 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Creatine supplementation is ubiquitously consumed by fitness enthusiasts due to its perceived advantages in enhancing athletic performance. Although there is an increasing concern within this demographic regarding its possible impact on renal function, there is still a lack of rigorous scientific investigations into this alleged association.
Methods
Data were collected through an online survey on the participants’ demographics, creatine usage and concerns related to renal function. The reliability and validity of the survey were assessed using SPSS software. A total of 1129 participants responded to the survey, and chi-square tests were utilized for data analysis. To explore the potential association between creatine levels (as the exposure) and renal function (as the outcome), we utilized open-access genetic databases, and Mendelian randomization (MR) techniques were used to confirm this correlation.
Results
Chi-square analysis revealed no significant association between creatine usage and renal function among the participants. Our MR analysis further supported this finding, demonstrating no significant association between creatine levels and six indicators assessing renal function (IVW, all with p values exceeding 0.05). Similar p values were consistently observed across other MR methods, confirming the absence of a statistical correlation.
Conclusions
This MR study offers compelling evidence indicating that creatine levels are not statistically associated with renal function, suggesting the potential to alleviate concerns within the fitness community and emphasizing the significance of evidence-based decision-making when considering nutritional supplementation.
Introduction
Creatine, an endogenous molecule synthesized within the human body, has attracted considerable attention in the field of sports nutrition and exercise physiology. As a dietary supplement, creatine has been praised for its potential to enhance athletic performance, particularly in activities characterized by high intensity and short duration, such as soccer and sprinting. Its effectiveness in increasing phosphocreatine stores in muscles, thereby facilitating rapid regeneration of adenosine triphosphate (ATP) during physical exertion, has been extensively documented in numerous studies. As a result, creatine supplementation has become a routine practice for many athletes and individuals involved in physical training, solidifying its status as one of the most commonly ergogenic aids used worldwide.
Renal function is a vital aspect of physiological well-being and refers to the kidneys’ capacity to filter blood, eliminate waste products, and regulate electrolyte balance. Impaired renal function can result in various health complications, including chronic kidney disease (CKD), hypertension, and, in severe instances, and kidney failure. The repercussions of compromised renal function are significant, posing substantial risks of morbidity and mortality. Moreover, the economic and societal burdens associated with renal-related conditions are considerable, underscoring the imperative of preserving renal health.
The question of whether creatine supplementation affects renal function remains debatable in existing research. Some studies propose that high doses of creatine may have detrimental effects on renal function, particularly in individuals with preexisting renal conditions. Conversely, other research suggests that consuming creatine at recommended doses does not result in renal damage in healthy individuals. Given the widespread use of creatine among athletes and fitness enthusiasts, a comprehensive understanding on the potential impacts of creatine supplementation on renal function is essential.
In this study, we hypothesized that creatine supplementation might adversely affect renal function. Considering that Mendelian Randomization (MR) can reveal causal relationships between exposures and outcomes, bypassing many confounders and biases often present in observational studies, particularly when utilizing genetic variants as proxies, this study employs MR to investigate the possible link between creatine levels and renal function to address this growing concern within the fitness community.
Materials and methods
Survey design
Data collection was conducted through an online survey via Wenjuanxing (https://www.wjx.cn/vm/). The survey comprised 31 questions, with respondents providing yes/no answers or selecting from predetermined response options. Participants were recruited using a random sampling approach at fitness centers via WeChat (https://weixin.qq.com/). To ensure participant confidentiality, all responses were anonymized and aggregated for analysis. This study was approved by the Jiaxing Hospital of Traditional Chinese Medical, and participants provided informed consent before accessing the survey, indicating their voluntary participation.
Survey
The survey comprises five items divided into two main categories: (1) demographic information and creatine usage, and (2) scale questions covering awareness and usage of creatine, reasons for use and barriers, effects and impacts, knowledge, and recommendations. We included questions regarding participants’ perceptions of their renal health, specifically asking if they have been diagnosed with any renal function issues by a healthcare provider and their general feelings about their renal health. Only respondents confirming creatine usage are eligible to answer the questions in part two. Validity analysis of the questionnaire was conducted using SPSS software. The factor analysis method, specifically rotational principal component analysis, was employed to automatically screen the extracted factors. Cronbach’s alpha was utilized to evaluate the consistency and reliability of the questionnaire.
Statistical analysis
Comparisons between groups for normally distributed quantitative data were conducted using the independent samples t-test. Categorical data are presented as frequencies and percentages [n(%)]. A p value less than 0.05 was considered statistically significant.
Mendelian randomization research approach
Utilizing a two-sample MR technique, we examined the potential causal relationship between creatine levels and six indicators evaluating renal function. In MR, genetic variations serve as proxies for risk determinants. To ensure a valid causal interpretation through instrumental variables (IVs), three essential criteria must be met: (1) There must be a direct relationship between the genetic variation and the exposure; (2) There should be no association between the genetic variation and any confounders of the exposure-outcome relationship, and (3) The genetic variation must influence the outcome only through the exposure.
Genomic insights into creatine levels
Summary statistics regarding Creatine levels were obtained from Genome-wide association studies (GWAS), accessible via the designated repository (https://gwas.mrcieu.ac.uk/). This research conducted a GWAS analysis involving a cohort of 291 individuals, assessing 6,847,545 distinct SNPs. The study population mainly comprised individuals of European descent.
Genomic analysis of renal function metrics
Summary statistics for six renal function metrics were retrieved from the recognized GWAS repository (https://gwas.mrcieu.ac.uk/). The metrics used for renal function assessment are as follows: (1) Serum creatinine levels: Analyzed in a GWAS with a cohort of 344,104 individuals, evaluating 19,034,241 distinct SNPs; (2) Estimated glomerular filtration rate (eGFR): Studied in a GWAS with a cohort of 143,658 individuals, evaluating 5,961,601 distinct SNPs; (3) Serum uric acid levels: Investigated in a GWAS with a cohort of 343,836 individuals, evaluating 19,041,286 distinct SNP; (4) Microalbumin in urine: Examined in a GWAS with a cohort of 3,036 individuals, evaluating 15,523,141 distinct SNPs; (5) Creatinine enzymatic in urine: Assessed in a GWAS with a cohort of 396,837 individuals, evaluating 10,783,704 distinct SNPs, and (6) Blood urea nitrogen levels: Investigated in a GWAS with a cohort of 344,052 individuals, evaluating 19,049,084 distinct SNPs.
Instrumental variables (IVs) selection
Based on recent scientific findings, we set a significance level for IVs associated with each characteristic at 1 × 10 − 5. Utilizing the TwoSampleMR package in R, we optimized the selection of single nucleotide polymorphisms (SNPs). The clumping parameter was activated, with a secondary significance level set at 1 × 10 − 5. We defined a criterion for linkage disequilibrium (LD) r^2 at 0.001 and specified a clumping proximity of 10,000 kb. To mitigate bias that could arise from suboptimal instruments, we computed R^2 and F statistics for each SNP using the following formula:
Here:
βexposure represents the beta coefficient of exposure,
eafexposure is the effect of allele frequency of exposure.
seexposure represents the standard error of exposure.
samplesizeexposure indicates the sample size of the exposure group.
We excluded SNPs with an F-statistic less than 10 from our analysis to avoid weak instrument bias, which can inflate type I error rates and yield unreliable estimates.
Statistical approach
Analyses were conducted using the R programming environment version 4.3.1 (Vienna, Austria). The causal relationship between Creatine levels and renal function was evaluated using methods, such as inverse variance weighting (IVW), weighted median, and mode-based techniques, primarily through the TwoSampleMR package. Heterogeneity among IVs was assessed using Cochran’s Q statistic, with a p value greater than 0.05 considered indicative of non-significant heterogeneity. In instances of significant heterogeneity, a random-effects IVW model was employed instead of the fixed-effects approach. The MR-Egger method was utilized to address potential horizontal pleiotropy, with significance in its intercept indicating the presence of pleiotropy. The MR-PRESSO technique further refined the analysis by identifying and excluding potential pleiotropic outliers. Funnel plots were included to assess publication bias and validate the consistency and robustness of the results, respectively.
Results
Sample characteristics and use of creatine
The study comprised 1129 individuals and had a Cronbach’s α coefficient of 0.973 for the 31-item questionnaire. The validity analysis of the questionnaire was conducted using SPSS software, employing factor analysis through rotational principal component analysis. The results indicated high validity, as evidenced by characteristic root values, variance explanation rates, and cumulative variance explanation rates, all suggesting a clear factor structure and item attribution to each factor. The Kaiser–Meyer–Olkin (KMO) measure was 0.974, indicating strong inter-variable correlations suitable for factor analysis. Additionally, Bartlett’s test of sphericity yielded a value of 83,245.626 with a significance level of 0.000, indicating significant construct validity for the questionnaire (Raw questionnaire data in Supplemental Table S6).
Among the 1129 valid respondents, 92.03% were male, and 7.97% were female, indicating a male majority in the survey. Among these respondents, 968 (85.74%) were aware of creatine, while 161 (14.26%) were unaware. Of those aware, only 29.55% reported using creatine.
Further analysis on the association between creatine use and renal function revealed a chi-square statistic (χ2) of 0.96 (p = 0.619), indicating no significant difference in renal function between creatine users and non-users. Additionally, the analysis revealed that there are no significant differences in creatine usage based on age groups or gender (p > 0.05). However, creatine users reported a significantly lower BMI compared to non-users (χ2 = 136.41, p < 0.001, degrees of freedom = 5), suggesting that creatine may have a positive effect on BMI reduction (Supplemental Table S7).
Probing the impact of creatine levels on renal function
Based on the predefined criteria for selecting IVs, a comprehensive set of 114 SNPs was utilized as IVs in this study. The detailed information regarding these selected SNPs and their characteristics are shown in Supplemental Table S1.
Investigations into the causal relationship between creatine levels and the risk of renal function were based on the potential implications of creatine supplementation and were assessed using various MR methodologies. The data obtained from the Inverse Variance Weighted (IVW) method did not demonstrate a significant correlation between creatine levels and the risk of six indicators evaluating renal function. Supplementary MR techniques also revealed no association, displaying varying degrees of statistical significance, as illustrated in and Supplemental Table S2.
Figure 1. Forest Plots showed the causal associations between creatine levels and six indicators evaluating renal function traits. IVW: inverse variance weighting; CI: confidence interval.
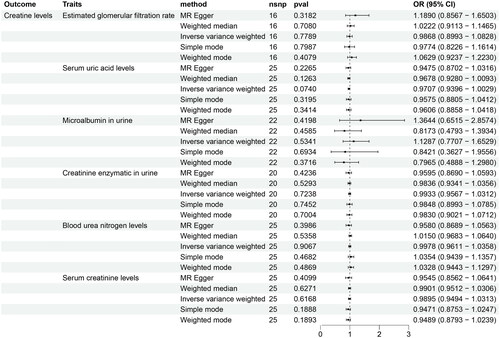
The results of Cochran’s IVW Q test indicated a lack of significant heterogeneity among the IVs (Supplemental Table S3). Moreover, the MR-Egger regression intercept analysis did not show any significant directional horizontal pleiotropy (Supplemental Table S4). Additionally, the MR-PRESSO global test did not identify any significant outliers (Supplemental Table S5), implying a minimal presence of horizontal pleiotropy in the association.
Discussion
The complex interplay between creatine levels and renal function has garnered significant attention from both the fitness and medical communities. In this study, we conducted a comprehensive survey among fitness center attendees to assess a broad range of experiences and perceptions regarding creatine usage and its perceived effects on renal function. We combined this observational data with MR analysis, which offers a genetic basis that enhances our understanding of the biological causality behind these observations. This integrative approach leverages both immediate community-based perceptions and robust genetic insights, providing a scientifically comprehensive perspective on the potential causal relationships. This methodology enriches the depth and credibility of the obtained results and strengthens the validity of our findings.
In this study, we used six different biomarkers, which included blood urea nitrogen, urinary creatinine enzymatic, eGFR, urinary microalbumin, serum creatinine, and serum uric acid levels, to comprehensively evaluate renal function, with each biomarker offering a unique perspective on kidney function, collectively providing a robust framework for assessing renal health. Particularly noteworthy is the combined use of blood urea nitrogen, urinary microalbumin, and eGFR, which is essential for the early detection of kidney damage, underscoring their clinical significance. Moreover, monitoring serum uric acid levels is crucial for identifying potential renal alterations, especially in the context of metabolic syndromes.
Several studies have investigated the relationship between creatine supplementation and renal function. For instance, an investigation on the common misconceptions about creatine supplementation was conducted and confirmed the link between creatine intake and renal function. However, the causal nature of these associations has remained largely unexplored. Herein, our results, which indicate no significant causal link between creatine levels and renal function, align with the associative patterns observed in previous research and provide a causal perspective. This causal insight refines the existing results, providing a clearer understanding of the role of creatine in renal function risk. Our findings also suggest that concerns regarding creatine supplementation as a potential trigger for renal issues may not be justified, which could potentially alleviate apprehensions within the fitness community, promoting evidence-based decision-making regarding creatine supplementation.
The rigorous methodological framework, utilizing a two-sample MR approach, stands as a pivotal strength of this study, bolstering the validity of the causal inferences drawn. However, like all research endeavors, this study has its limitations. The applicability of the findings might be restricted to specific demographic groups, highlighting the need for further investigations across a wider demographic spectrum. While the survey method of self-reporting renal function provides valuable insights into participants’ behaviors and perceptions, it inherently carries limitations due to its reliance on respondents’ subjective feelings and personal health awareness. This reliance may introduce biases as individuals could underestimate or overestimate their health issues based on personal judgment or lack of medical knowledge.
Taken together, this study provides important insights into the growing literature on the relationship between creatine and renal function by shedding light on the causal dynamics, highlighting the importance of evidence-based approaches for understanding the implications of creatine supplementation and marking a significant advancement toward informed decision-making in both fitness supplementation and renal health.
Conclusions
In conclusion, this study elucidates the potential causal relationship between creatine levels and the risk of renal function, revealing no significant association between the two. By combining survey data with MR analysis, we offer a comprehensive assessment that aligns public perceptions with genetic causality. These findings alleviate concerns within the fitness community regarding creatine supplementation as a trigger for renal issues. The results highlight the importance of evidence-based decision-making in both fitness supplementation and renal health. Through comprehensive assessments of the creatine-renal function etiological connection, this study contributes to the broader efforts of promoting informed health choices among fitness enthusiasts and individuals predisposed to renal issues.
Institutional review board statement
Not applicable, as this research did not involve human subjects or animal experiments.
Informed consent statement
Not applicable, as this research did not involve human subjects.
Author contributions
Bing Zhou and Minping Hong: Responsible for data analysis and took the lead in writing the majority of the manuscript. Liqin Jin: Contributed to data collection and manuscript review. Keng Ling: Guided the project, proposed creative ideas, and revised the manuscript critically for important intellectual content.
Supplemental Material
Download ()Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The datasets analyzed during this study are available in the GWAS repository. The specific datasets used are:
Creatine levels: ebi-a-GCST90026143
Blood urea nitrogen levels: ebi-a-GCST90018948
Creatinine enzymatic in urine: ebi-a-GCST90013987
Estimated glomerular filtration rate: ieu-a-1284
Microalbumin in urine: ukb-e-30500_AFR
Serum creatinine levels: ebi-a-GCST90018979
Serum uric acid levels: ebi-a-GCST90018977
Additional information
Funding
References
- Almeida D, Colombini A, Machado M. Creatine supplementation improves performance, but is it safe? Double-blind placebo-controlled study. J Sports Med Phys Fitness. 2020;60(7):1–7. doi: 10.23736/S0022-4707.20.10437-7.
- Antonio J, Candow DG, Forbes SC, et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J Int Soc Sports Nutr. 2021;18(1):13. doi: 10.1186/s12970-021-00412-w.
- Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian Randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965.
- Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26(5):2333–2355. doi: 10.1177/0962280215597579.
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the Mr-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x.
- Chen JH, Zeng LY, Zhao YF, et al. Causal effects of gut microbiota on sepsis: a two-sample Mendelian randomization study. Front Microbiol. 2023;14:1167416. doi: 10.3389/fmicb.2023.1167416.
- D, Souza E, Silva A, A, Pertille, et al. Effects of creatine supplementation on renal function: a systematic review and meta-analysis. J Ren Nutr. 2019;29(6):480–489. doi: 10.1053/j.jrn.2019.05.004.
- Elsherbiny NM, Said E. Editorial: insights in renal endocrinology: 2021. Front Endocrinol (Lausanne). 2022;13:1003683. doi: 10.3389/fendo.2022.1003683.
- Forbes SC, Candow DG, Neto JHF, et al. Creatine supplementation and endurance performance: surges and sprints to win the race. J Int Soc Sports Nutr. 2023;20(1):2204071.
- Hall M, Trojian TH. Creatine supplementation. Curr Sports Med Rep. 2013;12(4):240–244. doi: 10.1249/JSR.0b013e31829cdff2.
- Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol. 2017;956:307–325. doi: 10.1007/5584_2016_84.
- Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102.
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate Gfr without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953.
- Kim HJ, Kim CK, Carpentier A, et al. Studies on the safety of creatine supplementation. Amino Acids. 2011;40(5):1409–1418. doi: 10.1007/s00726-011-0878-2.
- Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156–165. doi: 10.1038/s41588-020-00763-1.
- Lanhers C, Pereira B, Naughton G, et al. Creatine supplementation and lower limb strength performance: a systematic review and meta-analyses. Sports Med. 2015;45(9):1285–1294. doi: 10.1007/s40279-015-0337-4.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006.
- Maiuolo J, Oppedisano F, Gratteri S, et al. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;387:131126. doi: 10.1016/j.ijcard.2015.08.109.
- Mielgo-Ayuso J, Calleja-Gonzalez J, Marqués-Jiménez D, et al. Effects of creatine supplementation on athletic performance in soccer players: a systematic review and meta-analysis. Nutrients. 2019;11(4):757. doi: 10.3390/nu11040757.
- Olsen E, van Galen G. Chronic renal failure-causes, clinical findings, treatments and prognosis. Vet Clin North Am Equine Pract. 2022;38(1):25–46. doi: 10.1016/j.cveq.2021.11.003.
- Owen L, Sunram-Lea SI. Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients. 2011;3(8):735–755. doi: 10.3390/nu3080735.
- Peng Z, Saito S. Creatine supplementation enhances anti-tumor immunity by promoting adenosine triphosphate production in macrophages. Front Immunol. 2023;14:1176956. doi: 10.3389/fimmu.2023.1176956.
- Poortmans JR, Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30(3):155–170. doi: 10.2165/00007256-200030030-00002.
- Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. doi: 10.1038/nrdp.2017.88.
- Tidmas V, Brazier J, Hawkins J, et al. Nutritional and non-nutritional strategies in bodybuilding: impact on kidney function. Int J Environ Res Public Health. 2022;19(7):4288. doi: 10.3390/ijerph19074288.
- Vega J, Huidobro EJ. [Effects of creatine supplementation on renal function]. Rev Med Chil. 2019;147(5):628–633. doi: 10.4067/S0034-98872019000500628.
- Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7.
- Wang V, Vilme H, Maciejewski ML, et al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36(4):319–330. doi: 10.1016/j.semnephrol.2016.05.008.
- Wax B, Kerksick CM, Jagim AR, et al. Creatine for exercise and sports performance, with recovery considerations for healthy populations. Nutrients. 2021;13(6):1915. doi: 10.3390/nu13061915.
- Yun Z, Guo Z, Li X, et al. Genetically predicted 486 blood metabolites in relation to risk of colorectal cancer: a Mendelian randomization study. Cancer Med. 2023;12(12):13784–13799. doi: 10.1002/cam4.6022.
