Abstract
Background
Podocytes, as intrinsic renal cells, can also express MHC-II and costimulatory molecules under inflammatory conditions, suggesting that they may act as antigen-presenting cells (APCs) to activate immune cell responses and then lead to immune-mediated renal injury. They are already recognized as main targets in the pathogenic mechanism of hepatitis B virus (HBV)-associated glomerulonephritis (HBV-GN). Previous studies also have indicated that inflammatory cells infiltration and immune-mediated tissue injury are evident in the kidney samples of patients with HBV-GN. However, the role of podocytes immune disorder in the pathogenic mechanism of HBV-GN remains unclear.
Methods
Renal function and inflammatory cells infiltration were measured in HBV transgenic (HBV-Tg) mice. In vitro, podocytes/CD4+ T cells or macrophages co-culture system was established. Then, the expression of HBx, CD4, and CD68 was determined by immunohistochemistry, while the expression of MHC-II, CD40, and CD40L was determined by immunofluorescence. Co-stimulatory molecules expression was examined by flow cytometry. The levels of inflammatory factors were detected by ELISA.
Results
In vivo, renal function was obviously impaired in HBV-Tg mice. HBx was significantly upregulated and immune cells infiltrated in the glomerulus of HBV-Tg mice. Expression of MHC-II and costimulatory molecule CD40 increased in the podocytes of HBV-Tg mice; CD4+ T cells exhibited increased CD40L expression in glomerulus. In vitro, CD40 expression was markedly elevated in HBx-podocytes. In co-culture systems, HBx-podocytes stimulated CD4+ T cells activation and caused the imbalance between IFN-γ and IL-4. HBx-podocytes also enhanced the adhesion ability of macrophages and induced the release of proinflammatory mediators.
Conclusion
Taken together, these podocyte-related immune disorder may be involved in the pathogenic mechanism of HBV-GN.
Introduction
Persistent HBV infection may cause HBV-associated glomerulonephritis (HBV-GN), which has been considered as one of the main secondary kidney diseases in China [Citation1]. HBV-GN is mainly characterized by glomerular damage [Citation2]. As an important part of the glomeruli, podocytes play a crucial role in the glomerular filtration barrier, and act as main damage targets in the progression of HBV-GN [Citation3]. Podocytes are often seen as the “victim” of immune cells infiltration, while they can also aggravate immune-mediated renal injury [Citation4,Citation5]. Under inflammatory stimuli, podocytes can exhibit upregulation of MHC-II and co-stimulatory molecules, suggesting that podocytes may function as professional antigen-presenting cells (APCs) and contribute to the inflammatory defense and immune reactions, ultimately resulting in renal damage [Citation5–8]. However, whether podocyte dysimmunity can regulate inflammatory immune responses causing glomerular damage in HBV-GN is unclear.
HBV transactivator protein, HBx, is a small soluble protein composed of 154 amino acids, which has a pivotal role in regulating the transcription and replication of HBV [Citation9,Citation10]. It can affect cellular transcription, signal transmission, cell proliferation regulation, DNA repair, and then result in the immune cells dysfunction and release of proinflammatory cytokines [Citation3,Citation11–14]. Previously conducted research has shown that HBx can be identified in the podocytes of HBV-GN patients [Citation3,Citation15,Citation16], indicating the role of HBx in podocyte injury. Our earlier studies have also found that HBx can induce the MHC-II and costimulatory molecules upregulation in human podocytes [Citation17], but whether HBx-infected podocytes can promote immune cell responses and aggravate the immune-mediated renal injury of HBV-GN is still obscure. Therefore, our study investigated the role of podocytes in regulating immune cell responses in HBV-GN.
Materials and methods
Animals
Fourteen 6-week-old male HBV transgenic (HBV-Tg) mice were purchased from Vitalstar Biotechnology Co., Ltd. (Beijing, PR China). Fourteen age-matched male wild-type (WT) C57BL/6 mice were used as controls. All mice were reared in SPF environment at 22 °C with a 12 h/12 h light/dark cycle. Mice were allowed under specific pathogen-free conditions. At the age of 24 weeks, 24 h urine and blood were collected. We detected 24 h proteinuria, serum creatinine (Scr) and albumin (Alb) using an automatic biochemical analyzer. Meanwhile, kidneys samples were collected for immunohistochemistry and immunofluorescence analysis. All animal procedures were approved by the Animal Care and Use Committee of Qingdao Municipal Hospital, University of Health and Rehabilitation Sciences, and performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Mice kidneys were removed and immediately fixed in 4% paraformaldehyde, then embedded in paraffin, and sliced into sections (4 μm). The 4 μm sections were deparaffinized, rehydrated, blocked with 5% BSA and then incubated with primary antibodies to HBx, CD4, CD68 (Abcam, Cambridge, MA, UK) overnight at 4 °C. The sections were then incubated with secondary antibodies and analyzed. Images were taken by a light microscope (Olympus, DP70, Tokyo, Japan). Image Pro Plus 6.0 (Media Cybemetics, USA) was used to make semi-quantitative analysis by sum of integrated optical density (IODsum).
Immunofluorescence
The 4 μm sections were blocked with 5% BSA and then incubated overnight at 4 °C with primary antibodies to MHC-II, CD40, CD4, CD40L (Abcam, Cambridge, MA, UK). After three times washing, the sections were incubated with the corresponding secondary antibodies for 2 h at room temperature, and then stained DAPI at room temperature for 3 min. Images were taken under a fluorescent microscope (Olympus, BX51TF, Tokyo, Japan). Semi-quantitative analysis of mean optical density in each group.
Cell culture
The human podocytes were kindly given by Prof. Chuanming Hao (Huashan Hospital, Fudan University) and cultured as previously reported [Citation3]. The cells were grown to permissive temperature at 33 °C. When the cells reached approximately 70%–80% confluence, they were transferred to 37 °C for differentiation in the medium without insulin, transferrin or selenium for approximately one week. The medium for podocytes consisted of RPMI 1640 medium (HyClone, USA) added with 10% FBS (Gibco, USA). At both temperatures, the cells were fed with fresh medium 3 times per week.
Plasmids and transfection
The HBx-expressing plasmid was constructed and obtained from Genomeditech (Shanghai, China). The HBx gene sequences of primers were described previously [Citation3]. Human podocytes were transfected with HBx plasmid using Lipofectamine 3000 (Invitrogen, USA). The podocytes transfected with HBx plasmid were named as HBx-podocytes; podocytes transfected with empty vector were called negative control (NC) cells. The untreated podocytes were used as control (Cont) cells.
Human podocyte/CD4+ T cell co-culture system
Peripheral blood mononuclear cells (PBMCs) were kindly provided by Prof. Weijie Yuan (Shanghai General Hospital, Shanghai Jiao Tong University) and CD4+ T cells (> 95%) were further separated by microbeads (Miltenyi Biotech, Germany). Co-cultured procedures were carried out as previously reported [Citation13,Citation18]. Human podocytes transfected with HBx plasmid were raised in 6-well plate, then newly isolated CD4+ T cells (6 × 105) were appended. These cells were co-cultured for 24 h, supernatants then were obtained to detect inflammatory mediators using ELISA. Podocytes and CD4+ T cells were obtained respectively to test CD40 and CD40L expression with flow cytometry.
Flow cytometry
Fluorescence-activated cell sorting (FACS) analysis was performed using a Kaluza flow cytometer (Beckman Coulter, Miami, USA). Cells were collected and then incubated using antibodies against CD40 (Santa Cruz, CA, USA), CD40L (e-Bioscience, San Diego, USA). CD40 and CD40L were detected by flow cytometry with a fluorescence-activated cell sorter (FACS Calibur cytometer, USA).
Human podocyte/macrophage co-culture system
Macrophages were produced from monocytes isolated from PBMCs kindly provided by Prof. Weijie Yuan (Shanghai General Hospital, Shanghai Jiao Tong University). The CD14+ monocytes (> 98%) were enriched with magnetic bead sorting and then cultured for one week in the RPMI-1640 medium (Gibco, USA) with 10% FBS and 50 U/mL GM-CSF (Sangon Biotech, Shanghai, China) to generate macrophages. Macrophages were cultured at 106/mL in 24-well plate, permitted to adhere, and then cultured for 12 h with human podocytes supernatants. The supernatants were collected and levels of inflammatory cytokines were detected using ELISA.
Adhesion ability of macrophages assay
The adhesion ability of macrophages assay was performed as previously reported [Citation19]. After co-culture for 6 h with human podocytes supernatants, 0.5 mL macrophages suspension (106/ml) were cultured in EP tubes or 96-well plates (pretreated with fibronectin) at 37 °C and shaken for 1 h. The 10 μL suspension were obtained to count the cells without adhesion in cell counting chamber with a light microscope. Adhesion indexes (AI) were calculated as follows: AI = 100 – [(macrophages/ml supernatant)/(macrophages/ml original sample)] × 100. Alternatively, macrophages were seeded into 96-well plates at a density of 5 × 104 cells/well for 6 h. Then, CCK-8 solution (10 μL) was added into each well. Optical density was measured at 450 nm, and the cell adhesion ability was calculated as follows: cell adhesion (%) = [(OD value of adherent cells)/(OD value of total cells)] × 100.
ELISA
The IFN-γ, IL-4, MCP-1, IL-1β, and TNF-α levels in the supernatants were detected by ELISA kits according to manufacturer’s recommendations (eBioscience, San Diego, CA, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). The results were analyzed using Student’s t-test, χ2 test, or one-way analysis of variance. Statistical analysis was performed by SPSS 18.0 software (Chicago, IL, USA). P-value < 0.05 was considered statistically significant.
Results
Renal impairment in HBV-Tg mice
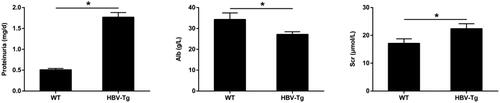
To measure renal function in vivo, WT mice and HBV-Tg mice were used for the following studies. 24 h urine protein and serum creatinine were markedly increased in HBV-Tg mice than in WT mice at 24 weeks of age. Conversely, serum albumin was remarkably decreased in HBV-Tg mice compared to WT mice (shown in ). The results indicated that renal function is obviously impaired in HBV-Tg mice.
HBx upregulation and immune cells infiltration in HBV-Tg mice
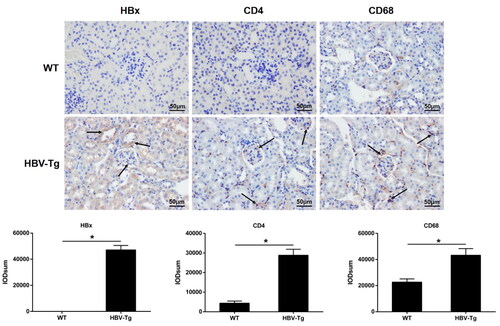
In WT mice, HBx expression wasn’t apparent. But, in HBV-Tg mice, its expression could be identified in glomeruli and renal tubules (shown in ). Then, the infiltration of CD4+ T cells and macrophages was measured in HBV-Tg mice. Immunohistochemistry analysis indicated that CD4 and CD68 expression were significantly higher in HBV-Tg mice than in WT mice (shown in ). The results showed that HBV-Tg mice have notable glomerular immune cells infiltration.
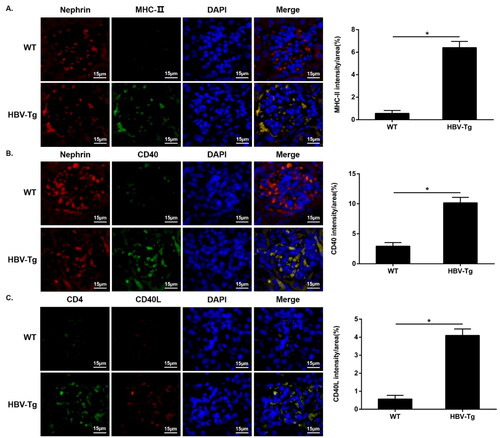
Upregulated MHC-II and costimulatory molecule CD40 expression in the podocytes of HBV-Tg mice
Next, we tested the expression of MHC-II and CD40 in glomeruli by immunofluorescence. Immunofluorescence analysis revealed that MHC-II and CD40 were colocalized at the podocytes, and their expression was significantly increased in the podocytes of HBV-Tg mice (shown in ). We then stained CD4 and CD40L using the same method. The data showed that CD4 and CD40L were also colocalized at the glomeruli of HBV-Tg mice; and infiltrated CD4+ T cells exhibited increased level of CD40L in HBV-Tg mice than in WT mice (shown in ).
Figure 3. Upregulated MHC-II and CD40 expression in the podocytes of HBV-Tg mice, and infiltrated CD4+ T cells exhibited increased CD40L expression in the glomeruli. Scale bar: 15 μm. (A-C) Immunofluorescence for MHC-II (a), CD40 (B), CD4 and CD40L (C) in WT and HBV-Tg mice. Analysis of mean optical density in each group. Data are presented as the mean ± SD. *p < 0.05.
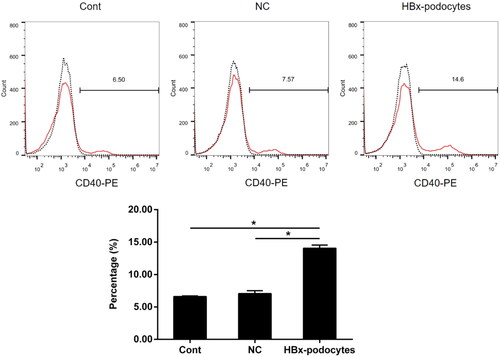
Significantly upregulated costimulatory molecule CD40 expression in HBx-podocytes
Previous studies have showed that podocytes can express costimulatory molecules [Citation7,Citation17]. To analyze the effect of HBx on CD40 expression in podocytes, we firstly transfected HBx plasmid into human podocytes, and confirmed the overexpression of HBx by western blotting [Citation3]. We then determined the expression of CD40 on the surface of podocytes with or without HBx transfection by Flow cytometry. The data revealed that HBx markedly increased the cell-surface CD40 expression in human podocytes (shown in ).
Figure 4. Significantly upregulated the CD40 expression in HBx-podocytes. Flow cytometry analysis of CD40 expression in human podocytes with empty vector lentivirus (NC) or HBx-expressing lentivirus (HBx-podocytes) transfection and without transfection (cont) for 24 h. Data are presented as the mean ± SD (n = 3). *p < 0.05.
HBx-podocytes modulate the CD40L expression in CD4+ T cells
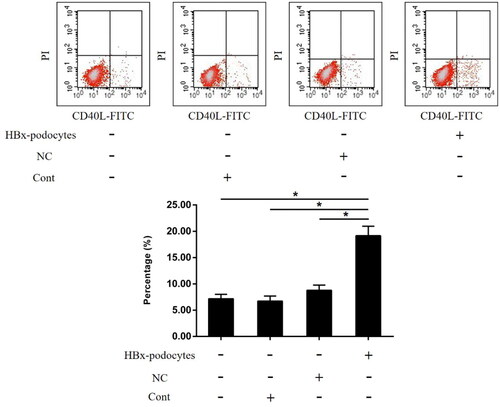
CD40 ligand (CD40L) is primarily expressed in the CD4+ T cells after activation. And the CD40/CD40L interactions have previously been demonstrated to activate APCs and modulate T-cell function. To analyze whether HBx-podocytes regulate the expression of CD40L, CD4+ T cells were co-cultivated with human podocytes with or without HBx transfection. We then confirmed the expression of CD40L in CD4+ T cells co-cultured with podocytes under the same stimulating conditions by Flow cytometry. HBx-podocytes remarkably increased CD40L expression in CD4+ T cells compared with those controls (shown in ). Based on these findings, we suspected that HBx-podocytes may regulate specific T cells function by the CD40/CD40L interactions.
HBx-podocytes alter the cytokines production of activated CD4+ T cells
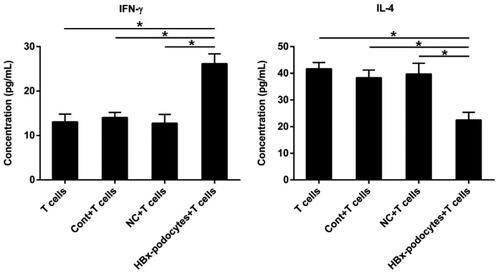
Glomerular infiltrating CD4+ T cells have long been considered to lead to renal impairment, so we explored whether HBx-podocytes also affected the activation of CD4+ T cells. We next detected the levels of IFN-γ and IL-4 in the supernatants of co-culture systems consisting of human podocytes and CD4+ T cells using ELISA. The results indicated that podocytes with or without empty vector couldn’t induce IFN-γ production compared with IFN-γ produced only by CD4+ T cells (shown in ). However, podocytes with HBx plasmid obviously promoted the release of IFN-γ in the supernatants of co-cultured systems. On the contrary, CD4+ T cells generated much fewer IL-4 after co-culturing with podocytes with the same conditions (shown in ). These results demonstrated that HBx can stimulate podocytes to activate CD4+ T cells and result in the imbalance between IFN-γ and IL-4.
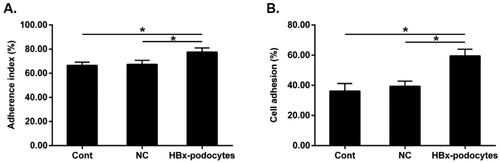
Enhanced macrophage adherence after HBx-podocytes supernatants treatment
We next investigated the adhesion ability of macrophages in vitro. The results showed that the adhesion ability of macrophages increased in all groups. However, the adhesion ability of macrophages treated with HBx-podocytes supernatants was much higher compared to controls (shown in ), suggesting that HBx-podocytes can enhance macrophages adherent capacity.
Figure 7. Enhanced macrophage adherence after HBx-podocytes supernatants treatment. Macrophages adhesion ability after co-culture with supernatants of cont, NC, and HBx-podocytes groups for 6 h was analyzed. (A) Microscopic counting method; (B) CCK-8 assay. Data are presented as the mean ± SD (n = 3). *p < 0.05.
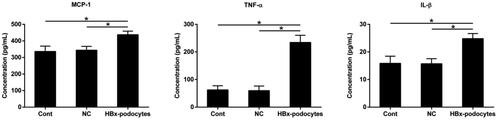
HBx-podocytes stimulate the inflammatory cytokines secretion of macrophages
It has been proved that macrophages can contribute to the modulation of immune response, we then tested whether HBx-podocytes supernatants could stimulate the activation of macrophages. Macrophages were co-cultured with human podocytes supernatants after transfection with or without HBx plasmid for 12 h. The releases of MCP-1, IL-1β, and TNF-α in culture medium were measured by ELISA. This data indicated that the levels of inflammatory cytokines were remarkably elevated in culture medium of macrophages stimulated with supernatants of HBx-podocytes compared to control groups (shown in ).
Discussion
HBV infection is prevalent in China, and persistent infection with this virus can lead to the progression and development of HBV-GN. The pathogenic mechanism of HBV is closely related to the deposition of HBV-related immune complex, direct HBV infection of intrinsic renal cells, or immune dysfunction resulting from HBV infection [Citation20]. However, the specific pathogenesis is still not fully elucidated. HBx plays a crucial regulatory role in the HBV replication and transcription. And it has been detected in the glomerulus of patients with HBV-GN, implying that HBx may be implicated in glomerular injury. However, the exact mechanism of HBx-induced renal injury in HBV-GN remains unknown.
Earlier studies have demonstrated that HBx induces the MHC-II and co-stimulating molecules expression in renal tubular cells, resulting in the inflammation defense and immune response [Citation13]. HBx also enhances the expression of MHC-II and co-stimulating molecules in podocytes [Citation17]. These findings suggest that intrinsic renal cells exhibit immune cell-like functions when infected by HBx. However, more evidence has indicated that impaired podocytes may recruit inflammatory and immune cells or even act as APCs to result in immune-mediated renal injury [Citation21,Citation22]. In present study, immunohistochemistry analysis of HBV-Tg mice kidney sections indicated that the expression of HBx and infiltration of CD4+ T cells and macrophages were significantly evident in glomeruli. Importantly, analysis of HBV-GN patients renal biopsies also revealed that CD4+ T and macrophages infiltration was much more obvious, and their infiltration was associated with glomerular and tubulointerstitial injury [Citation1,Citation3,Citation12,Citation14]. Thus, we hypothesize that HBx-infected podocytes can function as APCs, and subsequently induce immune cell responses, which may contribute to HBV-GN progression.
Podocytes have been recognized as the main antigen targets in variety of glomerular diseases [Citation5,Citation7]. Recent studies have found that disorder podocytes may act as APCs, activate T cells and lead to the local release of chemokines, thereby contributing to immune microenvironment disorders in local renal tissue [Citation5,Citation7,Citation17,Citation22,Citation23]. To activate T cells, T cell antigen receptor-peptide-MHC signaling and co-stimulating molecule presented by APCs are needed [Citation24]. The fact that intrinsic renal cells expressing MHC-II are also needed for immune-mediated renal injury was reported previously [Citation25]. Our study showed that MHC-II and CD40 were co-expressed in podocytes, and their expression were obviously upregulated in the podocytes of HBV-Tg mice. And CD4+ T cells exhibited increased expression of CD40L in glomerulus. To further explore whether HBx-stimulated podocytes can activate T cell responses, we co-cultured human podocytes with CD4+ T cells. Then, we confirmed that HBx stimulated the upregulation of CD40 in podocytes. Additionally, HBx-stimulated podocytes promoted the expression of CD40L of CD4+ T cells. It has been reported that CD40 is expressed in professional APCs and epithelial cells, and it can regulate the activation and proliferation of T cells [Citation26,Citation27]. Based on these results, we believe that HBx-stimulated podocytes can modulate the activation of CD4+ T cells, which may be associated with the activation of CD40-CD40L signaling. In addition, we found that activated CD4+ T cells could significantly increase IFN-γ production while reducing IL-4 production. Previous reports have also indicated that the balance between IFN-γ and IL-4 plays an important role in Th0 cells differentiating into Th1 cells or Th2 cells. So, we speculate that the imbalance between IFN-γ and IL-4 might disrupt the balance of Th1 and Th2 cells, which is critical under persistent infection with HBV [Citation28].
Researchers have discovered that podocytes are also closely related to innate immune responses, resulting in glomerular immune injury [Citation21]. As it is known, macrophages play an essential role in the innate immune responses resisting virus infection [Citation29]. We further assessed whether macrophages could respond to treatment with HBx-podocytes supernatants. In present study, we revealed that the adhesion indexes of macrophages were remarkably increased after treatment with HBx-podocytes supernatants, suggesting that HBx-podocytes promote the initial phase of inflammatory response. HBx-podocytes also increased the production of MCP-1. It is clear that MCP-1 can stimulate more macrophages and lymphocytes activation, and these activated cells then infiltrated into the renal tissue, resulting in immune-mediated damage [Citation30]. Also, activated macrophages can induce the release of proinflammatory mediators, then contributing to the proliferation and activation of B cells [Citation31,Citation32], consequently promote inflammation and exacerbate HBV-GN progression.
Based on our findings, we propose an unexpectedly active role for glomerular podocytes in local immune responses under viral infection, which may have broader importance for the progression and immunotherapies of immunologic kidney diseases, such as membranous glomerulopathy, minimal change disease, and FSGS as well as other viral nephropathy. However, this study has certain limitations. For example, the corresponding experiments were carried out only in the cultured human podocyte line. Renal biopsy samples from HBV-GN patients were not collected for verification. Furthermore, the specific molecular mechanisms behind it have not been further explored. Although there are shortcomings in the present study, it may still provide new clues for the pathogenic mechanism of HBV-GN.
In conclusion, our studies demonstrate that HBx can stimulate podocytes to act as professional APCs, thereby promote immune cell responses and lead to inflammatory cytokines release or even imbalance, probably contributing to HBV-GN progression.
Ethics approval and consent to participate
This experimental study was reviewed and approved by the ethics committee of Qingdao Municipal Hospital, University of Health and Rehabilitation Sciences. All mice were kept in specific pathogen-free conditions at the Animal Resource Center at Qingdao Municipal Hospital. All experiments were performed in accordance with relevant guidelines and regulations. We obtained informed consent from the owner to use the animals in our study.
Author contributions
Yitong Yang and Luyan Bian conceived the study and wrote the manuscript; Luyan Bian, Yuchao Niu and Huasheng Du performed most of the experiments and analyzed the data. Weijie Yuan provided expertise and advice. Huasheng Du and Yitong Yang oversaw the project. All authors reviewed the manuscript.
Acknowledgements
We highly appreciate Chuanming Hao (Huashan Hospital, Fudan University) for providing the human podocyte cell line required for this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Yang YT, Wang X, Zhang YY, et al. The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis. Cell Death Dis. 2019;10(4):1. doi: 10.1038/s41419-019-1514-4.
- Shah AS, Amarapurkar DN. Spectrum of hepatitis B and renal involvement. Liver Int. 2018;38(1):23–11. doi: 10.1111/liv.13498.
- Guan H, Zhu N, Tang G, et al. DNA methyltransferase 1 knockdown reverses PTEN and VDR by mediating demethylation of promoter and protects against renal injuries in hepatitis B virus-associated glomerulonephritis. Cell Biosci. 2022;12(1):98. doi: 10.1186/s13578-022-00835-1.
- Bruno V, Mühlig AK, Oh J, et al. New insights into the immune functions of podocytes: the role of complement. Mol Cell Pediatr. 2023;10(1):3. doi: 10.1186/s40348-023-00157-3.
- Li S, Liu Y, He Y, et al. Podocytes present antigen to activate specific T cell immune responses in inflammatory renal disease. J Pathol. 2020;252(2):165–177. doi: 10.1002/path.5508.
- Coers W, Brouwer E, Vos JT, et al. Podocyte expression of MHC class I and II and intercellular adhesion molecule-1 (ICAM-1) in experimental pauci-immune crescentic glomerulonephritis. Clin Exp Immunol. 1994;98(2):279–286. doi: 10.1111/j.1365-2249.1994.tb06138.x.
- Goldwich A, Burkard M, Olke M, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol. 2013;24(6):906–916. doi: 10.1681/ASN.2012020133.
- Cai M, Zhou T, Wang X, et al. DC-SIGN expression on podocytes and its role in inflammatory immune response of lupus nephritis. Clin Exp Immunol. 2016;183(3):317–325. doi: 10.1111/cei.12723.
- Kim GW, Siddiqui A. Hepatitis B virus X protein recruits methyltransferases to affect cotranscriptional N6-methyladenosine modification of viral/host RNAs. Proc Natl Acad Sci U S A. 2021;118(3):e2019455118. doi: 10.1073/pnas.2019455118.
- Liu W, Yao Q, Su X, et al. Molecular insights into Spindlin1-HBx interplay and its impact on HBV transcription from cccDNA minichromosome. Nat Commun. 2023;14(1):4663. doi: 10.1038/s41467-023-40225-w.
- Xia LM, Huang WJ, Wu JG, et al. HBx protein induces expression of MIG and increases migration of leukocytes through activation of NF-kappaB. VIROLOGY. 2009;385(2):335–342. doi: 10.1016/j.virol.2008.11.042.
- Wang X, Zhou Y, Zhu N, et al. The deposition of Notch1 in hepatitis B virus-associated nephropathy and its role in hepatitis B virus X protein-induced epithelial-mesenchymal transdifferentiation and immunity disorder in renal tubular epithelial cells. J Viral Hepat. 2014;21(10):734–743. doi: 10.1111/jvh.12244.
- Wang X, Wang L, Zhu N, et al. Hepatitis B virus X protein modulates renal tubular epithelial cell-induced T-cell and macrophage responses. Immunol Cell Biol. 2016;94(3):266–273. doi: 10.1038/icb.2015.85.
- Yang Y, Wang X, Zhang Y, et al. Hepatitis B virus X protein and proinflammatory cytokines synergize to enhance TRAIL-induced apoptosis of renal tubular cells by upregulation of DR4. Int J Biochem Cell Biol. 2018;97:62–72. doi: 10.1016/j.biocel.2018.02.006.
- He P, Zhang D, Li H, et al. Hepatitis B virus X protein modulates apoptosis in human renal proximal tubular epithelial cells by activating the JAK2/STAT3 signaling pathway. Int J Mol Med. 2013;31(5):1017–1029. doi: 10.3892/ijmm.2013.1295.
- Feng M, Yu Y, Chen Y, et al. HBx-induced PLA(2)R overexpression mediates podocyte pyroptosis through the ROS-NLRP3 signaling pathway. Ren Fail. 2023;45(1):2170808. doi: 10.1080/0886022X.2023.2170808.
- Yang YT, Du Y, Yuan WJ, et al. [Role of histone demethylase KDM6B in HBx-mediated podocyte-macrophage transdifferentiation]. Zhonghua Yi Xue Za Zhi. 2021;101(12):866–871. doi: 10.3760/cma.j.cn112137-20210119-00170.
- Chen Y, Zhang J, Li J, et al. Triptolide inhibits B7-H1 expression on proinflammatory factor activated renal tubular epithelial cells by decreasing NF-kappaB transcription. Mol Immunol. 2006;43(8):1088–1098. doi: 10.1016/j.molimm.2005.07.026.
- Ortega E, García JJ. De la Fuente M: modulation of adherence and chemotaxis of macrophages by norepinephrine. Influence of ageing. Mol Cell Biochem. 2000;203(1–2):113–117.
- Ren J, Wang L, Chen Z, et al. Gene expression profile of transgenic mouse kidney reveals pathogenesis of hepatitis B virus associated nephropathy. J Med Virol. 2006;78(5):551–560. doi: 10.1002/jmv.20575.
- Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19(4):704–713. doi: 10.1681/ASN.2007040395.
- Peng W, Pei GQ, Tang Y, et al. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J Transl Med. 2019;17(1):406. doi: 10.1186/s12967-019-02157-2.
- Xia H, Bao W, Shi S. Innate immune activity in glomerular podocytes. Front Immunol. 2017;8:122. doi: 10.3389/fimmu.2017.00122.
- Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2(6):439–446. doi: 10.1038/nri823.
- Li S, Kurts C, Köntgen F, et al. Major histocompatibility complex class II expression by intrinsic renal cells is required for crescentic glomerulonephritis. J Exp Med. 1998;188(3):597–602. doi: 10.1084/jem.188.3.597.
- Rogers NJ, Jackson IM, Jordan WJ, et al. CD40 can costimulate human memory T cells and favors IL-10 secretion. Eur J Immunol. 2003;33(4):1094–1104. doi: 10.1002/eji.200323475.
- Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L). Crit Rev Immunol. 2017;37(2–6):371–420. doi: 10.1615/CritRevImmunol.v37.i2-6.100.
- Jiang Y, Ma Z, Xin G, et al. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm. 2010;2010:143026. doi: 10.1155/2010/143026.
- Unanue ER, Allen PM. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987;236(4801):551–557. doi: 10.1126/science.2437650.
- Segerer S, Nelson PJ, Schlöndorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11(1):152–176. doi: 10.1681/ASN.V111152.
- Sabel MS, Arora A, Su G, et al. Synergistic effect of intratumoral IL-12 and TNF-alpha microspheres: systemic anti-tumor immunity is mediated by both CD8+ CTL and NK cells. SURGERY. 2007;142(5):749–760. doi: 10.1016/j.surg.2007.05.008.
- Watanabe H, Gehrke S, Contassot E, et al. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J Immunol. 2008;180(9):5826–5832. doi: 10.4049/jimmunol.180.9.5826.