Abstract
Background
This study aims to establish a simplified and effective animal model of catheter malfunction caused by omental wrapped using negative pressure suction.
Method
The peritoneal dialysis catheter outlet was linked to a negative-pressure (0-75mmHg) suction pump to intensify the negative pressure. Different negative pressures were tested for model construction in vitro. In vivo, a model of peritoneal catheter malfunction caused by omental wrapped was constructed in five beagles after catheter placement. Catheter drainage conditions and related complications were monitored before and after the model establishment.
Results
In the vitro experiment, the overall success rate of constructed models was 90% (36/40). The total malfunction rate was higher in 62.5 mmHg (10/10) and 75 mmHg (10/10) than in 12.5 mmHg (8/10) and 37.5 mmHg (8/10). The outflow velocity of dialysate at 62.5 mmHg was significantly lower than that at 12.5 mmHg and 37.5 mmHg, without a statistically significant difference compared to 75 mmHg. In the in vivo experiment, catheter outflow velocity increased, and residual fluid volume decreased after omental wrapped (99.6 ± 6.7 ml/min vs. 32.6 ± 4.6 ml/min at initial five minutes, p < 0.0001; 69.2 ± 16.3 ml vs. 581.0 ± 109.4 ml, p < 0.001). And the outflow velocity was finally below 2 ml/min. No severe related complications (such as infection, organ damage, or bleeding) were observed through laparoscopic examination and dialysate tests seven days post-operation.
Conclusion
Utilizing negative pressure suction to increase negative pressure around catheter tip is a simple, safe, and effective method for establishing an animal model of omental wrapped leading to catheter malfunction.
Introduction
Peritoneal dialysis (PD) serves as a critical treatment strategy for patients suffering from end-stage renal disease. However, PD is often compromised by catheter malfunction (CM), predominantly triggered by omental wrap and catheter obstruction, leading to technical failure in PD [Citation1–2]. Previous studies have reported that omental wrap is responsible for 3.7% to 13% of CM [Citation3–5]. Furthermore, a high incidence of mechanical omental wrapped of the peritoneal dialysis catheter (PDC) has been observed in patients undergoing laparoscopy for CM, with rates spanning between 57.1% to 88.9% [Citation6–7]. This clinical complication often requires laparoscopy or open surgical technique to recover the function of PDC, as conservative treatments yield limited success. Given these circumstances, the establishment of an appropriate animal model for omental wrapped-caused catheter malfunction (OWCM) is crucial for propelling fundamental research. However, few studies have addressed animal models of PDC wrapped by omentum. Jack Moncrief et al. [Citation8] detailed an animal model developed by altering the position of PDC. This procedure appears labor-intensive, time-consuming, and lacks definitive confirmation of catheter wrapped by the omentum in the abdominal cavity. In response to these challenges, we propose a novel animal model for PD omental wrapping employing a negative pressure suction technique, which is closely similar to the actual occurrence of omental wrapped. This model provides a reliable foundation for conducting basic research on the management of mechanical omental obstruction of the PDC.
Materials and methods
Experimental animals
This study followed the guiding principles of the Chinese Animal Protection Committee, complied with the ARRIVE guidelines (https://arriveguidelines.org), and was approved by the Ethics Committee of 900th Hospital of Joint Logistics Support Force (IRB approval number 2022-014). For in vivo experiments, five healthy, clean-grade adult male Beagles, weighing between 10 kg and 15 kg, were selected. The animals were housed in cages larger than 0.74 m2, provided with clean feed and water, and maintained at an indoor temperature of 23 ± 2 °C and 50% relative humidity under a 12-h light-dark cycle. The animals were given a fast of 12 h before the experiment, injected intramuscularly consisting of a mixture of xylazine (6 mg/kg) intramuscularly 15 min before surgery, and maintained with intravenous Zoletil 50 (6 mg/kg) for anesthetic. All of the animals were used to construct OWCM models after PDC placement. Collecting relatively intact omentum from two healthy, clean-grade adult male beagles in vitro experiments.
Materials and equipment
A suction pump with the capacity to generate a maximum negative pressure of 75 mmHg (Fujian Zhuge Liang Electronics Co., Ltd), a vacuum gauge (Suzhou Leipai Electric Science and Technology Co., Ltd), an animal laparoscopic system (Chengdu Xinxing Endoscope Science and Technology Co., Ltd), straight double-cuff Tenckhoff catheters with a length of 30 cm (product number 8888423111, Covidien), gastroscope biopsy forceps (product number 20182021546, Micro-Tech (Nanjing) Co., Ltd), a five-liter Bucket Bag (Yiwu Xinghua Outdoor Products Co., Ltd), and a 1.5% dextrose peritoneal dialysis solution (BAXTER, USA) were utilized in the study.
In vitro testing of negative pressures
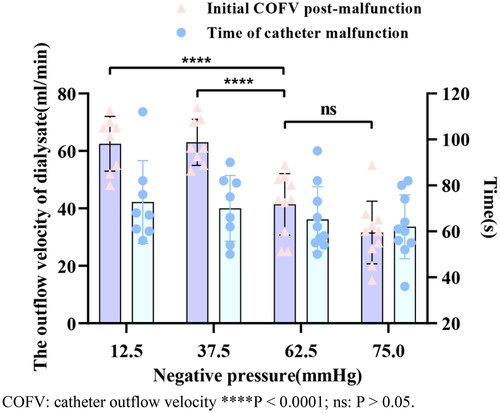
We divided into four groups (12.5 mmHg, 37.5 mmHg, 62.5 mmHg, and 75 mmHg) with 10 cases per group to assess the model construction under different negative pressures.
A successful OWCM model was characterized that the omentum was visually examined for tip blockage, tip encapsulation or catheter encapsulation, along with discontinuous, droplet-like or halted dialysate outflow, and drainage amount of less than 450 mL within five minutes, and residual dialysate volume of more than 100 mL. Compliance with the above indicates successful model.
The PDC was connected to a negative pressure suction device with the flow velocity regulator was adjusted to the target negative pressures (12.5 mmHg, 37.5 mmHg, 62.5 mmHg, and 75 mmHg). A total of four-liter of 1.5% peritoneal dialysate was filled into five-liter bag containing omentum, and the PDC was positioned within the omentum. Left and right oscillation of the bag caused the floating omentum to move. See Supplementary Figure 1 for details.
The following data were collected: The time of CM, which is the duration from the initiation of negative pressure suction to the point when the dialysate outflow became discontinuous. The average catheter outflow velocity (COFV) of dialysate post-catheter malfunction is identified as the mean COFV, as measured over a five-minute period, following the transition of the dialysate outflow to a discontinuous pattern. The obstruction conditions around the catheter were carefully observed.
In vivo establishment of omental wrapped-caused catheter malfunction model
Five beagles were utilized as the subjects of this study. All beagles underwent open surgical placement of catheters to establish a PD model. The level of negative pressure used were selected from those with a high success rate in in vitro modeling.
The open surgery method was used to insert PDC and laparoscopic operation were carried out in accordance with standard operating procedure. With the animal in a supine position, a surgical incision was made five to six centimeters below the xiphoid process and one to two centimeters to the right of the abdominal midline. The straight double-cuff catheter was then inserted. The COFV and the volume of residual dialysate were recorded after infusion of 1000 mL dialysate and subsequent complete drainage following catheter placement.
Following this, 1000 mL of 1.5% dextrose peritoneal dialysate was infused into the abdominal cavity and a negative pressure of 62.5 mmHg was established by connecting the system to a negative pressure suction device. We gently pressed the lateral abdomen, and recorded the average COFV for five minutes when the dialysate outflow became discontinuous. Once the COFV dropped below two ml/min, the negative pressure suction was sustained for an additional five minutes before turned off. Thereafter, the volume of residual dialysate and COFV were recorded. The obstruction conditions around the catheter were carefully observed by laparoscopy. If these criteria are not met, the process is repeated with another cycle of negative pressure suction.
A successful model was identified if it satisfied the previously defined criteria. If these criteria are not met, the process is repeated with another instance of negative pressure suction. The construction was considered failed if the CM was caused by tissues other than the omentum or if severe intraoperative complications occurred.
Following the successful modeling, we created a subcutaneous tunnel with the tunnel exit located in the lower left abdomen to embed the external limb of the PDC. The PDC was then placed subcutaneously. The skin at the catheter exits and each layer of tissue was sutured in layers. Subsequently Penicillin sodium was injected into the abdominal cavity.
Data collection as follow:
Mean COFV post-implantation, measured during the initial five minutes post-implantation.
Volume of residual dialysate, calculated by subtracting the volume of dialysate outflow from 1000 ml when the outflow velocity fell below two ml/min over a five-minute interval.
Time of catheter malfunction, determined as the duration from the start of negative pressure suction until the dialysate outflow became discontinuous.
Initial mean COFV post-malfunction, recorded over a five-minute period after dialysate outflow changes to a discontinuous state.
Final mean COFV post-malfunction, measured over a five-minute period upon cessation of the negative pressure.
Laparoscopic examination findings, detailing any potential damage to the intraperitoneal organs and the tissue surrounding the catheter.
Dialysate analyses, including the dialysate white blood cell (WBC) count, dialysate red blood cell (RBC) count. The dialysate was collected for five minutes when the COFV dropped below two ml/min, and another five minutes after the negative pressure turned off.
Postoperative evaluation of catheter functionality
On the third and seventh postoperative days, the catheter opening of the subcutaneous tunnel was extracted and a 50 mL volume of heparin sodium saline solution was infused and subsequently drained. on the 7th day post-surgery, the position of the catheter tip was monitored by laparoscopy, followed by the removal of the catheter.
The data collected included parameters such as COFV, dialysate WBC count, dialysate RBC count.
Statistical analyses
SPSS 16.0 statistical software was used for data analysis and processing, and Prism (GraphPad software, CA, USA) was used for data graphing. Measurement data following a normal distribution were expressed as mean ± SD, and a paired samples T-test was utilized for comparison between two groups in a paired design. A one-way ANOVA was used for comparing multiple groups. and Post hoc comparisons were performed using LSD test to compare the differences among groups. Count data were represented by the number of cases (%), with Fisher’s exact test utilized for group comparisons. p < 0.05 indicated that the difference was statistically significant.
Results
Comparing negative pressure in in vitro modeling
Our study had a higher success rate in inducing OWCM, with a success rate of 90% (36/40). and reaching a success rate of 100% at negative pressure of 62.5 mmHg and 75 mmHg.
The post-malfunction COFV of the 62.5 mmHg group was lower than that of the 12.5 mmHg group and the 37.5 mmHg group (p < 0.0001), and the 75 mmHg group was lower than that of the 12.5 mmHg group and the 37.5 mmHg group (p < 0.0001). But the post-malfunction COFV in the 62.5 mmHg group compared to that in the 75 mmHg group was not statistically different The comparisons of the time of catheter malfunction in the 12.5 mmHg, 37.5 mmHg, 62.5 mmHg, and 75 mmHg groups were not statistically significant (p > 0.05). see .
Catheter malfuntion induced by omental wrapped after in vivo modeling
Based on the results of the in vitro experiments, we selected the negative pressure of 62.5 mmHg for the in vivo experiment to establish the animal model.
The results indicated that the incidence of catheter malfunctions was 100% (5/5) in the five Beagles after one instance of suction. The time of catheter malfunction was 115.1 ± 38.3 s and the initial post-malfunction COFV was 32.6 ± 4.6 mL/min. The initial mean COFV post-malfunction was slower compared to the mean COFV post-implantation (p < 0.0001), and the final COFV post-malfunction fell below five ml/min. Furthermore, the residual dialysate volume post-malfunction was significantly larger than post-implantation (p < 0.001). These findings are presented in .
Table 1. Comparison of catheter function after catheter implantation and omental-wrapped in Beagles.
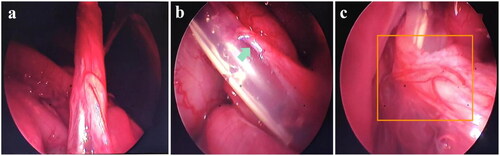
Laparoscopic verification of the modeling effect consistently revealed omental encapsulation or obstruction of the PDC by the side holes () and catheter tip () in all models. The positioning of the catheter tip was confirmed to be satisfactory.
Ineffectiveness of conservative methods in improving model catheter function
Despite the application of conservative treatments, catheter function remained impaired. Forceful saline flushes of the catheter resulted in a drip-shaped dialysate outflow in all five beagles. In two instances, treatment with gastroscopic biopsy forceps resulted in an intermittent linear dialysate outflow, while in three cases, the outflow continued to be drip-shaped ().
Table 2. Assessment of catheter malfunction in beagle models.
No obvious injury to abdominal organs
Laparoscopy revealed no instances of the intestinal tube entering the catheter or wrapping around it, no organ injury or severe bleeding complications were found ().
Table 3. Catheter functionality and complications assessment 7 days post-modelling.
Postoperative complications
On the 3rd and 7th days post-surgery, all five Beagles demonstrated continued issues with the smooth flow of dialysate. Nonetheless, all five Beagles had good activity levels and normal diets. All indicators, including dialysate RBC count, dialysate WBC count, were within the normal range, presenting no symptoms of peritonitis, tunnel exit-site infection, incision infection, or bleeding. In four Beagles, the catheter positions were confirmed to be in place under laparoscopy, while in one Beagle, catheter displacement was observed on the 7th day post-surgery ().
Discussion
CM due to omental wrapped is a significant cause of technical failure in PD patients. Current management strategies for this complication are challenging. While laparoscopic techniques and open surgery are effective in addressing omental wrapping complications, they pose invasiveness and entail anesthetic risks. Notably, the previous reports indicated omental wrapping is one of the major factors for the failure of catheter recanalization by conservative methods which treat it via catheter lumen [Citation9–10]. Consequently, establishing a model for OWCM becomes paramount in the quest for an effective and safe treatment. Additionally, with recent advancements in interventional technology, Paul Li et al. employed two guidewires which showed promise in restoring catheter malposition with the high success rate while circumventing the risks associated with open surgery and laparoscopic techniques [Citation11]. The safety of treating omental wrapping can be enhanced through noninvasive treatment methods. Therefore, modeling OWCM is crucial in finding an efficient method via lumen to successfully address omental wrapping.
However, there is a lack of research focused on developing a reliable animal model for studying CM due to omental wrapped. Existing studies have reported low success rates and limited evidence for the effectiveness of such models. In our study, we successfully established an animal model of OWCM using negative pressure suction. The intra-abdominal abnormalities observed during laparoscopy after modeling closely resembled clinical findings, providing a solid foundation for further fundamental research on the prevention and treatment of omental wrapped in PD.
Numerous studies have highlighted the suitability of Beagles for constructing uremic PD animal models [Citation12–13]. Beagles possess ample omental tissue and a spacious abdominal cavity, making them ideal for simulating catheter-related mechanical complications such as omental wrapping, catheter migration, and dialysate leakage. Earlier investigations have indicated that Beagles serve as appropriate experimental subjects for developing PDC malfunction due to omental wrapping [Citation8]. Considering the size of a Beagle’s abdominal cavity, a pediatric PDC was used in this study. The primary characteristics of omental wrapped of PDC are that the side holes and tip of PDC are encapsulated and obstructed by omentum [Citation14–16]. The negative pressure may attract free omental tissue gathering around the PDC and even block catheter during the dialysis fluid discharge process [Citation2,Citation17,Citation18].
Thus, we att empted to establish a model of OWCM by negative pressure suction. Meanwhile, we found that negative pressure had a significant effect on omental wrapped leading to catheter drainage function in vitro experiments. Our result showed that OWCM could easily be induced with a negative pressure over 62.5 mmHg with a success rate of 100%. Thus, we opted for a negative pressure setting of 62.5 mmHg for the experiments involving Beagles.
In subsequent animal experiments, we take routine open operation to insert straight PDC and used a negative pressure of 62.5 mmHg to build OWCM model. Firstly, we conducted a comparison of the COFV and the volume of residual dialysate before and after modeling. Following 115.1 ± 38.3 s of negative pressure suction, a significant decrease in the COFV was observed. The results indicated that the initial post-malfunction COFV was 32.6 ± 4.6 mL/min, which further declined to below 5 mL/min finally. The outflow of dialysate was observed in the form of drips or intermittent threads. Additionally, there was a significant increase in the volume of residual dialysate, meeting the criteria for catheter dysfunction as mentioned previously. Through laparoscopy, we observed that omental tissue adhered to, wrapped the tube wall, some omental tissue, sucked into the side hole and tip that lead to block of the catheter eventually. All five dogs were successfully modeled, resulting in a 100% success rate in inducing CM. Meanwhile, catheter position was assessed on the day of surgery, the 3rd postoperative day, and the 7th postoperative day. One Beagle was combined with catheter displacement on postoperative day 7.
At days 0, 3, and 7, we evaluated the safety of the modeling procedure by examining dialysate indicators, including WBC and RBC, which remained within normal limits. Simultaneously, laparoscopy was conducted throughout the entire process, revealing no occurrences of bleeding, injury, or complications involving the intestine or other solid organs. The results indicated no signs of inflammation or bleeding. Based on these findings, it can be concluded that the modeling procedure is safe in the short term.
In this study, we successfully established a model of OWCM using negative-pressure suction method which is easy to perform, time-efficient, and safety. However, several limitations of our study deserve mention. In our study, the negative pressure we used was not continuous. The relationship between the negative pressure and omental wrapping has not been clarified. More negative pressure groupings and in vivo experiments still need to verify this. The validation of results was confined to a seventh-day postoperative period and thus, longer-term observations are warranted. Moreover, we did not investigate CM specifically related to coiled catheters, an avenue that future studies may wish to explore. Additionally, in clinical practice, omental wrapped of catheters often co-occurs with catheter migration. Hence, future research should consider developing a combined model that simulates both catheter migration and omental wrapped. Lastly, while our method successfully induced omental wrapping under negative pressure without observed complications such as hemorrhage or organ damage, the anatomical differences between animals and humans may lead to varied potential complications.
In conclusion, the present study offers a straightforward, time-efficient, and safe method for establishing an animal model of OWCM. This model provides a firm foundation for basic research and could serve as a platform for developing clinical treatments for OWCM in PD.
Ethical approval
The animal experiment protocols were approved by the Ethics Committee of 900th Hospital of Joint Logistics Support Force (IRB approval number 2022-014).
Authors’ contributions
YG, XW, WW contributed to the study conception and design. Material preparation and animal experiment were performed by XW, LY, JC, QS, MQ, QW, JY. Data collection, and analysis were performed by XW, LY, JC, QS. The first draft of the manuscript was written by XW. WW, LY, JC performed the statistical analysis. All authors read and approved the final manuscript. All authors searched the literature.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgements
The authors are grateful to Dr. Laien Xue for technical support in animal experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Li J-R, Cheng C-h, Chiu K-y, et al. Minilaparotomy salvage of malfunctioning catheters in peritoneal dialysis. Perit Dial Int. 2013;33(1):1–7. doi: 10.3747/pdi.2011.00237.
- Khan SF. Updates on infectious and other complications in peritoneal dialysis: core curriculum 2023. Am J Kidney Dis. 2023;82(4):481–490. doi: 10.1053/j.ajkd.2023.03.011.
- Martínez-Mier G, Luna-Castillo M, Ortiz-Enríquez JJ, et al. Factors associated with early peritoneal dialysis catheter replacement in Veracruz, Mexico. Nefrologia. 2012;32(3):353–358. doi: 10.3265/Nefrologia.pre2012.Jan.11295.
- Li J-R, Chen C-H, Cheng C-L, et al. Five-year experience of peritoneal dialysis catheter placement. J Chin Med Assoc. 2012;75(7):309–313. doi: 10.1016/j.jcma.2012.06.001.
- Lemoine C, Keswani M, Superina R. Factors associated with early peritoneal dialysis catheter malfunction. J Pediatr Surg. 2019;54(5):1069–1075. doi: 10.1016/j.jpedsurg.2019.01.042.
- Yang PJ, Lee CY, Yeh CC, et al. Mini-laparotomy implantation of peritoneal dialysis catheters: outcome and rescue. Perit Dial Int. 2010;30(5):513–518. doi: 10.3747/pdi.2009.00033.
- Santarelli S, Zeiler M, Marinelli R, et al. Videolaparoscopy as rescue therapy and placement of peritoneal dialysis catheters: a thirty-two case single centre experience. Nephrology Dialysis Transplantation. 2006;21(5):1348–1354. doi: 10.1093/ndt/gfk041.
- Moncrief J, Popovich R, Simmons E, et al. Catheter obstruction with omental wrap stimulated by dialysate exposure. Perit Dial Int. 1993;13 Suppl 2:S127–S129.
- Kim HJ, Lee TW, Ihm CG, et al. Use of fluoroscopy-guided wire manipulation and/or laparoscopic surgery in the repair of malfunctioning peritoneal dialysis catheters. Am J Nephrol. 2002;22(5–6):532–538. doi: 10.1159/000065292.
- Ohira N, Yorioka N, Ito T, et al. Correction of capd catheter displacement using gastric biopsy forceps: the push-pull method. Int J Artif Organs. 1999;22(4):202–204. doi: 10.1177/039139889902200404.
- Li P, Choo D, Deved V, et al. Salvage of malfunctioning peritoneal dialysis catheters: an algorithm for recanalization and repositioning. J Vasc Interv Radiol. 2021;32(6):902–906. doi: 10.1016/j.jvir.2021.03.522.
- Cooper RL, Labato MA. Peritoneal dialysis in veterinary medicine. Vet Clin North Am Small Anim Pract. 2011;41(1):91–113. doi: 10.1016/j.cvsm.2010.10.002.
- Dupré G, Čoudek K. Laparoscopic‐assisted placement of a peritoneal dialysis catheter with partial omentectomy and omentopexy in dogs: an experimental study. Vet Surg. 2013;42(5):579–585. doi: 10.1111/j.1532-950X.2013.01097.x.
- Ogura S, Bristol G, Burchman M, et al. Omental wrap: radiographic diagnosis confirmed surgically – Report of two cases in Grenada. Perit Dial Int. 2021;41(6):581–583. doi: 10.1177/0896860820982221.
- Zeiler M, Federico A, Lentini P, et al. Diagnostic capability of ultrasound in peritoneal catheter malfunction compared to videolaparoscopy. Perit Dial Int. 2021;41(6):564–568. doi: 10.1177/0896860821993946.
- Jheng J-S, Chang C-T, Huang C-C. Omental wrapping of a peritoneal dialysis catheter. Kidney Int. 2012;82(7):827. doi: 10.1038/ki.2012.126.
- Shao XJ, Zhang LY. Peritoneal dialysis catheter malfunction caused by wrapping of the catheter by the sigmoid mesocolon: a case report. Ren Fail. 2021;43(1):313–314. doi: 10.1080/0886022X.2021.1879854.
- Hu J, Liu Z, Liu J, et al. Reducing the occurrence rate of catheter dysfunction in peritoneal dialysis: a single-center experience about CQI. Ren Fail. 2018;40(1):628–633. doi: 10.1080/0886022X.2018.1515084.