Abstract
Background
Currently, several studies have explored the association between the modified creatinine index (mCI) and prognosis in patients on hemodialysis (HD). However, some of their results are contradictory. Therefore, this study was conducted to comprehensively assess the role of mCI in predicting prognosis in HD patients through meta-analysis.
Methods
We searched and screened literature from PubMed, Embase, Web of Science, and Cochrane databases from their establishment until March 2024. Relevant data were extracted. The statistical analysis was performed using Stata 15.0, RevMan 5.4, and Meta DiSc 1.4 software.
Results
The results showed a positive association between mCI and nutritional status in HD patients (BMI r = 0.19, 95% CI: 0.1–0.28, p = .000; albumin r = 0.36, 95% CI: 0.33–0.39, p = .000; normalized protein catabolic rate (nPCR) r = 0.25, 95% CI: 0.13–0.38, p = .000). In addition, mCI in deceased HD patients was significantly lower than that in HD survivors (SMD = −0.94, 95% CI: −1.46 to −0.42, p = .000). A low mCI was associated with an increased risk of all-cause death in HD patients (HR = 1.95, 95% CI: 1.57–2.42, p = .000). In addition, a low mCI was significantly associated with decreased overall survival (OS) in HD patients (HR = 3.01, 95% CI: 2.44–3.70, p = .000). mCI showed moderate diagnostic accuracy for sarcopenia in both male and female HD patients (male AUC = 0.7891; female AUC = 0.759).
Conclusions
The mCI can be used as a prognostic marker for HD patients, and monitoring mCI may help to optimize the management of HD and improve overall prognosis in patients.
1. Introduction
Hemodialysis (HD), a form of renal replacement therapy (RRT), is currently the main choice for patients with end-stage renal disease (ESRD). Currently, nearly 4 million people in the world are undergoing RRT, and HD patients account for 69% of them [Citation1]. Most ESRD patients still require long-term and even lifelong HD, although it relieves their disease. During HD, patients often present with symptoms of malnutrition including loss of body mass, muscle loss, and protein energy wasting (PEW) [Citation2], which are accompanied by a higher risk of infection/death and a lower survival rate, as compared with those in the normal population [Citation3]. In addition, with the increasing trend of global aging, the incidence of ESRD has risen, which in turn has led to a change in the age distribution of HD patients [Citation4]. In Japan, one of countries with the most severely aging population, the mean age of HD patients reached 69 years as early as 2017 [Citation5]. This undoubtedly makes it more difficult to manage HD patients. Given these serious prognostic and societal burdens, there is a need to identify a valid and accurate indicator to screen HD patients for adverse clinical events.
In 1995, Canaud et al. obtained a normalized creatinine production rate, the creatinine index (CI), which is equal to the sum of the estimated creatinine excretion rate and the metabolic degradation rate and was derived from creatinine kinetic modeling (CKM) and used to assess nutritional status in HD patients [Citation6]. Subsequently, in a study by Desmeules et al., CI became a predictor of long-term survival and mortality in HD patients [Citation7]. However, its cumbersome calculation formula has become a major problem for clinical use. Until 2014, Canaud et al. succeeded in simplifying the CI formula (modified CI, termed as mCI) simply by using some usual data, i.e., age, gender, pre-dialysis serum creatinine level, and urea clearance index (Kt/V) [Citation7]. Consequently, in the following 10 years or so, mCI were frequently used in clinical studies in HD patients, but whether it is associated with prognosis in HD patients is still controversial. For example, a study by Bataille et al. stated that mCI could not predict sarcopenia in HD patients [Citation8], whereas studies by Tian et al. and Kakita et al. stated that mCI could be used to predict sarcopenia in HD patients [Citation9,Citation10].
Therefore, we summarized currently available literature relevant to the topic and initially performed a systematic review and meta-analysis of it to assess the association between mCI and prognosis in HD patients.
2. Methods
2.1. Search strategy
This study was already registered on PROSPERO (CRD42024529310) and strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Using the same search strategy, two investigators conducted independent comprehensive search in Web of Science, Embase, Cochrane Library, and PubMed from inception to March 2024. Search terms included the subject headings ‘Renal Dialysis’ and ‘Creatinine’ and the free-text terms ‘haemodialysis, hemodialysis, Kreatinine, Krebiozen’, as shown in Attachment 1. Subsequently, no other publications were found after reference lists and citations were searched. Independent comprehensive search was conducted in Web of Science, Embase, Cochrane Library, PubMed, and Scopus from inception to March 2024. Search terms included the subject headings ‘Renal Dialysis’ and ‘Creatinine’ and the free-text terms ‘haemodialysis, hemodialysis, Kreatinine, Krebiozen’, as shown in Attachment 1. Subsequently, no other publications were found after reference lists and citations were searched.
2.2. Inclusion and exclusion criteria
Inclusion criteria: (1) study type: a cohort study; (2) study population: (2) adults (≥18 years) treated with HD; (3) formula for calculation of mCI: modified Cr index (mg/kg/day) = 16.21 + 1.12 × [1 if male; 0 if female] − 0.06 × age (years) − 0.08 × single-pooled Kt/V for urea + 0.009 × serum Cr level before dialysis (µmol/L); (4) studies evaluating the association between mCI and prognosis in HD patients. Exclusion criteria: (1) reviews, letters, pathology reports, conference abstracts, meta-analyses, or animal studies; (2) studies conducted by the same authors using the same data; (3) unavailable full texts or insufficient data.
2.3. Literature screening
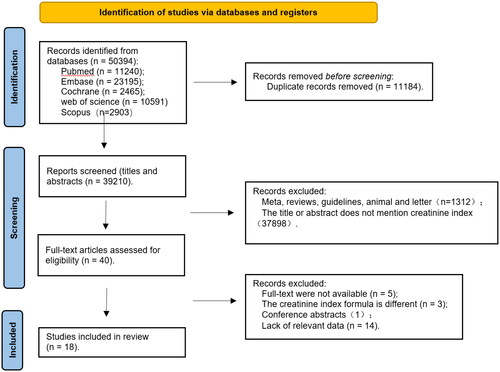
In this study, duplicate references were first removed, and then, the titles and abstracts of literature were independently read by two investigators for initial screening based on pre-established inclusion and exclusion criteria. Finally, the full texts were read to exclude nonconforming literature, thereby identifying final literature to be included and extracting relevant data.
2.4. Data extraction
Data extraction in this study was performed independently by two investigators. The main data extracted included the first author, year of publication, region where a study was conducted, specific type of a study, sample size, mean age, sex ratio, and relevant outcome measures, such as risk of all-cause death, and sensitivity and specificity to predict sarcopenia. If disagreements arose between the two investigators (Tao Ye and Jingfang Du) during this process, they would be resolved by a third investigator (Pian Li).
2.5. Quality assessment
In this study, two investigators (Tao Ye and Jingfang Du) independently assessed the risk of bias in included studies using the Newcastle Ottawa Scale (NOS) and Joanna Briggs Institute Practical Application of Clinical Evidence System (JBI PACES) [Citation11]. NOS assessment contains the following: adequacy of the case definition, representativeness of the cases, selection of controls, comparability of cases and controls, and ascertainment of exposure. In addition, based on the corresponding scores, the included studies were classified as high quality (7–9), medium quality (4–6), and low quality (1–3). JBI assessment contains 10 items: ① Was the purpose of the study clear? Was the study fully justified? ② How was the study population selected? ③ Were the inclusion and exclusion criteria for samples clearly described? ④ Were the sample characteristics clearly described? ⑤ Were the data collection instruments reliable and valid? ⑥ What were the measures to verify the authenticity of the data? ⑦ Were ethical issues taken into account? ⑧ Were the statistical methods correct? ⑨ Were the results of the study presented appropriately and accurately? ⑩ Was the value of the study clearly stated? Each item was assessed by ‘non-compliant (0),’ ‘mentioned, but not described in detail (1)’, and ‘described fully and accurately in detail (2)’. When the score was > 70% of the total score, i.e., a score >14, it was considered that the study had a low risk of bias. If disagreements arose between the two investigators, they would be resolved via negotiation with a third investigator (Ping Li).
2.6. Statistical analysis
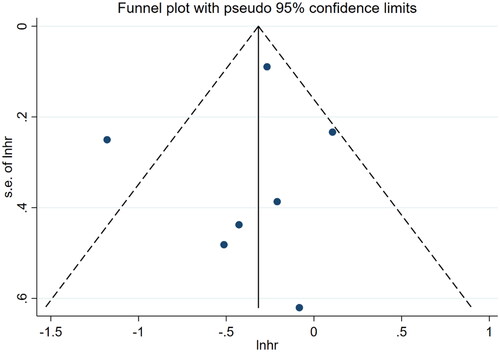
All statistical analyses were performed using Stata 15.0 (StataCorp, College Station, TX), RevMan 5.4 (The Cochrane Centre Collaboration, Copenhagen, Denmark), and Meta-DiSc 1.4 (Madrid, Spain). Multivariable-adjusted effect estimates (odds ratio (OR)/hazard ratio (HR)) were extracted for all included studies to calculate the pooled estimates and 95% confidence intervals (CIs). In addition, the standardized mean difference (95% CI) of mCI between groups such as the surviving group and the dead group was assessed. The I2 statistic was used to measure the level of heterogeneity. I2 > 50% indicated significant heterogeneity among the included studies. For it, a random-effects model was used, and sensitivity analysis (one-by-one elimination method), meta-regression (n ≥ 5) and subgroup analysis (n ≥ 2) were performed to find the source of heterogeneity. If I2 < 50%, a fixed-effects model would be used. Publication bias in included studies was assessed by a funnel plot (n > 8), Egger’s test and Begg’s test. Egger’s or Begg’s p < .05 was considered to indicate the presence of publication bias. If publication bias was present, publication bias would be corrected by the trim-and-fill method.
3. Results
3.1. Literature search results
According to the search strategy, a total of 50,394 references were obtained. Of them, 11,240 were published in PubMed, 23,195 published in EMBASE, 2465 published in Cochrane, 10,591 published in Web of Science, 2903 published in Scopus, and 11,184 duplicates removed. Forty references were obtained after the titles and abstracts were read. Their quality was then assessed based on their full texts. Twenty-two of them were excluded, including five references without full texts available, three references containing different CI formulas, one conference abstract, and 13 references without relevant data. In the end, 18 references [Citation8–10,Citation12–26] were obtained. The literature screening process is shown in .
3.2. Characteristics of the included studies
Of the 80 references (studies) included, 15 were published in Asia, two were published in Europe, and one was published in Oceania. They were published from 2016 to 2023. They involved 32,160 patients. They were all cohort studies. The included studies were given NOS scores and JBI scores separately. NOS scores showed 15 high-quality studies [Citation8–10,Citation12,Citation14–24,Citation26] and three medium-quality studies [Citation13,Citation15,Citation25]. JBI scores showed all the included studies were scored >14, with a low risk of bias. Data are detailed in .
Table 1. General characteristics of included studies.!!
3.3. Association between mCI and the duration of maintenance of dialysis in HD patients
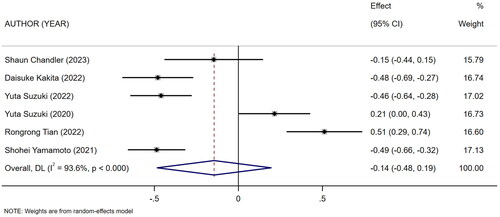
Six studies [Citation9,Citation10,Citation14,Citation21,Citation22,Citation26] described the duration of maintenance of dialysis in HD patients in high and low mCI groups. The heterogeneity test showed high heterogeneity (I2 = 93.6%). Therefore the random-effects model was used for meta-analysis. The results showed that there was no association between high and low levels of mCI and the duration of maintenance of dialysis in HD patients (SMD = −0.14, 95% CI: −0.48 to 0.19, p = .404), as shown in . Sources of heterogeneity were subsequently explored based on sensitivity analysis (one-by-one elimination method), meta-regression (mean age of the study population, mCI cutoff value), and subgroup analysis (mean age of the study population, type of the study). No sources of heterogeneity sensitivity were shown by sensitivity and subgroup analyses (Attachment 1), and meta-regression (mean age, p = .275; mCI cutoff value, p = .803). Tests for publication bias were performed. The results showed no publication bias (Egger’s test, p = .323; Begg’s test, p = .06).
3.4. Association between mCI and nutritional status in HD patients
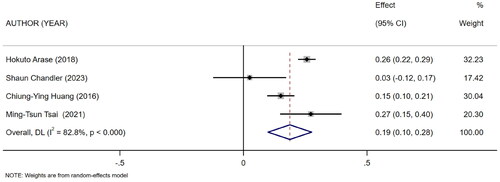
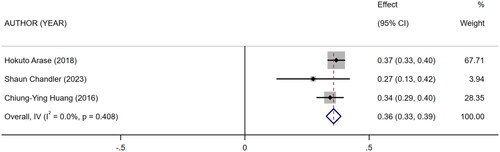
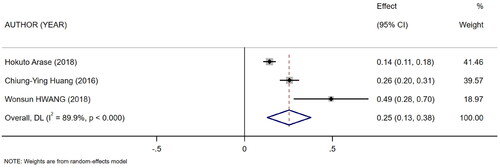
Five references [Citation11–15] described the association between mCI and indicators of nutritional status in HD patients: body mass index (BMI), albumin, and normalized protein catabolic rate (nPCR). Of them, four [Citation11–13,Citation15] reported the association between mCI and BMI (I2 = 82.8%), three [Citation11,Citation13,Citation14] reported the association between mCI and nPCR (I2 = 89.9%), and three [Citation11–13] reported the association between mCI and albumin (I2 = 0.0%). The tests for heterogeneity in BMI and nPCR were performed using the random-effects model. The test for heterogeneity in albumin was performed using the fixed-effects model. The results showed that mCI was positively associated with BMI (r = 0.19, 95% CI: 0.1–0.28, p = .000), albumin (r = 0.36, 95% CI: 0.33–0.39, p = .000), and nPCR (r = 0.25, 95% CI: 0.13–0.38, p = .000), as shown in . Subsequently, sensitivity analysis and a test for publication bias were performed. No sources of heterogeneity were found (BMI: Egger’s test, p = .451, Begg’s test, p = .734; nPCR, Egger’s test, p = .324, Begg’s test, p = 1; albumin, Egger’s test, p = .122, Begg’s test, p = .296).
3.5. Difference in the level of mCI between surviving and dead HD patients
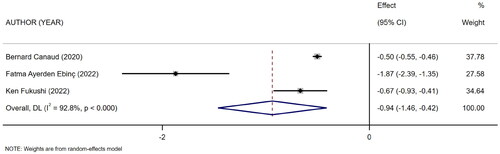
Three references [Citation16–18] described the level of mCI in surviving and dead HD patients. The heterogeneity test showed high heterogeneity (I2 = 92.8%). Therefore, the random-effects model was used for analysis. The results showed a difference in the level of mCI between surviving and dead HD patients (SMD = −0.94, 95% CI: −1.46 to −0.42, p = .000), as shown in . Subsequently, sensitivity analysis and a test for publication bias were performed. The results showed no sources of heterogeneity, and absence of publication bias (Egger’s test, p = .322, Begg’s test, p = .296).
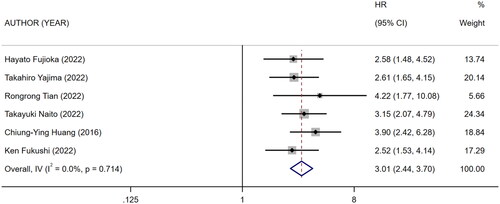
3.6. Association between mCI and risk of all-cause death/OS in HD patients
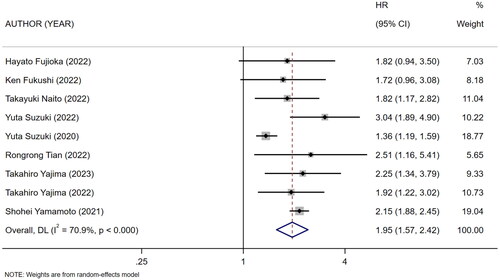
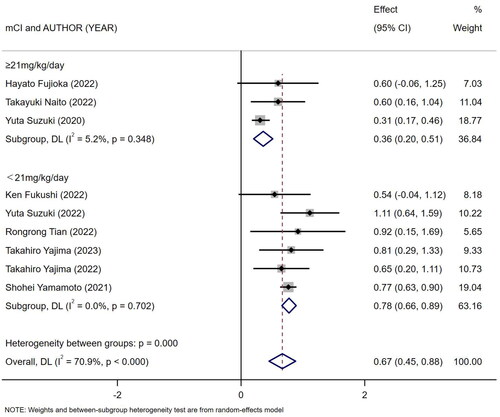
Nine references [Citation10,Citation18–25] described the association between mCI and risk of all-cause death in HD patients, and the heterogeneity test showed high heterogeneity in risk of all-cause death (I2 = 70.9%). Six references [Citation10,Citation13,Citation18–20,Citation24] described the association between mCI and overall survival (OS) in HD patients, and the heterogeneity test showed no heterogeneity in OS (I2 = 0%). The association between mCI and OS in HD patients was assessed using the fixed-effects model. The association between mCI and risk of all-cause death in HD patients was assessed using the random-effects model. The results showed that mCI was associated with risk of all-cause death (HR = 1.95, 95% CI: 1.57–2.42, p = .000) and OS (HR = 3.01, 95% CI: 2.44–3.70, p = .000) in HD patients. Subsequently, sensitivity analysis, meta-regression (mean age of the study population, mCI cutoff value) and subgroup analysis (mCI cutoff value) were performed regarding the risk of all-cause death in mCI and HD patients. As a result, sensitivity analysis and meta-regression showed that mCI was associated with the risk of all-cause mortality in HD patients (mean age of the study population, p = .065, mCI cutoff value, p = .004). Subgroup analysis (mCI cutoff value) showed mCI ≥21 mg/kg/day: I2 = 5.2%, HR = 0.36, 95% CI 0.20–0.51, p = .000; mCI <21 mg/kg/day: I2 = 0%, HR = 0.78, 95% CI 0.66–0.89, p = .000. Tests for publication bias showed the absence of publication bias (association between mCI and risk of all-cause death in HD patients: Egger’s test, p = .471, Begg’s test, p = 1; association between mCI and OS in HD patients: Egger’s test, p = .598, Begg’s test, p = 1), as shown in .
Figure 8. Forest plot for the association between mCI (21 mg/kg day) and risk of all-cause death in HD patients.
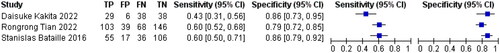
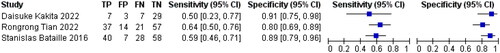
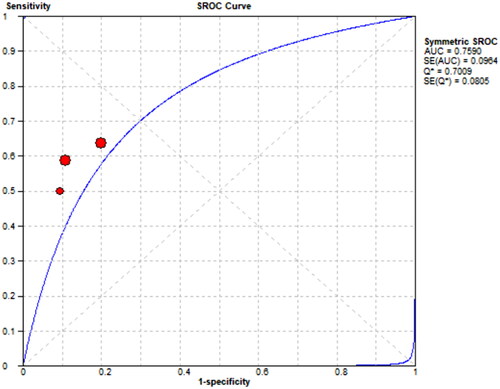
3.7. Sensitivity and specificity of mCI in predicting sarcopenia in HD patients
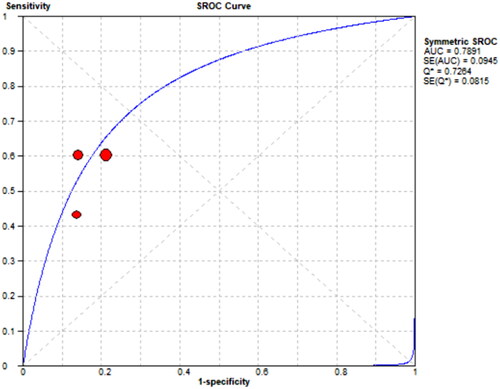
Three references [Citation8–10] respectively described the accuracy of mCI used to diagnose sarcopenia in HD patients. The results showed a sensitivity of 0.57 (95% CI: 0.51–0.62), specificity of 0.82 (95% CI: 0.78–0.86), and diagnostic odds ratio (DOR) of 6.43 (95% CI: 4.51–9.19; SROC curve AUC = 0.7891) for mCI in predicting sarcopenia in male HD patients, as well as a sensitivity of 0.60 (95% CI: 0.51–0.68), specificity of 0.86 (95% CI: 0.79–0.91), and DOR of 8.97 (95% CI: 5.12–15.74; SROC curve AUC = 0.759) for mCI in predicting sarcopenia in female HD patients, as shown in .
4. Discussion
4.1. Nutritional status in HD patients
For half a century, HD has remained the mainstay of life-sustaining treatment (LST) for ESRD patients, but it is not a complete substitute for renal function. With long-term treatment with HD, such patients will have to face a serious problem with a prevalence of 28–54% – malnutrition [Citation26]. Low-quality nutritional status may cause metabolic acidosis, altered intestinal microbial flora, chronic inflammation, accelerated degradation of muscle proteins, hormonal imbalance, and other alterations [Citation27]. This not only impairs muscle mass but also increases the risk of infection and hospitalization in HD patients, thereby causing poor prognosis such as a decreased survival rate, increased risk of death, and occurrence of sarcopenia [Citation28,Citation29]. Currently, nutritional status in HD patients is often assessed clinically by parameters such as BMI, nPCR, and serum albumin [Citation30]. In particular, the decreased level of serum albumin has been generally believed to be strongly associated with poor prognosis and higher risk of death in HD patients [Citation31]. Our results showed that mCI was positively associated with BMI, nPCR, and serum albumin in HD patients. Therefore, it can be used for assessment of nutritional status in HD patients.
4.2. All-cause death and survival in HD patients
Currently, HD has improved dramatically, both technically and in terms of care management, but HD patients still face a high risk of death. In Poland only, HD patients had a mortality of up to 15–20%, which was 4–7 times that in the general population [Citation32]. In contrast, the mortality was up to 25% within the first year of treatment with HD [Citation33]. It is particularly significant in the first 90 days, with a staggeringly high percentage of 32–44% [Citation34,Citation35]. Meanwhile, the age-standardized incidence of ESRD treated with HD has increased by 43% with the global aging trend [Citation36]. Statistics showed that patients older than 65 years of age accounted for 67.7% of dialysis patients in Portugal, which has the highest prevalence and incidence of ESRD in Europe [Citation37]. Compared with young HD patients, older HD patients have a higher burden involving comorbidities, malnutrition and the efficiency of fistulae, thereby posing a higher risk of death and lower survival rate to older HD patients [Citation1]. Therefore, there is an urgent need for an accurate parameter for predicting the probability of an outcome and for understanding how patients’ condition changes over time, so as to intervene in and improve the outcome in HD patients.
mCI is a parameter derived by Canaud et al. using the simplified CI formula. In a study by Huang et al., mCI was first proposed as a good parameter for predicting the survival rate in HD patients, and the level of mCI was found to be associated with the risk of death in HD patients [Citation13]. Similar conclusions were also drawn from several subsequent cohort studies in Japan [Citation19,Citation20,Citation23]. We pooled all currently available studies for meta-analysis. The results showed that mCI predicted all-cause death in HD patients. Therefore, it can be a potential predictor of survival in HD patients. In short, a higher level of mCI in HD patients predicts longer survival in them.
4.3. Sarcopenia in HD patients
Sarcopenia is a condition in which there is a progressive loss of skeletal muscle mass and function with age [Citation38]. In HD patients, PWE is prevalent due to chronic metabolic dysregulation, oxidative stress, chronic inflammation, and malnutrition. Sarcopenia, in turn, is one of main manifestations of PWE and thus tends to occur at a higher rate in the HD population. According to statistics, the prevalence of sarcopenia in HD patients has reached 28.5% [Citation39].
As a result of sarcopenia, HD patients often manifest a significant decrease in muscle strength and physical function, which greatly increases the risk of falls and fractures in them [Citation40]. At the same time, patients will consciously reduce their physical activities due to fear brought about by falls, which in turn leads to further development of sarcopenia and ultimately a vicious circle [Citation41]. In addition, there is an increase in risk for cardiovascular events and death as sarcopenia affects the quality of life and physical function in patients [Citation42]. There is still no consensus on the diagnosis of sarcopenia. There are independent expert consensuses in various regions, e.g., those proposed by the Asian Working Group for Sarcopenia (AWGS), Foundation for the National Institutes of Health (FNIH), and European Working Group on Sarcopenia in Older People (EWGSOP). In Asia, ‘possible sarcopenia’ has been developed as an additional diagnostic criterion [Citation43]. These cumbersome diagnostic criteria not only require specially trained healthcare professionals, but also take up a significant amount of medical time to complete a series of tests they require, and as a result, only less than 25% of physicians report having used some of such criteria to diagnose sarcopenia [Citation44]. In contrast, mCI only requires the collection of some routine data from HD patients, together with a simple formula to accomplish diagnosis of sarcopenia [Citation10]. We summarized three currently published references relevant to topic and performed a meta-analysis. The results showed that mCI had good diagnostic accuracy for sarcopenia in both male and female HD patients. Hence, it can be a potential tool for identifying sarcopenia in HD patients.
Currently, most patients treated with long-term HD choose to undergo it in secondary or local hospitals. However, there may be a discrepancy between them and large medical institutions with respect to the skills and expertise of healthcare professionals, the sophistication of medical equipment, and the level of management in the hospitals. This discrepancy may result in failure to detect change in HD patients’ condition in a timely manner, thereby delaying treatment and increasing the risk of poor prognosis. Our study proposed an mCI derived from substituting patients’ conventional clinical indicators into a simple formula. The mCI can effectively predict the prognosis of HD patients, including nutritional status, risk of death, survival rate, and sarcopenia. The mCI is simple to calculate and easy to apply in clinical practice, providing clinicians with a powerful tool to more accurately assess and manage HD patients. In addition, to effectively integrate the mCI into clinical practice, we recommended that ① relevant healthcare professionals be trained regarding the mCI as a means of ensuring that they understand the importance and use of the mCI; ② mCI be included as part of the routine care of HD patients, and the mCI be monitored and recorded on a regular basis; and ③ the treatment plan be adjusted and the therapeutic effects be monitored according to the mCI.
Limitations: ① This study analyzed multiple predefined outcome measures separately by including 18 studies. However, not all outcome measures are well supported by the literature, although its broad information provides a study with comprehensive data support. For outcome measures included in a limited amount of literature, the results of analysis of them are less reliable. ② In this study, most of the included studies were from Asia, and this geographic bias may pose a limitation on the generalizability of our findings. ③ Some of the meta-analysis results in a study were highly heterogeneous. This may pose a challenge to the consistency and reliability of the results. Although this study used a random-effects model and conducted sensitivity analysis, meta-regressions and subgroup analysis to find out the sources of heterogeneity, these efforts did not fully address the heterogeneity problem. ④ This study was unable to adequately discuss possible confounders, which may not have been adequately identified or controlled during analysis. Future studies should consider these potential confounders in greater depth and adjust for these key variables in analysis to enhance the precision of findings.
5. Conclusions
The results of this study suggest that mCI can be used as a prognostic marker for HD patients. However, the number of the included studies is still limited, and the generalizability and accuracy of their results still need to be further validated and confirmed by more high-quality studies. It is also hoped that future studies will not only focus on the prognostic role of the mCI in HD patients, but also track the interventional outcomes of the mCI in clinical application.
Author contributions
Tao Ye: conceptualization, formal analysis, methodology, writing – original draft preparation. Jingfang Du: conceptualization, formal analysis, investigation, writing – review and editing. Pian Li: conceptualization, formal analysis, writing – review and editing. Dan Rong: conceptualization, supervision, writing – review and editing. Wang Gu: conceptualization, resources, writing – review and editing. Yao Yao: conceptualization, methodology, writing – review and editing. Na Shen: conceptualization, formal analysis, methodology, funding acquisition, writing – review and editing. All authors read and approved the final manuscript.
Ethical approval
All analyses were based on previous published studies, thus no ethical approval is required.
Consent form
All analyses were based on previous published studies, thus no patient consent is required.
Supplemental Material
Download MS Word (441 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18(6):378–395. doi: 10.1038/s41581-022-00542-7.
- Inaba M, Mori K. Extension of healthy life span of dialysis patients in the era of a 100-year life. Nutrients. 2021;13(8):2693. doi: 10.3390/nu13082693.
- Song KK, Zhao DL, Wang YD, et al. Analysis of factors associated with death in maintenance hemodialysis patients: a multicenter study in China. Chin Med J. 2017;130(8):885–891. doi: 10.4103/0366-6999.204103.
- Hoshino J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients. Nutrients. 2021;13(5):1444. doi: 10.3390/nu13051444.
- Inaba M, Okuno S, Ohno Y. Importance of considering malnutrition and sarcopenia in order to improve the QOL of elderly hemodialysis patients in Japan in the era of 100-year life. Nutrients. 2021;13(7):2377. doi: 10.3390/nu13072377.
- Canaud B, Garred LJ, Argiles A, et al. Creatinine kinetic modelling: a simple and reliable tool for the assessment of protein nutritional status in haemodialysis patients. Nephrol Dial Transplant. 1995;10(8):1405–1410.
- Desmeules S, Levesque R, Jaussent I, et al. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004;19(5):1182–1189. doi: 10.1093/ndt/gfh016.
- Bataille S, Serveaux M, Carreno E, et al. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin Nutr. 2017;36(6):1654–1660.
- Kakita D, Matsuzawa R, Yamamoto S, et al. Simplified discriminant parameters for sarcopenia among patients undergoing haemodialysis. J Cachexia Sarcopenia Muscle. 2022;13(6):2898–2907. doi: 10.1002/jcsm.13078.
- Tian R, Chang L, Liu D, et al. Association of the modified creatinine index with muscle strength and mortality in patients undergoing hemodialysis. Ren Fail. 2022;44(1):1732–1742.
- Arase H, Yamada S, Yotsueda R, et al. Modified creatinine index and risk for cardiovascular events and all-cause mortality in patients undergoing hemodialysis: the Q-Cohort study. Atherosclerosis. 2018;275:115–123. doi: 10.1016/j.atherosclerosis.2018.06.001.
- Chandler S, MacLaughlin H, Wolley M. Creatinine index: a retrospective cohort study in an urban Australian dialysis context. Intern Med J. 2023;53(12):2291–2297. doi: 10.1111/imj.16054.
- Huang CY, Lee SY, Yang CW, et al. A simpler creatinine index can predict long-term survival in Chinese hemodialysis patients. PLOS One. 2016;11(10):e0165164. doi: 10.1371/journal.pone.0165164.
- Hwang W, Cho MS, Oh JE, et al. Comparison of creatinine index and geriatric nutritional risk index for nutritional evaluation of patients with hemodialysis. Hemodial Int. 2018;22(4):507–514.
- Tsai MT, Tseng WC, Ou SM, et al. Comparison of simplified creatinine index and systemic inflammatory markers for nutritional evaluation of hemodialysis patients. Nutrients. 2021;13(6):1870. doi: 10.3390/nu13061870.
- Canaud B, Ye X, Usvyat L, et al. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients—results from the International Monitoring Dialysis Outcome Initiative. Nephrol Dial Transplant. 2020;35(12):2161–2171. doi: 10.1093/ndt/gfaa098.
- Ebinç FA, Okyay GU, Değer SM, et al. Comparison of geriatric nutritional risk index and creatinine index in short-term mortality prediction in maintenance hemodialysis patients. Turk J Med Sci. 2022;52(3):641–648.
- Fukushi K, Okamoto T, Ozaki Y, et al. Butyrylcholinesterase level as an independent prognostic factor for overall survival in patients on maintenance hemodialysis: a single-center retrospective study. Clin Exp Nephrol. 2022;26(2):190–197. doi: 10.1007/s10157-021-02140-6.
- Fujioka H, Koike T, Imamura T, et al. Impact of geriatric nutritional risk index and modified creatinine index combination on mortality in hemodialysis patients. Nutrients. 2022;14(4):801. doi: 10.3390/nu14040801.
- Naito T, Doi T, Morii K, et al. Synergistic effects of the geriatric nutritional risk index and the modified creatinine index for predicting mortality in patients on hemodialysis. Nutrients. 2022;14(12):2398. doi: 10.3390/nu14122398.
- Suzuki Y, Matsuzawa R, Hoshi K, et al. Comparative analysis of simplified, objective nutrition-associated markers in patients undergoing hemodialysis. J Ren Nutr. 2022;32(4):458–468.
- Suzuki Y, Matsuzawa R, Kamiya K, et al. Trajectory of lean body mass assessed using the modified creatinine index and mortality in hemodialysis patients. Am J Kidney Dis. 2020;75(2):195–203.
- Yajima T, Yajima K. Ratio of extracellular water to intracellular water and simplified creatinine index as predictors of all-cause mortality for patients receiving hemodialysis. PLOS One. 2023;18(3):e0282864. doi: 10.1371/journal.pone.0282864.
- Yajima T, Yajima K, Arao M. Combined evaluation of geriatric nutritional risk index and modified creatinine index for predicting mortality in patients on hemodialysis. Nutrients. 2022;14(4):752. doi: 10.3390/nu14040752.
- Yamamoto S, Matsuzawa R, Hoshi K, et al. Modified creatinine index and clinical outcomes of hemodialysis patients: an indicator of sarcopenia? J Ren Nutr. 2021;31(4):370–379.
- Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28(6):380–392.
- Carrero JJ, Severs D, Aguilera D, et al. Intradialytic parenteral nutrition for patients on hemodialysis: when, how and to whom? Clin Kidney J. 2023;16(1):5–18. doi: 10.1093/ckj/sfac171.
- Kurajoh M, Mori K, Miyabe M, et al. Nutritional status association with sarcopenia in patients undergoing maintenance hemodialysis assessed by nutritional risk index. Front Nutr. 2022;9:896427. doi: 10.3389/fnut.2022.896427.
- Lodebo BT, Shah A, Kopple JD. Is it important to prevent and treat protein-energy wasting in chronic kidney disease and chronic dialysis patients? J Ren Nutr. 2018;28(6):369–379. doi: 10.1053/j.jrn.2018.04.002.
- Ipema KJ, Struijk S, van der Velden A, et al. Nutritional status in nocturnal hemodialysis patients – a systematic review with meta-analysis. PLOS One. 2016;11(6):e0157621. doi: 10.1371/journal.pone.0157621.
- Graterol Torres F, Molina M, Soler-Majoral J, et al. Evolving concepts on inflammatory biomarkers and malnutrition in chronic kidney disease. Nutrients. 2022;14(20):4297. doi: 10.3390/nu14204297.
- Jeznach-Steinhagen A, Boniecka I, Rymarz A, et al. Higher muscle mass and higher serum prealbumin levels are associated with better survival in hemodialysis patients during a five-year observation period. Nutrients. 2023;15(5):1237. doi: 10.3390/nu15051237.
- Himmelfarb J, Vanholder R, Mehrotra R, et al. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573–585. doi: 10.1038/s41581-020-0315-4.
- Yazawa M, Kido R, Ohira S, et al. Early mortality was highly and strongly associated with functional status in incident Japanese hemodialysis patients: a cohort study of the Large National Dialysis Registry. PLOS One. 2016;11(6):e0156951. doi: 10.1371/journal.pone.0156951.
- McQuillan R, Trpeski L, Fenton S, et al. Modifiable risk factors for early mortality on hemodialysis. Int J Nephrol. 2012;2012:435736. doi: 10.1155/2012/435736.
- Yip W, Ng SHX, Kaur P, et al. Risk factors for short-term all-cause mortality in patients with end stage renal disease: a scoping review. BMC Nephrol. 2024;25(1):71. doi: 10.1186/s12882-024-03503-3.
- Santos J, Fonseca I, Malheiro J, et al. End-stage renal disease versus death in a Portuguese cohort of elderly patients: an approach using competing event analysis. J Investig Med. 2017;65(7):1041–1048. doi: 10.1136/jim-2017-000480.
- Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533.
- Shu X, Lin T, Wang H, et al. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):145–158. doi: 10.1002/jcsm.12890.
- Veronese N, Smith L, Barbagallo M, et al. Sarcopenia and fall-related injury among older adults in five low- and middle-income countries. Exp Gerontol. 2021;147:111262. doi: 10.1016/j.exger.2021.111262.
- Shirai N, Inoue T, Ogawa M, et al. Relationship between nutrition-related problems and falls in hemodialysis patients: a narrative review. Nutrients. 2022;14(15):3225. doi: 10.3390/nu14153225.
- Kim JK, Kim SG, Oh JE, et al. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J Intern Med. 2019;34(3):599–607. doi: 10.3904/kjim.2017.083.
- Xie D, Zhu Q, Lu J, et al. Development and validation of a diagnostic nomogram for sarcopenia in Chinese hemodialysis patients. Nephrol Dial Transplant. 2023;38(4):1017–1026. doi: 10.1093/ndt/gfac260.
- Guralnik JM, Cawthon PM, Bhasin S, et al. Limited physician knowledge of sarcopenia: a survey. J Am Geriatr Soc. 2023;71(5):1595–1602. doi: 10.1111/jgs.18227.