Abstract
Introduction
A reduction in platelet count in critically ill patients is a marker of severity of the clinical condition. However, whether this association holds true in acute kidney injury (AKI) is unknown. We analyzed the association between platelet reduction in patients with AKI and major adverse kidney events (MAKE).
Methods
In this retrospective cohort, we included AKI patients at the Hospital Civil of Guadalajara, in Jalisco, Mexico. Patients were divided according to whether their platelet count fell >21% during the first 10 days. Our objectives were to analyze the associations between a platelet reduction >21% and MAKE at 10 days (MAKE10) or at 30-90 days (MAKE30-90) and death.
Results
From 2017 to 2023, 400 AKI patients were included, 134 of whom had a > 21% reduction in platelet count. The mean age was 54 years, 60% were male, and 44% had sepsis. The mean baseline platelet count was 194 x 103 cells/µL, and 65% of the KDIGO3 patients met these criteria. Those who underwent hemodialysis (HD) had lower platelet counts. After multiple adjustments, a platelet reduction >21% was associated with MAKE10 (OR 4.2, CI 2.1-8.5) but not with MAKE30-90. The mortality risk increased 3-fold (OR 2.9, CI 1.1-7.7, p = 0.02) with a greater decrease in the platelets (<90 x 103 cells/µL). As the platelets decreased, the incidence of MAKE was more likely to increase. These associations lost significance when accounting for starting HD.
Conclusion
In our retrospective cohort of patients with AKI, a > 21% reduction in platelet count was associated with MAKE. Our results are useful for generating hypotheses and motivating us to continue studying this association with a more robust design.
PLAIN LANGUAGE SUMMARY
A reduction in platelet count in critically ill patients has been associated with a worse prognosis, but it is not yet known whether this relationship also exists in patients with acute kidney injury, who are more susceptible to platelet decrease due to the syndrome or due to the onset of hemodialysis. In our study of acute kidney injury patients, we found that those whose platelet count decreased >21% during the first days were more likely to experience a major kidney event. In addition, the greater the decrease in platelet count was, the more likely these events were to occur. The significance of this association was lost in patients who start hemodialysis. Our conclusions could serve to generate hypotheses about this interesting relationship.
Introduction
In critically ill patients, variations in platelet count, a phenomenon associated with disease severity and behaving as an acute-phase reactant, are typically observed [Citation1]. The kinetics of platelet formation in these patients have long been an enigma, as various inflammatory factors promote platelet production by directly stimulating bone marrow [Citation2,Citation3] while simultaneously reducing platelet counts through the formation and destruction of microthrombi [Citation4,Citation5]. This dichotomy is observed through platelet consumption manifested by bleeding events [Citation6] and dysregulated formation of thrombi, which contribute to ischemic events and organ failure, such as acute kidney injury (AKI). In patients undergoing cardiac surgery, a relationship has been found between decreased platelet counts and the development of AKI, an event probably associated with the generation of microthrombi with subsequent tissue ischemia [Citation7]. AKI occurs in up to 23% of critically ill patients, approximately 10% of whom require kidney replacement therapy (KRT), and near to 50% of them die during follow-up [Citation8,Citation9]. No treatment for AKI exists, so we are limited to addressing its sequelae with KRT, necessary to alleviate complications at peak severity. Intermittent hemodialysis (IHD) is the most widely employed modality for this purpose worldwide [Citation10]. The process involves the use of a filter and an extracorporeal circuit through which blood is circulated. Despite its popularity, IHD is not free from complications, one of which is the shearing of blood cells in the extracorporeal circuit. This adversely impacts platelet morphology and function, and platelets are already susceptible to this condition due to the pathophysiological process of illness, which can lead to fragmentation. This all results in a reduction in platelet count [Citation11]. However, the impact of platelet count changes during AKI on major kidney adverse events (MAKEs) has not been determined. We believe that the reduction in platelet count in patients with AKI may be related to MAKE. To help fill this information gap, we conducted a retrospective cohort study comprising AKI patients in which the trajectory of platelet counts was monitored to analyze the relationship between a reduction in total platelet count and MAKE.
Methods
Study design and patient population
This was a retrospective cohort study conducted at the Hospital Civil de Guadalajara Fray Antonio Alcalde, a tertiary referral academic center, located in Mexico, between August 2017 and March 2023.
All patients included in this study were treated by a primary medical or surgical team. We included only patients with AKI who were receiving care from the nephrology staff. The decision to consult with a nephrologist was at the discretion of the attending physician.
The patients’ platelet counts were monitored for the first ten days after their AKI diagnosis. We chose this ten-day follow-up period since most AKI patients started KRT during this time [Citation12]. For a total follow-up picture, we assessed MAKE10 and MAKE30-90 frequencies and risk.
AKI was diagnosed using the serum creatinine (sCr) KDIGO criterion, and chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73m2 for more than three months [Citation12].
For patients without baseline sCr values, we estimated this value by back-calculating the Modification of Diet in Renal Disease (MDRD) equation, assuming an eGFR of 75 mL/min/1.73 m2 [Citation12]. Patients included in the study had available admission platelet levels and at least three platelet measurements during hospitalization, and they were all diagnosed with AKI.
The exclusion criteria were common causes of thrombocytopenia in AKI patients, such as lupus, antiphospholipid syndrome, or hematologic malignancies; CKD stage 5; chronic dialysis; hospitalization stay less than 48 h; transplant patients; pregnancy; and missing data that would render analysis incomplete.
The study was approved by the Hospital Civil de Guadalajara Fray Antonio Alcalde Institutional Review Board (HCG/CEI-0550/15) and was conducted in accordance with the Declaration of Helsinki. All the subjects provided informed consent. The study protocol adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Citation13].
Data collection
Clinical characteristics, demographic information, and laboratory data were collected prospectively via automated retrieval from the institutional electronic medical records system. The primary focus of this study was to determine the predictive value of in-hospital changes in platelets during the first 10 days of hospitalization. To create this metric, platelet level values were obtained on various days throughout the hospital stay (as not all values were available daily). The mean and percentage changes in platelet counts were then calculated to represent the index day. Platelet counts were analyzed using the Cell-Dyn Ruby device, and demographic and clinical data (including age, diabetes status, hypertension status, hypothyroidism status, CKD grade, smoking status, cerebrovascular disease status, and incidence of ischemic heart disease) were collected. The baseline serum creatinine level was defined as the most recent value within a year prior to admission, and contributing factors of AKI, such as sepsis (Sepsis-3 criteria) [Citation14], clinical hypovolemia, cardiorenal syndrome [Citation15], nephrotoxic drugs and shock, as well as biochemical data such as hemoglobin, platelets, leukocytes, glucose, urea, creatinine, sodium, potassium, chloride, phosphate, calcium, arterial pH, PCO2, PO2, bicarbonate and lactate levels, were prespecified. The indications for KRT included fluid overload that was resistant to diuretics, severe hyperkalemia, severe metabolic acidosis, and uremic manifestations, such as encephalopathy, pericarditis, and seizures [Citation12,Citation14].
Study outcomes
The primary outcome was MAKE at 10 days (MAKE10), which was composed of death, new requirement for dialysis, and worsening of kidney function (WKF), defined by a ≥ 25% decrease in the estimated glomerular filtration rate (eGFR) from baseline. As a secondary outcome, we considered MAKE at 10 and 30-90 days (MAKE10 and MAKE30-90). We chose the MAKE criteria because of the recommendation to assess homogeneous results in studies conducted in AKI patients [Citation15].
Statistical analysis
The distribution of the quantitative variables was examined visually through histograms and confirmed using the Kolmogorov–Smirnov and Shapiro–Wilk tests for nonnormally distributed data. Thus, continuous variables are presented as medians with interquartile ranges, while categorical variables are presented as counts and proportions. To analyze differences in categorical variables between the IHD and non-IHD groups, the χ2 test or Fisher’s exact test was used, as appropriate. Continuous variables were compared using the Wilcoxon rank test. A box graph was generated to display platelet counts for the IHD group daily. We estimated the average treatment effect by nearest-neighbor matching, imputing missing potential outcomes by averaging the outcomes of similar subjects who received the other exposure level. Similarity was measured based on a weighted function of observation covariates that included clinically relevant variables. Exact matching of platelet reduction was performed. The treatment effect was computed using the difference between the observed and imputed potential outcomes. We estimated the effects of IHD on MAKE10, MAKE30-90, and death. Logistic regression analysis was used to determine the risk of MAKE10 expression, worsening renal function, and mortality according to three different models. Model 1 was adjusted for age and sex, Model 2 was adjusted for baseline characteristics and clinically relevant characteristics, with p < 0.05 between groups, and Model 3 included KRT modality. Odds ratios for mortality across different platelet reduction ranges were calculated for the entire population and for those treated with IHD. The odds ratio was plotted against the change in platelet count as a continuous variable.
To analyze the primary and secondary objective, logistic regression was performed on several levels. Individual odds ratios were calculated for MAKE10 and MAKE30-90 among patients with a ≥ 21% reduction in platelet count using bivariate analysis. Multivariate logistic regression was performed, including age and sex in Model 1; comorbid conditions (primary diagnosis, hypertension, and chronic heart failure) in Model 2; and IHD as the treatment in Model 3. Finally, based on Model 3, odds ratios for death and WKF were estimated. A platelet reduction cutoff of >21% was chosen based on a previous study that observed a 20% reduction in platelet count in hemodialysis patients [Citation16]. p < 0.05 was considered to indicate statistical significance. All the statistical calculations were performed with Stata version 16.1 (StataCorp, College Station, TX, USA).
Results
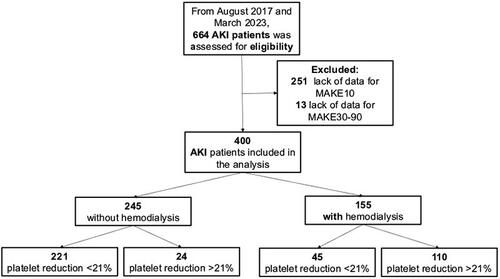
In total, 664 AKI patients were assessed as candidates for the study; 264 were excluded because of a lack of data on any of the outcomes. Ultimately, 400 patients were included in the analysis, 155 (38.7%) of whom required IHD. A flow chart of the study population is shown in .
AKI patients were divided into those who received IHD and those who did not. Differences in demographic characteristics between these groups are presented in . After the proper analysis, patients who received IHD had significantly lower heart rates [86 (78-100) vs. 90 (80-100)]; included a greater proportion of hypertensive patients [98 (40%) vs. 45 (29%)] and had a lower eGFR of 10.0 mL/min [6.5-13.2] vs. 18.7 mL/min [12.0-27.9]; a greater proportion of obstructive nephropathy [35 (22.5%) vs. 26 (10.6%)]; a greater hemoglobin level 9.9 (8.2-12.4) vs. 9.0 (7.8-10.4); a greater urea level 178.8 (138-235.3) vs. 137.7 (90.5-189); a greater serum creatinine level 5.71 (4.12-7.93) vs. 3.17 (2.33-4.39); and a greater phosphorus 6.3 (4.6-7.8) vs. 5 (3.9-6.2), p < 0.05 for all. Importantly, more patients who required IHD had a platelet reduction ≥21% (110 [70.97%] vs. 24 [9.8%], p < 0.01). There was also a greater percentage of MAKE10, MAKE30-90 and mortality in the IHD group.
Table 1. Baseline clinical characteristics of AKI patients according to the hemodialysis treatment.
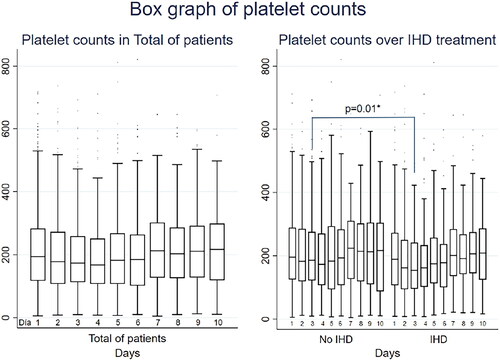
A complete representation of platelet counts during the first 10 days is presented in and . AKI patients who received IHD had consistently lower platelet counts throughout the follow-up period. A statistically significant difference in platelet count was observed on Day 3, with patients who received IHD having lower platelet counts than did those who did not [154 × 103 cells/µL (96-242) vs. 185.75 × 103 cells/µL (122-275), p = 0.01].
Table 2. AKI patients platelet count on every day comparing those without IHD and IHD group.
We found that the percentage change in platelet count was associated with the primary objective MAKE10 (). Patients in the more stable group (< 21% reduction) had a 73% lower risk of MAKE10, which was significant in Models 1 and 2, but its significance was no longer present in Model 3, mainly due to the presence of IHD. Conversely, patients in the >21% reduction group had 3-4 times greater risk of MAKE10, which was no longer significant in Model 3 when IHD was considered.
Table 3. Odds ratio of platelet reduction for the primary and secondary objectives.
The other secondary endpoint MAKE30-90 was not associated with the percentage change in platelet count. However, the need for IHD was found to increase the risk of death in patients with a platelets reduction <21% (). Patients in the highest platelet drop group (>90 × 103 cells/µL), segregated into intervals of 20 × 103 cells/µL, had an almost 3-fold increase in the risk of death during hospitalization, with or without the inclusion of IHD in Model 2.
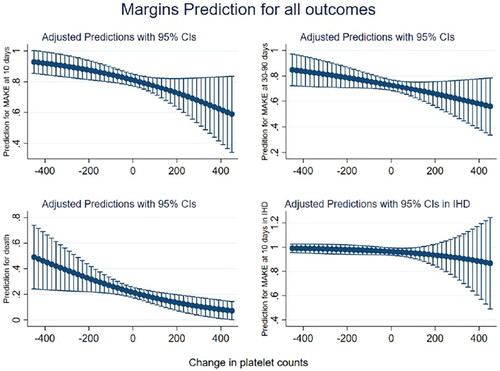
In Model 3, when the dependent variable was restricted only to patients who received IHD, none of the changes in platelet counts were associated with the risk of death (p > 0.05 for all) (). The adjusted predictions of MAKE10, MAKE30-90, and death monotonically increased as the platelet count decreased and vice versa. However, this association was again not observed in IHD patients, in whom the prediction of MAKE10 was similar regardless of platelet kinetics ( and ).
Table 4. Platelets changes in groups of 30 (103cel/µL) and or for mortality risk.
Nearest-neighbor matching
An analysis was also conducted to examine the impact of IHD on MAKE10 and MAKE30-90 scores and on mortality outcomes; patients were matched by platelet reduction using nearest-neighbor matching. Covariates such as primary diagnosis, comorbidities (hypertension and heart failure), heart rate, eGFR, and hemoglobin level were also used to match neighbors with different changes in platelet count (as a binary variable: more than or less than a 21% reduction). The results showed a significant OR for MAKE10 (1.24, CI 1.21-1.38, p < 0.001) and MAKE30-90 (1.21, CI 1.07-1.39, p = 0.003). These findings indicate that the odds of MAKE were greater when IHD was utilized, even when adjusted for demographic characteristics that differed between the groups.
Discussion
In this cohort of patients with AKI, we found that a platelet reduction >21% during the first days of hospitalization was associated with a 2-fold increase in the risk of MAKE in the short and medium term, even after adjusting for multiple variables or matching propensity scores; this association was no longer significant with the onset of IHD, where the prognosis was worst regardless of platelet kinetics.
Platelets play a fundamental role in the inflammatory response. In critically ill patients, platelet levels can increase or decrease, both of which are important and relevant. Increased platelet counts are related to both aggregation and inflammation [Citation17] and may reflect platelet hyperactivation [Citation18]. Once activated, platelets secrete inflammatory mediators and chemokines that synergize with vasoactive amines, IL-1, and proteolytic enzymes to augment the inflammatory reaction [Citation19,Citation20] and recruit leukocytes to the blood vessel wall [Citation18,Citation21] through P-selectin and the subsequent formation of platelet–leukocyte aggregates, thereby upregulating the proinflammatory function of leukocytes.
Decreases in platelet counts are especially common in patients with sepsis, and these decreases can occur through different mechanisms: platelet TLR4 expression in sepsis is closely related to thrombocytopenia [Citation22]; the pathogen and its metabolic products directly damage megakaryocytes, reduce platelet production and destroy them; endotoxins cause platelet adhesion; and macrophage colony-stimulating factor and hemophagocytic syndrome remove platelets [Citation11].
We must account for that sepsis was the etiology responsible for AKI in almost half of our patients. Additionally, multiple drugs that our patients had taken, such as quinolones, vancomycin, sulfamethoxazole, and linezolid, have also been associated with thrombocytopenia [Citation23].
We found that the more the platelet count decreased, the greater the probability of MAKE and mortality. This association could be explained by the following in AKI patients: an increase in creatinine has been associated with a decrease in platelet count in critically ill patients [Citation24]. In our cohort, which included only patients with AKI, it is intuitive to think that this syndrome was also associated with thrombocytopenia due to its pathogenic mechanism. Urea is always elevated during AKI [Citation25], a change that has been associated with a significant reduction in platelet count [Citation26] through mechanisms that involve up to 31 uremic toxins, but the most commonly studied uremic toxins have been cyanate [Citation27], guanidine compounds [Citation28], methylamines [Citation29], polyamines [Citation30], urea [Citation31], hippurates [Citation32], and indoles [Citation33], among others. These uremic toxins could reduce the number of platelets through different mechanisms such as increasing their destruction, their adhesion and limiting their production in the bone marrow [Citation26]. In the case of adhesion to the endothelium and the formation of microthrombi, it may be a pathophysiological explanation that causes MAKE during AKI, through the ischemia that it would cause to the renal parenchyma.
We observed that the platelet concentrations were notably lower in AKI patients requiring IHD than in those not requiring IHD; obviously, these patients represent the sickest individuals in the entire cohort. IHD can cause further platelet abnormalities and substantial platelet activation, thus further increasing the risk of hemorrhage and thrombosis [Citation34]. The platelet number of IHD patients can be reduced by up to 20% [Citation16]. This decrease is very similar to what we found in our cohort of patients with AKI who did receive HD. Thrombocytopenia is an adverse event during IHD since unfractionated heparin (UFH) and low-molecular weight heparins (LMWHs) are commonly used anticoagulants, and both increase the risk of heparin-induced thrombocytopenia (HIT), a rare complication (0.2-5% of patients exposed to heparin) usually observed 5-14 days after exposure [Citation35]. Platelets usually decrease by >50% when there are new thrombotic or hemorrhagic events [Citation36]. Due to the nature of our cohort, it was not possible to associate thrombocytopenia with HIT. In addition, the filters used in IHD are associated with a reduction in platelet count despite their high biocompatibility [Citation37] due to complement activation, pulmonary leukostasis, hypersensitivity to ethylene oxide, interactions between the AN69 membrane and angiotensin-converting enzyme inhibitors [Citation38,Citation39], sterilization methods and the hydrophilizing agent of the filter [Citation40]. One cohort study associated thrombocytopenia (<50 × 103cel/µL) with the initiation of KRT in patients who underwent liver transplantation, and the need for KRT increased the probability of thrombocytopenia 2.3-fold [Citation41]. Comparing and contrasting our findings with these results is difficult because, in our cohort, only 12 (3%) patients had cirrhosis, and none of our patients received transplants. Liver transplant patients are inherently more at risk of thrombocytopenia; therefore, the incidence of thrombocytopenia was greater (65%) in the Ben Hamida et al. study. The start of KRT with the hemofiltration modality reduces the platelet count in critically ill patients. In a clinical trial, it was observed that the platelet count decreased from a mean value of 225 × 103cel/µL (35.5) at the beginning of KRT to 63 × 103cel/µL (25.8) at the end [Citation42]. Our patients underwent IHD, which is a different modality from hemofiltration that involves less anticoagulant therapy and a shorter therapy time and therefore less exposure of the blood to the filter.
The findings of our cohort add to the evidence that associates the reduction in platelet count with negative outcomes, an association recently confirmed in a meta-analysis of 70 studies carried out in intensive care units [Citation43]. Between 13 and 44% of critically ill patients have thrombocytopenia [Citation44].
The relationship between platelet decreases and the need for KRT has been investigated for decades; some studies have noted an association [Citation45–47], while others have not [Citation48]. These studies differ from ours, mainly in terms of the characteristics of the patients analyzed. Our cohort included only patients with AKI, unlike previous studies, who were mainly critically ill but had diseases other than AKI. To our knowledge, this is the first study in which platelet reduction has been associated with MAKE in patients with AKI.
Our study has important limitations. As a single-center study, the external validity of our findings is unknown, although our findings are in line with previous smaller or unadjusted studies and can be viewed as confirmatory. Excluding more than 30% of patients because of lack of outcome data is another important limitation. No effort was made to investigate whether there were cases of heparin-induced thrombocytopenia in patients with AKI plus IHD who took anticoagulants. The doses of anticoagulants used during IHD sessions were not determined. Bleeding or thrombosis events associated with platelet changes were not recorded, nor was the need to use blood products for the management of thrombocytopenia. The main strength of our cohort study lies in our finding of an association between thrombocytopenia and MAKE in a cohort of patients with only AKI even after adjustment for important confounders.
Conclusions
In our retrospective cohort of patients with AKI, we found that a > 21% reduction in platelet count during the first days of hospitalization was associated with MAKE. Our findings are useful for generating hypotheses and motivate us to continue studying this association in a larger population with a more robust design.
Author contributions
Ramón Medina-González, Jonathan S. Chávez-Íñiguez, Jose J. Zaragoza and Eduardo M. Hernández-Barajas designed the study, analyzed the data, made the tables and figures, and wrote the manuscript. Rolando Claure-Del Granado, Armando Vazquez-Rangel, Gael Chávez-Alonso, Liliana M. Pineda-Segura, Karina Franco-Garcia, Juan A. Gómez-Fregoso, Francisco G. Rodríguez-García, Guillermo Navarro-Blackaller, Luz Alcantar-Vallin, Alejandro Martínez Gallardo-González, and Gabriela J. Abundis-Mora collected the data and wrote the manuscript. Guillermo García-García wrote the manuscript and supervised the entire process.
Consent to participate
Written informed consent was obtained from the participants.
Statement of ethics
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Hospital Civil de Guadalajara Fray Antonio Alcalde Institutional Review Board (HCG/CEI-0550/15).
Acknowledgment
If it were not for all the Social Service students of medicine who have been in the nephrology service, these articles would not have been possible.
Disclosure statement
The authors have no conflicts of interest to declare.
Availability of data
The files and data are in the physical and electronic archive of the Civil Hospital of Guadalajara Fray Antonio Alcalde and can be requested with prior authorization. All the data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):1–11. PMID: 26293514. doi:10.1160/TH14-12-1067.
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. PMID: 26109205; PMCID: PMC4520875. doi:10.1182/blood-2014-08-531582.
- Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371(3):567–576. PMID: 29178039; PMCID: PMC5820397. doi:10.1007/s00441-017-2727-4.
- Rivera J, Lozano ML, Navarro-Núñez L, et al. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94(5):700–711. PMID: 19286885; PMCID: PMC2675683. doi:10.3324/haematol.2008.003178.
- Estevez B, Du X. New concepts and mechanisms of platelet activation signaling. Physiology (Bethesda). 2017;32(2):162–177. PMCID: PMC5337829. doi:10.1152/physiol.00020.2016.
- Jain N, Corken AL, Kumar A, et al. Role of platelets in chronic kidney disease. J Am Soc Nephrol. 2021;32(7):1551–1558. PMID: 34140394; PMCID: PMC8425650. doi:10.1681/ASN.2020121806.
- Kertai MD, Zhou S, Karhausen JA, et al. Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology. 2016;124(2):339–352. PMCID: PMC5040517. doi:10.1097/ALN.0000000000000959.
- Chávez-Iñiguez JS, García-García G, Lombardi R. [Epidemiología y desenlaces de la lesión renal aguda en latinoamérica]. Gac Med Mex. 2018;154(Supp 1):S6–S14. Spanish. doi:10.24875/GMM.M18000067.PMID: 30074021.
- Chávez-Íñiguez JS, Madero M. Global perspectives in acute kidney injury: Mexico. Kidney360. 2022; 3(4):737–739. PMID: 35721615; PMCID: PMC9136888. doi:10.34067/KID.0006592021.
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. PMID: 26162677. doi:10.1007/s00134-015-3934-7.
- Guo Q, Lou Y, Liu L, et al. How can I manage thrombocytopenia in hemodialysis patient? A review. Ther Apher Dial. 2020;24(4):352–360. PMID: 31661590. doi:10.1111/1744-9987.13448.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
- von Elm E, Altman DG, Egger M, et al. Declaración de la iniciativa STROBE (strengthening the reporting of observational studies in epidemiology): directrices Para la comunicación de estudios observacionales [The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies]. Rev Esp Salud Publica. 2008;82(3):251–259. Spanish. PMID: 18711640. doi:10.1590/s1135-57272008000300002.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. PMID: 26903338; PMCID: PMC4968574. doi:10.1001/jama.2016.0287.
- Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;82(2):147–157. PMID: 22592187. doi:10.1038/ki.2012.130.
- Liu D, Czigany Z, Heij LR, et al. The value of platelet-to-lymphocyte ratio as a prognostic marker in cholangiocarcinoma: a systematic review and meta-analysis. Cancers (Basel). 2022;14(2):438. PMID: 35053599; PMCID: PMC8773915. doi:10.3390/cancers14020438.
- Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. PMID: 30515177; PMCID: PMC6255980. doi:10.3389/fimmu.2018.02712.
- van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. PMID: 30429532. doi:10.1038/s41569-018-0110-0.
- Rubenstein DA, Yin W. Platelet-Activation mechanisms and vascular remodeling. Compr Physiol. 2018;8(3):1117–1156. doi:10.1002/cphy.c170049.PMID: 29978900.
- Koupenova M, Clancy L, Corkrey HA, et al. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122(2):337–351. PMID: 29348254; PMCID: PMC5777300. doi:10.1161/CIRCRESAHA.117.310795.
- Wang YQ, Wang B, Liang Y, et al. Role of platelet TLR4 expression in pathogensis of septic thrombocytopenia. World J Emerg Med. 2011;2(1):13–17. PMID: 25214976; PMCID: PMC4129734. doi:10.5847/wjem.j.1920-8642.2011.01.002.
- Kam T, Alexander M. Drug-induced immune thrombocytopenia. J Pharm Pract. 2014;27(5):430–439. Epub 2014 Aug 17. PMID: 25134884. doi:10.1177/0897190014546099.
- Baughman RP, Lower EE, Flessa HC, et al. Thrombocytopenia in the intensive care unit. Chest. 1993;104(4):1243–1247. PMID: 8404200. doi:10.1378/chest.104.4.1243.
- Chávez-Íñiguez JS, Maggiani-Aguilera P, González-Barajas D, et al. Urea reduction in acute kidney injury and mortality risk. Kidney Blood Press Res. 2023;48(1):357–366. Epub ahead of print. PMID: 36972576. doi:10.1159/000530237.
- Baaten C, Sternkopf M, Henning T, et al. Platelet function in CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32(7):1583–1598. PMID: 33941607; PMCID: PMC8425648. doi:10.1681/ASN.2020101440.
- Hu L, Tian K, Zhang T, et al. Cyanate induces oxidative stress injury and abnormal lipid metabolism in liver through Nrf2/HO-1. Molecules. 2019;24(18):3231. PMID: 31491954; PMCID: PMC6767610. doi:10.3390/molecules24183231.
- Horowitz HI, Stein IM, Cohen BD, et al. Further studies on the platelet-inhibitory effect of guanidinosuccinic acid and its role in uremic bleeding. Am J Med. 1970;49(3):336–345. doi:10.1016/s0002-9343(70)80025-0.PMID: 5455565.
- Zhu W, Buffa JA, Wang Z, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16(9):1857–1872. PMID: 29981269; PMCID: PMC6156942. doi:10.1111/jth.14234.
- Corona-de-la-Peña N, Uribe-Carvajal S, Barrientos-Rios R, et al. Polyamines inhibit both platelet aggregation and glycoprotein IIb/IIIa activation. J Cardiovasc Pharmacol. 2005;46(2):216–221. PMID: 16044034. doi:10.1097/01.fjc.0000171753.43564.7c.
- Linthorst GE, Avis HJ, Levi M. Uremic thrombocytopathy is not about urea. J Am Soc Nephrol. 2010;21(5):753–755. PMID: 20360312; PMCID: PMC2865735. doi:10.1681/ASN.2009111181.
- Ostertag LM, O’Kennedy N, Horgan GW, et al. In vitro anti-platelet effects of simple plant-derived phenolic compounds are only found at high, non-physiological concentrations. Mol Nutr Food Res. 2011;55(11):1624–1636. doi:10.1002/mnfr.201100135.PMID: 21898791.
- Shashar M, Belghasem ME, Matsuura S, et al. Targeting STUB1-tissue factor axis normalizes hyperthrombotic uremic phenotype without increasing bleeding risk. Sci Transl Med. 2017;9(417):eaam8475. PMID: 29167396; PMCID: PMC5854487. doi:10.1126/scitranslmed.aam8475.
- Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30(5):579–589. PMID: 15497100. doi:10.1055/s-2004-835678.
- Onwuemene O, Arepally GM. Heparin-induced thrombocytopenia: research and clinical updates. Hematol Am Soc Hematol Educ Program. 2016;2016(1):262–268. PMID: 27913490; PMCID: PMC6142447. doi:10.1182/asheducation-2016.1.262.
- Bui VC, Nguyen TH. The role of Single-Molecule force spectroscopy in unraveling typical and autoimmune heparin-induced thrombocytopenia. Int J Mol Sci. 2018;19(4):1054. PMID: 29614814; PMCID: PMC5979551. doi:10.3390/ijms19041054.
- Kobari E, Terawaki H, Takahashi Y, et al. Dialyzer-related thrombocytopenia due to a polysulfone membrane. Intern Med. 2016;55(8):965–968. PMID: 27086813. doi:10.2169/internalmedicine.55.5636.
- Yang RC, Lindsay RM. Dialyzer reactions in a patient switching from peritoneal dialysis to hemodialysis. Hemodial Int. 2005;9(2):120–126. PMID: 16191059. doi:10.1111/j.1492-7535.2005.01123.x.
- Posadas MA, Hahn D, Schleuter W, et al. Thrombocytopenia associated with dialysis treatments. Hemodial Int. 2011;15(3):416–423. PMID: 21711442. doi:10.1111/j.1542-4758.2011.00561.x.
- Bacelar Marques ID, Pinheiro KF, de Freitas do Carmo LP, et al. Anaphylactic reaction induced by a polysulfone/polyvinylpyrrolidone membrane in the 10th session of hemodialysis with the same dialyzer. Hemodial Int. 2011;15(3):399–403. PMID: 21624039. doi:10.1111/j.1542-4758.2011.00553.x.
- Reeves JH, Cumming AR, Gallagher L, et al. A controlled trial of low-molecular-weight heparin (dalteparin) versus unfractionated heparin as anticoagulant during continuous venovenous hemodialysis with filtration. Crit Care Med. 1999;27(10):2224–2228. PMID: 10548211. doi:10.1097/00003246-199910000-00026.
- Ben Hamida C, Lauzet JY, Rézaiguia-Delclaux S, et al. Effect of severe thrombocytopenia on patient outcome after liver transplantation. Intensive Care Med. 2003;29(5):756–762. PMID: 12677370. doi:10.1007/s00134-003-1727-x.
- Jonsson AB, Rygård SL, Hildebrandt T, et al. Thrombocytopenia in intensive care unit patients: a scoping review. Acta Anaesthesiol Scand. 2021;65(1):2–14. PMID: 32916017. doi:10.1111/aas.13699.
- Hui P, Cook DJ, Lim W, et al. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271–278. PMID: 21071526. doi:10.1378/chest.10-2243.
- Williamson DR, Albert M, Heels-Ansdell D, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207–1215. PMID: 23788287. doi:10.1378/chest.13-0121.
- Strauss R, Wehler M, Mehler K, et al. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30(8):1765–1771. PMID: 12163790. doi:10.1097/00003246-200208000-00015.
- Lin J, Gallagher M, Bellomo R, et al. SOFA coagulation score and changes in platelet counts in severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level (RENAL) study. Nephrology (Carlton). 2019;24(5):518–525. PMID: 29693303. doi:10.1111/nep.13387.
- Vandijck DM, Blot SI, De Waele JJ, et al. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung. 2010;39(1):21–26. PMID: 20109983. doi:10.1016/j.hrtlng.2009.07.005.
- Xu X, Wang H, Wu X, et al. [Risk factors of early septic shock-related thrombocytopenia and its impact on prognosis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(8):938–943. PMID: 34590560. doi:10.3760/cma.j.cn121430-20210112-00036.