Abstract
Background
Exercise therapy can effectively manage chronic kidney disease (CKD) risk factors and improve renal function and physical fitness, but the challenge lies in choosing the right exercise type tailored to patients’ condition.
Methods
An electronic search of databases including PubMed, The Cochrane Library, EMBASE, Web of Science, VIP, WanFang, and CNKI was performed. The random effects model was used. Mean difference was employed as the effect size for continuous variables, with 95% confidence interval (CI) provided.
Results
A total of 36 RCTs were included in this study. Compared to conventional therapy (CT), the combination of three exercise therapies with CT resulted in notable benefits in enhancing six minutes walk test (6MWT) capacity, 24-h urinary protein quantity (24hUTP), systolic blood pressure (SBP), diastolic blood pressure (DBP). Resistance exercise therapy (RT) + CT were more effective than CT to reduce serum creatinine (Scr), body mass index (BMI), and hemoglobin A1c (HbA1c) and improve estimated glomerular filtration rate (eGFR). In terms of improving peak oxygen uptake (VO2 peak), only two exercise modalities were involved, aerobic exercise therapy (AT) and combined (Resistance-Aerobic) exercise therapy (CBT), both of which were more efficacious than CT. The efficacy ranking overall demonstrated clear benefits for RT in enhancing eGFR and 6MWT, decreasing Scr, BMI, SBP, DBP, and HbA1c, while AT was more suitable for boosting VO2 peak, and CBT had greater potential for reducing 24hUTP.
Conslusions
Exercise therapy combined with CT offers significant advantages over CT in many cases, but no single exercise modality is universally effective for all indicators.
1. Introduction
Chronic kidney disease (CKD) has emerged as a significant global public health concern, threatening health worldwide. According to the global burden of disease study, the worldwide prevalence of CKD was estimated to be 9.1% in 2017 [Citation1]. CKD typically exhibits a subtle onset, and progression to end-stage kidney disease necessitates renal replacement therapy. However, this therapy significantly impairs patient quality of life [Citation2]. There is an imperative to enhance comprehensive management strategies for CKD, aiming to retard disease progression and mitigate the risk of cardiovascular events. Current international therapy approaches for CKD primarily encompass lifestyle and dietary modifications, aggressive control of blood pressure [Citation3], blood glucose, and other cardiovascular risk factors, as well as pharmacological interventions for managing comorbidities and complications. While pharmacotherapy plays a crucial role in the management of CKD, lifestyle modifications, serving as the cornerstone in controlling the disease’s progression, should be integral to the entire disease management continuum [Citation4].
As renal function declines, CKD patients experience a decrease in cardiopulmonary function and the onset of muscle atrophy [Citation5,Citation6], which significantly impacts prognosis and quality of life. Engaging in regular exercise can mitigate the limitations of medication, enhance physical function and muscle strength in individuals with CKD, reduce systemic inflammation, and slow the progression of renal impairment [Citation7,Citation8]. An observational study found that higher levels of physical activity were associated with a slower decline in glomerular filtration rate among CKD patients at Stages 3 and 4 [Citation9]. Another study also suggested that long-term moderate exercise training may protect renal function and delay the progression of kidney injury [Citation10]. Despite this, epidemiological studies indicated that CKD patients engage in physical exercise only 0–9 d per month [Citation11]. Therefore, proactively providing health education to patients, enhancing adherence to exercise therapy, and scientifically determining exercise modes, as well as the timing, intensity, and frequency, represent one of the most challenging and pressing issues that must be addressed.
Currently, published network meta-analysis (NMA) mainly addressed the impact of exercise therapy on the dialysis patient population [Citation12,Citation13]. However, for non-dialysis CKD (ND-CKD) patients, exercise therapy shows great potential in slowing down the disease process, controlling related risk factors, and delaying the initiation of dialysis treatment [Citation14,Citation15]. But, to date, there have been no studies that compare the benefits of different types of exercise in managing the risk factors influencing the progression of CKD and in protecting cardiorespiratory function. Different exercise modalities may have varying effects, and a rational selection of exercise modality can be targeted to managing the clinical concerns of diverse CKD patients. NMA can obtain the superiority and inferiority results of any two exercise modes through direct or indirect comparisons, while also ranking different interventions, which can play a role in guiding clinical physicians in developing exercise programs for ND-CKD patients. In view of the lack of evidence-based guidelines for selecting appropriate exercise modalities for ND-CKD patients, this study used NMA to evaluate the efficacy advantages of different exercise modes, with the aim of providing guidance for clinical practice.
2. Methods
2.1. Registration
This study was registered with PROSPERO, number: CRD42023483372. The registration date is 26 November 2023 (https://www.crd.york.ac.uk/prospero/).
2.2. Search strategy
An electronic search of databases including PubMed, The Cochrane Library, EMBASE, Web of Science, VIP, WanFang, and CNKI was performed from inception to 1 May 2024. The searches were conducted using subject terms and free words. English search terms include: exercise, resistance training, physical activity, aerobic exercise, CKD, chronic renal failure, and randomized controlled trial (RCT). The search strategy was adapted to the characteristics of different databases, as described in Supplementary Appendix 1. We also combed the references of included RCTs and related meta-analyses to ensure comprehensive access to eligible RCTs.
2.3. Study selection
Detailed inclusion criteria are listed below: (1) Participants: Individuals aged 18 or older with a definitive diagnosis of CKD as per the clinical practice guidelines for the evaluation and management of CKD published by the Kidney Disease Improving Global Outcomes Organization in 2012 [Citation16], who are not undergoing renal replacement therapy, and with any stage. No other significant conditions that could impact renal function, e.g. cancer or severe heart failure. (2) Experimental group: exercise therapy on the basis of conventional therapy (CT). The intensity and frequency of the exercises were not limited. Among conventional therapies, including dietary control and basic medications, such as antihypertensives, hypoglycemics, and lipid-lowering drugs, conventional drugs for cardiovascular diseases, and other comorbidities, such as aspirin, as well as drugs for CKD complications. (3) Control group: given CT, keeping the original lifestyle unchanged, or exercise different from that of the experimental group. (4) Outcome indicators: renal function-related indicators: estimated glomerular filtration rate (eGFR), serum creatinine (Scr), 24 h urinary protein quantity (24hUTP); general risk factors: body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglyceride (TG), hemoglobin A1c (HbA1c); physical function-related indicators: six minutes walk test (6MWT), peak oxygen uptake (VO2 peak). (5) Type of study: RCT. (6) Language: English and Chinese. Exclusion criteria: (1) duplicate publications, incomplete or obviously incorrect data, and unavailable literature; (2) summaries of personal experience, purely theoretical investigations, animal experiments, or case reports; (3) studies in which the experimental and control groups contained other therapies that might have affected comparison of results.
2.4. Data extraction
HDL searched and summarized the literature using EndNote X9 software for literature management. CL and JJY independently screened the literature, extracted data on information, such as the first author, year of publication, country, as well as details about the study including age of subjects, gender, sample size, and intervention protocol. During the literature screening and data extraction process, when disagreements occurred between the two researchers, they sought the advice of MZZ to ensure consensus was reached.
2.5. Risk of bias assessment
The included RCTs underwent risk of bias assessment by YYD, PFH, and JZ using the RCT risk of bias assessment tool recommended by the Cochrane Handbook [Citation17], which includes: random allocation method, allocation scheme concealment, blinding of subjects and implementers, blinding of outcome evaluators, data completeness, selective reporting, and other biases. After the assessment, the investigators cross-referenced the findings. If there were any discrepancies, they reviewed the results collectively or sought external input to achieve a unanimous agreement.
2.6. Statistical analysis
RevMan version 5.4 software (Copenhagen, Denmark) was used to assess the risk of bias, and Stata version 16.0 (StataCorp LLC, College Station, TX) was used to conduct NMA. Mean difference (MD) was employed as the effect size for continuous variables, with 95% confidence interval (CI) provided. p < 0.05 was considered to indicate statistical significance. The network and mvmeta packages were used to conduct the analysis. First, the network of evidence was plotted. When a closed loop was formed in the graph, consistency testing was performed using the Z test, and the inconsistency factor (IF) value was calculated using the ifplot command. If the IF value was closer to 0 and the lower limit of the 95% CI included 0, it indicated that the results of direct and indirect comparisons were more consistent. In this case, consistency model analysis was employed. Conversely, inconsistent model analysis is used. Publication bias and the effect of small-sample studies were assessed using a comparison-adjusted funnel plot. A Bayesian random-effects model was utilized to compare various exercise modalities. The interventions were ranked in terms of efficacy by plotting the surface under the cumulative ranking curve (SUCRA). The SUCRA value ranges from 0% to 100%, where 100% represents the highest probability that the intervention is the best among all options, and 0% represents the lowest probability that the intervention is the best.
3. Results
3.1. Literature screening process and basic characteristics of the included RCTs
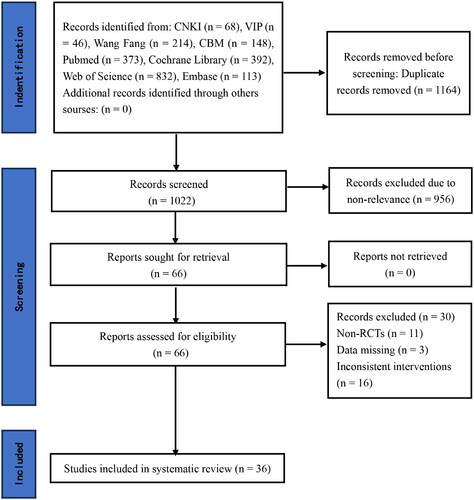
The initial search yielded 2,186 publications, and after gradual screening, 36 literatures [Citation18–53] with a total of 1,876 ND-CKD patients were finally included. Based on the results of the literature screening, the kappa value for representational consistency was calculated to be 0.83, indicating good consistency between the literature screening results of the two researchers. Twenty-eight of the included RCTs were published in English, eight in Chinese. Ten RCTs were conducted in China, eight in Switzerland, seven in the United States, four in Japan, three in the United Kingdom, two in Australia, one in Belgium, and one in Canada, the baseline information of the patients in all the RCTs was comparable, and all of them assessed at least one of the outcome indicators. For the interventions, three different types of exercise were involved: aerobic exercise therapy (AT), resistance exercise therapy (RT), and combined (Resistance-Aerobic) exercise therapy (CBT), and the frequency of exercise was predominantly three times per week, with a period of 12–52 weeks. The literature screening process and results are shown in . The basic characteristics of the literature are shown in , and the specific details of the interventions are shown in .
Table 1. Basic characteristics of included randomized controlled trials.
Table 2. The specific details of the interventions.
3.2. The evaluation of study quality
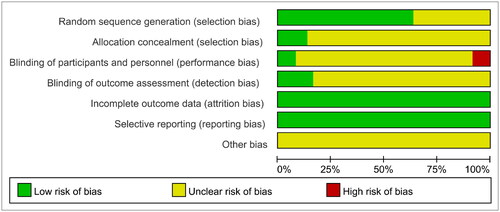
The quality of the included literature was evaluated using the ‘Risk Assessment Tool’ recommended by the Cochrane Collaboration: 23 RCTs described specific randomization methods and were assessed as ‘Low risk’, the rest 13 mentioned random allocation only and were rated as ‘unclear’, four mentioned allocation concealment, three mentioned blinding and were rated as ‘Low risk’, three explicitly stated that subjects or researchers were not blinded due to the specificity of the intervention, six mentioned blinding of the outcome measures and were rated as ‘Low risk’. All RCTs explicitly reported outcome indicators. No duplicate publications or published biases were found in any of the RCTs and were evaluated as ‘Low risk’; other biases were unknown and were evaluated as ‘Unclear risk’ ().
3.3. Results of network meta-analysis
3.3.1. Network evidence relationship diagram
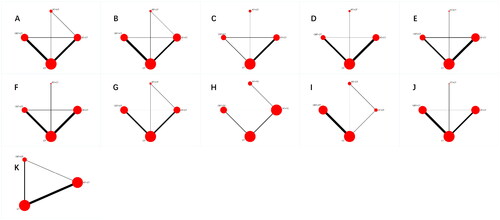
The network evidence relationship of different exercise modalities is shown in . Each node represents an intervention, and the size of the node corresponds to the sample size of the intervention. Solid lines between nodes indicate direct comparisons between two interventions, with the thickness of the solid line indicating the amount of evidence from these direct comparisons. Among the outcome, eGFR, Scr, TC, and TG have formed two closed loops, while the rest have formed one closed loop. The results of the inconsistency test revealed that the IF values were all close to 0 and the lower limits of the 95% CI were zero, indicating good consistency across each closed loop.
Figure 3. Network diagrams of comparisons on different outcomes of treatments in different groups of non dialysis chronic kidney disease patients. A: eGFR; B: Scr; C: 24hUTP; D: BMI; E: SBP; F: DBP; G: TC; H: TG; I: HbA1c; J: 6MWT; K: VO2 peak; AT: aerobic exercise therapy; RT: resistance exercise therapy; CBT: combined (Resistance-Aerobic) exercise therapy; CT: conventional therapy.
3.3.2. Renal function: eGFR
A total of 24 RCTs [Citation18–20,Citation22–25, Citation27, Citation29,Citation32–34,Citation37–39,Citation42–48,Citation50,Citation51] evaluated the changes of eGFR. Comparative to CT, a significant increase of eGFR was observed in patients who received treatment with RT + CT [MD = 5.47, 95% CI (1.40, 9.55), p < 0.05]. The differences in other pairwise comparisons were not statistically significant (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 89.6%) > CBT + CT (SUCRA = 60.1%) > AT + CT (SUCRA = 46.3%) > CT (SUCRA = 3.9%) ().
Figure 4. Pooled estimates of the network meta-analysis. A: eGFR; B: Scr; C: 24hUTP; D: BMI; E: SBP; F: DBP; G: TC; H: TG; I: HbA1c; J: 6MWT; K: VO2 peak; AT: aerobic exercise therapy; RT: resistance exercise therapy; CBT: combined (Resistance-Aerobic) exercise therapy; CT: conventional therapy.
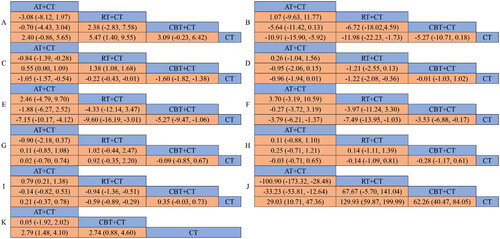
Figure 5. Surface under the cumulative ranking area for outcomes. A: eGFR; B: Scr; C: 24hUTP; D: BMI; E: SBP; F: DBP; G: TC; H: TG; I: HbA1c; J: 6MWT; K: VO2 peak; AT: aerobic exercise therapy; RT: resistance exercise therapy; CBT: combined (Resistance-Aerobic) exercise therapy; CT: conventional therapy.
3.3.3. Renal function: Scr
The effect of exercise on Scr was evaluated in 15 RCTs [Citation18–20,Citation24,Citation26,Citation27,Citation29,Citation34,Citation38,Citation41,Citation43,Citation44,Citation46,Citation50,Citation52]. The results showed that both AT + CT [MD = −10.91, 95% CI (-15.90, −5.92), p < 0.05] and RT + CT [MD = −11.98, 95% CI (-22.23, −1.73), p < 0.05] were superior to CT. No statistically significant differences were found in other pairwise comparisons (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 81.5%) > AT + CT (SUCRA = 79.9%) > CBT + CT (SUCRA = 37.2%) > CT (SUCRA = 1.4%) ().
3.3.4. Renal function: 24hUTP
24hUTP had been used in 7 RCTs [Citation19,Citation24,Citation26,Citation32,Citation41,Citation43,Citation51] for comparison. There was a significant effect that AT + CT [MD = −1.05, 95% CI (−1.57, −0.54), p < 0.05], CBT + CT [MD = −1.60, 95% CI (−1.82, −1.38), p < 0.05], and RT + CT [MD = −0.22, 95% CI (−0.43, −0.01), p < 0.05] were all superior to CT. The results of other pairwise comparisons showed: CBT + CT was superior to RT + CT, and AT + CT was superior to RT + CT (). The best probability ranking results showed: CBT + CT (SUCRA = 99.3%) > AT + CT (SUCRA = 67.4%) > RT + CT (SUCRA = 32.6%) > CT (SUCRA = 0.8%) ().
3.3.5. General risk factors: BMI
There were 17 RCTs [Citation18,Citation20,Citation23,Citation30, Citation32, Citation39,Citation42,Citation44–49, Citation51,Citation52] that examined the effect of exercise on BMI. The results showed that RT + CT was superior to CT [MD = −1.22, 95% CI (−2.08, −0.36), p < 0.05]. The other pairwise comparisons did not reveal any notable statistical discrepancies (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 87.0%) > AT + CT (SUCRA = 75.9%) > CBT + CT (SUCRA = 19.3%) > CT (SUCRA = 17.8%) ().
3.3.6. General risk factors: SBP
A total of 20 RCTs [Citation18,Citation19,Citation22,Citation24,Citation29–33,Citation35,Citation36,Citation42–47,Citation49–51]investigated the effects of different exercise modalities on SBP. AT + CT [MD = −7.15, 95% CI (−10.17, −4.12), p < 0.05], CBT + CT [MD = −5.27, 95% CI (-9.47, −1.06), p < 0.05], and RT + CT [MD = −9.60, 95% CI (−16.19, −3.01), p < 0.05] were all superior to CT with significant difference. There were no statistically significant differences in other pairwise comparisons (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 86.9%) > AT + CT (SUCRA = 68.6%) > CBT + CT (SUCRA = 44.2%) > CT (SUCRA = 0.3%) ().
3.3.7. General risk factors: DBP
There were 20 RCTs [Citation18,Citation19,Citation22,Citation24,Citation29–33,Citation35,Citation36,Citation42–47, Citation49–51] that investigated the effects of exercise on DBP. The NMA of these RCTs revealed that AT + CT [MD = −3.79, 95% CI (−6.21, −1.37), p < 0.05], CBT + CT [MD = −3.53, 95% CI (−6.88, −0.17), p < 0.05], and RT + CT [MD = −7.49, 95% CI (−13.95, −1.03), p < 0.05] were all superior to CT. No statistically significant differences were found in other pairwise comparisons (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 90.1%) >AT + CT (SUCRA = 57.0%)>CBT + CT (SUCRA = 51.8%) > CT (SUCRA = 1.1%) ().
3.3.8. General risk factors: TC
11 RCTs [Citation19,Citation33,Citation38,Citation39,Citation42,Citation43,Citation45–47,Citation50,Citation51] reported the effect of exercise on TC. The results showed that there were no significant differences between any of the groups when compared pairwise (p > 0.05) (). The best probability ranking results showed: CBT + CT (SUCRA = 69.4%) > CT (SUCRA = 61.7%) > AT + CT (SUCRA = 60.6%) > RT + CT (SUCRA = 8.3%) ().
3.3.9. General risk factors: TG
6 RCTs [Citation19,Citation38,Citation39,Citation42,Citation43,Citation50] measured the TG between the different exercise modes. The results of the NMA indicated that no significant differences were observed among any of the groups in pairwise comparisons (p > 0.05) (). The best probability ranking results showed: CBT + CT (SUCRA = 66.7%) > RT + CT (SUCRA = 53.5%) > AT + CT (SUCRA = 41.9%) > CT (SUCRA = 37.9%) ().
3.3.10. General risk factors: HbA1c
Nine RCTs [Citation19,Citation21,Citation27,Citation28,Citation32,Citation38,Citation42,Citation46,Citation52] were included to examined the impacts of different exercise modalities on HbA1c. RT + CT has a significant impact on HbA1c compared to CT [MD = −0.59, 95% CI (−0.89, −0.29), p < 0.05]. There were no statistically significant differences identified in other pairwise comparisons (p > 0.05) (). The best probability ranking results showed: RT + CT (SUCRA = 99.9%) > CT (SUCRA = 57.3%) > AT + CT (SUCRA = 30.6%) > CBT + CT (SUCRA = 12.3%) ().
3.3.11. Physical function: 6MWT
A total of 10 RCTs [Citation19,Citation21,Citation22,Citation32,Citation34,Citation40,Citation42,Citation44,Citation46,Citation48] used the 6MWT as an outcome. There was an obvious rise in the 6MWT in patients with ND-DKD after receive AT + CT [MD = 29.03, 95% CI (10.71, 47.36), p < 0.05], CBT + CT [MD = 62.26, 95% CI (40.47, 84.05), p < 0.05], and RT + CT [MD = 129.93, 95% CI (59.87, 199.99), p < 0.05]. The results of other pairwise comparisons showed: RT + CT was superior to AT + CT and CBT + CT was superior to AT + CT (). The best probability ranking results showed: RT + CT (SUCRA = 98.7%) > CBT + CT (SUCRA = 67.8%) > AT + CT (SUCRA = 33.4%) > CT (SUCRA = 0.1%) ().
3.3.12. Physical function: VO2 peak
There were 17 RCTs [Citation20,Citation21,Citation30–32,Citation35,Citation39,Citation41,Citation42,Citation44,Citation45,Citation47–49,Citation51–53] that reported the influence of exercise mode on VO2 peak. The results showed that AT + CT [MD = 2.79, 95% CI (1.48, 4.10), p < 0.05], and CBT + CT [MD = 2.74, 95% CI (0.88, 4.60), p < 0.05] were all superior to CT. No statistically significant differences were found in other pairwise comparisons (p > 0.05) (). The best probability ranking results showed: AT + CT (SUCRA = 75.5%) > CBT + CT (SUCRA = 74.4%) > CT (SUCRA = 0.1%) ().
3.4. Detection of publication bias
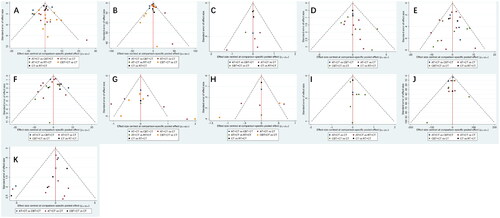
Publication bias was examined for each outcome indicator by plotting a funnel plot. Each dot represents a comparison between two therapies and the number of identically colored dots shows how many RCTs included that two-way comparison. If the funnel plot shows no significant small-sample effect or publication bias, the dots are evenly distributed on both sides of the vertical line X = 0. In this study, the dots on the funnel plot for each result did not create perfect symmetry (). This could indicate some publication bias and scattered dots that lie beyond the 95% CI of the funnel plot, which could suggest a small-sample effect. The reasons for this may be associated with the general quality of the research, limited sample sizes, variations in therapeutic procedures, and the patient’s disease progression stage.
4. Discussion
This study, to the best of our knowledge, represents the first NMA to examine the efficacy of various exercise modalities in managing general risk factors, enhancing renal function, and improving physical capacity in patients with ND-CKD, covering three common exercise modalities and incorporating the largest array of outcomes to date, thus providing more nuanced recommendations for tailoring exercise programs to ND-CKD patients.
In terms of improving renal function, we chosed eGFR, Scr, and 24hUTP as the outcome. Based on the results, RT + CT showed the best effect in improving eGFR and reducing Scr, while CBT + CT seemed to be the most appropriate exercise form for decreasing 24hUTP levels. A study demonstrated that RT could increase muscle strength and up-regulate the expression of anti-inflammatory factors, including IL-4 and IL-10, while down-regulating the levels of IL-6 [Citation54]. This modulation of cytokine expression profiles was associated with a reduction in renal fibrosis and inflammation. In addition, RT could improve renal fibrosis by increasing muscle mass [Citation55], slow the progression of CKD [Citation56]. Impaired renal function is manifested by increases in Scr and the presence of proteinuria. Studies have shown that AT could up-regulate the expression levels of transforming growth factor beta and platelet-derived growth factor BB, which in turn promotes renal cell survival, proliferation, and partial recovery of renal function [Citation57]. This mechanism may contribute to the reduction of Scr and proteinuria. Additionally, proteinuria levels are known to be influenced by blood pressure [Citation16]. In our study, RT was found to have the most significant impact on blood pressure reduction. Consequently, CBT may represent the optimal exercise regimen to decrease proteinuria levels.
We selected BMI, SBP, DBP, TC, TG, and HbA1c as indicators for assessing risk factors. Hypertension is a significant risk factor for the development and progression of CKD [Citation58]. Abnormalities of lipid metabolism are more common in patients with CKD, can induce and exacerbate kidney injury. Hyperglycemia can directly damage kidney vessels and glomerular structures, leading to gradual decline in renal function [Citation59]. Our results showed that exercise had better efficacy than CT alone in reducing SBP, and DBP, all with the best efficacy of RT + CT, and only RT + CT was superior to CT in lowering BMI and HbA1c. We speculate that this may be associated with the potential of RT to enhance endothelial function [Citation60], thereby improving overall circulatory health and alleviating the workload on the heart. The beneficial effects of RT on reducing BMI may stem from the fact that muscle tissue consumes more calories than fat tissue at rest. Therefore, increasing muscle mass can elevate the basal metabolic rate, facilitating the burning of more calories, and subsequently lowering the BMI [Citation61,Citation62]. CKD patients are more prone to exercise pressure response in daily life such as lifting heavy objects, and RT + CT blunts this response by enhancing muscle strength, effectively reducing the risk of cardiovascular-related events [Citation63,Citation64]. Our study results are consistent with a meta-analysis, showing that exercise helps to lower the blood pressure and BMI in patients with ND-CKD [Citation65]. However, differently from other systematic reviews [Citation66–68], this could be due to the number of included studies, the selection of study participants, or the differences in the medications taken. Notably, our study revealed no significant difference in the efficacy of exercise compared to CT in lowering TC and TG. But another meta-analysis demonstrated that a 3-month short-term exercise intervention significantly decreased TG [Citation65], while having no impact on TC, it should be noted that this outcome only comprised four studies with a smaller sample size. Due to the lack of sufficient research on blood glucose levels, we have chosen HbA1c as a substitute indicator and found that RT + CT helps to reduce HbA1c. However, Pan’s research suggested that CBT has a more significant effect on improving HbA1c levels [Citation69]. The variations between the studies might be due to the NMA combining direct and indirect comparisons with a larger sample size, reducing the CI for the effect size. The number of studies included in our research was limited, which may lead to small-sample effect biases. We therefore exercised caution regarding these findings and emphasized the need for further studies to validate these results.
VO2 peak and 6MWT were selected as outcome to assess the physical function of CKD patients. VO2 peak has emerged as the benchmark for cardiorespiratory fitness and exhibits an inverse correlation with both cardiovascular risk and all-cause mortality [Citation70]. The widely used 6MWT measures submaximal gait, indicating levels of ambulatory tolerance reflective of real-world mobility. Patients with CKD perform over 30% worse than healthy adults on this particular test, which has a high predictive value for short and long-term mortality in individuals with chronic conditions, including CKD [Citation71]. Our results showed that AT + CT had the highest efficacy in improving VO2 peak. This result is consistent with the majority of previous research. Aerobic exercise has a favorable impact on the cardiovascular system and cardiorespiratory fitness, and significantly reduces the risk of related diseases. However, the specific mechanisms in the ND-CKD population are not yet clear [Citation47], RT has rarely been used to enhance cardiorespiratory function, and few relevant studies have been published to date. RT + CT is more advantageous for improving 6MWT capacity. Decreased mitochondrial respiration in CKD is linked to a reduction in muscle mass [Citation72,Citation73]. RT is expected to improve physical fitness by enhancing muscle strength and mass [Citation74], leading to better performance in 6MWT.
Our research findings are in line with the exercise guidelines for ND-CKD (Stages 1–5), which recommend that individuals with ND-CKD should engage in daily physical activity [Citation75]. This should begin with low-intensity aerobic exercises and progressively increase to high-intensity or combined muscle-strengthening training, which can help improve patients’ blood pressure, weight, and mental health. In terms of frequency of exercise, most studies show higher compliance with a frequency of 3 times a week. The exercise time is generally around 30 min each time. The intervention period ranges from 12 to 52 weeks, with an average of 25 weeks. Regarding exercise intensity for elderly ND-CKD patients, researchers typically recommend a gradual increase in intensity, with AT performed at 50%–80% of heart rate reserve or 40%–60% of VO2 peak, and RT performed at 50%–70% of one-repetition maximum or Borg 12–15 [Citation76]. Based on our research findings, the following recommendations are provided for the development of an exercise prescription for patients with ND-CKD: firstly, reasonable control of exercise intensity, as excessive exercise intensity may lead to increased blood pressure and cardiac burden. Patients unable to complete exercises may also experience psychological stress. Second, a longer exercise regimen should be established, with regular checks on the patient’s physical condition to allow for adjustments as needed. Third, Patients with advanced CKD may need to adjust their exercise plans to avoid excessive fatigue and muscle damage. Exercise prescriptions should be tailored to the patient’s personal preferences, and clinical practitioners should provide sufficient health education to improve patient adherence to exercise.
This study had the following advantages. Not only is our study the first NMA comparing the efficacy of three classical exercise modalities in slowing the progression of ND-CKD, this study also conducted a comprehensive and systematic search of databases to include study populations from multiple countries and ethnicities, thereby increasing the external validity of the findings and remedying the shortcomings of traditional meta-analyses. This study also had a few limitations. Most of the included literature has a lower quality, which may imply certain small-sample effects and publication bias. The potential bias risks could reduce the power of testing. While all three exercise modalities are commonly used, the number of studies on RT is comparatively low compared to AT and CBT, the ranking of overall efficacy may be biased. And all comparisons between different interventions are indirect, which may affect the accuracy and credibility of the results. Additionally, due to the lack of specification of the CKD stage in most studies, there is an insufficient number of studies available for subgroup analysis.
5. Conclusion
Developing an appropriate exercise prescription is effective in controlling risk factors and slowing down the course of the disease. There is not one exercise modality that is ideal for all indicators. RT offers significant benefits in enhancing eGFR, reducing BMI, blood pressure, and Scr, and enhancing 6MWT capacity. AT appears to be more effective in improving cardiorespiratory function, whereas CBT shows promise in reducing urinary protein. To delve deeper into the impact of exercise on patients with ND-CKD, it is suggested that future research be designed based on the different stages of CKD. These studies should aim to identify the appropriate intensity, frequency, and duration of exercise for CKD patients at each stage, thereby providing a more solid scientific basis for exercise therapy in ND-CKD patients.
Ethics statement
None.
Author contributions
The study was designed by CL and JY. HL searched and screened the literature for inclusion, with input from SC. YY, PH, and JZ assessed the quality of the literature. Data were extracted from the literature by CL and JY. JY performed the NMA and created the figures. CL drafted the manuscript, which was then revised by SC and XW. Final revisions and critical review of the study results were carried out by MZ. All personnel were familiar with the manuscript’s contents, and CL and JY’s contributions to this study were consistent.
Supplemental Material
Download MS Word (20.6 KB)Disclosure statement
All authors have no conflict of interest of any kind.
Data availability statement
The original data involved in this study can be requested by contacting the corresponding author (MZ) or the first author.
Additional information
Funding
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):1–14. doi: 10.1016/S0140-6736(20)30045-3.
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1.
- Tomson C, Cheung AK, Mann J, et al. Management of blood pressure in patients with chronic kidney disease not receiving dialysis: synopsis of the 2021 KDIGO clinical practice guideline. Ann Intern Med. 2021;174(9):1270–1281. doi: 10.7326/M21-0834.
- Coyne E, Briggs J, Loud F, et al. Achieving consensus on psychosocial and physical rehabilitation management for people living with kidney disease. Clin Kidney J. 2023;16(11):2185–2193. doi: 10.1093/ckj/sfad116.
- Chatrenet A, Piccoli G, Audebrand JM, et al. Analysis of the rate of force development reveals high neuromuscular fatigability in elderly patients with chronic kidney disease. J Cachexia Sarcopenia Muscle. 2023;14(5):2016–2028. doi: 10.1002/jcsm.13280.
- Perl J, Karaboyas A, Morgenstern H, et al. Association between changes in quality of life and mortality in hemodialysis patients: results from the DOPPS. Nephrol Dial Transplant. 2017;32(3):521–527. doi: 10.1093/ndt/gfw233.
- Villanego F, Naranjo J, Vigara LA, et al. Impact of physical exercise in patients with chronic kidney disease: systematic review and meta-analysis. Nefrologia (Engl Ed). 2020;40(3):237–252. doi: 10.1016/j.nefroe.2020.06.012.
- Kanbay M, Copur S, Yildiz AB, et al. Physical exercise in kidney disease: a commonly undervalued treatment modality. Eur J Clin Invest. 2024;54(2):e14105. doi: 10.1111/eci.14105.
- Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399–406. doi: 10.1681/ASN.2013040392.
- Toyama K, Sugiyama S, Oka H, et al. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56(2):142–146. doi: 10.1016/j.jjcc.2010.06.007.
- Avesani CM, Trolonge S, Deléaval P, et al. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant. 2012;27(6):2430–2434. doi: 10.1093/ndt/gfr692.
- Zang W, Fang M, He H, et al. Comparative efficacy of exercise modalities for cardiopulmonary function in hemodialysis patients: a systematic review and network meta-analysis. Front Public Health. 2022;10:1040704. doi: 10.3389/fpubh.2022.1040704.
- Hu H, Wu C, Kwok J, et al. Effects of different exercises on physical function, dialysis adequacy, and health-related quality of life in maintenance hemodialysis patients: a systematic review and network meta-analysis. Am J Nephrol. 2023;54(9–10):379–390. doi: 10.1159/000532109.
- Nakamura K, Sasaki T, Yamamoto S, et al. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: a systematic review and meta-analysis. Sci Rep. 2020;10(1):18195. doi: 10.1038/s41598-020-75405-x.
- Wu L, Liu Y, Wu L, et al. Effects of exercise on markers of inflammation and indicators of nutrition in patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(4):815–826. doi: 10.1007/s11255-021-02949-w.
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007.
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. doi: 10.1002/14651858.ED000142.
- Kimura T, Washida N, Ohtsuki S, et al. A multi-center randomized controlled trial to investigate potential effects of exercise therapy on renal function stratified by renal disorders and renal pathology: beneficial or harmful effect in immunoglobulin a nephropathy. Clin Exp Nephrol. 2024;28(6):539–546. doi: 10.1007/s10157-024-02461-2.
- de Araújo TB, de Luca Corrêa H, de Deus LA, et al. The effects of home-based progressive resistance training in chronic kidney disease patients. Exp Gerontol. 2023;171:112030. doi: 10.1016/j.exger.2022.112030.
- Pu L. Clinical application of fitt-based five-element palm progression exercise workout in patients with chronic kidney disease (CKD) stages 1–4. Chin Sci Technol J Database (Citation Edition) Med Hygiene. 2023;9(07):58–60.
- Weiner DE, Liu CK, Miao S, et al. Effect of long-term exercise training on physical performance and cardiorespiratory function in adults with CKD: a randomized controlled trial. Am J Kidney Dis. 2023;81(1):59–66. doi: 10.1053/j.ajkd.2022.06.008.
- Yuan C, Li W. Effects of aerobic-resistance exercise on renal function, cognitive function, and motor function in chronic kidney disease patients. Shenzhen J Integrated Trad Chin Western Med. 2023;33(05):76–79.
- Corrêa HL, Neves RVP, Deus LA, et al. Low-load resistance training with blood flow restriction prevent renal function decline: the role of the redox balance, angiotensin 1–7 and vasopressin(✰,✰✰). Physiol Behav. 2021;230:113295. doi: 10.1016/j.physbeh.2020.113295.
- Corrêa HL, Neves RVP, Deus LA, et al. Blood flow restriction training blunts chronic kidney disease progression in humans. Med Sci Sports Exerc. 2021;53(2):249–257. doi: 10.1249/MSS.0000000000002465.
- de Deus LA, Neves R, Correa HL, et al. Improving the prognosis of renal patients: the effects of blood flow-restricted resistance training on redox balance and cardiac autonomic function. Exp Physiol. 2021;106(4):1099–1109. doi: 10.1113/EP089341.
- Dong J. Effect of exercise therapy on renal function and fatigue in patients with chronic kidney disease. Reflexol Rehabil Med. 2021;2(23):103–105.
- Otobe Y, Yamada M, Hiraki K, et al. Physical exercise improves cognitive function in older adults with stage 3–4 chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2021;52(12):929–939. doi: 10.1159/000520230.
- Uchiyama K, Adachi K, Muraoka K, et al. Home-based aerobic exercise and resistance training for severe chronic kidney disease: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2021;12(6):1789–1802. doi: 10.1002/jcsm.12775.
- Zhao W, Shi X, Zhao J. Effects of moderate-intensity aerobic training on ambulatory blood pressure levels and renal function in elderly patients with chronic renal insufficiency combined with hypertension. Int J of Transpl Blood Purificat. 2021;19(4):37–39.
- Huppertz N, Beetham KS, Howden EJ, et al. A 12-month lifestyle intervention does not improve cardiac autonomic function in patients with chronic kidney disease. Auton Neurosci. 2020;224:102642. doi: 10.1016/j.autneu.2020.102642.
- Kirkman DL, Ramick MG, Muth BJ, et al. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol. 2019;316(5):F898–F905. doi: 10.1152/ajprenal.00539.2018.
- Aoike DT, Baria F, Kamimura MA, et al. Home-based versus center-based aerobic exercise on cardiopulmonary performance, physical function, quality of life and quality of sleep of overweight patients with chronic kidney disease. Clin Exp Nephrol. 2018;22(1):87–98. doi: 10.1007/s10157-017-1429-2.
- Barcellos FC, Del VF, Reges A, et al. Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens. 2018;32(6):397–407. doi: 10.1038/s41371-018-0055-0.
- Liang F, Huo W, Ou Y, et al. Effects of different types of exercise on motor function in patients with chronic kidney disease. Chin J Rehabil Theory Pract. 2018;24(02):208–213.
- Watson EL, Gould DW, Wilkinson TJ, et al. Twelve-week combined resistance and aerobic training confers greater benefits than aerobic training alone in nondialysis CKD. Am J Physiol Renal Physiol. 2018;314(6):F1188–F1196. doi: 10.1152/ajprenal.00012.2018.
- Headley S, Germain M, Wood R, et al. Blood pressure response to acute and chronic exercise in chronic kidney disease. Nephrology (Carlton). 2017;22(1):72–78. doi: 10.1111/nep.12730.
- Hiraki K, Shibagaki Y, Izawa KP, et al. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: a randomized pilot and feasibility trial. BMC Nephrol. 2017;18(1):198. doi: 10.1186/s12882-017-0613-7.
- Kong Y, Wang J, Zhou Y, et al. The effect of resistance training on early diabetic nephropathy in patients with type 2 diabetes mellitus. Sci Rep. 2017;14(1):2761.
- Miele EM, Headley S, Germain M, et al. High-density lipoprotein particle pattern and overall lipid responses to a short-term moderate-intensity aerobic exercise training intervention in patients with chronic kidney disease. Clin Kidney J. 2017;10(4):524–531. doi: 10.1093/ckj/sfx006.
- Tang Q, Yang B, Fan F, et al. Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: a randomized controlled trial in China. Int J Nurs Pract. 2017;23:e12519.
- Zhou F, Zhao Z, Wang L, et al. The effects of mid-intense aerobic training on peak oxygen uptake and anaerobic threshold in patients with chronic kidney disease. Chin J Rehabil Med. 2017;34:525–529.
- Leehey DJ, Collins E, Kramer HJ, et al. Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2016;44(1):54–62. doi: 10.1159/000447703.
- Liang F, Chao M, Wang Z, et al. The effects of exercises of different types on renal function and the risk factors associated with cardiovascular disease in patients with chronic kidney disease. Chin J Rehabil Med. 2016;30:1234–1238.
- Aoike DT, Baria F, Kamimura MA, et al. Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol. 2015;47(2):359–367. doi: 10.1007/s11255-014-0894-8.
- Greenwood SA, Koufaki P, Mercer TH, et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis. 2015;65(3):425–434. doi: 10.1053/j.ajkd.2014.07.015.
- Howden EJ, Coombes JS, Strand H, et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65(4):583–591. doi: 10.1053/j.ajkd.2014.09.017.
- Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, et al. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3–4: a randomized controlled trial. Am J Kidney Dis. 2015;66(2):285–296. doi: 10.1053/j.ajkd.2015.03.015.
- Baria F, Kamimura MA, Aoike DT, et al. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant. 2014;29(4):857–864. doi: 10.1093/ndt/gft529.
- Headley S, Germain M, Wood R, et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis. 2014;64(2):222–229. doi: 10.1053/j.ajkd.2014.02.022.
- Shi ZM, Wen HP, Liu FR, et al. The effects of tai chi on the renal and cardiac functions of patients with chronic kidney and cardiovascular diseases. J Phys Ther Sci. 2014;26(11):1733–1736. doi: 10.1589/jpts.26.1733.
- Headley S, Germain M, Milch C, et al. Exercise training improves HR responses and V O2peak in predialysis kidney patients. Med Sci Sports Exerc. 2012;44(12):2392–2399. doi: 10.1249/MSS.0b013e318268c70c.
- Gregory SM, Headley SA, Germain M, et al. Lack of circulating bioactive and immunoreactive IGF-I changes despite improved fitness in chronic kidney disease patients following 48 weeks of physical training. Growth Horm IGF Res. 2011;21(1):51–56. doi: 10.1016/j.ghir.2010.12.005.
- Mustata S, Groeneveld S, Davidson W, et al. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43(4):1133–1141. doi: 10.1007/s11255-010-9823-7.
- Souza MK, Neves R, Rosa TS, et al. Resistance training attenuates inflammation and the progression of renal fibrosis in chronic renal disease. Life Sci. 2018;206:93–97. doi: 10.1016/j.lfs.2018.05.034.
- Hanatani S, Izumiya Y, Araki S, et al. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol. 2014;25(12):2800–2811. doi: 10.1681/ASN.2013091025.
- Flahault A, Metzger M, Chassé J-F, et al. Low serum creatine kinase level predicts mortality in patients with a chronic kidney disease. PLoS One. 2016;11(6):e0156433. doi: 10.1371/journal.pone.0156433.
- Peng CC, Chen KC, Lu HY, et al. Treadmill exercise improved adriamycin-induced nephropathy. J Biol Regul Homeost Agents. 2012;26(1):15–28.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066.
- Pedralli ML, Marschner RA, Kollet DP, et al. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial Exercise, endothelium and blood pressure. Sci Rep. 2020;10(1):10564. doi: 10.1038/s41598-020-64365-x.
- Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring). 2009;17(0 3):S27–S33. doi: 10.1038/oby.2009.385.
- McPherron AC, Guo T, Bond ND, et al. Increasing muscle mass to improve metabolism. Adipocyte. 2013;2(2):92–98. doi: 10.4161/adip.22500.
- Dankel SJ, Loenneke JP, Loprinzi PD. Determining the importance of meeting muscle-strengthening activity guidelines: is the behavior or the outcome of the behavior (strength) a more important determinant of all-cause mortality? Mayo Clin Proc. 2016;91(2):166–174. doi: 10.1016/j.mayocp.2015.10.017.
- Lopez-Jaramillo P, Lopez-Lopez JP, Tole MC, et al. Muscular strength in risk factors for cardiovascular disease and mortality: a narrative review. Anatol J Cardiol. 2022;26(8):598–607. doi: 10.5152/AnatolJCardiol.2022.1586.
- Zhang L, Wang Y, Xiong L, et al. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: evidence from a meta-analysis. BMC Nephrol. 2019;20(1):398. doi: 10.1186/s12882-019-1586-5.
- Pei G, Tang Y, Tan L, et al. Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int Urol Nephrol. 2019;51(10):1787–1795. doi: 10.1007/s11255-019-02234-x.
- Thompson S, Wiebe N, Padwal RS, et al. The effect of exercise on blood pressure in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14(2):e0211032. doi: 10.1371/journal.pone.0211032.
- Chen CC, Huang YY, Li XQ, et al. Impact of resistance exercise on patients with chronic kidney disease. BMC Nephrol. 2024;25(1):115. doi: 10.1186/s12882-024-03547-5.
- Pan B, Ge L, Xun YQ, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. doi: 10.1186/s12966-018-0703-3.
- Loe H, Nes BM, Wisløff U. Predicting VO2peak from submaximal- and peak exercise models: the HUNT 3 fitness study, Norway. PLoS One. 2016;11(1):e0144873. doi: 10.1371/journal.pone.0144873.
- Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi: 10.1681/ASN.2012070702.
- Tamaki M, Hagiwara A, Miyashita K, et al. Improvement of physical decline through combined effects of muscle enhancement and mitochondrial activation by a gastric hormone ghrelin in male 5/6Nx CKD model mice. Endocrinology. 2015;156(10):3638–3648. doi: 10.1210/en.2015-1353.
- Yazdi PG, Moradi H, Yang JY, et al. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int J Clin Exp Med. 2013;6(7):532–539.
- Mendes S, Leal DV, Baker LA, et al. The potential modulatory effects of exercise on skeletal muscle redox status in chronic kidney disease. Int J Mol Sci. 2023;24(7):6017. doi: 10.3390/ijms24076017.
- Baker LA, March DS, Wilkinson TJ, et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 2022;23(1):75. doi: 10.1186/s12882-021-02618-1.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381.