Abstract
IgA nephropathy (IgAN) is one of the most common primary glomerulonephritis, and serum Helicobacter pylori (H. pylori) antibody levels are increased in patients with IgA N, but the role of H. pylori infection in the pathogenesis of IgAN is unclear. In this study, we investigated whether there is a causal relationship and reverse causality between IgAN and H. pylori infection by using a bidirectional two-sample Mendelian randomization (MR) analysis. This study was estimated using inverse variance weighted (IVW), MR-Egger and weighted median methods, with the IVW method having the strongest statistical efficacy. Seven common serum H. pylori antibodies were selected as exposure factors for positive MR analysis. The results showed that there was no evidence of a causal relationship between H. pylori infection and IgAN. Reverse MR analysis showed that there was also no evidence that the occurrence of IgAN leads to an increased risk of H. pylori infection.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that colonizes the mucus layer of the human stomach and is usually transmitted during childhood. If left untreated, H. pylori infections can persist throughout life [Citation1]. H. pylori infections are not only involved in the pathogenesis of gastritis and peptic ulcers. Still, they are also inextricably linked to renal diseases [Citation2–4].
IgA nephropathy(IgAN) is one of the most common forms of glomerulonephritis worldwide [Citation5], affecting mainly young adults in their 20s and 40s [Citation6]. Patients present with microscopic hematuria, proteinuria, nephrotic syndrome, or acute progressive glomerulonephritis[Citation7]. Between 30% and 45% of patients may progress to end-stage renal disease within 20 to 25 years of diagnosis of IgAN [Citation8–10].
Evidence from epidemiology suggests that H. pylori infection is associated with an increased risk of developing kidney disease, with a cross-sectional study showing H. pylori positivity in up to 50% of 293 patients undergoing kidney transplantation [Citation2]. A study from Pan W suggested that H. pylori infection may be a risk factor for kidney injury, which was positively correlated with altered albumin/creatinine ratio, which decreased after H. pylori eradication [Citation3], and a prospective study found that IgA was detected in 38 out of 43 (88.4%) patients with tonsil H. pylori positivity, and it was hypothesized that H. pylori is one of the causative antigens of IgAN [Citation11]. However, the results of most observational studies are not entirely consistent, and a cross-sectional study did not find any significant difference in the prevalence of proteinuria between the H. pylori-infected and non-infected groups [Citation12]. In addition, multiple linear regression analysis showed that there was still no significant difference in eGFR and odds of proteinuria reduction between H. pylori-positive and negative subjects. Liu XZ et al. showed the experimental results that type I H. pylori-infected patients showed a tendency toward lower eGFR but did not reach statistical significance [Citation13].
Conventional studies are inevitably subject to a variety of confounding factors and reverse causality bias. Therefore, it remains unknown whether H. pylori infection contributes to the development of IgAN. Although several studies have shown a correlation between H. pylori and IgAN, it may be affected by confounding factors such as age, gender, hypertension, diabetes, smoking, hyperlipidemia, etc., and thus it is crucial to study the correlation between H. pylori infection and IgAN. With the exponential growth and wide availability of genetic data, MR as an epidemiologic study reduces confounding strengthens exposure-outcome associations, and avoids reverse causation. In this study, we analyzed MR with seven indicators associated with H. pylori infection (Anti-H. pylori IgG, H. pylori CagA antibody, H. pylori CAT antibody, H. pylori GroEL antibody, H. pylori OMP antibody, H. pylori UreA antibody, and H. pylori VacA antibody) as exposure factors and IgAN as outcome. To further confirm whether there was a reverse causality between H. pylori infection and IgAN, we also performed reverse MR analysis ().
Materials and methods
Study design and hypothesis
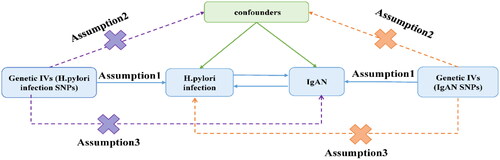
Mendelian randomization(MR), which uses single nucleotide polymorphisms(SNPs) as instrumental variables(IVs), is a method of causal inference based on genetic variation. Its basic principle is to use the effect of randomly assigned genotypes on phenotype in nature to infer the effect of genetic biological factors on disease. Since the genes are assigned following the principle of random assignment and the mutations precede the onset of the disease, MR can be regarded as a natural randomized controlled trial(RCT). Compared with conventional studies, the interference of reverse causality and confounding factors on the accuracy of results can be avoided. The present study is a bidirectional MR study, and MR must fulfill three major assumptions: 1. the assumption of correlation (the selected IVs are strongly correlated with exposure, with p < 1e-5 and F > 10); 2. the assumption of independence (there is no correlation between the IVs and the outcome); and 3. the assumption of exclusivity (the IVs cannot influence the outcome through confounders other than exposure) ().
Exposure GWAS data source
Suitable genetic variants were selected as IVs for H. pylori infection, and the genetic background of all cases and controls was of European ancestry, which reduces bias due to race-related confounders. A search of the IEU database (https://gwas.mrcieu.ac.uk/) for H. pylori-related phenotypes revealed that the seven phenotypes used in the analysis of exposure factors for this MR all came from the same study. A total of 8735 individual UK participants were enrolled in this study, with 55.9% female and 44.1% male participants, and the median age (interquartile range) at enrollment in the UKB was 58 (51–64) years, which analyzed seropositivity rates for 13 pathogen-related indicators. Anti-H. pylori IgG seropositivity included a total of 8,735 individuals containing 9,170,312 SNPs, H. pylori CagA antibody included 985 individuals containing 9,165,056 SNPs, H. pylori CAT antibody included 1,558 individuals containing 9,167,570 SNPs, H. pylori GroEL antibody included 2,716 individuals containing 9,172,299 SNPs, H. pylori OMP antibody incorporated 2640 individuals containing 9,167,440 SNPs, H. pylori UREA antibody incorporated 2251 individuals containing 9,170,248 SNPs, and H. pylori VacA antibody incorporated 1571 individuals containing 9,178,635 SNPs ().
Table 1. Details of the GWAS data in this study.
Outcome GWAS data sources
Genetic variants associated with IgAN were extracted from the GWAS study, which included 15,587 cases and 462,197 controls, totaling 24,182,646 SNPs, and all participants were of European ancestry. Instrumental SNPs were associated with IgAN at the genome-wide significance (p < 1e-5).
Selection of IVs
In this study, IVs were rigorously screened for SNPs. SNPs with genome-wide association (p < 1e-5) were extracted, and SNPs with weak IVs for exposure were excluded. in addition, the F statistic indicated the strength of association between the IVs and the exposure factors, and SNPs with F > 10 were selected. Then, chain-unbalanced SNPs were excluded (r^2 < 0.001, k > 10,000kb) to ensure that the SNPs were independent. Selected SNPs that are strongly correlated with exposure, independent of each other. Then remove the SNPs that are strongly correlated with the outcome from these selected SNPs. Next, these SNPs should be sequentially searched using PhenoScannerV2 and LDtrait to identify and remove confounding factors that affect the occurrence of IgAN, such as age, weight, hypertension, proteinuria, dental caries, periodontitis, tonsillitis, and other infections, including respiratory pathogens (e.g., Mycoplasma pneumoniae, herpes virus, influenza), gut microbiota. Remove SNPs associated with confounding factors prior to conducting MR analysis to minimize their interference with results. If there were no corresponding SNPs in the outcome, a proxy SNP could be found in the outcome based on the LDR^2 > 0.8. Finally, the exposure SNPs were synergized with the outcome SNPs to eliminate palindromic sequences and incompatible SNPs.
MR analysis
Forward MR analysis was performed using H. pylori-related IVs as the exposure and IgAN as the outcome to detect the causal relationship between H. pylori infection and IgAN at the gene level. Reverse MR analysis was performed using SNPs strongly associated with IgAN as IVs and seven phenotypes associated with H. pylori infection as outcomes to explore whether there was a causal relationship between IgAN and H. pylori infection at the gene level.
In the bidirectional MR analysis, the IVW method had the highest statistical efficacy, and IVW was used as the primary analysis method, with MR-Egger and weighted median methods as complementary methods for MR analysis.
Heterogeneity and horizontal pleiotropy of IVs may lead to bias in MR results, and the selection of appropriate statistical methods is of particular importance. Before analyzing the data, heterogeneity among IVs was assessed using the Cochranes’ Q test and the I2 test. The presence of heterogeneity is indicated by a p value > 0.05, while a p value < 0.05 suggests the absence or lesser degree of heterogeneity. The I2 statistic was calculated using the formula I2 = 100% × (Q –df)/Q, reflecting the proportion of the heterogeneity component in the total variance of the effect sizes as a complement to the sensitivity analysis. An I2 value > 50% indicates more pronounced heterogeneity. If no heterogeneity was detected, the analysis was conducted using either the IVW fixed-effects model or the IVW random-effects model. When heterogeneity was present and no horizontal pleiotropy was detected, the analysis was conducted using the IVW random-effects model. Horizontal pleiotropy was examined using the MR-Egger intercept test, with statistical significance determined by the p value of the intercept term. A p value > 0.05 indicates no horizontal pleiotropy, suggesting reliable results. When a p value < 0.05, indicating horizontal pleiotropy, the MR-PRESSO global test method was further applied to correct for it by eliminating outlier variables. Outlier-corrected and leave-one-out methods were employed to detect outliers. If outliers were identified, the outlier SNPs needed to be removed and the analysis repeated. Additionally, the Raw test from the MR-PRESSO analysis can serve as a complement to the primary IVW outcome. It is generally accepted that a causal relationship between exposure and outcome may exist if the MR analysis results are significant (p < 0.05), and the results are considered robust when horizontal pleiotropy is absent. We applied the MR-Steiger analysis to monitor the direction of potential causality between H. pylori infection and IgAN risk and used reverse MR analysis to further validate the absence of reverse causality between H. pylori infection and IgAN.
Statistical processing
All analyses were performed using the ‘Two Sample MR’ (version 0.5.11) package in R software (version 4.2.3). Results are presented as odds ratio (OR) and corresponding 95% confidence interval (CI), with a difference of p < 0.05 considered statistically significant. All analyses were based on publicly available data and did not require ethical approval.
Results
Selection of IVs
In the forward MR analysis with H. pylori infection as the exposure factor, p < 1e-5, F > 10 between the IVs and the exposure factor was used as the screening condition, and chain imbalance and palindromic alleles were removed. H. pylori-IgG screened 17 SNPs as instrumental variables and excluded those that might be associated with confounding factors: rs10488625, rs11139381, rs11201988, rs17232730. A total of 13 SNPs were retained. H. pylori CagA antibody screened 24 SNPs and excluded SNPs related to confounding factors: rs1571534, rs3998182, rs56264437, rs6530847, leaving 20 SNPs retained. H. pylori-CAT screened a total of 16 SNPs, excluding those related to confounding factors: rs2946532, rs6456714, with 14 SNPs remaining. H. pylori GroEL antibody screened 9 SNPs with no SNPs associated with confounders. H. pylori OMP antibody screened 16 SNPs, removing those related to confounding factors: rs3104361, and retaining 15 SNPs. H. pylori UreA antibody screened 24 SNPs, removing confounding-related SNPs: rs9273219, rs111488068, and retaining 22 SNPs. H. pylori VacA antibody screened 24 SNPs, removing SNPs. rs11844235, and retaining 23 SNPs. These phenotypes were analyzed by MR as exposure factor versus outcome IgAN, respectively. In the reverse MR analysis, with IgAN as the exposure factor and H. pylori-associated antibody as the outcome, the same method was used to identify the instrumental variables. H. pylori CagA antibody and H. pylori VacA antibody screened 32 SNPs each, and 5 SNPs that might be associated with confounding factors were deleted: rs10786766, rs2058367, rs2524075, rs4434650, rs9266216, with 27 SNPs retained as instrumental variables. The remaining H. pylori infection-associated serum antibodies were screened for 33 SNPs each, and 5 SNPs associated with confounding were similarly deleted: rs10786766, rs2058367, rs2524075, rs4434650, rs9266216, with 28 SNPs remaining for analysis by reverse MR.
Forward MR analysis
After the rigorous screening of IVs, the Cochran Q test results for both the IVW and MR-Egger methods indicated that some heterogeneity for H. pylori CAT antibody as an exposure factor. The Cochran Q test result of MR-Egger method was p = 0.009, with an I2 of 55.1%, and for the IVW method, it was p = 0.011, with an I2 of 52.5%. These results were analyzed using an IVW random-effects model. Horizontal pleiotropy was assessed using the MR-Egger intercept test and the MR-PRESSO global test; all p values were > 0.05, indicating no horizontal pleiotropy and thus the results are considered reliable. The MR-Egger regression results were further validated using outlier-corrected and leave-one-out methods, which showed no evidence of outliers. Additionally, the Raw test from the MR-PRESSO served as a complement to the primary IVW outcome, with a p value > 0.05 (), further validating the lack of evidence for a causal association between H. pylori infection and IgAN. Forward MR analysis of each of these seven H. pylori infection-associated phenotypes as exposure factors showed no evidence that any of the genetic variations.
Table 2. Results of the sensitivity analysis between H. pylori infection and IgAN estimated by the forward MR method.
The results of the IVW method showed the following odds ratios (ORs) and confidence intervals (CIs) for Anti-H. pylori IgG (OR = 0.834; 95% CI, 0.685 to 1.015; p = 0.070), H. pylori CagA antibody (OR = 0.999; 95% CI, 0.973 to 1.025; p = 0.933), H. pylori CAT antibody (OR = 1.002; 95% CI, 0.950 to 1.057; p = 0.951), H. pylori GroEL antibody (OR = 1.003; 95% CI, 0.929 to 1.084; p = 0.929), H. pylori OMP antibody (OR = 0.969; 95% CI, 0.923 to 1.018; p = 0.208), H. pylori UREA antibody (OR = 0.998; 95% CI, 0.962 to 1.034; p = 0.891), and H. pylori VacA antibody (OR = 1.027; 95% CI, 0.997 to 1.058; p = 0.081) (). There was no statistically significant association between these H. pylori antibodies and increased risk of IgAN; the funnel plots of the phenotypic IVW method for all 7 studies were largely symmetric and did not affect the estimated causal effect. Removal of individual SNPs by the leave-one-out method did not significantly alter the overall results, suggesting that the results were stable and reliable (Additional file1: Figures 1–S7). Steiger-MR analyses were used to test the robustness of the causal effect estimates. Steiger-MR found that SNPs explained more of the exposure than the outcome, indicating no reverse causality.
Table 3. Forward MR of serum H. pylori antibodies with IgAN.
Reverse MR analysis
Inverse MR analysis was performed with IgAN as an exposure factor and H. pylori as an outcome, and again negative results were obtained: there was no evidence of a causal relationship between IgAN and H. pylori infection. Cochran Q test results and I2 statistics indicated no significant heterogeneity (all p > 0.05) in MR analysis using IgAN as exposure and serum H. pylori antibodies as outcomes. Horizontal pleiotropy was assessed using the MR-Egger intercept test and the MR-PRESSO global test; the results showed no evidence of horizontal pleiotropy (all p > 0.05), confirming the reliability of the MR analysis conclusions. The MR-Egger regression results were further validated by using Outlier-corrected and leave-one-out methods. For H. pylori VacA antibody, an outlier (rs112048665) was detected; subsequent MR analysis was conducted after its removal. The remaining phenotypes showed no evidence of outliers (). The IVW results provided no evidence that the occurrence of IgAN leads to increased expression of H. pylori antibodies. Anti-H. pylori IgG (OR = 0.992; 95% CI 0.954, 1.042; p = 0.759), H. pylori CagA antibody (OR = 0.992; 95% CI0.745,1.320; p = 0.955, H. pylori CAT antibody (OR = 1.099; 95%CI0.882,1.369; p = 0.402) H. pylori GroEL antibody (OR = 1.024; 95%CI0.836,1.254; p = 0.820) H. pylori OMP antibody (OR = 0.993; 95%CI0.836-1.181; p = 0.940) H. pylori UREA antibody (OR = 1.111; 95%CI0.923-1.337; p = 0.267) H. pylori VacA antibody (OR = 0.984; 95%CI0.753,1.284; p = 0.903) (). The Raw test from the MR-PRESSO analysis, which complements the primary IVW result, had a p-value > 0.05, further validating the lack of evidence for a causal association between H. pylori infection and IgAN. Funnel plots for all IVW method analyses were largely symmetric and did not affect the estimated causal effect. The overall results remained stable and reliable after the removal of individual SNPs using the leave-one-out method, with no significant change in the outcomes (Additional file 1: Figure S8–S14).
Table 4. Results of the sensitivity analysis between H. pylori infection and IgAN estimated by the reverse MR method.
Table 5. Reverse MR of serum H. pylori antibodies with IgAN.
Discussion
To the best of our knowledge, this is the first MR study to focus on a causal relationship between H. pylori and IgAN. The results of our analysis showed no evidence to support a causal relationship between H. pylori infection and increased IgAN, which contradicts the results of some previous experiments. On this basis, we added a reverse MR analysis to explore whether H. pylori infection was associated with the occurrence of IgAN, but still obtained negative results.
IgG antibodies are a common test for detecting H. pylori infection, and in a study of H. pylori infection in adolescents the sensitivity and specificity of IgG antibody testing were found to be 91.2% and 97.4%, respectively [Citation14]. CagA is the coding product of the H. pylori CagA pathogenic island gene, which is present in most H. pylori-infected patients with IgAN [Citation15]. It promotes glomerular plasma cell proliferation and extracellular matrix secretion by inhibiting apoptotic signaling pathways, and can also lead to severe inflammatory responses in the host [Citation16]. VacA is a toxin protein secreted by H. pylori [Citation17], and it can cause morphological alterations, such as vacuolization, apoptosis, cytoskeletal rearrangement, and even cell death [Citation18,Citation19]. GroEL is a homolog of heat shock protein (HSP 60) in prokaryotes, which is an important pathogenesis-associated antigenic component, and its main function is to promote protein transport, complete the correct folding and restore the natural conformation. Previous studies have shown that more than 80% of the H. pylori-infected population produces H. pylori GroEL antibodies [Citation20,Citation21]. Serum H. pylori antibody test is a commonly used method for detecting H. pylori infection, but it is not the gold standard for H. pylori infection, which may lead to inconsistent results in detecting the association between the two by different methods. In this study, we used Anti-H. pylori IgG antibody, H. pylori CagA antibody, H. pylori CAT antibody, H. pylori GroEL antibody, H. pylori OMP antibody, H. pylori UREA antibody, H. pylori VacA antibody as exposure factors to maximize the application of GWAS data related to H. pylori infection, and a significant causal relationship between H. pylori infection and IgAN was still not detected, and more substantial evidence is needed to elucidate the role of H. pylori infection in the role of IgAN.
Previous studies have shown some association between H. pylori infection and IgAN, with patients with IgAN having higher serum H. pylori antibodies compared to controls without renal disease [Citation22], and a significantly higher prevalence of type I H. pylori infection in patients with IgAN compared to controls (44.4 vs. 28.3%, p < 0.040). Patients with type I H. pylori infection had higher levels of systolic blood pressure, 24-h proteinuria, and blood urea nitrogen compared to uninfected patients [Citation13]. The pathogenesis may be that H. pylori infection induces the expression of inflammatory cytokines, chemokines, growth factors, etc., which leads to an inflammatory microenvironment, damage to the endothelial structure of the renal vasculature, and an increase in the urinary albumin excretion rate. In addition, H. pylori infection induces the release of cytokines and vasoactive substances, such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6), interleukin 8 (IL-8), and heat shock proteins (HSPs), which elicit both local and systemic immune responses and exacerbate microvascular injury [Citation23]. Hypersensitive C-reactive protein (hs-CRP) is increased after H. pylori infection [Citation24], and high levels of CRP are considered to be one of the key risk factors for chronic kidney injury [Citation23,Citation25,Citation26]. TNF-α induces the expression of vascular endothelial cell adhesion molecules, leading to the proliferation of glomerular tunica albuginea cells [Citation27]. Therefore, H. pylori infection can lead to chronic kidney damage or accelerate renal impairment by causing a systemic inflammatory response [Citation28,Citation29].
Multiple potential biological mechanisms may explain the relationship between H. pylori infection and IgAN. H. pylori stimulates acute and chronic inflammatory immune responses, and IgAN patients are more prone to develop a robust mucosal immune response upon exposure to H. pylori. Higher levels of IgA1 bound to lectin in IgAN patients with mucosal H. pylori infection suggest that mucosal H. pylori infection induces underglycosylation of IgA1 [Citation30]. This incomplete glycosylation of IgA1 may act as a self-antigen, triggering autoantibody production [Citation31]. It has been reported that circulating immune complexes containing IgA autoantibodies, with the possible assistance of IgA receptors and combined with polymerized galactose-deficient IgA1 (Gd-IgA1), are deposited in the glomerular mesangial zone, leading to glomerular mesangial cell proliferation, extracellular matrix accumulation, overproduction of cytokine and chemokine, and glomerular injury. Previous studies have also shown elevated levels of Gd-IgA1 in H. pylori-infected patients with IgAN [Citation13]. One study showed that of 71 antibodies that reacted with H. pylori in the gastric mucosa, 11 cross-reacted with renal tubular cells [Citation32]. Another report found that monoclonal anti-H. pylori and anti-CagA antibodies exclusively reacted with renal tubules. CagA, a key virulence factor of H. pylori, is implicated in the pathogenesis of IgAN by stimulating the proliferation of the B-cell line DAKIKI cells (a surface IgA 1-positive human B-lymphoma cell line), which produce IgA1 and exhibit underglycosylation of IgA1. CagA promotes cell proliferation at low concentrations and inhibits it at high concentrations [Citation28].
In patients with IgAN, acute pharyngeal tonsillitis is the most common extrarenal clinical manifestation [Citation33]. The onset of pharyngeal tonsillitis is closely related to hematuria and proteinuria [Citation34]. Specific pathogens can directly deposit in renal tissue, participating in the onset and progression of IgAN. In the renal tissue of IgAN patients, various pathogens can be detected, including bacteria such as Haemophilus parainfluenzae, Staphylococcus aureus, as well as viruses like cytomegalovirus and EB virus [Citation35–39]. Two recent Japanese studies have reported finding H. pylori in the palatal tonsils of all studied IgAN patients, with 78.6% testing positive for CagA. This suggests that H. pylori in the tonsils may be a pathogenic agent for IgAN [Citation10,Citation14]. This phenomenon is thought to result from the substantial IgA system response to infectious antigens present in the tonsils. Seventy percent of IgAN patients exhibit positive staining for anti H. pylori antibodies, which are distributed in a granular pattern along the glomerular capillary wall [Citation40]. It is hypothesized that H. pylori and anti H. pylori IgA antibodies form an immune complex in the tonsils, circulate through the bloodstream, and deposit in the glomeruli. However, further mechanistic research is warranted.
Recently, Some studies have suggested that H. pylori is more prevalent in patients with IgAN, but the current view remains controversial [Citation11,Citation22]. Observational studies are subject to unavoidable confounding factors, and reverse causality cannot be ruled out. In addition, the sample sizes of these studies are small, so the results are somewhat biased and the level of evidence is lower than that of RCT studies. The causal relationship between the two still needs to be further verified by RCTs with large sample sizes. The use of RCTs to determine reliable and accurate causality can be very expensive and unethical, whereas MR has no research costs or ethical issues, and the random assignment of IV alleles at conception makes the MR method a ‘naturally RCT,’ making MR a good tool for causal inference. In conclusion, we used a two-sample bidirectional MR analysis to investigate the relationship between H. pylori infection and IgAN. This MR study has several strengths. First, the seven phenotypic summary statistics associated with H. pylori infection were utilized to expand the list of H. pylori infections, and the use of the most recent and complete GWAS data as IVs maximized statistical efficacy. Second, the application of strict IVs prerequisites and bidirectional two-sample MR analysis ensured that confounders were less biased and excluded the effect of reverse causality. Finally, the large sample size of the study and the sample populations used were all European, avoiding bias from population heterogeneity. We refined the bidirectional magnetic resonance analysis to exclude the interference of reverse causality, and the results were reliable. Although our study obtained significant results, some limitations should be noted: first, to ensure that the working variables were present in all study populations, we set the IVs significance threshold of 1e-5 instead of the classical 5e-8. Second, the serum H. pylori antibody test is not considered the gold standard for diagnosing H. pylori infection. However, current GWAS data do not incorporate histological, molecular, or breath test data for H. pylori infection, so serum H. pylori antibodies are often used as an exposure factor for MR study [Citation41, Citation42]. Third, the study population included only individuals of European origin; therefore, the results of this study may not be generalized to populations of other ancestries. The prevalence of IgAN is highest in Asia and the Pacific Rim, followed by European[Citation6], and the genetic risk alleles for IgAN are reportedly more common in Chinese individuals than in Europeans[Citation43]. However, our search did not yield specific GWAS data on H. pylori infection in Asian populations. Consequently, more studies are needed in the future to determine whether there are any regional or racial differences in the results of MR analyses. Fourth, there was some sample overlap between exposure factors and outcomes, however, the calculated maximum overlap was <1%, which did not affect the results obtained from the analysis. Fifth, due to the use of summary level data, we are unable to analyze non-linear correlations or stratified effects. Finally, the sample size of H. pylori CagA is relatively small, which may diminish the statistical ability to detect causal relationships. If more comprehensive data become available, future research will continue to explore the causal relationship between H. pylori CagA antibody and IgAN to verify the reliability of the results.
Conclusion
Our study using MR showed no evidence of a significant causal relationship between genetically predicted H. pylori infection and IgAN. Further longitudinal studies using more adequate data and populations from different sources are still needed to explore causality, refine subgroup stratification, and elucidate how H. pylori infection affects the onset or progression of IgAN.
Authors’ contributions
S.J. designed the study and wrote the manuscript. L.L. performed the data extraction. J.L. and J.P. analyzed and interpreted the data. X.Q. performed the project management, comparison and review. All authors read and approved the final manuscript.
Supplemental Material
Download ()Acknowledgements
We thank all the participants in this study. We thank all contributors to the GWAS database.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Data availability statement
The data we used are publicly available summary statistics and can be obtained upon reasonable request.
Additional information
Funding
References
- Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN Guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64(6):1–11. doi: 10.1097/MPG.0000000000001594.
- Niknam R, Barfei M, Mahmoudi L. Helicobacter pylori, endoscopic, and histologic features among kidney transplant candidates in Southern Iran. Infect Drug Resist. 2019;12:3687–3693.
- Pan W, Zhang H, Wang L, et al. Association between Helicobacter pylori infection and kidney damage in patients with peptic ulcer. Ren Fail. 2019;41(1):1028–1034. doi: 10.1080/0886022X.2019.1683029.
- Shin SP, Bang CS, Lee JJ, et al. Helicobacter pylori infection in patients with chronic kidney disease: a systematic review and meta-analysis. Gut Liver. 2019;13(6):628–641. doi: 10.5009/gnl18517.
- Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: core Curriculum 2021. Am J Kidney Dis. 2021;78(3):429–441. doi: 10.1053/j.ajkd.2021.01.024.
- Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38(5):435–442. doi: 10.1016/j.semnephrol.2018.05.013.
- Galla JH. IgA nephropathy. Kidney Int. 1995;47(2):377–387. doi: 10.1038/ki.1995.50.
- Reich HN, Troyanov S, Scholey JW, et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177–3183. doi: 10.1681/ASN.2007050526.
- D’Amico G, Colasanti G, Belgioioso GBd, et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;7(4):355–358.
- Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLOS One. 2014;9(3):e91756. doi: 10.1371/journal.pone.0091756.
- Kusano K, Inokuchi A, Fujimoto K, et al. Coccoid Helicobacter pylori exists in the palatine tonsils of patients with IgA nephropathy. J Gastroenterol. 2010;45(4):406–412. doi: 10.1007/s00535-009-0169-9.
- Kong X, Xu D, Li F, et al. Association of H. pylori infection with chronic kidney disease among Chinese adults. Int Urol Nephrol. 2017;49(5):845–850. doi: 10.1007/s11255-016-1498-2.
- Liu XZ, Zhang YM, Jia NY, et al. Helicobacter pylori infection is associated with elevated galactose-deficient IgA1 in IgA nephropathy. Ren Fail. 2020;42(1):539–546. doi: 10.1080/0886022X.2020.1772295.
- Ueda J, Okuda M, Nishiyama T, et al. Diagnostic accuracy of the E-plate serum antibody test kit in detecting Helicobacter pylori infection among Japanese children. J Epidemiol. 2014;24(1):47–51. doi: 10.2188/jea.JE20130078.
- Zhu TT, Wang L, Wang HL, et al. Helicobacter pylori participates in the pathogenesis of IgA nephropathy. Ren Fail. 2016;38(9):1398–1404. doi: 10.1080/0886022X.2016.1216713.
- Wang L, Tan RZ, Chen Y, et al. CagA promotes proliferation and secretion of extracellular matrix by inhibiting signaling pathway of apoptosis in rat glomerular mesangial cells. Ren Fail. 2016;38(3):458–464. doi: 10.3109/0886022X.2016.1138831.
- Chmiela M, Kupcinskas J. Review: pathogenesis of Helicobacter pylori infection. Helicobacter. 2019;24(S1):e12638. doi: 10.1111/hel.12638.
- Galmiche A, Rassow J, Doye A, et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. Embo j. 2000;19(23):6361–6370. doi: 10.1093/emboj/19.23.6361.
- Molinari M, Salio M, Galli C, et al. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187(1):135–140. doi: 10.1084/jem.187.1.135.
- Fernández-de-Larrea N, Michel A, Romero B, et al. Antibody reactivity against Helicobacter pylori proteins in a sample of the Spanish adult population in 2008–2013. Helicobacter. 2017;22(5):e12401. doi: 10.1111/hel.12401.
- Michel A, Pawlita M, Boeing H, et al. Helicobacter pylori antibody patterns in Germany: a cross-sectional population study. Gut Pathog. 2014;6(1):10. doi: 10.1186/1757-4749-6-10.
- Barratt J, Bailey EM, Buck KS, et al. Exaggerated systemic antibody response to mucosal Helicobacter pylori infection in IgA nephropathy. Am J Kidney Dis. 1999;33(6):1049–1057. doi: 10.1016/S0272-6386(99)70141-1.
- Kanbay M, Kasapoglu B, Akcay A. An occult risk factor for proteinuria: helicobacter pylori infection. Med Hypotheses. 2007;69(3):709–710. doi: 10.1016/j.mehy.2007.01.010.
- Rahmani A, Moradkhani A, Hafezi Ahmadi MR, et al. Association between serum levels of high sensitive C-reactive protein and inflammation activity in chronic gastritis patients. Scand J Gastroenterol. 2016;51(5):531–537. doi: 10.3109/00365521.2015.1102318.
- Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. WJG. 2014;20(36):12781–12808. doi: 10.3748/wjg.v20.i36.12781.
- Umit H, Umit EG. Helicobacter pylori and mean platelet volume: a relation way before ımmune thrombocytopenia? Eur Rev Med Pharmacol Sci. 2015;19(15):2818–2823.
- Sulikowska B, Rutkowski B, Marszałek A, et al. The role of interstitial changes in the progression of chronic kidney disease. Postepy Hig Med Dosw. 2015;69:830–837. doi: 10.5604/17322693.1162570.
- Yang M, Li FG, Xie XS, et al. CagA, a major virulence factor of Helicobacter pylori, promotes the production and underglycosylation of IgA1 in DAKIKI cells. Biochem Biophys Res Commun. 2014;444(2):276–281. doi: 10.1016/j.bbrc.2014.01.050.
- Lin SY, Lin CL, Liu JH, et al. Association between Helicobacter pylori infection and the subsequent risk of end-stage renal disease: a nationwide population-based cohort study. Int J Clin Pract. 2015;69(5):604–610. doi: 10.1111/ijcp.12602.
- Smith AC, Molyneux K, Feehally J, et al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol. 2006;17(12):3520–3528. doi: 10.1681/ASN.2006060658.
- Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22(10):1795–1803. doi: 10.1681/ASN.2011050464.
- Ko GH, Park HB, Shin MK, et al. Monoclonal antibodies against Helicobacter pylori cross-react with human tissue. Helicobacter. 1997;2(4):210–215. doi: 10.1111/j.1523-5378.1997.tb00090.x.
- Caraman PL, Azoulay E, Kessler M, et al. Mucosal infections and allergy in IgA nephropathy. A retrospective study. Association des Néphrologues de l’Est de la France. Contrib Nephrol. 1993;104:24–30.
- Béné MC, Ligny BHd, Kessler M, et al. Tonsils in IgA nephropathy. Contrib Nephrol. 1993;104:153–161.
- Park JS, Song JH, Yang WS, et al. Cytomegalovirus is not specifically associated with immunoglobulin A nephropathy. J Am Soc Nephrol. 1994;4(8):1623–1626. doi: 10.1681/ASN.V481623.
- Suzuki S, Nakatomi Y, Sato H, et al. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet. 1994;343(8888):12–16. doi: 10.1016/S0140-6736(94)90875-3.
- Sharmin S, Shimizu Y, Hagiwara M, et al. Staphylococcus aureus antigens induce IgA-type glomerulonephritis in Balb/c mice. J Nephrol. 2004;17(4):504–511.
- Iwama H, Horikoshi S, Shirato I, et al. Epstein-Barr virus detection in kidney biopsy specimens correlates with glomerular mesangial injury. Am J Kidney Dis. 1998;32(5):785–793. doi: 10.1016/S0272-6386(98)70134-9.
- Tomino Y, Yagame M, Omata F, et al. A case of IgA nephropathy associated with adeno- and herpes simplex viruses. Nephron. 1987;47(4):258–261. doi: 10.1159/000184520.
- Nagashima R, Maeda K, Yuda F, et al. Helicobacter pylori antigen in the glomeruli of patients with membranous nephropathy. Virchows Arch. 1997;431(4):235–239. doi: 10.1007/s004280050094.
- Zhu Z, Yang Y, Han X, et al. Causality of Helicobacter pylori infection on eosinophilic esophagitis and potential pathogenesis: a Mendelian randomization study. Front Immunol. 2024;15:1365604. doi: 10.3389/fimmu.2024.1365604.
- Huang J, Liu Y, Xu D, et al. Causal associations between Helicobacter pylori infection and pregnancy and neonatal outcomes: a two-sample Mendelian randomization study. Front Cell Infect Microbiol. 2024;14:1343499. doi: 10.3389/fcimb.2024.1343499.
- Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology. 2019;24(9):885–895. doi: 10.1111/nep.13592.