Abstract
Background and objective
Chronic kidney disease (CKD) is a global health concern that is frequently associated with hypertension. Inflammation is an important factor in the development of both illnesses. The Dietary Inflammation Index (DII) has evolved as a way to measure how much a diet can cause inflammation, which may impact CKD, especially in hypertensive persons. The study’s goal is to investigate the link between DII and the occurrence of CKD in hypertensive individuals.
Methods
This study examined data from 22940 hypertensive patients from 1999 to 2018 of the National Health and Nutrition Examination Survey (NHANES). The DII was computed using 28 dietary components. CKD was diagnosed based on the estimated glomerular filtration rate and urine albumin-to-creatinine ratio. The link between DII and CKD was explored using sampling-weighted logistic regression and restricted cubic splines.
Results
Higher DII scores were shown to be strongly related with an increased risk of CKD. In the fully adjusted model, this connection remained consistent across demographic and clinical categories.
Conclusions
The study found a strong association between a pro-inflammatory diet and an elevated risk of CKD in hypertensive individuals, emphasizing the potential of dietary changes in CKD management.
Introduction
Chronic kidney disease (CKD) is a major public health issue around the world, with a direct relationship to hypertension [Citation1]. In recent years, the incidence of CKD has steadily increased, aggravated by lifestyle changes and an aging population [Citation2]. Hypertension, as a key risk factor for CKD, not only deteriorates renal function but also raises the risk of cardiovascular disease [Citation3]. Despite increasing research in CKD and hypertension, mysteries remain in pathogenic processes, early detection, and effective therapies, demanding ongoing exploration to address these gaps.
Inflammation is a complicated biological reaction that acts as the body’s natural defense against damage and infection [Citation4]. However, prolonged or severe inflammatory reactions can cause the development of numerous disorders, including CKD and hypertension [Citation5,Citation6]. Recent studies have highlighted the involvement of inflammation in the etiology of CKD and hypertension. Chronic inflammation not only exacerbates renal impairment but may also increase hypertension by altering vascular function [Citation7]. While the significance of inflammation in both disorders is well understood, successfully regulating and modifying the inflammatory response to reduce the impact on CKD and hypertension remains an unsolved difficulty.
Diet, a major element determining individual health, has received increased scientific focus due to its link to chronic illnesses. The DII has been used to investigate how various dietary patterns impact inflammation levels [Citation8]. Studies show that diets with high DII scores may increase inflammatory responses, increasing the risk of CKD and hypertension [Citation9,Citation10]. However, current research on the link between DII and CKD, particularly in hypertensive individuals, is limited.
Given this context, we postulate that pro-inflammatory foods may enhance the risk of CKD in hypertension patients. Thus, this study aims to look into the relationship between the DII and CKD in hypertensive individuals.
Materials and methods
Data and population source
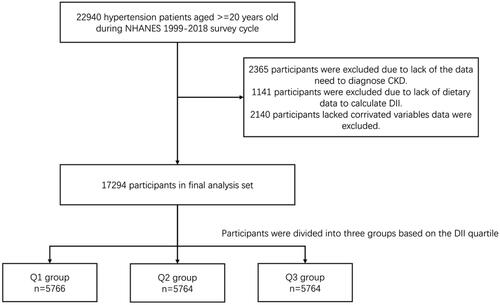
This study used data from the National Health and Nutrition Examination Survey (NHANES) database, focusing on the United States. The cohort included 22,940 hypertensive patients aged 20 and above, from 1999 to 2018. Individuals with preexisting CKD, a history of acute kidney injury (AKI), nephritis, or other significant risk factors for CKD were excluded to ensure the study population consisted of hypertensive patients at risk of developing CKD ().
Hypertension definition
Participants who reported hypertension or were taking antihypertensive medication, as well as those whose systolic/diastolic blood pressure exceeded the criteria (140 mmHg or 90 mmHg), were classified as hypertensive.
Primary outcome
The principal consequence was CKD. Patients were diagnosed with CKD if they met either of the subsequent criteria: (1) eGFR less than 60 mL/min/1.73 m2 or (2) uACR greater than 30 mg/g.
DII Calculation
In our work, we calculated DII using 28 nutrients. The nutrients included cholesterol, fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, n-3 fatty acids, n-6 fatty acids, niacin, retinol, thiamin, riboflavin, pyridoxine, cobalamin, ascorbic acid, vitamin D, tocopherol, iron, magnesium, zinc, selenium, folic acid, and β-carotene. The DII formula also took into account carbs, protein, total fat, alcohol, fiber, caffeine, and energy. The equations from earlier research were utilized to determine DII. NHANES experts estimated nutritional intake information based on participants’ 24-h dietary recall, and specific intake levels are available on the NHANES. We acknowledge the limitation of using a 24-h recall and will discuss this in the manuscript. Detailed information on the DII calculation is provided in the supplement section.
Definition of confounding variable
Participants provided their age, gender, race, education, family income, and body mass index (BMI). Patients also described their smoking and drinking behaviors. Diabetes was defined as being advised by a medical expert that you had Type 2 Diabetes (T2D), or satisfying one of the following criteria: (1) HbA1c >6.5%. (2) Fasting blood glucose ≥7.0 mmol/L. (3) Random blood glucose ≥11.1 mmol/L. (4) 2-h Oral Glucose Tolerance Test (OGTT) blood glucose ≥11.1 mmol/L. (5) Diabetes medication or insulin use.
Grouping method
DII tertiles were determined, and patients were grouped by these values: Group Q1 (DII < 0.96), Group Q2 (DII 0.96–2.66), and Group Q3 (DII ≥ 2.66).
Statistical analysis
According to NHANES rules, suitable sample weights were applied to individuals using the specified formulae, and all statistical computations took these weights into account. We utilized means (standard error) to describe continuous variables and counts and percentages (weighted) for categorical variables. ANOVA and χ2 tests were employed to assess baseline differences between groups for continuous and categorical variables, respectively.
A sampling-weighted logistic regression analysis was used to determine if DII is connected with CKD prevalence. Model 1 reflected uncorrected raw data. Model 2 accounted for age, gender, race, education level, and household income. Model 3 was fully adjusted for age, gender, race, education, family income, smoking status, alcohol intake, and diabetes. Restricted cubic splines (RCS) were employed to illustrate the relationship between DII and CKD. In addition, stratified analyses were performed taking into account age, gender, race, education, family income, smoking status, alcohol use, diabetes, and CVD.
Data was analyzed using R(version 4.2.2) and related package. A p-value of <0.05 was judged statistically significant.
Results
Baseline characteristics
The baseline characteristics table in this research of CKD and hypertension patients included a number of critical statistics (). The average age of the entire sample was 59.78 years, with significant age differences across groups (p = 0.01). The sample’s gender distribution was nearly equal, with females accounting for 50.03% and men for 49.97%. However, significant disparities were identified across groups (p < 0.0001). There were substantial disparities in racial distribution between groups (p < 0.0001). In the whole sample, roughly 29.39% of patients had diabetes, with a significant 70.61% having hypertension. Furthermore, 31.99% of patients had CKD, with significant variations seen between DII quartile groups (p < 0.0001). These statistical variations in baseline characteristics indicated variability across distinct subgroups within the sample, giving valuable background for future examination of the research outcomes.
Table 1. Baseline study population characteristics.
Relationship between DII and chronic kidney disease
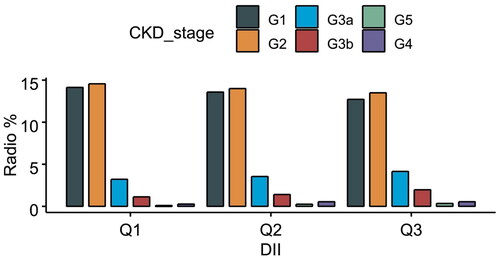
The distribution of each type of CKD is illustrated in . In this investigation on the link between DII and the risk of CKD, several statistical models indicated variable degrees of correlation (). In the Crude Model, DII had a significant positive connection with CKD incidence, with a 95% confidence interval (CI) of 1.11 (1.08, 1.14) and a p-value <0.0001. This implies a strong link between elevated DII and increased CKD risk. After adjusting for age, gender, race, and education level, the correlation remained significant with a 95% confidence interval of 1.09 (1.05, 1.13), and a P-value of <0.0001. In Model 2, when smoking, alcohol use, physical activity, BMI, and other characteristics were taken into account, the connection between DII and CKD was further decreased, with a 95% confidence interval of 1.07 (1.03, 1.11) and a P-value of 0.001. Despite the similar distribution across DII tertiles, our statistical models indicated significant associations between higher DII scores and increased CKD risk after adjusting for potential confounders. These findings emphasize the potential impact of dietary inflammation on CKD risk, even when the raw distributions appear similar.
Table 2. The association between the DII and CKD in patients with hypertension (weighted).
RCS regression
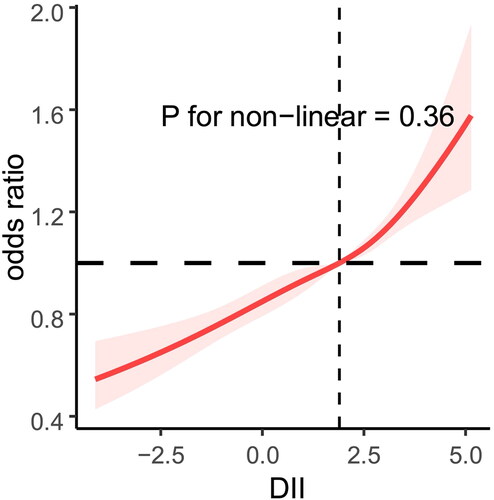
In hypertensive individuals, the RCS revealed a linear connection between DII and CKD (non-linear p = 0.036). The prevalence of chronic renal disease among hypertensive individuals increased in tandem with the DII ().
Subgroup-based analysis
When individuals were classified according to age, gender, race, education, family income, and diabetes, the connection between DII and CKD remained constant. Furthermore, no interaction between stratification characteristics and DII was identified, except for education ().
Table 3. Association between the DII and CKD by the selected subgroups (weighted).
Discussion
The current study systematically explores the relationship between the DII and the incidence of CKD in individuals with hypertension, using a large dataset from NHANES. Our data show a substantial positive relationship between higher DII scores and increased CKD risk in hypertensive individuals, providing insight on the complicated interaction of food, inflammation, and renal function.
Previous research has shown a relationship between diet-induced inflammation and the development of CKD. For example, a study by Chen et al. found that diets heavy in pro-inflammatory components are linked to decreasing kidney function over time [Citation11]. This is consistent with our findings of increased CKD risk with higher DII scores, highlighting the negative influence of pro-inflammatory diets on renal health.
Hypertension’s function in hastening CKD development is widely known. In a recent investigation by Bi and colleagues, hypertension was discovered as an important factor increasing renal injury [Citation12]. Our findings contribute to this story by illustrating how dietary patterns influence this connection, underlining the need of dietary control in hypertension patients to reduce CKD risk.
The relationship between chronic inflammation, CKD, and hypertension has been extensively studied. Kadatane et al. conducted a comprehensive study that highlighted the involvement of inflammation in the development of both illnesses [Citation13]. Our findings mirror similar comments, suggesting that high-inflammatory diets may play a role in this complicated dynamic.
Our findings contribute to a growing body of data supporting the use of DII as a CKD prediction tool. A recent study by Wu et al. established the usefulness of DII in predicting renal health outcomes in various groups [Citation14]. Our work broadens this information to especially hypertensive individuals, providing a more nuanced understanding of how food increases CKD risk in this cohort.
Given the global burden of CKD and hypertension, our study’s findings have broad implications. Paterson and colleagues’ research emphasized the worldwide heterogeneity in dietary patterns and its influence on the prevalence of chronic kidney disease [Citation15]. This global perspective highlights the significance of our findings and the necessity for specific dietary guidance to treat CKD in various populations.
By focusing on hypertensive patients, our study provides nuanced insights into how dietary inflammation affects kidney health in a high-risk group. This contributes to a more targeted approach in managing CKD risk. However, as an observational cross-sectional study, causality cannot be established. Longitudinal studies are needed to confirm the directionality of the relationship between DII and CKD in hypertensive patients.
In conclusion, our study highlights the importance of dietary inflammation in the development of CKD among hypertensive patients. These findings suggest that dietary interventions aimed at reducing inflammation could be a viable strategy for CKD prevention and management in this population. Future research should aim to establish causality and explore the efficacy of dietary modifications in reducing CKD risk in hypertensive individuals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are publicly available from the NHANES database https://wwwn.cdc.gov/nchs/nhanes/.
Additional information
Funding
References
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007.
- Akchurin OM. Chronic kidney disease and dietary measures to improve outcomes. Pediatr Clin North Am. 2019;66(1):247–267. doi:10.1016/j.pcl.2018.09.007.
- Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–379. doi:10.1007/s40265-019-1064-1.
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi:10.1038/nature07201.
- Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103(2):282–296. doi:10.1016/j.kint.2022.10.030.
- Xiao L, Harrison DG. Inflammation in Hypertension. Can J Cardiol. 2020;36(5):635–647. doi:10.1016/j.cjca.2020.01.013.
- Widyarini T, Indarto D, Soetrisno S, et al. Modulation effects of Etlingera elatior ethanol extract as anti-inflammatory on chronic kidney disease in mice with hypertension and diabetes. J Popul Ther Clin Pharmacol. 2022;29(4):e140–e149. doi:10.47750/jptcp.2022.988.
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi:10.1017/S1368980013002115.
- Mazidi M, Shivappa N, Wirth MD, et al. Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. 2018;120(2):204–209. doi:10.1017/S0007114518001071.
- Zhou N, Xie ZP, Liu Q, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. 2022;13:1097228. doi:10.3389/fimmu.2022.1097228.
- Chen S, Chen J, Li S, et al. High-fat diet-induced renal proximal tubular inflammatory injury: emerging Risk Factor of Chronic Kidney Disease. Front Physiol. 2021;12:786599. doi:10.3389/fphys.2021.786599.
- Bi Q, Kuang Z, Haihong E, et al. Research on early warning of renal damage in hypertensive patients based on the stacking strategy. BMC Med Inform Decis Mak. 2022;22(1):212. doi:10.1186/s12911-022-01889-4.
- Kadatane SP, Satariano M, Massey M, et al. The role of inflammation in CKD. Cells. 2023;12(12):1581. doi:10.3390/cells12121581.
- Wu J, Yu C, Shivappa N, et al. Dietary inflammatory index and renal cancer risk: a prospective study. Food Funct. 2023;14(20):9287–9294. doi:10.1039/d3fo02158k.
- Paterson EN, Neville CE, Wallace SM, et al. Dietary patterns associated with renal impairment in the Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA). Eur J Nutr. 2021;60(7):4045–4054. doi:10.1007/s00394-021-02579-z.