Abstract
Background and objectives
In clinical practice, some patients are diagnosed with diabetic nephropathy (DN) combined with acute tubulointerstitial nephritis (ATIN) through renal biopsy. There is relatively little research on the treatment and prognosis of such patients, and no consensus exists on the use of glucocorticoid for treatment. Therefore, our study explores the progression of DN combined with ATIN and the renal outcomes after treatment with glucocorticoid.
Methods
This study retrospectively analyzed patients diagnosed with DN combined with ATIN through renal biopsy at our center from January 1, 2015, to December 31, 2021. We collected general patient information, laboratory indicators, renal pathology indicators, and the glucocorticoid usage after kidney biopsy. Follow-up data were collected from medical records. Statistical analysis methods included t-tests, non-parametric tests, and chi-square tests. Univariate and multivariate Cox regression analyses were used to evaluate the risk factors for renal endpoint events in patients. Statistical significance was defined as p-values < 0.05.
Results
In this study, a total of 67 patients were included. The subjects were divided into two groups based on whether they received glucocorticoid treatment: 33 patients in the steroid group and 34 in the non-steroid group. In the steroid group, 19 patients reached the renal endpoint event, which was significantly higher than in the non-steroid group (57.58% vs. 29.41%, p = 0.038). Univariate Cox regression analysis showed that serum creatinine (HR = 1.008, p < 0.001), albumin (HR = 0.919, p < 0.001), 24-h urinary protein (HR = 1.093, p = 0.002), hemoglobin (HR = 0.964, p = 0.001), triglycerides (HR = 1.12, p = 0.04), and the use of glucocorticoid (HR = 2.507, p = 0.019) were influencing factors for renal endpoint events in patients with DN combined with ATIN. Multivariate Cox regression analysis showed that albumin (HR = 0.863, p = 0.003) was an independent risk factor for renal endpoint events in patients with DN combined with ATIN.
Conclusions
The use of glucocorticoid in treatment does not improve renal prognosis in patients with DN combined with ATIN. Lower levels of albumin are associated with a worse renal prognosis.
Introduction
Diabetic nephropathy (DN) is one of the primary microvascular complications of diabetes, affecting approximately 40% of diabetic patients [Citation1]. Traditionally, DN has been considered a typical metabolic disorder [Citation2]. Currently, the progression of the disease is primarily managed through dietary management, controlling blood pressure and blood glucose levels, and using medications such as Renin-Angiotensin System receptor blockers and Sodium-Glucose Co-transporter 2 inhibitors [Citation3,Citation4]. In recent years, new treatment approaches for DN have gradually come into focus [Citation5]. Despite these developments, DN remains a leading cause of end-stage renal disease (ESRD), necessitating renal replacement therapy [Citation1], approximately 30%-50% of ESRD cases are attributed to DN [Citation2].
Acute tubulointerstitial nephritis (ATIN) is one of the common causes of acute kidney injury (AKI), and it can progress to chronic kidney disease (CKD) if not diagnosed and treated promptly [Citation6]. The main histological features of ATIN are the infiltration of inflammatory cells in the renal interstitium [Citation7]. The causes of ATIN are diverse, commonly including drugs, autoimmune diseases, infections, genetic factors, and tumors. Most studies consider drug-related ATIN the most common [Citation6,Citation8,Citation9]. In addition to removing the causative factors, glucocorticoid is usually applied early unless there are contraindications to treatment. This is a common method for treating ATIN, effectively improving symptoms and renal prognosis in most cases [Citation6,Citation10–12]. In clinical practice, we observed that some patients with renal dysfunction were diagnosed with DN combined with ATIN through renal biopsy. The effective treatment plan for such cases is a topic worthy of discussion. When treating DN combined with ATIN, it is a feasible treatment option to consider applying glucocorticoid based on the experience of treating pure ATIN. However, patients with DN often have various complications, and inappropriate use of glucocorticoid can lead to multiple side effects, affecting the renal prognosis of patients. Hence, there is currently no consensus on the use of glucocorticoid for treating DN combined with ATIN. This study aims to explore the impact of glucocorticoid on the progression and prognosis of DN combined with ATIN, in hopes of finding a more comprehensive treatment plan. Through this research, we hope to provide more scientific evidence for clinical treatment decisions.
Methods
Study patients
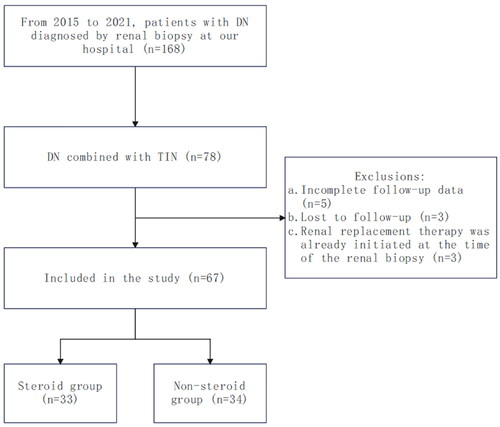
This study retrospectively analyzed the renal pathology records and medication records of 78 patients diagnosed with DN combined with ATIN through renal biopsy at the China-Japan Friendship Hospital from 1 January 2015, to 31 December 2021. Several exclusion criteria were applied: participants with missing clinical or pathological data (5 cases), participants lost to follow-up (3 cases), and participants who had already undergone renal replacement therapy at the time of renal biopsy (3 cases), with a final total of 67 cases included in the study. Subjects were divided into a steroid group and a non-steroid group based on whether they received glucocorticoid treatment, with 33 and 34 cases respectively (). The grouping of patients was decided collaboratively by the attending physicians and the patients themselves, based on the patients’ conditions and individual circumstances. This study was approved by the Medical Ethics Committee of the China-Japan Friendship Hospital.
Clinical data
Clinical data were obtained by reviewing patient medical records, including diagnostic age, gender, body mass index (BMI), date of renal biopsy, renal biopsy pathology results, medical history, medication usage, and cumulative glucocorticoid dose. Biological data were collected before the renal biopsy and at the end of the follow-up, including serum creatinine, estimated glomerular filtration rate (eGFR), serum albumin, 24-h urinary protein, urinary red blood cell count, blood eosinophil count and percentage, hemoglobin, total cholesterol, triglycerides, uric acid, and glycated hemoglobin.
Renal tissue samples were obtained from all patients via percutaneous renal biopsy. The standard processing method for renal biopsy specimens included examination using light microscopy and immunofluorescence. Light microscopy was used to observe pathological changes in renal tissue, and patients’ glomerular pathology grading, interstitial fibrosis and tubular atrophy (IFTA) were recorded. The pathological results of all patients indicated inflammatory cell infiltration composed of lymphocytes, monocytes, and eosinophils in the renal tubules and interstitium.
glucocorticoid treatment: Oral administration of 0.5 mg/kg/day prednisone or an equivalent dose of methylprednisolone for 2-4 weeks, followed by gradual tapering of the dose depending on the disease condition, with the total glucocorticoid treatment period being ≤3 months.
Outcomes
The renal endpoint is defined as: ①eGFR < 15 mL/(min·1.73 m2) or ②eGFR declines by ≥50% from the baseline level at the time of renal biopsy or ③initiation of regular renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplantation). The end of follow-up is defined as 6 months after the renal biopsy or upon reaching the above renal endpoint.
Statistical analysis
All statistical processing and analysis were performed using SPSS software version 19.0. A p-value < 0.05 was considered statistically significant. Data conforming to a normal distribution are expressed as mean ± standard deviation, while data not conforming to normal distribution are expressed as median (interquartile range). For continuous variables, independent sample t-tests and paired t-tests were used for those conforming to a normal distribution, while non-parametric tests were used for those not conforming to normal distribution. Categorical variables were analyzed using chi-square tests. Univariate and multivariate COX regression analyses were used to identify factors influencing the occurrence of renal endpoint events in patients with DN combined with ATIN.
Results
In this study, 67 patients diagnosed with DN combined with ATIN through renal biopsy were included and divided into a steroid group of 33 patients and a non-steroid group of 34 patients. Statistical analyses were performed on these two groups. shows the differences in general characteristics between the two groups. In both the steroid and non-steroid groups, patients were predominantly male, with a higher proportion of males in the steroid group (87.88% vs. 64.71%, p = 0.026). However, in terms of age, BMI, comorbidities, glomerular pathology staging, and IFTA, the two groups were roughly similar, showing no statistically significant differences.
Table 1. Baseline characteristics of patients with DN combined with ATIN.
We demonstrated the changes in clinical indicators at baseline and at the end of follow-up for the two groups of patients (). In the steroid group, the patients’ 24-h urinary protein and urinary red blood cell count were reduced at the end of follow-up compared to baseline; however, there was an increase in serum creatinine levels and a downward trend in eGFR. In the non-steroid group, the patients’ 24-h urinary protein, total cholesterol, uric acid, and glycated hemoglobin all decreased at the end of follow-up compared to baseline, while serum creatinine levels increased. Further comparing the baseline data of the two groups, we found that the steroid group had higher serum creatinine, 24-h urinary protein, and urinary red blood cell count than the non-steroid group, while having lower levels of hemoglobin and total cholesterol. At the end of follow-up, the steroid group’s serum creatinine, 24-h urinary protein, and uric acid levels were significantly higher than those of the non-steroid group, while the hemoglobin level remained lower than that of the non-steroid group.
Table 2. Changes in Laboratory Indicators of patients with DN combined with ATIN.
summarizes the incidence of treatment-related complications and prognosis at the end of follow-up for two groups of patients: In the steroid group, during the follow-up period, the incidence of increased blood glucose and abnormal blood pressure was 36.36% and 24.24%, respectively, with p < 0.001. No treatment-related complications were found in the non-steroid group. Additionally, in the steroid group, three patients developed infections, one had gastrointestinal symptoms, and another exhibited neuropsychiatric symptoms, but these incidences were not statistically significant. Notably, 19 patients in the steroid group experienced the renal endpoint event, a significantly higher rate than that of non-steroid group (57.58% vs. 29.41%, p = 0.038).
Table 3. Complications and prognosis of patients with DN combined with ATIN at the end of follow-up.
Our study conducted a univariate COX regression analysis with the renal endpoint event in patients with DN combined with ATIN as the dependent variable and the time from kidney biopsy to the endpoint event or the end of follow-up as the survival time. The results showed that serum creatinine (HR 1.008; 95% CI 1.005–1.011; p < 0.001), serum albumin (HR 0.919; 95% CI 0.869–0.973; p < 0.001), 24-h urinary protein (HR 1.093; 95% CI 1.033–1.155; p = 0.003), hemoglobin (HR 0.964; 95% CI 0.942–0.985; p = 0.001), triglycerides (HR 1.12; 95% CI 1.005–1.249; p = 0.04), and glucocorticoid treatment (HR 2.507; 95% CI 1.164–5.401; p = 0.019) are risk factors for renal endpoint events in patients with DN combined with ATIN ().
Table 4. Univariate COX regression analysis of renal endpoint event in patients with DN combined with ATIN.
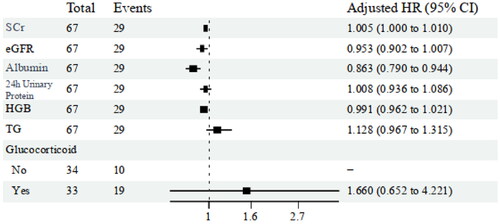
Building upon the univariate Cox regression analysis, we incorporated additional indicators, including serum creatinine, serum albumin, 24-h urinary protein, hemoglobin, triglycerides, and glucocorticoid treatment, into a multivariate Cox regression analysis. The results of the analysis indicated that a reduction in serum albumin levels (HR 0.863; 95% CI 0.790–0.944; p = 0.003) is an independent risk factor for renal endpoint events in patients with DN combined with ATIN ().
Discussion
DN combined with ATIN is not uncommon in clinical settings, but currently, there is a lack of standardized treatment protocols for these patients. Internationally, there is controversy over whether to use glucocorticoids for treatment and their efficacy. Hence, we conducted this study. In this study, although we could not determine the cause of ATIN through detailed medical history and comprehensive examinations, all patients in the steroid group were confirmed to have eosinophilic infiltration in the renal interstitium through renal biopsy, which typically indicates the presence of an immune-mediated renal hypersensitivity reaction. Therefore, the doctors at the time chose to treat the patients with glucocorticoid. After organizing the data, we found that there were 33 patients in the steroid group. Among them, four patients showed a decrease in serum creatinine levels compared to the baseline after glucocorticoid treatment, indicating improved renal function. However, the final statistical results showed that, regardless of glucocorticoid use, the median serum creatinine levels at the end of the follow-up were significantly higher than at baseline, while 24-h urinary protein were significantly lower than baseline. The increase in serum creatinine mainly reflects a significant decrease in eGFR, which may be due to a substantial reduction in kidney filtration function, leading to the accumulation of creatinine and other waste products in the blood. In contrast, the reduction in urinary protein may be associated with glomerulosclerosis and fibrosis. During the process of glomerulosclerosis, the number of functional glomeruli decreases, leading to a reduction in the glomeruli capable of producing proteinuria, thus lowering urinary protein levels. Furthermore, the use of certain medications may reduce proteinuria to some extent, but they cannot completely prevent the further deterioration of kidney function.
Additionally, some patients in the steroid group developed conditions such as hypertension and hyperglycemia after treatment. Furthermore, 57.58% of the patients required renal replacement therapy at the end of follow-up, which was significantly higher than the non-steroid group. The study results indicate that the use of glucocorticoid in treating DN combined with ATIN did not achieve the positive outcomes we anticipated. This is clearly different from the good renal outcomes obtained in treating most cases of pure ATIN [Citation13–17].
There may be several reasons for obtaining such research results: first, it is closely related to the pathogenesis of the disease. The etiologies of ATIN are diverse and complex [Citation17,Citation18]. Drug-related ATIN is considered one of the main types, possibly accounting for more than two-thirds of ATIN cases [Citation19], followed by immune-related ATIN. It is widely believed that the pathogenesis of drug-related ATIN involves renal immunological allergic reactions, related to cellular and humoral immunity [Citation6,Citation7,Citation10,Citation11]. In contrast, the pathogenesis of immune-related ATIN is more complex, possibly including the production of autoantibodies, deposition of immune complexes in the renal interstitium, and activation of inflammatory pathways [Citation11,Citation20]. Glucocorticoid, due to their immunosuppressive and anti-inflammatory effects, can effectively intervene in some of the main pathogenic mechanisms of ATIN, thereby playing a positive role in improving renal function. However, the pathogenesis of DN is also highly complex, with hyperglycemia being the primary cause of renal damage in DN. Hyperglycemia can have varying effects on different parts of the kidney, and the mechanisms by which it induces renal dysfunction are not yet fully understood [Citation21]. It is widely accepted that the onset and progression of DN are associated with a variety of factors, including hemodynamic abnormalities, metabolic disorders, immune dysregulation, oxidative stress, and inflammatory responses [Citation22,Citation23]. Hemodynamic abnormalities can activate intracellular second messengers and various growth factors, while glucose-dependent pathways within the kidney are also activated, leading to increased oxidative stress, polyol pathway activity, and accumulation of advanced glycation end products (AGEs) [Citation24]. These processes ultimately result in glomerulosclerosis and tubulointerstitial fibrosis. Although glucocorticoid can regulate the immunological dysregulation and inflammatory responses in patients with DN combined with ATIN, their effects on other potential pathogenic factors, such as hemodynamic abnormalities, metabolic disorders, and infections, may not be significant.
Secondly, in this study, the baseline IFTA of patients with DN combined with ATIN was mostly moderate to severe. This finding suggests that the patients may have more severe tubulointerstitial damage. Most were also found to have significant glomerular damage, indicating that the kidneys had suffered multifaceted damage. It is important to note that the injuries to the glomeruli, tubules, interstitium, and renal vasculature are not independent of each other; they have a mutually exacerbating relationship. This complex process may lead to and aggravate tissue hypoxia, while damaged cells may activate multiple fibrogenic signaling pathways [Citation25], which cannot be improved with glucocorticoid treatment. On the other hand, the overall high baseline IFTA indicates that in some patients, tubulointerstitial lesions had progressed to a certain extent at the time of diagnosis. This may be related to the typically asymptomatic early stages of ATIN or the lack of specific symptoms. Additionally, DN patients often have multiple comorbidities, which may lead them to overlook new symptoms, or these symptoms may be masked by the manifestations of other complications. Therefore, by the time patients seek medical attention, the tubulointerstitial lesions have already developed to a certain extent, making it difficult for glucocorticoid intervention to effectively reverse the tubulointerstitial damage. This was prominently highlighted in our study, emphasizing the importance of early diagnosis and intervention in clinical practice.
Thirdly, the use of glucocorticoid may exacerbate metabolic disorders, thereby accelerating the progression of DN and ATIN, particularly in terms of glucose and lipid metabolism. Hyperglycemia is one of the main causes of renal damage in DN, and disorders in glucose metabolism keep the body in a high glucose environment for extended periods. Research shows [Citation26,Citation27] that in such a high-glucose environment, the expression of certain pro-inflammatory and pro-fibrotic injury factors significantly increases. Meanwhile, disorders in lipid metabolism may promote tubulointerstitial fibrosis through various pathways, including oxidative stress, release of pro-inflammatory factors, changes in cell signaling, and lipid deposition [Citation28,Citation29]. Existing studies indicate that lipid metabolism disorder is an independent risk factor for tubulointerstitial fibrosis [Citation30], particularly as it disrupts the existing cholesterol homeostasis. This imbalance is closely associated with the progression of tubulointerstitial lesions [Citation31]. More attention and emphasis should be given to this aspect in future research and treatment strategies.
In our study, through multivariable COX regression analysis, we identified serum albumin as an independent risk factor for renal endpoint events. We observed that a decrease in albumin levels is closely associated with poorer renal outcomes, consistent with the results of existing studies [Citation32–34]. Zhang et al. [Citation35] reported a study on 188 patients with DN, in which they found that serum albumin levels were associated with proteinuria, renal function, and glomerular lesions. The COX regression analysis results showed a significant correlation between hypoalbuminemia and adverse renal prognosis. Hypoalbuminemia is closely linked to a variety of factors, including malnutrition, chronic inflammation, proteinuria, decreased colloid osmotic pressure, impaired drug metabolism, and cardiovascular diseases. These factors may collectively constitute part of the mechanism by which hypoalbuminemia leads to poor renal prognosis.
This study has certain limitations. First, it is a retrospective, single-center study with a relatively small sample size, which may limit the generalizability of our research findings. Second, our study primarily focused on patients with DN combined with ATIN. Although all patients involved in the study were confirmed to have DN and ATIN through renal biopsy, we do not have a reliable method to determine the sequence of occurrence of these two renal pathological changes. It is possible that some patients with tubulointerstitial lesions secondary to DN were mistakenly included in the study, thereby introducing some bias. Lastly, considering that DN is a chronic complication requiring long-term observation, the assessment of disease progression depends on extended follow-up periods. However, the follow-up period in our study was six months, which may introduce certain errors in the prognosis analysis due to the short follow-up duration.
In summary, the use of glucocorticoid in treatment does not improve renal prognosis in patients with DN combined with ATIN. The reduction in serum albumin levels is an independent risk factor for the occurrence of renal endpoint events in patients with DN combined with ATIN. Future research should focus on conducting more large-scale, multi-center, prospective randomized controlled trials (RCTs) to provide more optimized and personalized treatment plans for patients.
Ethics approval and consent to participate
This study was approved by the local ethics committee of the China-Japan Friendship Hospital. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All the enrolled patients have signed the renal biopsy and research consents before the renal biopsy was performed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Megumi O, Tadashi T, Masakazu H, J.], et al. Estimated glomerular filtration rate decline and risk of end-stage renal disease in type 2 diabetes. PLOS One. 2018;13(8):e0201535. doi: 10.1371/journal.pone.0201535.
- Ruiz-Ortega M, Rodrigues-Diez R, Lavoz C, et al. Special issue “diabetic nephropathy: diagnosis, prevention and treatment”. J Clin Med. 2020;9(3):813. doi: 10.3390/jcm9030813.
- Buse J, Wexler D, Tsapas A, et al. 2019 Update to: management of Hyperglycemia in Type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066.
- Hu Q, Chen Y, Deng X, et al. Diabetic nephropathy: focusing on pathological signals, clinical treatment, and dietary regulation. Biomed Pharmacother. 2023;159:114252. doi: 10.1016/j.biopha.2023.114252.
- Bakris G, Agarwal R, Anker S, et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845.
- Joyce E, Glasner P, Ranganathan S, et al. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring . Pediatr Nephrol. 2017;32(4):577–587. doi: 10.1007/s00467-016-3394-5.
- Ruebner R, Fadrowski J. Tubulointerstitial nephritis. Pediatr Clin North Am. 2019;66(1):111–119. doi: 10.1016/j.pcl.2018.08.009.
- Nakaosa N, Tsuboi N, Okabayashi Y, et al. Tubulointerstitial nephritis: a biopsy case series of 139 Japanese patients . Clin Exp Nephrol. 2022;26(5):435–444. doi: 10.1007/s10157-021-02178-6.
- Yun D, Jang M, An J, et al. Effect of steroids and relevant cytokine analysis in acute tubulointerstitial nephritis . BMC Nephrol. 2019;20(1):88. doi: 10.1186/s12882-019-1277-2.
- Schurder J, Buob D, Perrin P, et al. Acute interstitial nephritis: aetiology and management . Nephrol Dial Transplant. 2021;36(10):1799–1802. doi: 10.1093/ndt/gfz262.
- Caravaca-Fontán F, Fernández-Juárez G, Praga M. Acute kidney injury in interstitial nephritis. Curr Opin Crit Care. 2019;25(6):558–564. doi: 10.1097/MCC.0000000000000654.
- Huang L, Liang S, Dong J, et al. Prognosis of severe drug-induced acute interstitial nephritis requiring renal replacement therapy. Ren Fail. 2021;43(1):1020–1027. doi: 10.1080/0886022X.2021.1942914.
- Amaro D, Carreño E, Steeples L, et al. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a review. Br J Ophthalmol. 2020;104(6):742–747. doi: 10.1136/bjophthalmol-2019-314926.
- Arai H, Ogata S, Ozeki T, et al. Long-term changes in renal function after treatment initiation and the importance of early diagnosis in maintaining renal function among IgG4-related tubulointerstitial nephritis patients in Japan . Arthritis Res Ther. 2020;22(1):261. doi: 10.1186/s13075-020-02320-x.
- Prendecki M, Tanna A, Salama A, et al. Long-term outcome in biopsy-proven acute interstitial nephritis treated with steroids . Clin Kidney J. 2017;10(2):233–239. doi: 10.1093/ckj/sfw116.
- Fernandez-Juarez G, Perez J, Caravaca-Fontán F, et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. 2018;13(12):1851–1858. doi: 10.2215/CJN.01390118.
- Su T, Gu Y, Sun P, et al. Etiology and renal outcomes of acute tubulointerstitial nephritis: a single-center prospective cohort study in China. Nephrol Dial Transplant. 2018;33(7):1180–1188. doi: 10.1093/ndt/gfx247.
- Huang J, Su T, Tan Y, et al. Serum anti-CRP antibodies differentiate etiology and predict relapse in acute tubulointerstitial nephritis. Clin Kidney J. 2022;15(1):51–59. doi: 10.1093/ckj/sfab119.
- Gérard A, Merino D, Laurain A, et al. Drug-induced tubulointerstitial nephritis: insights from the World Health Organization safety database. Kidney Int Rep. 2022;7(7):1699–1702. doi: 10.1016/j.ekir.2022.04.090.
- Mose F, Birn H, Hoffmann-Petersen N, et al. Prednisolone treatment in acute interstitial nephritis (PRAISE) - protocol for the randomized controlled trial. BMC Nephrol. 2021;22(1):161. doi: 10.1186/s12882-021-02372-4.
- Thomas H, Ford Versypt A. Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J Biol Eng. 2022;16(1):19. doi: 10.1186/s13036-022-00299-4.
- Bonner R, Albajrami O, Hudspeth J, et al. Diabetic kidney disease . Prim Care. 2020;47(4):645–659. doi: 10.1016/j.pop.2020.08.004.
- Zheng W, Guo J, Liu Z. Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective. Clin Epigenetics. 2021;13(1):87. doi: 10.1186/s13148-021-01079-5.
- Arya A, Aggarwal S, Yadav H. Pathogenesis of diabetic nephropathy. Int J Pharm Pharm Sci. 2010;2(4):24–29.
- Slyne J, Slattery C, Mcmorrow T, et al. New developments concerning the proximal tubule in diabetic nephropathy: in vitro models and mechanisms. Nephrol Dial Transplant. 2015;30 Suppl 4:iv60–iv67. doi: 10.1093/ndt/gfv264.
- Salti T, Khazim K, Haddad R, et al. Glucose Induces IL-1α-dependent inflammation and extracellular matrix proteins expression and deposition in renal tubular epithelial cells in diabetic kidney disease Front Immunol. 2020;11:1270. doi: 10.3389/fimmu.2020.01270.
- Giancarlo T, Sara C. Tubulointerstitial disease in diabetic nephropathy . Int J Nephrol Renovasc Dis. 2014;7:107–115.
- Qu L, Jiao B. The interplay between immune and metabolic pathways in kidney disease . Cells. 2023;12(12):1584. doi: 10.3390/cells12121584.
- Gonçalves L, Andrade-Silva M, Basso P, et al. Vitamin D and chronic kidney disease: insights on lipid metabolism of tubular epithelial cell and macrophages in tubulointerstitial fibrosis . Front Physiol. 2023;14:1145233. doi: 10.3389/fphys.2023.1145233.
- Liu B, Zhao L, Yang Q, et al. Hyperuricemia and hypertriglyceridemia indicate tubular atrophy/interstitial fibrosis in patients with IgA nephropathy and membranous nephropathy. Int Urol Nephrol. 2021;53(11):2321–2332. doi: 10.1007/s11255-021-02844-4.
- Hu Z, Lu J, Chen P, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10(6):2803–2816. doi: 10.7150/thno.40571.
- Cheng T, Wang X, Han Y, et al. The level of serum albumin is associated with renal prognosis and renal function decline in patients with chronic kidney disease. BMC Nephrol. 2023;24(1):57. doi: 10.1186/s12882-023-03110-8.
- Song H, Wei C, Hu H, et al. Association of the serum albumin level with prognosis in chronic kidney disease patients. Int Urol Nephrol. 2022;54(9):2421–2431. doi: 10.1007/s11255-022-03140-5.
- Majoni S, Barzi F, Hoy W, et al. Baseline liver function tests and full blood count indices and their association with progression of chronic kidney disease and renal outcomes in Aboriginal and Torres Strait Islander people: the eGFR follow- up study. BMC Nephrol. 2020;21(1):523. doi: 10.1186/s12882-020-02185-x.
- Zhang J, Zhang R, Wang Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res. 2019;2019:7825804–7825809. doi: 10.1155/2019/7825804.