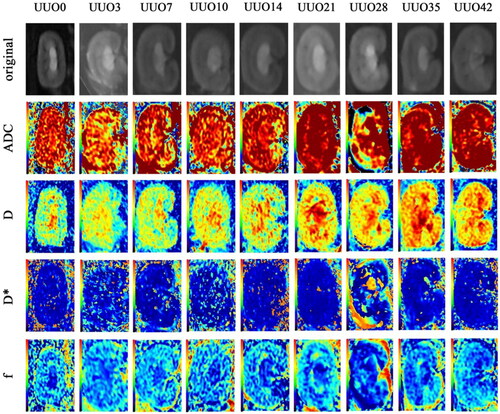
ADC, D, D*, and f values were measured using a postprocessing workstation (Functool, AW4.5, GE, America). The regions of interests (ROIs) were manually delineated on the cortex and medulla of the right kidneys (). Each parameter was measured three times and averaged.
Figure 1. We selected the optimal slice from the original image to visualize the renal cortex and medulla for each quantitative parameter. IVIM diffusion weighted image obtained with b = 0 s/mm2 (the top row) and IVIM-derived parameters (ADC; D; D*; f) of contralateral kidney (the 2nd to 5th row). These IVIM parameter maps of the right kidney depicted rat study case prior to left kidney ligation and at 3, 7, 10, 14, 21, 28, 35, 42 days postligation. Over time, in the unobstructed side, the color gradient deepened between the cortex and medulla in ADC, D, and f parameter images, whereas the color change was less pronounced in the D* parameter image.
Histopathology
Following each imaging session, rats were euthanized with an overdose of intraperitoneal pentobarbital sodium. Kidney tissue specimens were harvested and immediately immersed in formalin (G1101-500ML) for 24 h. Subsequently, the kidney specimens were dehydrated and processed for paraffin embedding. Serial sections, 4 μm thick, were prepared and stained with hematoxylin and eosin (H&E), as well as Masson staining. All staining was conducted by an experienced pathologist blinded to the experimental protocol.
Statistical analysis
Analyses were conducted using SPSS software (v. 22.0 software Chicago, IL, USA), with significance set at p ≤ 0.05. Measurement data are expressed as mean ± standard deviation. One-way ANOVA was employed to compare data across multiple groups at each time point, followed by post hoc analysis using the least significant difference test.
Results
Histopathology
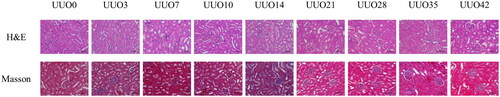
The right kidneys exhibited swelling of the renal tubular epithelial cells and a progressive accumulation of inflammatory cells. As the duration of obstruction increased, the renal tubules of the right kidneys became extensively dilated and deformed, accompanied by the onset of glomerulosclerosis. Masson staining a gradual escalation in fibrosis within the right kidney with prolonged left ureteral obstruction, with more pronounced fibrotic changes observed in the renal medulla ().
Figure 2. Histological analysis (H&E & Masson staining, × 200) of contralateral kidneys from the UUO models revealed varying degrees of pathological changes. These included swelling of the renal tubular epithelial cells, increasing numbers of inflammatory cells, dilation and deformation of renal tubules, and progressive glomerulosclerosis over time. Masson staining results demonstrated a corresponding increase in fibrosis severity in the contralateral kidney with the duration of left ureteral obstruction, particularly evident in the medulla.
Evaluation of the IVIM-derived parameters on the unobstructed kidney
Significant differences were observed in f, ADC, and D values at certain time points in the right kidney. However, no significant differences were noted in D* values at any time point on the same side ( and ).
Figure 3. The boxplots depicted the IVIM-derived parameters of contralateral kidney. (a) Cortical ADC values were statistically different between the UUO7 group and the sham group (p < 0.01). (b) Cortical D values exhibited a statistically significant difference between the UUO3 group and the sham group (p < 0.01). (c) Comparison of cortical D* values revealed no significant difference at any time point. (d) Cortical f values showed statistical significance between the UUO21 group and the sham group (p < 0.001). (e) Medullary ADC values in the UUO10 group differed significantly from those in previous groups (p < 0.01). (f) the difference of medullary D values between the UUO14 group and the sham group was statistically significant (p < 0.01). (g) Comparison of medullary D* values revealed no significant difference at any time point. (h) Medullary f values exhibited statistical significance between the UUO21 group and the sham group (p < 0.01).
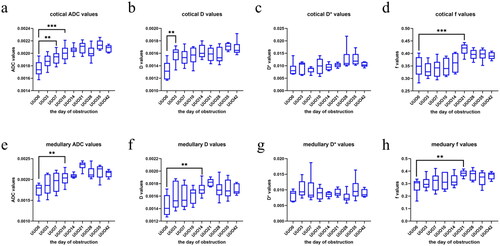
Table 1. Summary of mean values and standard deviations for the contralateral kidney cortex parameters (ADC, D*, D, and f) in a unilateral ureteral obstruction in rats.
Table 2. Summary of mean values and standard deviations for the contralateral kidney medulla parameters (ADC, D*, D, and f) in a unilateral ureteral obstruction in rats.
As the duration of obstruction increased, the ADC values of the cortex and medulla gradually rose. On the 7th day of obstruction, cortical ADC values exhibited a statistically significant difference between the UUO7 group and the sham group (p < 0.01). From the 21st day of obstruction onwards, ADC values in the cortex and medulla showed a trend toward stabilization, with no statistically significant differences observed among subsequent groups (). However, the medullary ADC values in UUO10 group differed significantly from those of previous groups (p < 0.01) ().
Statistically significant differences in cortical D values were observed between UUO3 group and sham group was statistically significant (p < 0.01) (). As the duration of obstruction prolonged, the cortical D value had little changes. Similarly, the difference of medullary D values between the UUO14 group and the sham group was statistically significant (p < 0.01) (). Medullary D value gradually increased with the duration of obstruction. However, from the 14th day of obstruction onwards, no significant differences were noted in the medullary D value among subsequent groups.
Both cortical and medullary f values exhibited statistically significant between the UUO21 group and the sham group (p < 0.001, p < 0.01) (). Especially, cortical f values demonstrated statistically significant between the UUO21 group and the UUO groups with shorter obstruction durations (at time point of 3, 7, 10, 14 day) (p < 0.01). However, beyond 21st day of unilateral ureteral obstruction, no statistically significant differences were observed in renal cortex f value.
Discussion
In our study, the cortical f value emerged as the most sensitive parameter for detecting changes in the renal microenvironment among the IVIM-derived parameters. Given that gadolinium-based contrast agents may compromise kidney function in chronic kidney disease (CKD) patients [Citation15], the f value holds promise for noninvasively assessing renal changes without the need for contrast agents. In this study, we established the UUO model on the right kidney under microscopic guidance, as the location of the right kidney remains relatively stable, and the MR images are less prone to gas-related disturbances. The UUO model serves as a pivotal animal model for elucidating the mechanisms underlying renal fibrosis induced by obstructive nephropathy and for evaluating potential therapeutic interventions to delay the progression of CKD. Pigs have been employed to establish UUO model, which faithfully reflects the structure and function alterations in the affected kidney [Citation16].
Normally, approximately 50% of cardiac output is directed to the renal artery and the distal aorta, with 25% of cardiac output reaching the kidney [Citation17]. Left unilateral ureteral obstruction leads to reduced blood flow in the left kidney. Consequently, 12.5% cardiac output is redirected to the contralateral renal artery and the distal aorta, augmenting the contralateral renal blood flow (RBF) [Citation17]. Over time, fibrin levels increase in glomerular endothelial cells and mesangial cells in the obstructed kidney, leading to pronounced glomerulosclerosis and interstitial fibrosis, further impairing glomerular blood flow [Citation18,Citation19]. Studies by Benitez [Citation20] and Dicker [Citation17] have demonstrated increased mitotic activity in the contralateral kidney and enhanced oxygen uptake in the contralateral renal cortex following unilateral ureteral ligation, respectively. Sigmund et al. [Citation21] suggested that the f value represents the ratio of cumulative volume of liquid within the renal tubules and capillaries to total fluid volume in the kidney. A higher f value indicates a more concentrated capillary distribution, signifying increased lumen diameters [Citation22,Citation23].
Our study revealed statistically significant differences in cortical f values between the UUO21 group and the UUO groups with shorter obstruction durations (at time point of 3, 7, 10, 14 day), indicating notable increase in contralateral renal blood flow on the 21st day postunilateral ureteral obstruction. However, cortical f values in the contralateral kidney remained relatively stable before 21 days. This stability may be attributed to the systemic blood pressure regulation system, which gradually adjusts blood flow to the contralateral kidney following unilateral ureteral obstruction. Wang et al. [Citation24] demonstrated the development of renal interstitial fibrosis in the contralateral kidney after unilateral ureteral obstruction. Masson staining revealed significant increases in collagen deposition and interstitial fibrosis in the contralateral kidney of UUO21 group, consistent with findings by Xiong [Citation25]. Beyond 21st days of unilateral ureteral obstruction, cortical f values remained unchanged, indicating concurrent contralateral renal interstitial fibrosis and cortical blood flow alterations affecting renal perfusion. With prolonged obstruction, the renal fibrosis and tubular atrophy in the affected kidney worsened. Meanwhile, long-term ureteral obstruction also led to severe fibrosis and chronic injury in the contralateral kidney [Citation24]. Pathological changes in the contralateral kidney impeded fluid flow in renal tubule, causing fluid accumulation [Citation26]. Therefore, compensatory growth of the contralateral kidney persisted with prolonged obstruction. However, medullary blood perfusion did not increase substantially due to partial collapse of medullary blood vessels following prolonged obstruction. Therefore, while there was a statistically significant difference in medullary f values between the UUO21 group and the sham group, no significant difference was observed between the UUO21 group and other groups.
During the obstruction process, cortical and medulla ADC values increased, indicating heightened water molecule dispersion attributed to significantly elevated contralateral RBF [Citation20]. The pronounced dispersion of water molecules has a greater impact on ADC values compared to perfusion, making ADC values more sensitive to renal interstitial fibrosis [Citation27]. Our study revealed a statistically significant difference between the UUO7 group and the sham group, indicating detectable interstitial fibrosis in the contralateral kidney by IVIM on 7th day of unilateral ureteral obstruction. As obstruction persisted, renal fibrosis in the contralateral kidney worsened, leading to continued elevation of cortical and medullary ADC values. Since the 21st day of obstruction, collagen deposition, and renal fibrosis in the contralateral kidney had reached advanced stages, with minimal changes in water molecule diffusion. Consequently, cortical, and medullary ADC value stabilized, with no statistical difference observed among subsequent groups.
Our study revealed a statistically significant difference in cortical D values between the UUO3 group and the sham group. Following unilateral ureteral obstruction, there was a rapid increase in blood flow to the contralateral kidney, resulting in heightened extracellular water molecule content and movement. Consequently, the diffusion of extracellular water molecules intensified. However, with prolonged obstruction, cortical D values exhibited minimal change, likely due to increased cell edema volume and collagen deposition in the contralateral kidney [Citation25], restricting the diffusion of extracellular water molecules. Histopathological analysis also demonstrated an increase in collagen fibers in the presence of inflammatory cells, such as neutrophils and macrophages, in the cortex and medulla of the contralateral kidney, indicating progressive inflammation and interstitial fibrosis with prolonged obstruction [Citation24]. Additionally, a statistically significant difference in medullary D values was observed between UUO14 group and the sham group, suggesting a slower rate of change in medullary D values compared to cortical D values. This discrepancy may be attributed to the lower water content and blood flow in the medulla compared to the cortex.
In our study, no significant difference was observed in D* in the contralateral renal cortex and medulla following unilateral ureteral obstruction, which may be attributed to the uneven distribution of RBF caused by renal interstitial fibrosis [Citation28]. As D* values are affected by various factors, they may not accurately reflect changes in the contralateral kidney. Further experimental research is required to elucidate the precise underlying cause.
Limitations of this study are as follows. Firstly, it is challenging to determine the trend of IVIM-derived parameters in the contralateral kidney following short-term unilateral ureteral obstruction. Further research in this area is warranted. Secondly, as the obstruction time increases, the demarcation between the renal cortex and the medulla becomes less distinct, probably resulting in bias in ROI selection. Additionally, immunohistochemical analysis was not conducted, limiting our ability to explore correlations between IVIM-derived parameters and the expression of proinflammatory cytokines such as TNF-α and IL-1β in the kidneys. Lastly, the urinary system anatomy of S-D rat differs from that of humans, potentially leading to variations in the pathophysiology of the contralateral kidney between rats and humans.
Conclusion
In conclusion, IVIM in rats with a left UUO model revealed contralateral renal changes in the morphology, pathology, and the real microenvironment. IVIM-derived parameters effectively capture renal microenvironment changes. Particularly, the f value serves as a reliable indicator for assessing contralateral kidney variations at different stages of ureteral obstruction, offering potential for preliminary evaluation of renal alterations before and after treatment.
Ethical approval
This study was approved by the Laboratory Animal Welfare and Ethics Committee of Jinan University (Ethical review NO. I ACUC-20191203-03).
Authorship contribution statement
Lingtao Zhang, Zijie Jiang and Wenfeng Mai wrote the article; Haiwei Su, Zhihua Zhang and Xukai Mo analyzed and interpreted the data; Xukai Mo revised the manuscript. Shunagquan Zhao and Changzheng Shi conceived and designed the experiments; Kunlin Ye and Dandan Fu performed the experiments. All authors reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Available from the authors upon reasonable request.
Additional information
Funding
References
- Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017; 3(1):1. doi: 10.1038/nrdp.2017.88.
- Martínez-Klimova E, Aparicio-Trejo OE, Tapia E, et al. Unilateral ureteral obstruction as a model to investigate fibrosis-attenuating treatments. Biomolecules. 2019;9(4):141. doi: 10.3390/biom9040141.
- Ranjit S, Dvornikov A, Levi M, et al. Characterizing fibrosis in UUO mice model using multiparametric analysis of phasor distribution from FLIM images. Biomed Opt Express. 2016; 7(9):3519–8.
- Narváez Barros A, Guiteras R, Sola A, et al. Reversal unilateral ureteral obstruction: a mice experimental model. Nephron. 2019;142(2):125–134. doi: 10.1159/000497119.
- Lee WG, Kim JH, Kim JM, et al. Renal uptakes of 99mTc-MAG3, 99mTc-DTPA, and 99mTc-DMSA in rabbits with unilateral ureteral obstruction. In Vivo. 2010;24(2):137–139.
- Ma G, Shao M, Xu B, et al. Glomerular filtration rate measured by 99mTc-DTPA gates method is not significantly affected by the premature or delayed initiation of image acquisition. Quant Imaging Med Surg. 2019; 9(6):1103–1109.
- Nguyen DL, de Labriolle-Vaylet C, Durand E, et al. Reproducibility of differential renal function measurement using technetium-99m-ethylenedicysteine dynamic renal scintigraphy: a french prospective multicentre study. Nucl Med Commun. 2018;39(1):10–15. doi: 10.1097/MNM.0000000000000769.
- Pelaez LI, Juncos LA, Stulak JM, et al. Non-invasive evaluation of bilateral renal regional blood flow and tubular dynamics during acute unilateral ureteral obstruction. Nephrol Dial Transplant. 2005;20(1):83–88. doi: 10.1093/ndt/gfh556.
- Pedersen M, Irrera P, Dastrù W, et al. Dynamic contrast enhancement (DCE) MRI-derived renal perfusion and filtration: basic concepts. Methods Mol Biol. 2021;2216:205–227.
- Irrera P, Consolino L, Dastrù W, et al. Dynamic contrast enhanced (DCE) MRI-derived renal perfusion and filtration: experimental protocol. Methods Mol Biol. 2021;2216:429–441. doi: 10.1007/978-1-0716-0978-1_25.
- Nicola S, Jason IB, Andreu FC, et al. Gadolinium-based contrast agents in kidney disease: a comprehensive review and clinical practice guideline issued by the Canadian association of radiologists. Can J Kidney Health Dis. 2018;5:1–17.
- Liang J, Li Z, Li J, et al. Application of IVIM-DWI in detecting the tumor vasculogenic mimicry under antiangiogenesis combined with oxaliplatin treatment. Front Oncol. 2020; 10:1376. doi: 10.3389/fonc.2020.01376.
- Feng Y-Z, Chen X-Q, Yu J, et al. Intravoxel incoherent motion (IVIM) at 3.0 T: evaluation of early renal function changes in type 2 diabetic patients. Abdom Radiol (NY). 2018; 43(10):2764–2773. doi: 10.1007/s00261-018-1555-7.
- Ebrahimi B, Rihal N, Woollard JR, et al. Assessment of renal artery stenosis using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging analysis. Invest Radiol. 2014;49(10):640–646. doi: 10.1097/RLI.0000000000000066.
- Rudnick MR, Wahba IM, Leonberg-Yoo AK, et al. Risks and options with Gadolinium-Based contrast agents in patients with CKD: a review. Am J Kidney Dis. 2021;77(4):517–528. doi: 10.1053/j.ajkd.2020.07.012.
- Liu Y, Sun J, Miao L, et al. A porcine model of relief of unilateral ureteral obstruction: study on self-repairing capability over multiple time points. Mol Cell Biochem. 2016;419(1-2):115–123. doi: 10.1007/s11010-016-2755-5.
- Dicker SE, Shirley DG. Compensatory hypertrophy of the contralateral kidney after unilateral ureteral ligation. J Physiol. 1972;220(1):199–210. doi: 10.1113/jphysiol.1972.sp009701.
- Löwen J, Gröne EF, Groß-Weißmann ML, et al. Pathomorphological sequence of nephron loss in diabetic nephropathy. Am J Physiol Renal Physiol. 2021;321(5):F600–F616. doi: 10.1152/ajprenal.00669.2020.
- Wilkening A, Krappe J, Mühe AM, et al. C-C chemokine receptor type 2 mediates glomerular injury and interstitial fibrosis in focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2020;35(2):227–239.
- Bianco M, Lopes JA, Beiral HJV, et al. The contralateral kidney presents with impaired mitochondrial functions and disrupted redox homeostasis after 14 days of unilateral ureteral obstruction in mice. PLoS One. 2019;14(6):e0218986. doi: 10.1371/journal.pone.0218986.
- Sigmund EE, Vivier PH, Sui D, et al. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology. 2012;263(3):758–769. doi: 10.1148/radiol.12111327.
- Togao O, Hiwatashi A, Yamashita K, et al. Measurement of the perfusion fraction in brain tumors with intravoxel incoherent motion MR imaging: validation with histopathological vascular density in meningiomas. Br J Radiol. 2018;91(1085):20170912. doi: 10.1259/bjr.20170912.
- Castellano G, Franzin R, Stasi A, et al. Complement activation during ischemia/reperfusion injury induces pericyte-to-Myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front Immunol. 2018;9:1002. doi: 10.3389/fimmu.2018.01002.
- Wang C-H, Wang Z, Liang L-J, et al. The inhibitory effect of eplerenone on cell proliferation in the contralateral kidneys of rats with unilateral ureteral obstruction. Nephron. 2017; Apr136(4):328–338. doi: 10.1159/000473702.
- Xiong Y, Chang Y, Hao J, et al. Eplerenone attenuates fibrosis in the contralateral kidney of UUO rats by preventing macrophage-to-Myofibroblast transition. Front Pharmacol. 2021;12:620433. doi: 10.3389/fphar.2021.620433.
- Liu BC, Tang TT, Lv LL, et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93(3):568–579. doi: 10.1016/j.kint.2017.09.033.
- Le Bihan D. What can we see with IVIM MRI? Neuroimage. 2019; Feb 15187:56–67. doi: 10.1016/j.neuroimage.2017.12.062.
- Woo S, Cho JY, Kim SY, et al. Intravoxel incoherent motion MRI-derived parameters and T2* relaxation time for noninvasive assessment of renal fibrosis: an experimental study in a rabbit model of unilateral ureter obstruction. Magn Reson Imaging. 2018; 51:104–112.
