?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To assess the association between kidney function and odds of having low skeletal muscle mass (LSMM) in Chinese adults on the basis of a community study.
Data and methods
In this cross-sectional study, we included 3726 Chinese older persons who participated in an ongoing prospective study, the China Health and Retirement Longitudinal Study(CHARLS). Fasting blood samples were collected in 2012 and analyzed for serum creatinine. Estimated glomerular filtration rate(eGFR) was computed using serum creatinine, gender, and age, according to the 2021 race-free Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). We classified the target population into three categories according to eGFR (normal eGFR;90mL/min/1.73m2, mildly-impaired eGFR;60 to < 90 mL/min/1.73 m2, moderate to severve impaired eGFR;<60 mL/min/1.73 m2). BMI-adjusted muscle mass was used to measure skeletal muscle mass.The association between eGFR(per interquartile range(IQR) increment) and the risk of low skeletal muscle mass was assessed using logistic regression model.
Results
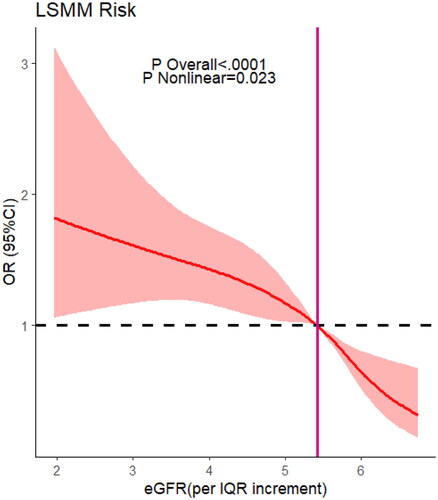
Worsening renal function was associated with being high risk for LSMM after adjusting for potential confounders:the odds ratios (ORs) 95% confidence intervals (CIs) were 0.76 (95% CI = 0.63 − 0.88) for male, and [0.71, (0.61-0.82)]in female, p < 0.001. Specifically, male participants with mildly renal impairment were more prone to develop LSMM (multiadjusted OR, 1.43, 95% CI(0.92 to 2.09), p = 0.1) than femal(multiadjusted OR, 1.32, 95% CI(0.85 to 2.00), p = 0.2), the gender difference was not significant in severe renal dysfunction.However, there was a non-linear relationship between eGFR(per IQR increment) and risk of LSMM(eGFR/IQR =5.42, knot = 4 OR =1, p for non-linear <0.001).
Conclusions
Lower levels of eGFR had a high likelihood of being high risk for LSMM. Older male patients with mildly renal insufficiency are more likely to experience a decrease in skeletal muscle mass compared to female.
Introduction
Renal failure refers to a clinical syndrome caused by severe impairment of glomerular filtration function, leading to a decrease in glomerular filtration rate (eGFR) and the retention of nitrogenous waste products (such as blood urea nitrogen and creatinine) in the body. Its initial manifestations include oliguria or anuria, or the mere detection of azotemia through laboratory tests. This is often accompanied or followed by water-electrolyte imbalance and acid-base disturbance. Renal failure is generally classified into two types: acute kidney injury and chronic kidney disease.Approximately 10% of adults worldwide [Citation1] are affected by some form of chronic kidney disease and 8.2% chronic kidney disease (CKD) in China [Citation2]. Declines in eGFR were associated with worsening of uremic symptom severity [Citation3]. The clinical manifestations of renal failure arise from persistent acidosis, uremia, anemia, electrolyte imbalances, mineral and bone disorders, and inevitably lead to death if left untreated [Citation4].
Skeletal muscle mass(SMM) wasting and dysfunction are common characteristics noted in people who suffer from chronic kidney failure [Citation5,Citation6]. Skeletal muscle damage in kidney failure occurs along with vulnerability from dynapenia (i.e. loss of muscle strength) to sarcopenia (i.e. loss of muscle size and strength), frailty, and cachexia. Lower skeletal muscle risk may be enhanced by concurrent renal failure, given described ubiquitin-mediated proteolysis in muscle and rapid development of exertional muscle fatigue [Citation7]. The etiology of skeletal muscle dysfunction in kidney failure is complex and multifactorial, with kidney dysfunction-related complications(e.g. metabolic acidosis, insulin and IGF1 resistance, changes in hormones, cytokines, inflammatory processes and decreased appetite.) and therapies (e.g. dialysis) all impacting skeletal muscle wasting [Citation8]. The magnitude or severity of skeletal muscle mass associated with kidney function remains unclear due to inconsistency in published literature for both clinical and preclinical models of kidney failure.
Currently, research on SMM losing in renal insufficiency primarily focuses on populations undergoing hemodialysis [Citation9,Citation10], and there is a lack of analytical studies examining the association between SMM and kidney function based on community population. Therefore, our research aims to explore the relationship between eGFR and low skeletal muscle mass(LSMM) in the older China individuals through a cross-sectional retrospective study utilizing data from CHARLS.
Materials and methods
Data source and study population
Our study is a large-scale community-based cross-sectional study based on data from CHARLS. The CHARLS aims to collect a high quality nationally representative sample of Chinese residents ages 45 and older to serve the needs of scientific research on the older population. The baseline national wave of CHARLS is being fielded in 2011 and includes about 10, 000 households and 17, 500 individuals in 150 counties/districts and 450 villages/resident committees, which includes assessment of demographic backgrounds, health status and functioning, social and economic status, as well laboratory examination and other relevant measurements [Citation11]. The CHARLS datasets can be downloaded at http://charls.pku.edu.cn/en, which was approved by the Biomedical Ethics Committee of Peking University, and all participants were required to sign informed consent. CHARLS was a reliable database for research on the health status and possible contributing factors for the older population.
This study used the baseline data from 2011. To research the association between LSMM and kidney function, a total of 3726 participants were selected in this cohort study after excluding those aged below 60 years and missing data.
Blood sample collection and analysis
The venous blood samples were collected by trained staff of the Chinese Center for Disease Control and Prevention(CDC). The samples were centrifugated before they were stored at the local laboratory. After that, the samples were transported to the CDC in Beijing within 2 weeks. Serum creatinine was tested by the rate-blanked and compensated Jaffe creatinine method. Cystatin C was tested by particle-enhanced turbimetric assay. Uric acid (UA) was assayed by the UA plus method. Hemoglobin A1c(HbA1c) was assayed by boronate affinity high-performance liquid chromatography. C-reactive protein(CRP) was tested by immunoturbidimetric assay.
Definition of kidney function
Compared with cystatin C levels, serum creatinine levels are more likely to be affected by muscle mass, and a decrease in eGFR may indicate a reduction in muscle mass [Citation12,Citation13]. eGFR(mL/min/1.73m2) was computed using serum creatinine(Scr), gender, and age, according to the 2021 race-free Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) [Citation14]:
Scr = mg/dL;k = 0.7 (females) or 0.9 (males);α = −0.241 (females) or −0.302 (males);min = indicates the minimum of Scr/k or 1;max = indicates the maximum of Scr/k or 1.
In order to analyze kidney function [Citation15], we classified the target population into three categories according to eGFR (normal:90mL/min/1.73m2, mildly-impaired:60 to < 90 mL/min/1.73 m2, moderate to severve impaired:<60 mL/min/1.73 m2).
Definition of skeletal muscle mass
Body Mass Index (BMI, kg/m2) was defined as the weight divided by the square of height. SMM was calculated using the equation [Citation16]: SMM = 0.244 * weight + 7.8* height − 0.098 * age + 6.6 * sex + race − 3.3 [where sex = 0 (female) and sex = 1 (male); race = 0 (White and Hispanic), race = 1.4 (Black) and race = −1.2 (Asian)] BMI-adjusted muscle mass was used to measure skeletal muscle mass [Citation17]. Similar to previous studies [Citation18,Citation19], the cutoff for LSMM was based on the sex-specific lowest 20% of the BMI-adjusted muscle mass (ASM/BMI) among the study population, with <4.89 kg/m2 in women and <6.79 kg/m2 in male.
Assessment of cvariates
According to prior knowledge, we also considered sociodemographic characteristics and health-related factors in our study.Using stepwise selection method(Stepwise regression) to select suitable covariates for inclusion in a model means to gradually add or remove variables from the model to find an optimal one that best explains the variation in the data while avoiding overfitting. Since age is used as a primary factor in the calculation of both the dependent variable LSMM and eGFR, it is excluded from the category of covariates to prevent overfitting [Citation20]. Sociodemographic characteristics included gender(male, female), residence (urban, rural), education (primary-, secondary+) and marital status (married, unmarried). Factors related to health include BMI, 5-times sit-to-stand test(chr5sec), grip, wspeed, and the number of comorbidities.
Statistical analysis
Chi-squared tests for categorical variables and Student’s t tests for continuous variables were employed to compare the baseline characteristics and incidence of kidney failure after grouping by staging of renal function(Normal, mildly-Impaired, severe-Impaired). We adjusted for marital status, education level, rsidence, smoking, drinking, BMI, 5 times sit-up test, grip, walk speed, and the number of comorbidities.We estimated the odds ratio (OR) and 95% confidence interval (CI) with multivariable GLM regression models fitting non-normally distributed continuous variable eGFR (per IQR increatment, IQR = 17.12 mL/min/1.73 m2). Additionally, the restricted cubic spline (RCS) model was applied to investigate the dose–response relationships between eGFR(per IQR increment) and LSMM.Utilizing the Akaike Information Criterion (AIC) for the purpose of selecting a superior curve-fitting model. Interaction analysis was performed to identify the effect modifications of sociodemographic characteristics, healthrelated behaviors, and anthropometric measurements in the relationship between eGFR(per IQR increment) and LSMM, using a product term in the main analysis [eGFR× (interaction term)].
The statistical analysis was undertaken using All statistical analyses were conducted using the R Studio software(2023.12.0.369), and the significance level of statistical testswas 0.05.
Results
The characteristics of the cohort on the basis of kidney failure status in 2011 are shown in . Approximately 38% participants(N = 1407) reported mildly renal impaired(60 to < 90 mL/min/1.73m2), approximately 4.0% participants(N = 150) reported moderate to severve impaired(eGFR < 60 mL/min/1.73m2). Of the population with renal impairment, approximately 24.4% developed LSMM, while 28.7% with severe renal dysfunction. According to the baseline data of CHARLS, we found that participants with poorer eGFR were more likely to be older, married, living in rural, drinking, higher cystain C, hyperuricemia, worse performance in the 5-times sit-to-stand test, number of comorbidities, and lower skeletal muscle mass.
Table 1. The basic characteristics in 2011 according to kidney function.
We observed that lower levels of eGFR were associated with a higher risk of developing LSMM (OR = 0.76, 95% CI = (0.63 − 0.88) in male, and OR = 0.71, 95% CI = (0.61-0.82) in female, p < 0.001) after adjusting for potential confounders (). According to the diagnostic criteria of renal dysfunction, male participants with mildly renal impairment are more prone to LSMM (multiadjusted OR, 1.43, 95% CI, 0.92 to 2.09, p = 0.1) than femal(multiadjusted OR, 1.32, 95% CI, 0.85 to 2.00, p = 0.2), but the gender difference is not significant in severe renal dysfunction.With the deterioration of eGFR(per IQR increment), the risk of LSMM gradually increases. (P for trend <0.05).
Table 2. The ORs and 95% CIs of the risk of LSMM based on renal dysfunction.
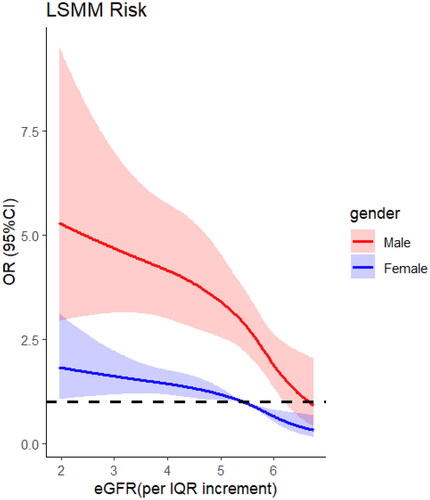
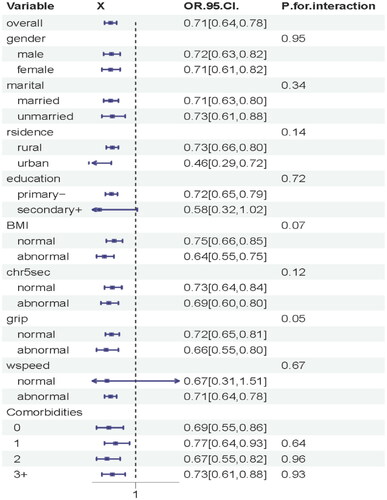
Restricted cubic spline regression () showed a non-linear relationship between eGFR and risk of LSMM(knot = 4, OR =1, eGFR/IQR = 5.42, p for non-linear <0.001). Upon further investigation (), we discovered that men were more susceptible to developing LSMM when experiencing kidney damage compared to women (knot = 4, OR =1, eGFR/IQR =6.62 in male and 5.42 in female, p for non-linear <0.001). We did not find significant interactions between gender, marital, rsidence, education, smoking, drinking, BMI, 5-times sit-up test, grip, wspeed, and the number of comorbidities (p interaction >0.05 for all) ().
Discussion
Loss of skeletal muscle mass is one of the primary diagnostic criteria for age-related sarcopenia, as defined by recent consensus [Citation17,Citation21,Citation22]. The presence of sarcopenia increases the risk of mortality and end-stage renal disease [Citation23]. It has been proposed that the decline in renal function is a condition associated with the process of ‘premature aging’ [Citation24], and that the catabolic alterations commonly associated with chronic kidney failure may explain why sarcopenia is such a prominent and typical feature in renal dysfunction [Citation25]. Similarly, higher muscle mass assessed by creatinine excretion rate are complementary in their association with lower risk of all-cause mortality in kidney transplant recipients [Citation26]. Muscle mass remains a conspicuous predictor of physical performance in chronic kidney disease [Citation27] and mortality in the dialysis patients [Citation28]. While previous research has indeed established a connection between renal impairment and the reduction of skeletal muscle mass [Citation29], our large-scale, community-based cross-sectional study offers the first detailed depiction of the nonlinear nature of this relationship. Our research pays particular attention to the gender differences in the correlation between renal dysfunction and skeletal muscle mass loss, uncovering that males tend to exhibit a more significant decline in skeletal muscle mass even with mild renal impairment. Our findings highlight the strong association between early-stage mild renal dysfunction and skeletal muscle decline, suggesting that proactive engagement in exercise and nutritional interventions at this early stage may help delay or prevent further deterioration of skeletal muscle mass. This provides a valuable reference for the management of chronic kidney disease in community health practices.
The non-linear relationship was particularly evident among male individuals with mild renal dysfunction. Gender differences in SMM during the early stages of renal failure can be attributed to both biological and sociocultural factors. Males and females exhibit variations in physiological structures, hormonal levels, and metabolic rates, which may influence muscle growth, development, and metabolism. Furthermore, the sociocultural environment significantly impacts individuals’ lifestyles, dietary habits, and physical activity levels. The differing social roles and expectations of males and females may lead to disparities in their physical activity, dietary structure, and nutritional intake, ultimately affecting SMM. However, as renal failure progresses and deteriorates, it leads to disturbances in the internal environment of the body, including imbalances in electrolyte levels and acid-base equilibrium. These pathological changes can have similar negative impacts on SMM for both males and females, thus obscuring the original gender differences. In the late stages of renal failure, patients may experience gastrointestinal symptoms such as anorexia, nausea, and vomiting, resulting in inadequate nutrient intake. Additionally, the metabolism and utilization of nutrients in the body may also undergo changes, further diminishing the significance of gender differences in SMM. Furthermore, some medications may have an effect on SMM, and these effects may not be gender-specific.
A significant correlation between serum creatinine levels and skeletal muscle mass index was reported, while significant decline in muscle mass in older males as serum levels of creatinine increasing but sustain stable or even increasing in females [Citation30]. The sharp decline in skeletal muscle mass among older male individuals is attributed to the decrease in testosterone levels and its anabolic effects [Citation31], as well as the reduction in protein synthesis [Citation32]. According to the research results, it can be stated that estrogens have a beneficial, protective effect on renal function in the persons 60 years and older, while androgens have the opposite effect [Citation33]. However, these factors are relatively absent in women, who are less affected by testosterone. As shown in our , the incidence of low skeletal muscle mass in older women is much lower than that in male patients.Despite the ever-increasing aging population globally [Citation34], unfortunately, the gender differences in the correlation between the decline of eGFR and the reduction of skeletal muscle mass have not received sufficient attention.In addition, it is well-known that renal function declines with age, the exact mechanisms of kidney senescence are still uncertain [Citation35]. Distinguishing between the physiological aging changes of the kidneys and those related to comorbidities associated with aging, such as the decrease in skeletal muscle content, poses a significant challenge.It is worth noting that the above discussions represent one of the possible mechanisms, and the specific mechanisms may vary depending on individual differences, disease types, and treatment approaches. To gain a more precise understanding of the impact of gender differences on SMM at different stages of renal failure and its underlying mechanisms, we recommend conducting further in-depth research, including multicenter clinical trials with large sample sizes and animal experiments.
Our study had several limitations. First, this study is cross-sectional, thus it does not permit the establishment of a causal relationship between the observed associations. We are unable to prospectively track the decline in muscle mass and eGFR levels for each individual.The relationship between a decrease in eGFR and low skeletal muscle mass may be bidirectional. Second, this study is based on a limited population from Chinese communities, therefore, its findings may not necessarily be generalized to other populations.Third, the SMM equation was derived from an anthropometric prediction mode, which is not the ‘gold standard’ in all cases. To further enhance the accuracy and reliability of our research, we propose the following measures for improving future studies: Conducting in-depth investigations to compare the accuracy and applicability of different SMM measurement methods, in order to select the most appropriate approach for diverse types of subjects. In future studies, we can consider utilizing more advanced measurement techniques, such as Dual-Energy X-ray Absorptiometry (DEXA) or Bioelectrical Impedance Analysis (BIA), to more precisely assess skeletal muscle mass. During the data analysis and interpretation phase, we will take a more nuanced approach in considering the impact of factors like obesity on SMM measurements, and adopt corresponding measures to ensure the accuracy and reliability of our results.
In conclusion, we found that participants with lower eGFR had a high likelihood of being high or very high risk for LSMM in this large-scale community-based study. With the decline of eGFR, older male patients with mild to moderate renal insufficiency are more likely to experience a decrease in skeletal muscle content compared to female patients. Male patients with renal insufficiency should undergo exercise and nutritional intervention as early as possible to prevent the loss of skeletal muscle.Future prospective studies are needed to clarify the temporal relationship between objectively measured skeletal muscle mass and eGFR.
Ethical approval
The CHARLS was ethically approved by the institutional review board at Peking University (Nu: IRB00001052–11015). All participants signed written informed consent.
Consent to publish
All authors agree with publication in this journal.
Acknowledgments
We thank the Peking National Center for Economic Research for providing the CHARLS data. We are grateful to all the participants and researchers in this project.
Disclosure statement
The authors of this study declare no conflicts of interest.
Data availability statement
The original datasets and questionnaires of this study are available at the CHARLS website (http://forum.charls.pku.edu.cn).
Additional information
Funding
References
- Collaboration GCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/s0140-6736(20)30045-3.
- Wang L, Xu X, Zhang M, et al. Prevalence of chronic kidney disease in china: results from the sixth china chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–310. doi: 10.1001/jamainternmed.2022.6817.
- Wulczyn KE, Zhao SH, Rhee EP, et al. Trajectories of uremic symptom severity and kidney function in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2022;17(4):496–506. doi: 10.2215/cjn.13010921.
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, et al. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–288. doi: 10.1038/s41581-019-0248-y.
- Troutman AD, Arroyo E, Lim K, et al. Skeletal muscle complications in chronic kidney disease. Curr Osteoporos Rep. 2022;20(6):410–421. doi: 10.1007/s11914-022-00751-w.
- Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380–392. doi: 10.1053/j.jrn.2018.08.006.
- Gregg LP, Bossola M, Ostrosky-Frid M, et al. Fatigue in CKD: epidemiology, pathophysiology, and treatment. Clin J Am Soc Nephrol. 2021;16(9):1445–1455. doi: 10.2215/cjn.19891220.
- Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat Rev Nephrol. 2022;18(3):138–152. doi: 10.1038/s41581-021-00498-0.
- Gamboa JL, Roshanravan B, Towse T, et al. Skeletal muscle mitochondrial dysfunction is present in patients with CKD before initiation of maintenance hemodialysis. Clin J Am Soc Nephrol. 2020;15(7):926–936. doi: 10.2215/cjn.10320819.
- Takata T, Mae Y, Yamada K, et al. Skeletal muscle mass is associated with erythropoietin response in hemodialysis patients. BMC Nephrol. 2021;22(1):134. doi: 10.1186/s12882-021-02346-6.
- Mori K. Maintenance of skeletal muscle to counteract sarcopenia in patients with advanced chronic kidney disease and especially those undergoing hemodialysis. Nutrients. 2021;13(5):1538. doi: 10.3390/nu13051538.
- Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/cjn.02870707.
- Potok OA, Ix JH, Shlipak MG, et al. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: a cohort analysis of the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis. 2020;76(6):765–774. doi: 10.1053/j.ajkd.2020.05.017.
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953.
- Podadera-Herreros A, Alcala-Diaz JF, Gutierrez-Mariscal FM, et al. Long-term consumption of a mediterranean diet or a low-fat diet on kidney function in coronary heart disease patients: the CORDIOPREV randomized controlled trial. Clin Nutr. 2022;41(2):552–559. doi: 10.1016/j.clnu.2021.12.041.
- Lee RC, Wang Z, Heo M, et al. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72(3):796–803. doi: 10.1093/ajcn/72.3.796.
- Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012.
- Tyrovolas S, Koyanagi A, Olaya B, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7(3):312–321. doi: 10.1002/jcsm.12076.
- Jacob L, Gyasi RM, Oh H, et al. Leisure-time physical activity and sarcopenia among older adults from low- and middle-income countries. J Cachexia Sarcopenia Muscle. 2023;14(2):1130–1138. doi: 10.1002/jcsm.13215.
- Williamson EJ, Aitken Z, Lawrie J, et al. Introduction to causal diagrams for confounder selection. Respirology. 2014;19(3):303–311. doi: 10.1111/resp.12238.
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169.
- Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010.
- Wilkinson TJ, Miksza J, Yates T, et al. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: a UK Biobank study. J Cachexia Sarcopenia Muscle. 2021;12(3):586–598. doi: 10.1002/jcsm.12705.
- O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–420. doi: 10.1681/asn.2015121308.
- Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29(9):1655–1665. doi: 10.1093/ndt/gft070.
- van Vliet IMY, Post A, Kremer D, et al. Muscle mass, muscle strength and mortality in kidney transplant recipients: results of the TransplantLines Biobank and Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13(6):2932–2943. doi: 10.1002/jcsm.13070.
- Wilkinson TJ, Gould DW, Nixon DGD, et al. Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant. 2019;34(8):1344–1353. doi: 10.1093/ndt/gfy139.
- Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–1728. doi: 10.2215/cjn.10261013.
- Zhou Y, Hellberg M, Svensson P, et al. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2018;33(2):342–348. doi: 10.1093/ndt/gfw466.
- Yim J, Son NH, Kyong T, et al. Muscle mass has a greater impact on serum creatinine levels in older males than in females. Heliyon. 2023;9(11):e21866. doi: 10.1016/j.heliyon.2023.e21866.
- Barone B, Napolitano L, Abate M, et al. The role of testosterone in the elderly: what do we know? Int J Mol Sci. 2022;23(7):3535. doi: 10.3390/ijms23073535.
- Franzke B, Neubauer O, Cameron-Smith D, et al. Dietary protein, muscle and physical function in the very old. Nutrients. 2018;10(7):935. doi: 10.3390/nu10070935.
- van der Burgh AC, Rizopoulos D, Ikram MA, et al. Determinants of the evolution of kidney function with age. Kidney Int Rep. 2021;6(12):3054–3063. doi: 10.1016/j.ekir.2021.10.006.
- Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. doi: 10.1038/s41586-018-0457-8.
- Dybiec J, Szlagor M, Młynarska E, et al. Structural and functional changes in aging kidneys. Int J Mol Sci. 2022;23(23):15435. doi: 10.3390/ijms232315435.