Abstract
Background
Patients with idiopathic membranous nephropathy (IMN) are more likely to be complicated by venous thromboembolism (VTE). The aim of the study was to investigate the potential association between anti-phospholipase A2 receptor (PLA2R) antibodies and hypercoagulability in patients with IMN.
Methods
A total of 168 patients with biopsy-proven IMN and 36 patients with biopsy-proven minimal change disease (MCD) were enrolled in this study. The clinical data, serum anti-PLA2R antibodies and coagulation-related indices of the patients were retrospectively analyzed.
Results
Patients with IMN were categorized into glomerular PLA2R staining-positive (GAg+) IMN group and glomerular PLA2R staining-negative (GAg-) IMN group in the study. Patients with IMN who were GAg + had lower PT, APTT and R time than patients with IMN who were GAg-, while the CI value was higher in patients with IMN who were GAg+. Patients with IMN who were GAg + were divided into the SAb+/GAg + group and the SAb-/GAg + group. Patients with IMN who were SAb+/GAg + had higher Fib and MA values than patients with IMN who were SAb-/GAg+. Correlation analysis showed that serum anti-PLA2R antibodies were positively correlated with fibrinogen, D-dimer, K time, CI value, α-angle, and MA value. Multiple linear regression analysis indicated that anti-PLA2R antibodies were independently correlated with fibrinogen and MA value.
Conclusion
Our study provides a new perspective on the underlying mechanisms of hypercoagulability in patients with IMN. Anti-PLA2R antibodies are associated with hypercoagulability in patients with IMN and may affect coagulation in patients with IMN by affecting platelet aggregation function and fibrinogen counts.
Introduction
Idiopathic membranous nephropathy (IMN) is a renal-limited autoimmune disease characterized by the deposition of immune complexes under the epithelial cells, accompanied by the thickness of the glomerular basement membrane (GBM), and it is one of the most common pathological types of adult nephrotic syndrome. The pathogenesis of IMN is that autoantibodies bind to antigens on the podocytes to form immune complexes, which activate complement to form membrane attack complex(MAC) leading to structural changes in the GBM and damage to the podocytes, resulting in proteinuria [Citation1]. In 2009, Beck et al. [Citation2] detected anti-phospholipase A2 receptor (PLA2R) antibodies in 70% of adult patients with IMN and demonstrated that PLA2R is the predominant podocyte antigen in patients with IMN. Antibodies against PLA2R are found in most patients, making a breakthrough in diagnosing and monitoring the disease without renal biopsy in certain situations [Citation3,Citation4].
The incidence of thrombosis is higher in IMN patients compared to other types of nephrotic syndromes. Thromboembolism, especially venous thromboembolism (VTE), is one of the most serious complications of adult nephrotic syndrome. VTE includes renal vein thrombosis (RVT), deep vein thrombosis (DVT) and pulmonary embolism (PE), with RVT having the highest incidence of 33% [Citation5]. Previous studies have indicated that hypercoagulability is the underlying cause of thromboembolism, and that hypercoagulability may be associated with disturbances in the patient’s anticoagulant system, platelet hyperactivity, and vascular endothelial damage [Citation6,Citation7]. Early diagnosis of hypercoagulability state and assessing the risk of thromboembolism are vital for effective clinical management and prognosis. Much of the current research on thromboembolism in patients with IMN has focused on identifying those at high risk for thromboembolism through the discovery of clinical markers and molecular targets, as well as prophylactic anticoagulation and therapeutic strategies. In this study, we retrospectively analyzed the clinical and coagulation data of patients with IMN, with the aim of investigating the role of anti-PLA2R antibodies in hypercoagulable state of patients with IMN.
Materials and methods
Patients
Biopsy-proven IMN and biopsy-proven minimal change disease (MCD) between October 2022 and April 2023 at the National Clinical Research Center for Kidney Diseases were enrolled in this study. The inclusion criteria include: (1) Age greater than 18 years; (2) Diagnosis of MN and MCD confirmed on the basis of light microscopy, immunofluorescence, and electron microscopy results. The exclusion criteria were as follows: (1) Presence of common causes of secondary MN, such as systemic lupus erythematosus (SLE), hepatitis B virus (HBV), drugs and tumors; (2) Presence of diseases affecting coagulation, such as severe infections, hematologic disorders, and severe hepatic function abnormalities. (3) Anticoagulant medications, such as heparin, low molecular heparin, or antiplatelet agents, prior to the TEG test and the routine coagulation tests; and (4) Hormonal and/or immunosuppressive therapy was prescribed for more than one month in the six months preceding the renal biopsy. All of those patients measured serum anti-PLA2R antibodies (SAb) and thromboelastogram (TEG) before renal biopsy.
Clinical evaluation
Clinical data were obtained by reviewing the patient’s previous medical records, including age, sex, serum albumin (Alb), quantification of proteinuria, platelet count (PLT), hemoglobin (Hb), hematocrit (HCT), serum creatinine (SCr), uric acid (UA), blood urea nitrogen (BUN), total cholesterol (TC), triacylglycerol (TG), high-density lipoproteins (HDL), low-density lipoproteins (LDL), estimated glomerular filtration rate (eGFR). The coagulation status was assessed using standard coagulation tests, which included prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (Fib), D-dimer, and TEG parameters (detected by TEG hemostasis system kaolin, purchased from HAEMONETICS corporation, Braintree, MA) such as R time, K time, CI value, α-angle, and MA value. The paraffin secd by a commercial enzyme-linked immunosorbent assay (ELISA) (EUROIMMUN, Lubeck, Germany) according to the manufacturer’s protocol, and the results were considered negative for <20 RU/ml and positive for ≥20 RU/ml.
Statistical analysis
Statistical software SPSS 25.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY) was employed for statistical analysis. Normally distributed data were expressed as mean ± standard deviation, and comparisons between groups were analyzed by t-tests. Non-normally distributed data were expressed as median and quartiles, comparisons between groups were made by the nonparametric test. The categorical variables were expressed as percentages, and comparisons between groups were made using the Chi-square test. In correlation analysis, Pearson’s correlation was used when both sets of data were measured and conformed to a normal distribution, otherwise Spearman’s correlation was used. Multiple linear regression was used to stepwise analyze the factors affecting the relevant indicators. Significance was defined as p < 0.05.
Results
Comparison of clinical and coagulation indices between patients with IMN and MCD
In this study, 168 patients with IMN and 36 patients with MCD were enrolled, and the comparative results between the two groups are shown in . There were no statistical differences in gender, serum creatinine, and eGFR between the two groups. Serum albumin (31 g/L vs 24.1 g/L, p < 0.001) was higher in patients with IMN than MCD, and urinary protein (4.1 g/24h vs 8.9 g/24h, p < 0.001) and platelet counts (250.7 × 10^9/L vs 291.3 × 10^9/L, p = 0.002) were lower in patients with IMN than MCD. In addition, patients with IMN had lower TC, HDL, and LDL than patients with MCD. The results of the comparison of coagulation indices between the two groups suggested that the APTT value (25s vs 26.5s, p < 0.001), TT value (16.9s vs 17.6s, p < 0.001), and R time (6.8 min vs 7.5 min, p = 0.02) were lower in patients with IMN than MCD. Patients with IMN had lower MA (69.3 mm vs 71.7 mm, p = 0.01) and Fib (4.3 g/L vs 6 g/L, p < 0.001) than MCD.
Table 1. Comparison of clinical and coagulation features in patients with IMN and patients with MCD.
Comparison of clinical and coagulation features between IMN patients with GAg + and GAg-
To evaluate the relationship between podocyte antigen and the hypercoagulable state in patients diagnosed with IMN. The patients were categorized based on their glomerular PLA2R staining results: those with positive staining (GAg+) consisted of 139 patients, as depicted in ; and those with negative staining (GAg-) included 29 patients. Patients in the GAg + group had lower serum albumin (30.2 g/L vs 34.6 g/L, p = 0.03) than patients in the GAg- group. In addition, there were no statistical differences in gender, age, 24h urine protein, PLT, serum creatinine, and eGFR between the two groups (). Patients in the GAg + group had lower PT (10.5s vs 11.1s, p < 0.001), APTT (24.7s vs 26.2s, p < 0.001) and R time (6.7 min vs 7.3 min, p = 0.03) than GAg- group, and the CI (1.1 vs 0.4, p = 0.03) was higher in the GAg + group than GAg- group.
Figure 1. Biopsy finding of patients with IMN who were glomerular PLA2R staining-positive (GAg+). immunofluorescence includes staining for PLA2R, IgG and C3.
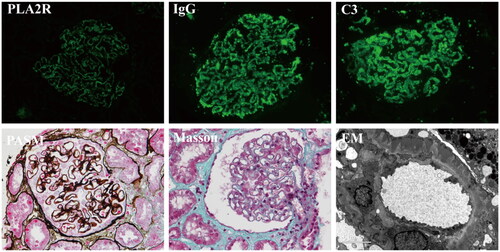
Table 2. Comparison of clinical and coagulation features in patients with IMN who were GAg + and patients with IMN who were GAg.
Comparison of clinical and coagulation features between IMN patients who were SAb+/GAg + and SAb-/GAg+
To further explore the effect of serum anti-PLA2R antibodies on coagulation function in patients with IMN who were GAg+, the patients with IMN who were GAg + were futured divided into the SAb+/GAg + group (SAb ≥20RU/mL, 85 cases) and the SAb-/GAg + group (SAb <20RU/mL, 54 cases). displays the comparison results between the two groups of patients. Patients with IMN who were SAb+/GAg + had lower eGFR (99 mL/min/1.73m2 vs 106 mL/min/1.73m2, p = 0.01) than patients with IMN who were SAb-/GAg+. Urinary protein (6 g/24h vs 3 g/24h, p < 0.001) and platelet count (262.9 × 109/L vs 232.3 × 109/L, p = 0.01) were higher in patients with IMN who were SAb+/GAg + than patients with IMN who were SAb-/GAg+. There were no differences in gender, age, serum albumin and serum creatinine between the two groups (p > 0.05). Among coagulation-related indices, patients with IMN who were SAb+/GAg + had higher Fib (4.5 g/L vs 3.4 g/L, p < 0.001) and MA value (70.4 vs 68, p = 0.01) than patients with IMN who were SAb-/GAg+.
Table 3. Comparison of clinical and coagulation features in patients with IMN who were SAb+/GAg + and patients with IMN who were SAb-/GAg+.
Correlation analysis of clinical and coagulation indices in patients with IMN who were SAb+/GAg+
In this study, we performed a one-way correlation analysis of clinical and coagulation indices in 85 patients with IMN who were SAb+/GAg+. The results suggested that anti-PLA2R antibodies did not correlate with PT, APTT, and TT, while it was positively correlated with Fib (r = 0.459, p < 0.001) and D-dimer (r = 0.262, p = 0.02) (). The results of correlation analysis between anti-PLA2R antibodies and TEG indexes showed that anti-PLA2R antibodies were positively correlated with CI value (r = 0.282, p = 0.01), α-angle (r = 0.23, p = 0.04), and MA value (r = 0.324, p = 0.002), and negatively correlated with K time (r=-0.246, p = 0.02). To further investigate the relationship between anti-PLA2R antibodies and coagulation indexes, multiple linear regression analysis was performed. The results suggested that after adjusting for related variables, anti-PLA2R antibodies were independently correlated with the MA value (β = 0.006, 95%CI (0.001, 0.012), p = 0.02) and Fib (β = 0.002, 95%CI (0.000, 0.003), p = 0.02) ( and ), and anti-PLA2R antibodies significantly positively predict the MA value and Fib. Anti-PLA2R antibodies were not significantly correlated with K time, CI value, α-angle, and D-dimer.
Figure 2. The scatter plots indicate the correlation between anti-PLA2R antibodies and the coagulation indices in patients with IMN who were SAb+/GAg+. anti-PLA2R antibody was highly skewed, so natural log transformation was used for the analysis.
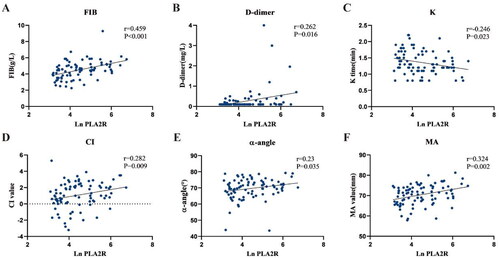
Table 4. Multiple linear regression analysis of fibrinogen and clinical indicators in patients with IMN who were SAb+/GAg+.
Table 5. Multiple Linear regression analysis of the MA value and clinical indicators in patients with IMN who were SAb+/GAg+.
Discussion
The pathogenesis of MN is complex, and recent studies on its pathogenesis have focused on the discovery of podocyte antigens. PLA2R was the most common podocyte antigen in MN, and 70–80% of patients with IMN have PLA2R-related MN [Citation2]. Previous studies have shown that anti-PLA2R antibodies are strongly correlated with disease activity, diagnosis, and prognosis of PLA2R-associated IMN [Citation3,Citation4]. Thromboembolism is one of the most serious and common complications of nephrotic syndrome. Patients with IMN are more prone to hypercoagulability as well as thrombosis than patients with other types of nephrotic syndrome. As the incidence of MN has increased in recent years, prevention of thrombosis and anticoagulation has become a priority in clinical care. The purpose of this study was to investigate the correlation between anti-PLA2R antibodies and hypercoagulability in patients with IMN. The results of our study showed that the anti-PLA2R antibodies correlated with fibrinogen, D-dimer, K time, CI value, α-angle and MA value. After adjusting for relevant variables, anti-PLA2R antibodies were independently correlated with fibrinogen and MA value. As can be seen, the effect of anti-PLA2R antibodies on coagulation in patients with IMN is mainly related to the function of platelets and the amount of fibrinogen.
The exact pathogenesis of hypercoagulability in patients with IMN is poorly understood. Previous studies [Citation6,Citation8–10] have suggested that the hypercoagulable state in patients with IMN is associated with massive proteinuria, which results in the loss of several anticoagulant factors with the urine, such as antithrombin III and proteins C and S, as well as a decrease in fibrinolytic substances, such as fibrinogen. In addition, hypoalbuminemia due to massive proteinuria leads to increased hepatic compensatory synthesis of coagulation factors, such as coagulation factors V, VIII, X, vWF, and fibrinogen, all of which contribute to an abnormal coagulation-fibrinolytic system in patients with membranous nephropathy. Moreover, the use of glucocorticoids in patients with IMN also poses a risk of thrombosis [Citation11,Citation12]. Glucocorticoids cause hypercoagulability mainly through elevated levels of procoagulant factors, such as coagulation factors VIII, IX, and vWF, and elevated expression of PAI-1 leading to a decrease in fibrinolytic function [Citation13]. The use of diuretics may also lead to insufficient effective circulating blood volume, viscous blood and blood stasis in patients with IMN. Despite both MCD and MN usually presenting with hypoalbuminemia, and even some patients with MCD having more severe hypoalbuminemia, previous reports suggest that the incidence of VTE in MN appears to remain higher than in MCD. Therefore, in order to correct the effect of hypoproteinemia, etc., patients with MCD who also presented with nephrotic syndrome were included in this study as a control group. APTT, TT, Fib and R time were lower in patients with IMN than MCD. However, the MA value was lower than those of patients with MCD. The MA value mainly reflect the function of platelet and is influenced by platelet counts [Citation14]. Patients with MCD have higher platelet counts than patients with IMN. In summary, patients with IMN were still more likely to have hypercoagulable states than patients with MCD. Therefore, this study hypothesis that there are additional factors contributing to the development of hypercoagulable state in patients with IMN.
Hamano et al. [Citation15] found that patients with membranous nephropathy had significantly more plasminogen activator inhibitor 1 (PAI-1) expression in renal tissues than patients with other pathologic types of nephrotic syndrome, suggesting that the hypercoagulable state of MN may be related to the disease itself. As the most common antigen in IMN is PLA2R, patients with IMN were divided into GAg + and GAg- in this study. Patients with IMN who were GAg + had lower PT, APTT and R time than patients with IMN who were GAg-, and the CI was higher in patients with IMN who were GAg + than patients with IMN who were GAg-. The results suggest that patients with IMN who were GAg + are more prone to hypercoagulation. Therefore, this study suggests that the hypercoagulable state observed in some patients with IMN may be linked to PLA2R. It has been found that circulating anti-PLA2R antibodies levels are significantly higher in patients with venous thrombosis than in those without venous thrombosis, and that anti-PLA2R antibodies levels are also an independent risk factor for venous thrombosis in patients with IMN [Citation16]. In our study, patients with IMN who were GAg + were further divided into SAb+/GAg + and SAb-/GAg + groups according to serum anti-PLA2R antibodies concentration. The results suggested that patients with IMN who were SAb+/GAg + had higher Fib and MA value than patients with IMN who were SAb-/GAg+, which suggest that serum anti-PLA2R antibodies may associated the plate activity. In this study, to further investigate the correlation between anti-PLA2R antibodies and hypercoagulable state of patients with IMN, correlation analysis and multiple linear regression analysis were performed on clinical and coagulation indexes of patients with IMN who were SAb+/GAg+. The results indicated that anti-PLA2R antibodies were independent influencing factor of MA value and fibrinogen levels, which suggested that anti-PLA2R antibodies have some effects on platelet function.
Numerous studies have shown that platelet hyperfunction is also involved in the development of the hypercoagulable state, including aberrant platelet activation and aggregation, increased release of reactive substances (P-selectin, platelet-activating factor, etc.), and an increase in surface-activated markers (phosphatidylserine, CD62P, CD63, etc.) [Citation17–19]. In addition, C-type lectin-like receptor 2(CLEC-2), expressed on the platelet surface, binds to podoplanin (PDPN) and can lead to platelet activation and promote thrombosis [Citation20,Citation21]. Under physiological conditions, PDPN expresses in a variety of cells, such as podocytes and lymphatic endothelial cells [Citation21]. It was found that serum levels of soluble PDPN were significantly elevated in patients with NS and significantly correlated with levels of glycoprotein VI (GPVI), a marker of platelet activation [Citation22].
In vivo, secretory phospholipase A2 (sPLA2) is the natural ligand for PLA2R and Group IB secretory phospholipase A2 (sPLA2 IB) has a high specific affinity for PLA2R. PLA2R binds to sPLA2 IB to activate multiple intracellular MAPK family signaling pathways and increase intracellular Ca2+ levels, which stimulate the phosphorylation of cytosolic-type phospholipase A2 (cPLA2), and leads to the generation of zymosynthesis activity, which in turn hydrolyzes cell membranes to produce arachidonic acid (AA). AA increases the generation of various platelet aggregating agents downstream by cyclooxygenase-1 (COX-1), e.g., thromboxane A2 (TXA2), which further promotes platelet activation and aggregation [Citation23]. Previous studies have shown that PLA2R expression is increased on podocytes in patients with PLA2R-associated MN. Moreover, it was reported that the level of sPLA2-IB was significantly elevated and correlated with the severity of the disease in patients with IMN [Citation24]. Anti-PLA2R antibodies may potentially affect the normal function of the PLA2R. The exact mechanism by which anti-PLA2R antibodies affect the function of PLA2R in membranous nephropathy is currently unknown. And the role that anti-PLA2R antibodies play in the interaction between PLA2R and sPLA2 still needs to be explored in further studies. High levels of anti-PLA2R antibodies also exacerbate patients’ hypoalbuminemia [Citation25], which increases the risk of thrombosis. The study by Schieppati et al. [Citation26] suggests that hypoalbuminemia results in less binding of albumin to arachidonic acid (AA), which further promotes platelet activation and aggregation. In addition, sPLA2-IB binds to PLA2R and activates cPLA2, causing it to catabolize membrane phospholipids to produce more AA, which promotes apoptosis in podocytes [Citation27,Citation28]. It was found that sPLA2-IB binding to PLA2R downregulates podocyte autophagy and promotes podocyte injury through activation of the p38MAPK/mTOR/ULK1ser757 signaling pathway, which further leads to increased proteinuria and hypoalbuminemia [Citation29]. Moreover, the findings suggest that coagulation dysfunction in patients with IMN is closely associated with platelet hyperactivity. However, due to the complexity of the specific mechanisms of hypercoagulation in patients with IMN, further investigation is still needed regarding the specific mechanisms, especially the role of the autoantibodies in thrombosis formation.
A study found that fibrinogen levels were significantly elevated in rats with Heymann nephritis [Citation30]. It is widely acknowledged that nephrotic syndrome results in significant protein loss through urine, prompting the liver to increase protein synthesis. Although fibrinogen is not excreted in urine, the liver still upregulates its synthesis. The presence of anti-PLA2R antibodies in patients with IMN correlates positively with the degree of proteinuria, leading to elevated levels of fibrinogen in affected individuals. In addition, previous studies have linked FIB levels to platelet activation [Citation31,Citation32]. Fibrinogen can form protein bridges between several platelets via glycoprotein Ib (GP Ib) receptors on the platelet membrane, promoting platelet aggregation into clusters increasing plasma viscosity and exacerbating hypercoagulability.
This study was a cross-sectional study, and its limitations included the lack of follow-up on the study population and the lack of statistics on whether the patients had experienced subsequent thrombotic events. Therefore, further statistics and analyses of the follow-up data of the patients, as well as assessment of the occurrence of thrombotic events, are needed in subsequent studies. In addition, the specific mechanism by which anti-PLA2R antibody affects hypercoagulability in IMN patients requires further experimental studies. The sample size for observation in this study was small, indicating the need for further observation and analysis after increasing the sample size. Moreover, in order to exclude the effect of drugs on the coagulation function, the enrollment subjects included in this study were those who had not used anticoagulant and antiplatelet drugs such as heparin and low molecular heparin prior to the TEG test, as well as those who had not been treated with hormones and/or immunosuppressant prior to the renal biopsy, but patients who had a significant risk of hypercoagulable or thrombotic development were also excluded.
In summary, our study provide a new insights for a potential mechanism underlying the hypercoagulable state observed in patients with IMN, specifically involving PLA2R and anti-PLA2R antibodies. Patients with IMN who were GAg + are more likely to have hypercoagulable states compared to patients with IMN who were GAg-. Anti-PLA2R antibodies are closely related to hypercoagulability in patients with IMN, mainly by affecting the aggregation function of platelets and the amount of fibrinogen. Therefore, clinical assessment of the risk of thromboembolism in patients with IMN, especially the GAg + and SAb+, and prophylactic anticoagulation and antiplatelet therapy are needed in a timely manner.
Ethical approval
This study was conducted in accordance with the principles of the Helsinki Declaration.
Authors’ contributions
Y. Liu wrote the manuscript and participated in the analyses. Y. Liu and Y. Tang contributed to data collection. Y. Zhong contributed to data analysis and discussion. D. Chen, D. Liang, F. Xu, S. Liang were responsible for methodology. C. Zeng were responsible for resources; C. Zeng and W. Le was responsible for funding acquisition; C. Zeng provided supervision; C. Zeng and Y. Zhong reviewed and edited the manuscript; and C. Zeng conceptualized the study and oversaw all clinical aspects of study conduct and manuscript preparation.
Acknowledgements
The authors thank all volunteers for their generous participation in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Additional information
Funding
References
- Hoxha E, Reinhard L, Stahl RAK. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. 2022; 18(7):1–8.
- Beck LH, Bonegio RGB, Lambeau G, et al. M-Type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009; 361(1):11–21. doi: 10.1056/NEJMoa0810457. PubMed PMID: 19571279; PubMed Central PMCID: PMC2762083.
- Kukuy OL, Cohen R, Gilburd B, et al. The prognostic value of anti-PLA2R antibodies levels in primary membranous nephropathy. Int J Mol Sci. 2023;24(10):9051.
- Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun Rev. 2016; 15(2):146–154.
- Li SJ, Guo JZ, Zuo K, et al. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome-a prospective study. Thromb Res. 2012; 130(3):501–505.
- Loscalzo J. Venous thrombosis in the nephrotic syndrome. N Engl J Med. 2013; 368(10):956–958.
- Roca N, Jatem E, Martín ML, et al. Relationship between soluble urokinase-type plasminogen activator receptor and serum biomarkers of endothelial activation in patients with idiopathic nephrotic syndrome. Clin Kidney J. 2021;14(2):543–549.
- Huang MJ, Wei RB, Wang ZC, et al. Mechanisms of hypercoagulability in nephrotic syndrome associated with membranous nephropathy as assessed by thromboelastography. Thromb Res. 2015;136(3):663–668.
- Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012; 7(3):513–520.
- Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118(3):397–407.
- Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743–752.
- Simion C, Campello E, Bensi E, et al. Use of glucocorticoids and risk of venous thromboembolism: a narrative review. Semin Thromb Hemost. 2021;47(6):654–661.
- Coelho MC, Santos CV, Vieira Neto L, et al. Adverse effects of glucocorticoids: coagulopathy. Eur J Endocrinol. 2015;173(4):M11–21.
- Gonzalez E, Pieracci FM, Moore EE, et al. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36(7):723–737.
- Hamano K, Iwano M, Akai Y, et al. Expression of glomerular plasminogen activator inhibitor type 1 in glomerulonephritis. Am J Kidney Dis. 2002;39(4):695–705.
- Zhu H, Xu L, Liu X, et al. Anti-PLA2R antibody measured by ELISA predicts the risk of vein thrombosis in patients with primary membranous nephropathy. Ren Fail. 2022; 44(1):594–600.
- Sirolli V, Ballone E, Garofalo D, et al. Platelet activation markers in patients with nephrotic syndrome. A comparative study of different platelet function tests. Nephron. 2002;91(3):424–430.
- Zwaginga JJ, Koomans HA, Sixma JJ, et al. Thrombus formation and platelet-vessel wall interaction in the nephrotic syndrome under flow conditions. J Clin Invest. 1994;93(1):204–211.
- Mirrakhimov AE, Ali AM, Barbaryan A, et al. Primary nephrotic syndrome in adults as a risk factor for pulmonary embolism: an up-to-date review of the literature. Int J Nephrol. 2014;2014:916760–916769.
- Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129(1):12–23.
- Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134(22):1912–1918.
- Ji Y, Wang YL, Xu F, et al. Elevated soluble podoplanin associates with hypercoagulability in patients with nephrotic syndrome. Clin Appl Thromb Hemost. 2022; 28:10760296221108967.
- Khan SA, Ilies MA. The phospholipase A2 superfamily: structure, isozymes, catalysis, physiologic and pathologic roles. Int J Mol Sci. 2023;24(2):1353.
- Li W, Zhang M, Guo Y, et al. Serum secretory phospholipase A2 group IB correlates with the severity of membranous nephropathy. Clin Chim Acta. 2018;482:178–184.
- Hofstra JM, Beck LH, Jr., Beck DM, et al. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6(6):1286–1291.
- Schieppati A, Dodesini P, Benigni A, et al. The metabolism of arachidonic acid by platelets in nephrotic syndrome. Kidney Int. 1984;25(4):671–676.
- Pan Y, Wan J, Liu Y, et al. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Sci Rep. 2014;4(1):6660.
- Lu X, Kan C, Zhang R. Phospholipase A2 receptor is associated with hypercoagulable status in membranous nephropathy: a narrative review. Ann Transl Med. 2022;10(17):938–938.
- Yang L, Wu Y, Lin S, et al. sPLA2-IB and PLA2R mediate insufficient autophagy and contribute to podocyte injury in idiopathic membranous nephropathy by activation of the p38MAPK/mTOR/ULK1(ser757) signaling pathway. Faseb J. 2021;35(2):e21170.
- Girot R, Jaubert F, Leon M, et al. Albumin, fibrinogen, prothrombin and antithrombin III variations in blood, urines and liver in rat nephrotic syndrome (Heymann nephritis). Thromb Haemost. 1983;49(1):13–17.
- Asch AS, Leung LL, Polley MJ, et al. Platelet membrane topography: colocalization of thrombospondin and fibrinogen with the glycoprotein IIb-IIIa complex. Blood. 1985;66(4):926–934.
- Perutelli P, Mori PG. The human platelet membrane glycoprotein IIb/IIIa complex: a multi-functional adhesion receptor. Haematologica. 1992;77(2):162–168.
