Abstract
Objectives
Geriatric Nutritional Risk Index (GNRI) is a new and simple index recently introduced to assess nutritional status, and its predictive value for clinical outcomes has been demonstrated in patients with chronic kidney disease. However, the association between the GNRI and prognosis has not been evaluated so far in patients with acute kidney injury (AKI), especially in those receiving continuous renal replacement therapy (CRRT).
Methods
A total of 1096 patients with severe AKI initiating CRRT were identified for inclusion in this retrospective observational study. Patients were divided into three groups according to GNRI tertiles, with tertile 1 as the reference. The outcomes of interest were the 28- and 90-days of all-cause mortality. The associations between GNRI and clinical outcomes were estimated using multivariate Cox proportional hazards model analysis.
Results
The overall mortality rates at 28- and 90-days were 61.6% (675/1096) and 71.5% (784/1096), respectively. After adjusting for multiple confounding factors, GNRI was identified as an independent prognostic factor for 28-days all-cause mortality (HR, 0.582; 95% CI, 0.467–0.727; p < .001 for tertile 3 vs. tertile 1) as well as 90-days all-cause mortality (HR, 0.540; 95% CI, 0.440–0.661; p < .001 for tertile 3 vs. tertile 1). The observed inverse associations were robust across subgroup analysis, and were more pronounced in elderly patients over 65 years of age. Finally, incorporating GNRI in a model with established risk factors might significantly improve its predictive power for the short-term death.
Conclusions
GNRI is considered to be a useful prognostic factor in patients with severe AKI initiating CRRT, especially in elderly patients.
Introduction
Acute kidney injury (AKI) affects the half of patients with critical illness admitted to intensive care units (ICUs) and is associated with worse outcomes and huge health care costs [Citation1,Citation2]. Continuous renal replacement therapy (CRRT) has been recommended as the preferred and indispensable modality for critically ill patients to treat AKI, particularly among those with hemodynamic instability [Citation3,Citation4]. However, despite improvements in CRRT techniques, the mortality rate of AKI patients undergoing CRRT remains as high as 40–60% [Citation3,Citation5,Citation6]. Therefore, it is important to identify AKI patients undergoing CRRT at high risk of death so that interventions can be taken in a timely manner, which may reduce this high mortality.
Malnutrition, characterized by loss of protein and energy stores, has been recognized as a significant risk factor for all cause death in patients with AKI [Citation7,Citation8]. The European Society for Clinical Nutrition and Metabolism (ESPEN) has presented the recommendations that any hospitalized patient with AKI, and especially those staying for more than 48 h in the ICU, should be screened for malnutrition [Citation9]. The subjective global assessment (SGA), based on medical history and clinical findings, has been used in AKI patients to predict poor outcomes [Citation7,Citation10]. However, the SGA is not widely employed and can be difficult to apply in the ICUs setting [Citation9].
Recently, a new nutritional screening tool, the Geriatric Nutritional Risk Index (GNRI), was specifically used in patients with CKD, showing a good sensitivity, specificity, and positive predictive value against the SGA [Citation11]. However, studies on the clinical implication of GNRI in critically ill patients with AKI are lacking. Therefore, we aimed to assess the predictive value of GNRI for all cause mortality in critically ill patients with AKI requiring CRRT in a retrospective cohort.
Materials and methods
Study population
This is a retrospective cohort study and the original data were obtained from a public dataset (https://datadryad.org), which was offered by Jung et al. [Citation12]. This study follows the tenets of the Declaration of Helsinki, with a waiver of informed consent owing to the anonymous nature of the data.
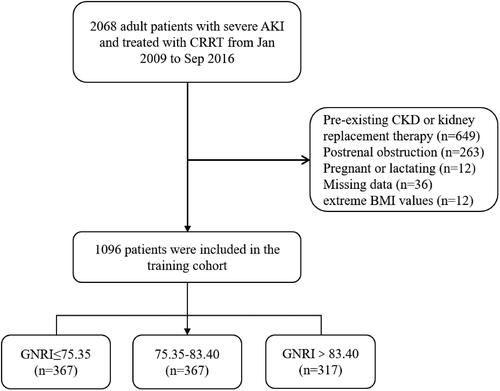
Details about the study design have been described elsewhere [Citation13]. Briefly, 2068 patients with severe AKI, who were at least 18 years of age, and received CRRT in the ICU at Yonsei University Health System Severance Hospital and National Health Insurance Service Medical Center Ilsan Hospital from January 2009 to September 2016, were initially enrolled. AKI and AKI stage were defined according to AKI network (AKIN) 2007 guidelines using serum creatinine (SCr) criteria and urine output criteria [Citation14]. AKI stage 1 was defined as mild AKI, and AKI stage 2 or 3 was defined as severe AKI. Exclusion criteria were as follows: (i) patients with CKD or had prior kidney replacement therapy (dialysis or kidney transplant) (n = 649); (ii) patient with AKI due to postrenal obstruction (n = 263); (iii) women who were pregnant or lactating during the study period (n = 12); (iv) individuals with missing available records for important variables including age, sex, body mass index (BMI), albumin (ALB), AKI cause, and AKI stage at baseline (n = 36); (v) patients with extreme BMI values of <15 or >55 kg/m2 (n = 12). Moreover, participants with an undefined survival status at the follow-up visit were also excluded. Ultimately, 1096 patients were included in our cohort ().
Clinical measurements
Demographic characteristics and comorbidities were collected from the hospital information system on admission to ICU, including data on age, sex, height, weight, mean arterial pressure (MAP), the history of cerebrovascular disease (CVD), heart failure (HF), peripheral vascular disease (PVD), myocardial infarction (MI), diabetes mellitus (DM), hypertension, chronic obstructive pulmonary disease (COPD), and dementia. Dementia was defined as a physician’s diagnosis of Alzheimer’s disease or other dementias. BMI is calculated as weight in kilograms divided by height in meters squared. Laboratory indicators measured at the time of CRRT initiation were used as baseline values, including ALB, hemoglobin, blood urea nitrogen (BUN), SCr, phosphate, potassium, C-reactive protein (CRP), and estimated glomerular filtration rate (eGFR) which were all determined by an automated analyzer using standard methods. In addition, acute physiologic and chronic health evaluation II (APACHE II) score and sequential organ failure assessment (SOFA) score were calculated for each individual by using established criteria.
The GNRI was used to evaluate nutritional status in our study. The GNRI, proposed by Bouillanne et al. [Citation15], was originally developed as a screening tool for malnutrition assessment and as a predictor of mortality in hospitalized elderly patients. The GNRI was calculated using the following equation: GNRI = 14.89 × serum ALB in grams per deciliter + (41.7 × actual body weight/ideal body weight). In line with recent studies [Citation16], the ideal body weight in our study was defined as the value calculated from the height and a BMI of 22 kg/m2, and the actual body weight was considered as ideal body weight if patient’s actual body weight was greater than the ideal.
CRRT protocol
In this study, patients in the ICU presenting with AKI were assessed by nephrologist for the initiation of CRRT. Indications for performing CRRT included oliguria or anuria, life-threatening volume overload, intractable hyperkalemia (blood potassium greater than 6.5 mmol/L) or metabolic acidosis. A temporary catheter is usually placed in the internal jugular vein, subclavian vein, or femoral vein to start continuous intravenous hemodialysis. In patients with critical AKI, local citrate anticoagulation is preferred, dialyzers with a small membrane surface area (1.0–1.4 m2) are used, and CRRT is maintained at a dose of 35 mL/kg/h of the total effluent volume, with a mean duration of the first CRRT session of more than 24 h. This approach is more conducive to maintaining hemodynamic stability and reducing complications in critically ill AKI patients.
Exposure and outcomes ascertainment
The main predictor of the present study was the value of the GNRI. To examine the relationship between GNRI and adverse outcomes, patients were divided into the following three groups according to tertiles of GNRI: ≤75.35, 75.35–83.40, and >83.40. In addition, we further analyzed this association with GNRI as a continuous variable in per-10 increments.
The outcomes of interest were all-cause mortality within 28- and 90-days after CRRT initiation. Regardless of visit schedule, deaths and the date of occurrence were thoroughly recorded.
Statistical analysis
Categorical variables were presented as counts (%), and continuous variables were expressed as mean (standard deviations) or median (interquartile ranges), as appropriate. The differences among the three groups stratified by the tertiles of GNRI were compared by Chi-squared test, analysis of variance (ANOVA), or Kruskal–Wallis followed with Bonferroni’s post hoc test. Kaplan–Meier’s plots were performed to calculate cumulative 28- and 90-days survival rates, and the differences among three groups were compared by the log-rank test. Multivariable Cox proportional hazards regression analyses were applied to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for associations of GNRI with all outcomes. The proportionality assumption was checked using Schoenfeld’s residuals. As mentioned before, GNRI was modeled both continuously and categorically based on tertiles and multiple clinically meaningful ranges. Potential covariates were selected based on their clinical relevance with the outcomes or if their inclusion in the models resulted in a 10% change in the risk estimates. For each analysis, four levels of hierarchical multivariate adjustment were examined: (i) crude model: without adjustment; (ii) model 1: adjusted for age and sex; (iii) model 2: adjusted for variables of the model 1 plus comorbidity history (MI, HF, CVD, PVD, DM, hypertension, COPD, and dementia); and (iv) model 3 (fully adjusted model): adjusted for covariates of the model 2 plus disease severity (SOFA score and APACHE II score), AKI stages, AKI causes, laboratory parameters (hemoglobin, phosphate, and CRP), and MAP levels. To test the robustness of the main findings, multiple sensitivity analyses and subgroup analyses were performed. In addition, to compare predictive ability of GNRI as a continuous variable, we calculated Harrell c-statistics, category net reclassification improvement (NRI), and integrated discrimination improvement (IDI) for models. Statistical analyses were conducted in R software version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p value <.05 was considered statistically significant.
Results
Patient characteristics
A total of 1096 patients with severe AKI who met our inclusion and exclusion criteria were involved in the final analysis (). The mean age was 63.2 years, 673 (61.4%) were men, 291 (26.6%) were AKI stage 2, and 805 (73.4%) were AKI stage 3. The median GNRI level was 78.93 mg/L (IQR 73.00–84.88), and all participants were classified into three groups according to the tertiles of GNRI. The comparison of baseline demographics, comorbidities, laboratory details, and clinical outcomes among three groups is described in . Patients in the highest GNRI tertiles had significantly higher levels of BMI, ALB, and hemoglobin, but had significantly lower levels of APACHE II score, SOFA score, and serum CRP (all p < .05, ). In addition, there was no difference in terms of AKI stage, eGFR, potassium, presence of diabetes and hypertension, but a lower level of CRRT dose and a lower prevalence of dementia in the highest GNRI tertiles compared to the lowest ().
Table 1. Baseline characteristics of the study patients, overall and stratified by the tertiles of GNRI.
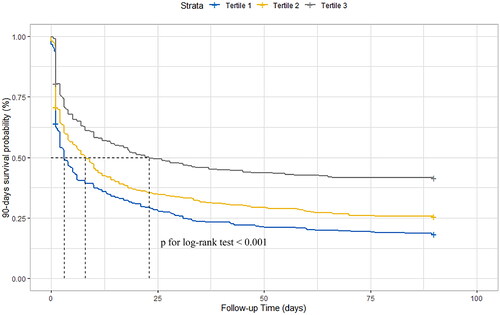
As shown in , the overall mortality rates among patients with severe AKI at 28- and 90-days after CRRT initiation were 61.6% (95% CI: 58.7–64.4%) and 71.5% (95% CI: 68.8–74.1%), respectively. Kaplan–Meier’s tests showed that the cumulative incidence rates of the 28- and 90-days all-cause death were consistently decreased alongside GNRI tertile (p for log-rank test <.001, ).
Association of GNRI levels with 28-days mortality
The relationship between GNRI level and risk of 28-days mortality was explored in different Cox proportional hazards regression models. First, when GNRI was included as a categorical variable. In the crude model, compared with those for individuals in the bottom tertile, the HRs for 28-days death were 0.85 and 0.59 for individuals with GNRI in the second and third tertiles, respectively (, p for trend <.001). After progressive adjustment for variables associated with demographic data (model 1), comorbidity history (model 2), and disease severity (model 3), high GNRI remained significantly associated with a decreased risk of all-cause mortality, despite the slight attenuation of the HRs (all p for trend <.001, ). Second, when GNRI was further modeled as a continuous variable, every 10 unit increase in GNRI was associated with a 26% reduction in the risk of 28-days death (fully-adjusted HR: 0.74; 95% CI: 0.67–0.81; p for trend <.001, and Table S1). In addition, restricted cubic spline curves further depict a linear association of GNRI levels with 28-days death after multivariable adjustment (Figure S1).
Table 2. Risk for 28- and 90-days all cause mortality according to the level of GNRI in overall participants.
Association of GNRI levels with 90-days mortality
In agreement with the result of 28-days all-cause death, Cox proportional hazards model analyses suggested that patients in the highest GNRI tertile experienced an approximate 48% reduction in the risk of 90-days death compared to those in the lowest tertile (fully-adjusted HR: 0.54; 95% CI: 0.44–0.66; p for trend <.001, and Table S1). Similarly, the association of GNRI and 90-days death was also confirmed when it was used as a continuous variable (all p < .05, and Figure S1).
Sensitivity and subgroup analyses
Sensitivity and subgroup analyses were also conducted to validate our main findings. First, we performed four sensitivity analyses among patients without a history of HF, MI, PVD, CVD, and COPD. In line with the main findings, there results showed that patients in the highest GNRI tertile were still associated with lower incidence of 28- and 90-days all cause death (Figure S2). Furthermore, subgroups analyses stratified by age (≥65 or <65 years), sex (men or women), hypertension status (absence or presence), diabetes status (absence or presence), SOFA score (≤12 or >13), APACHE II score (≤28 or >28), AKI stage (stage 2 or stage 3), and AKI cause (sepsis or non-sepsis) revealed similar results (all p < .05, Figure S2). In particular, this association between GNRI and 28- and 90-days death was more pronounced among older patients and those with diabetes (Figure S2).
In addition, since studies performed with GNRI are very limited, the use of diverse approaches will be very helpful. Thus, we regrouped all patients into two groups according to the maximum Youden index of the ROC curve. The results support our original conclusions that patients with higher GNRI (GNRI ≥79.22) at the time of CRRT initiation among AKI patients had a significantly lower risk of 28-days death (fully adjusted HR: 0.676; 95% CI: 0.572–0.800; p < .001) and 90-days death (fully adjusted HR: 0.631; 95% CI: 0.541–0.737; p < .001). Similar results were also obtained when using the GNRI cutoff of 78.93, as determined by restricted cubic spline regression (all p < .001, Table S2).
Predictive ability of GNRI for 28- and 90-days all cause mortality
The predictive ability of GNRI as a continuous variable for short-term death was estimated by comparing the c-statistics, categorized NRI, and IDI between base model and base + GNRI model (). In predicting 90-days mortality, the addition of GNRI into a clinical model, which consisted of age, sex, comorbidity history (MI, HF, CVD, PVD, DM, hypertension, COPD, and dementia), SOFA score, APACHE II score, AKI stages, AKI causes, hemoglobin, phosphate, CRP, and MAP levels at baseline, led to a significant improvement of c-statistics from 0.643 to 0.669 (p < .001). This was accompanied by improvement in both IDI (0.042, 95% CI 0.021–0.066; p < .001) and categorized NRI (0.094, 95% CI 0.037–0.153; p < .001). However, not discrimination (c-statistics) but reclassification (NRI and IDI) was significantly improved with the addition of GNRI to the base model for 28-days all-cause death ().
Table 3. The predictive performance of GNRI for 28- and 90-days death by the c-statistics, NRI, and IDI.
Discussion
In the present study, we noticed that a low GNRI was strongly related with an increased risk of 28- and 90-days all-cause death in patients with severe AKI undergoing CRRT, independent of other clinical risk factors, such as hemoglobin, CRP, APACHE II score, SOFA score, and multiple comorbidities. Interestingly, this observed inverse association was robust across subgroup analyses, and was more pronounced in elderly patients (age ≥ 65) and those with diabetes. Moreover, incorporating GNRI in a model with established risk factors might significantly improve its predictive power for the short-term death.
Malnutrition and its complications such as sarcopenia and frailty are prevalent in AKI patients. Negative energy balance caused by pro-inflammatory states, metabolic acidosis, and insulin resistance may be mechanisms underlying this pathological condition [Citation10,Citation17]. A recent meta-analysis estimated the overall prevalence of malnutrition evaluated by SGA among AKI patients to range from 67.9% to 82.1% [Citation7]. This condition is further exacerbated in those patients requiring CRRT treatment, which may be related to the loss of amino acids and proteins in the dialysate [Citation18]. Some studies with small samples reported that about 80% of the patients receiving CRRT were defined as malnourished [Citation19], and this proportion in our study cohort is as high as 91.4% when taking GNRI of 92 as a cutoff point as defined in previous studies [Citation11]. Therefore, early recognition of malnutrition and active nutritional interventions is crucial in this population.
The GNRI is a simplified evaluation tool for nutritional status. Its value in predicting adverse outcomes has been extensively validated in various chronic kidney diseases (CKDs) []. A longitudinal cohort study involving 2791 patients with CKD stages 1–4 revealed a significant inverse association between GNRI and renal progression, cardiovascular events and all-cause mortality [20]. Our previous research verified that low GNRI is an independent risk factor for worse health outcomes in newly initiated dialysis (including hemodialysis and peritoneal dialysis) patients [Citation16]. Similar results were also obtained in patients undergoing maintenance dialysis [Citation21,Citation22]. However, current knowledge on the role of GNRI in the prognosis of AKI patients is very limited. A recent study conducted by Xiong et al. [Citation23] demonstrated that GNRI is strongly associated with survival in patients in the ICU coexisting with AKI. However, this study did not focus on patients requiring CRRT for AKI. To our knowledge, this is the first study to elucidate the prognostic significance of GNRI for mortality in AKI patients requiring CRRT. Therefore, our study adds to the findings from previous literature by extending knowledge about associations of GNRI with poor outcomes to AKI patients undergoing CRRT.
GNRI is based on two objective parameters: BMI and ALB, and the superiority of GNRI in prognostic prediction has been well-verified, when compared with BMI or serum ALB level alone. The association between serum ALB and the prognosis of AKI has been widely reported. A meta-analysis with 17 studies suggested that every 1 g/dL decrease in ALB is associated with a 134% increase in risk for AKI development and a 147% increase in risk for subsequent death [Citation24]. A recent study further demonstrated that ALB infusion was found to be relatively effective for reducing 28-day mortality in patients with septic shock and AKI, especially in patients with age > 60 years [Citation25]. Meanwhile, some studies have also assessed the link between BMI and mortality in patients with AKI, while no consensus has been attempted. Druml et al. found that there was a U-shaped association between BMI and death in 5232 patients with severe AKI requiring CRRT [Citation26]. Kim et al. suggested a 30-days survival benefit associated with a higher BMI in 1144 AKI patients undergoing CRRT [Citation27]. Similarly, two recent cohort studies have shown that a higher BMI might have an additional positive modifying effect in short-term [Citation28] and long-term [Citation29] survival among patients post-percutaneous coronary intervention (PCI) with AKI. In contrast, other studies did not find a clear relationship between BMI and mortality [Citation30,Citation31]. The possible reason for these inconsistencies across studies is that BMI cannot distinguish between fat tissue and lean tissue [Citation32]. It is worth mentioning that the prevalence of sarcopenia is high in this group [Citation17]. Therefore, using GNRI, a surrogate indicator for muscle strength [Citation33,Citation34], can effectively solve this limitation. In addition, it is well known that elderly patients have poor nutritional status and are prone to develop sarcopenia [Citation35], this explains, in part, why the impact of GNRI on death among patients over 65 years of age is more pronounced in the present study. Overall, our findings confirm the value of GNRI in evaluating the nutrition condition and clinical outcomes.
Nutritional assessment by GNRI on CRRT initiation might help for earlier and more aggressive effective therapies in the management of high-risk patients. A series of studies have shown that ameliorating malnutrition promptly and effectively would be helpful to improve the prognosis of AKI patients [Citation8,Citation9,Citation36]. However, nutritional support for severe AKI patients is complicated, and CRRT further introduces new challenges. Based on reported amino acid losses during CRRT (10–15 g/per day) [Citation18] and the correlation between protein intake and neutral nitrogen balance [Citation37], the current ASPEN guidelines recommend a protein provision of 1.2–2.5 g/kg per day for patients receiving CRRT [Citation38]. However, a recent randomized controlled trial (RCT) compared higher (≥2.2 g/kg/day) vs. lower (≤1.2 g/kg/day) protein delivery and found that delivery of higher doses of protein to critically ill patients with AKI did not improve the time-to-discharge-alive (TTDA) from hospital and might have worsened outcomes, but its adverse effect on TTDA disappeared in patients who received dialysis [Citation39]. Therefore, further large-sample RCTs are required to determine the optimal dose of initial protein intake. In addition, the optimal timing of protein provision is also disputed, despite some observational research found that early (day 4) protein provision was associated with lower hospital mortality, which is worthy of further study [Citation40].
Indeed, our comprehensive study has a relatively large sample size. However, some shortcomings must be considered. First, due to the observational nature of this study, although we corrected for many potential confounders and conducted multiple subgroup analysis, residual confounding cannot be completely eliminated. Second, this study included only severe AKI patients admitted to ICU, whether this conclusion holds true for mild AKI patients requires further investigation. Finally, only 28- and 90-days death were measured in our study, clinical outcomes such as length of ICU stay, renal recovery, and 1-year mortality, etc., were not assessed.
Conclusions
In conclusion, GNRI, a relatively simple and effective noninvasive biomarker, may be an independent prognostic factor for 28- and 90-days death in patients with severe AKI undergoing CRRT. This association is evident in all subgroups considered and after careful adjustments. The present findings emphasized the prognostic importance of GNRI suggesting that AKI patients undergoing CRRT may benefit from a positive nutritional strategy, especially among those over 65 years of age.
Author contributions
Xue Zhao: data curation, methodology, writing – original draft, and investigation. Jie Li: data curation, writing – original draft, and investigation. Jie Li: data curation, methodology, and conceptualization. Hua Liu: data curation, methodology, and formal analysis. Kehui Shi: writing – review and editing, conceptualization, and methodology. Quan He: data curation, methodology, and conceptualization. Lingshuang Sun: writing – review and editing, conceptualization, and methodology. Jinhong Xue: writing – review and editing, conceptualization, and methodology. Hongli Jiang: supervision, writing – review and editing, conceptualization, and methodology. Limin Wei: writing – review and editing, writing – original draft, conceptualization, methodology, and investigation. All authors read and approved the final version of this manuscript.
Supplementary table 0528.doc
Download MS Word (83 KB)Acknowledgements
We thank the collaborator and patients from the datadryad databases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found here: http://datadryad.org/ with the doi: 10.5061/dryad.6v0j9
Additional information
Funding
References
- Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1–10. doi: 10.1007/s00134-015-3934-7.
- Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi: 10.1038/s41581-018-0052-0.
- Lee C-C, Kuo G, Chan M-J, et al. Characteristics of and outcomes after dialysis-treated acute kidney injury, 2009–2018: a Taiwanese multicenter study. Am J Kidney Dis. 2023;81(6):665–674.e1. doi: 10.1053/j.ajkd.2022.08.022.
- Teixeira JP, Neyra JA, Tolwani A. Continuous KRT: a contemporary review. Clin J Am Soc Nephrol. 2023;18(2):256–269. doi: 10.2215/CJN.04350422.
- Hwang S, Kang D, Park H, et al. Impact of renal replacement therapy on mortality and renal outcomes in critically ill patients with acute kidney injury: a population-based cohort study in Korea between 2008 and 2015. J Clin Med. 2022;11(9):2392. doi: 10.3390/jcm11092392.
- Pérez-Fernández X, Sabater-Riera J, Sileanu FE, et al. Clinical variables associated with poor outcome from sepsis-associated acute kidney injury and the relationship with timing of initiation of renal replacement therapy. J Crit Care. 2017;40:154–160. doi: 10.1016/j.jcrc.2017.03.022.
- Khor B-H, Tiong H-C, Tan SC, et al. Protein-energy wasting assessment and clinical outcomes in patients with acute kidney injury: a systematic review with meta-analysis. Nutrients. 2020;12(9):2809. doi: 10.3390/nu12092809.
- MacLaughlin HL, Friedman AN, Ikizler TA. Nutrition in kidney disease: core curriculum 2022. Am J Kidney Dis. 2022;79(3):437–449. doi: 10.1053/j.ajkd.2021.05.024.
- Fiaccadori E, Sabatino A, Barazzoni R, et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. 2021;40(4):1644–1668. doi: 10.1016/j.clnu.2021.01.028.
- Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28(6):380–392. doi: 10.1053/j.jrn.2018.08.006.
- Nakagawa N, Maruyama K, Hasebe N. Utility of Geriatric Nutritional Risk Index in patients with chronic kidney disease: a mini-review. Nutrients. 2021;13(11):3688. doi: 10.3390/nu13113688.
- Jung S-YJ, Kwon J, Park S, et al. Data from: phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy; 2019. DRYAD. Available from: doi: 10.5061/dryad.6v0j9.
- Jung S-Y, Kwon J, Park S, et al. Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One. 2018;13(2):e0191290. doi: 10.1371/journal.pone.0191290. PMC: 29415048.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713.
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi: 10.1093/ajcn/82.4.777.
- Wei L, Gao F, Chen L, et al. The joint association of malnutrition and activities of daily living dependence with adverse health outcomes among patients initiating maintenance dialysis. Clin Nutr. 2022;41(7):1475–1482. doi: 10.1016/j.clnu.2022.05.012.
- Teixeira JP, Mayer KP, Griffin BR, et al. Intensive care unit-acquired weakness in patients with acute kidney injury: a contemporary review. Am J Kidney Dis. 2023;81(3):336–351. doi: 10.1053/j.ajkd.2022.08.028.
- Honoré PM, De Waele E, Jacobs R, et al. Nutritional and metabolic alterations during continuous renal replacement therapy. Blood Purif. 2013;35(4):279–284. doi: 10.1159/000350610.
- Wong Vega M, Juarez Calderon M, Tufan Pekkucuksen N, et al. Feeding modality is a barrier to adequate protein provision in children receiving continuous renal replacement therapy (CRRT). Pediatr Nephrol. 2019;34(6):1147–1150. doi: 10.1007/s00467-019-04211-z.
- Xiong J, Wang M, Wang J, et al. Geriatric nutrition risk index is associated with renal progression, cardiovascular events and all-cause mortality in chronic kidney disease. J Nephrol. 2020;33(4):783–793. doi: 10.1007/s40620-019-00676-1.
- Matsukuma Y, Tanaka S, Taniguchi M, et al. Association of Geriatric Nutritional Risk Index with infection-related mortality in patients undergoing hemodialysis: the Q-Cohort Study. Clin Nutr. 2019;38(1):279–287. doi: 10.1016/j.clnu.2018.01.019.
- Lee MJ, Kwon YE, Park KS, et al. Changes in Geriatric Nutritional Risk Index and risk of major adverse cardiac and cerebrovascular events in incident peritoneal dialysis patients. Kidney Res Clin Pract. 2017;36(4):377–386. doi: 10.23876/j.krcp.2017.36.4.377.
- Xiong J, Yu Z, Huang Y, et al. Geriatric Nutritional Risk Index and risk of mortality in critically ill patients with acute kidney injury: a multicenter cohort study. J Ren Nutr. 2023;33(5):639–648. doi: 10.1053/j.jrn.2023.06.004.
- Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010;36(10):1657–1665. doi: 10.1007/s00134-010-1928-z.
- Ge C, Peng Q, Chen W, et al. Association between albumin infusion and outcomes in patients with acute kidney injury and septic shock. Sci Rep. 2021;11(1):24083. doi: 10.1038/s41598-021-03122-0.
- Druml W, Metnitz B, Schaden E, et al. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med. 2010;36(7):1221–1228. doi: 10.1007/s00134-010-1844-2.
- Kim H, Kim H, Lee M, et al. The impact of disease severity on paradoxical association between body mass index and mortality in patients with acute kidney injury undergoing continuous renal replacement therapy. BMC Nephrol. 2018;19(1):32. doi: 10.1186/s12882-018-0833-5.
- Schvartz R, Lupu L, Frydman S, et al. BMI modifies increased mortality risk of post-PCI STEMI patients with AKI. J Clin Med. 2022;11(20):6104. doi: 10.3390/jcm11206104.
- Kanic V, Suran D, Kompara G. Obesity and acute kidney injury in patients with ST-elevation myocardial infarction. J Clin Med. 2023;12(23):7311. doi: 10.3390/jcm12237311.
- Gameiro J, Gonçalves M, Pereira M, et al. Obesity, acute kidney injury and mortality in patients with sepsis: a cohort analysis. Ren Fail. 2018;40(1):120–126. doi: 10.1080/0886022X.2018.1430588.
- Pedersen AB, Gammelager H, Kahlert J, et al. Impact of body mass index on risk of acute kidney injury and mortality in elderly patients undergoing hip fracture surgery. Osteoporos Int. 2017;28(3):1087–1097. doi: 10.1007/s00198-016-3836-8.
- Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. 2018;360:k1274. doi: 10.1136/bmj.k1274.
- Takahashi F, Hashimoto Y, Kaji A, et al. Association between geriatric nutrition risk index and the presence of sarcopenia in people with type 2 diabetes mellitus: a cross-sectional study. Nutrients. 2021;13(11):3729. doi: 10.3390/nu13113729.
- Chen X, Han P, Zhu X, et al. Comparison of three nutritional screening tools for detecting sarcopenia in patients with maintenance hemodialysis. Front Public Health. 2022;10:996447. doi: 10.3389/fpubh.2022.996447.
- de Lucena Alves CP, de Almeida SB, Lima DP, et al. Muscle quality in older adults: a scoping review. J Am Med Dir Assoc. 2023;24(4):462–467.e12. doi: 10.1016/j.jamda.2023.02.012.
- Meyer D, Mohan A, Subev E, et al. Acute kidney injury incidence in hospitalized patients and implications for nutrition support. Nutr Clin Pract. 2020;35(6):987–1000. doi: 10.1002/ncp.10595.
- Bellomo R, Cass A, Cole L, et al. Daily protein intake and patient outcomes in severe acute kidney injury: findings of the randomized evaluation of normal versus augmented level of replacement therapy (RENAL) trial. Blood Purif. 2014;37(4):325–334. doi: 10.1159/000363175.
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863.
- Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi: 10.1186/s13054-023-04663-8.
- van Ruijven IM, Stapel SN, Girbes ARJ, et al. Early high protein provision and mortality in ICU patients including those receiving continuous renal replacement therapy. Eur J Clin Nutr. 2022;76(9):1303–1308. doi: 10.1038/s41430-022-01103-8.