Abstract
Background
There have been some shifts in the frequency and distribution of biopsy-proven renal diseases in China over recent years. The aim of the study was to investigate the changing spectrum of renal diseases from the view of kidney biopsy data in a single center of China.
Methods and results
A total of 10,996 cases of native renal biopsies from patients aged ≥15 years old in Huashan Hospital, Fudan University, between 2008 and 2018 were analyzed retrospectively. The results showed that primary glomerular nephropathy (PGN) remained the most common biopsy-proven renal disease (69.42% of total), with IgA nephropathy (IgAN) accounting for 44.40% of PGN, membranous nephropathy (MN) for 28.55%, minimal change disease (MCD) for 13.26% and focal segmental glomerulosclerosis (FSGS) for 8.00%. During the study period, the proportion of MN in PGN appeared an increasing tendency, while that of IgAN and MCD remained stable and that of FSGS showed a decline. Secondary glomerular nephropathy (SGN) constituted 21.54% of total cases, among which the leading two diseases were lupus nephritis (LN) and Henoch-Schonlein purpura nephritis (HSN) which accounted for 41.08% and 19.11% respectively.
Conclusions
The 11-year retrospective study revealed that PGN was the predominant histologic diagnosis among patients undergoing renal biopsy and the most frequent type of PGN remained to be IgAN, followed by MN which increased dramatically.
Introduction
Renal biopsy provides essential diagnostic insights that are pivotal for precise diagnosis, tailored treatment selection, and prognostic forecasting for patients. The spectrum of biopsy-proven renal diseases exhibits substantial variation, shaped by a multitude of factors including geographic regions, socioeconomic status, ethnic backgrounds, and the clinical indications prompting renal biopsy. In recent years, retrospective analyses of renal biopsies, complemented by statistical comparisons across various regions, ethnicities, and time frames, have yielded significant and enlightening insights. IgA nephropathy (IgAN) has been reported to be the most common primary glomerulonephritis in most European and Asian areas [Citation1–6]. However, focal segmental glomerulosclerosis (FSGS) is the most frequent primary glomerular disease in Brazil [Citation7], the United States [Citation8,Citation9] and Pakistan [Citation10]. The detection rate of membranous nephropathy (MN) has tended to rise in China [Citation11], while remained stable in Korea [Citation6].
Previous studies revealed a predominance of primary glomerular diseases in China’s renal disease profile, in stark contrast to the more frequent occurrence of renal complications arising from diabetes and hypertension in economically advanced countries [Citation12]. The dramatic changes in economic status and life styles of China during recent decades could possibly drive the switch of risk factors of renal diseases to a pattern, in which metabolic disorder is predominant [Citation13]. A recent study revealed that the percentage of chronic kidney disease (CKD) related to diabetes exceeded that of CKD related to glomerulonephritis in China based on diagnosis codes from the International Statistical Classification of Diseases and Related Health Problems (ICD)[Citation14]. To investigate the changing pattern of renal diseases in China from the pathological perspective, we retrospectively evaluated the data of the patients who received renal biopsies in a single center of China from 2008 to 2018.
Methods
Study subjects
Patients aged ≥15 years old who underwent native renal biopsy in Huashan Hospital, Fudan University between 2008 and 2018 were enrolled in our study. The written informed consent was obtained from all patients. The demorgraphic and clinical data of the patients were collected up to the time of renal biopsy. All patients underwent percutaneous renal biopsy guided by ultrasound, with a preference for puncture at the lower pole of the kidney. All the biopsy specimens were examined in the pathology laboratory of Department of Nephrology, Huashan Hospital. For the patients with multiple biopsies, only the first one was used for analysis. Patients with incomplete records and inadequate biopsies were excluded.
Clinical indications for renal biopsy were as follows: hematuria of renal origin, usually in association with other factors such as proteinuria, hypertension, and presence of serum biomarkers (ANCA, ANA, dsDNA, antiGBM, et al.), nephrotic syndrome or nephrotic range proteinuria, unexplained renal impairment and renal involvement of systemic disease. The principles for the indications of renal biopsy remained largely unchanged throughout the study period.
All renal biopsy specimens were examined using light microscopy and immunofluorescence (IF). Over 97% of the biopsy specimens were also examined by electron microscopy. Tissues used for light microscopy were fixed with methonal and embedded in parrifin. They were all examined by hematoxylin and eosin (HE) stain, Periodic Acid-Schiff (PAS) stain, Periodic Acid-Silver Metheramine (PASM) stain and Masson stain. Special staining such as Congo red, Oil Red O and Sudan III staining were performed as indicated. For IF examination, frozen sections were used for the staining of IgG, IgA, IgM, light chains (kappa and lambda) and complements (C3, C4 and C1q) with rabbit anti-human fluorescent antibody labeled with isothiocyanate. Tissues used for electron microscopy examination were fixed by 2.5% pentanediol and stained by uranyl acetate and lead citrate. The pathological diagnosis was made mainly based on the Revised Protocol for Histological Typing of Glomerulopathy (WHO, 1995) and previous published literature (Supplementary Table 1)[Citation2]. The final diagnosis was made by a group of trained nephrologists and pathologists, combining both clinical data and pathological findings.
Statistical analyses
SPSS software was used for statistical analysis. Continuous variables are expressed as mean ± SD. Student’s two-tailed t-tests were used to compare continuous variables between the groups. One-way ANOVA analysis was used to analyze the trend of age changes over the years. All tests were two-tailed, and a P value <0.05 was defined statistically significant.
Results
Demographic information and pathological distribution of renal diseases
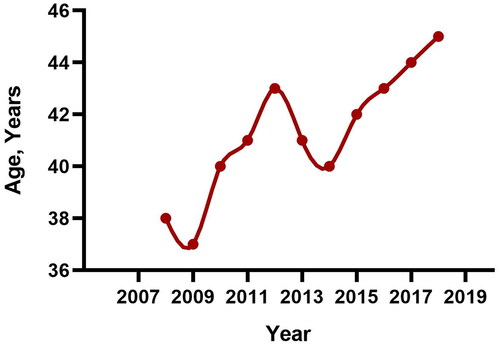
A total of 10996 cases between 2008 to 2018, with average age at the time of renal biopsy 42.01 ± 16.07 and a male to female ratio of 1.05 were analyzed. There was an increasing trend in the average age of patients over the period from 2008 to 2018. (p = 0.000) (). The distribution of biopsy-proven renal diseases and demographic information were shown in . Primary glomerular nephropathy (PGN) was the most common type of biopsy-proven renal diseases, accounting for 69.42% of all cases. The mean age of PGN patients at the time of biopsy was 41.98 ± 15.58 years, and 53.78% of them were male. Secondary glomerular nephropathy (SGN) accounted for 21.54%. The mean age of patients diagnosed with SGN at biopsy was 40.62 ± 17.40 years, and 40.12% of them were male. Approximately 2.92% of the cases exhibited co-occurrence of PGN and SGN. The prevalent combination was IgA nephropathy (IgAN) with benign nephrosclerosis (BNS), followed by membranous nephropathy (MN) with BNS. Tubulointerstitial injury and hereditary renal diseases accounted for 4.26% and 1.28% of all cases, respectively.
Table 1. Distribution of renal diseases and demographic information of the patients.
Distribution of primary glomerular nephropathy
The changing frequency of common histological categories in PGN was illustrated in and . IgAN constituted 44.40% of biopsy-proven PGN cases, making it the predominant cause of PGN. It was followed by MN, which accounted for 28.55% of the cases. The average proportion of IgAN in PGN during the periods of 2008–2013 and 2014–2018 were 46.40% and 42.28% respectively. The proportion of MN in PGN exhibited a marked increase, rising from 21.75% in the period from 2008 to 2013, to 35.70% in the subsequent years from 2014 to 2018. Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) accounted for 13.26% and 8.00% of PGN respectively. During the period from 2008 to 2013, there were 33 cases diagnosed with non-IgA mesangioproliferative glomerulonephritis (MsPGN). In contrast, no such cases were reported during the years 2014 to 2018.
Figure 2. (A) Changing frequency of common histological categories in primary glomerular nephropathy over the study period. (B) The frequency of the most common histological categories in primary glomerular nephropathy based on different age groups. Abbreviation: IgAN: IgA nephropathy; MN: membranous nephropathy; MCD: minimal change disease; FSGS: focal segmental glomerulosclerosis.
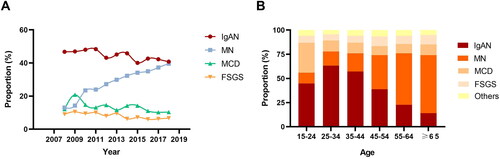
Table 2. Comparison of the proportions of common glomerular diseases between different periods.
To achieve a more refined comprehension of the distribution of biopsy-proven PGN across various age demographics, the patient cohort was categorized into six distinct age brackets: 15–24 years, 25–34 years, 35–44 years, 45–54 years, 55–64 years and those aged 65 years and above (). The proportion of IgAN in PGN was observed to peak within the 25–34 years age group and subsequently declined with advancing age. In contrast, the proportion of MN rose progressively with age, achieving its peak among individuals in the age group of 65 years and older. MCD was identified as being most prevalent among individuals in the 15 to 24 years age range.
Distribution of secondary glomerular nephropathy
The changing frequency of common histological categories in SGN was demonstrated in and . The most dominant type of SGN in the renal biopsy database was lupus nephritis (LN), which accounted for 41.08% of biopsy-proven SGN and displayed a declining trend with the proportion from 47.73% during 2008–2013 to 35.57% during 2014–2018. About 87% of the patients diagnosed with lupus nephritis were female. The most common subtype of lupus nephritis was Type IV (35.79%), followed by type IV + V (16.05%) and type III (16.05%) (). Henoch-Schonlein purpura nephritis (HSN) was the second most frequent form of biopsy-proven SGN in this database, which accounted for 24.67% during 2008–2013 and decreased to 14.66% during 2014–2018. In addition, the proportion of benign nephrosclerosis (BNS) as a subset of SGN saw a significant rise, escalating from 3.21% in the period from 2008 to 2013, to 13.89% in the years 2014 to 2018. Diabetic nephropathy (DN) and pauci-immune glomerulonephritis (pauci-immune GN) constituted 10.05% and 8.15% of biopsy-proven SGN respectively, both of which had not exhibited significant fluctuations in frequency. A total of 99 cases of paraprotein-related glomerular nephropathy were diagnosed in our center during the study period, accounting for 4.60% of SGN. Renal amyloidosis made up 76.14% of cases in the category of paraprotein-related kidney diseases (including paraprotein-related glomerular nephropathy and tubular damage) (). The remaining types, which include monoclonal immunoglobulin deposition disease (MIDD), cryoglobulinemic glomerulonephritis, proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID), light chain proximal tubulopathy (LCPT) and light chain cast nephropathy (LCCN) accounted for a collective 23.86%.
Figure 3. (A) Changing frequency of common histological categories in secondary glomerular nephropathy over the study period. (B) The frequency of the most common histological categories in secondary glomerular nephropathy based on different age groups. (C) Distribution of subtypes in lupus nephritis. (D) Distribution of subtypes in paraprotein-related glomerular nephropathy. Abbreviation: LN: lupus nephritis; HSN: Henoch-Schonlein purpura nephritis; DN: diabetic nephropathy; BNS: benign nephrosclerosis; pauci-immune GN: pauci-immune glomerulonephritis.
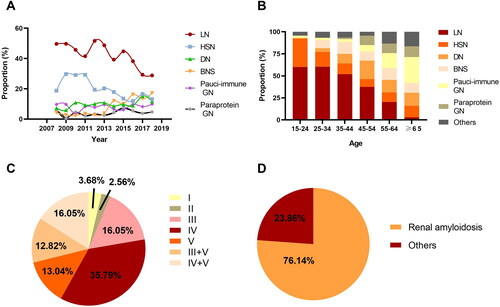
An analysis of the age distribution revealed that LN was most prevalent in the age groups of 15–24 and 25–34, accounting for over half of the proportion of SGN in these population. HSN was predominantly observed in individuals within the age bracket of 15 to 24 years old. The frequency of pauci-immune GN was observed to increase with age, reaching its zenith among individuals who are 65 years of age and older (). Paraprotein-related glomerular nephropathy (paraprotein GN) demonstrated a significant rise in proportion among individuals aged 45 years and above, as opposed to those below the age of 45.
Discussion
In this retrospective study, we found that PGN was the most common type of histologic diagnosis in patients who underwent renal biopsy with a proportion about 70%, and the most frequent types of biopsy-proven PGN was IgAN and MN. The proportion of MN in PGN appeared a significantly increasing tendency, while that of IgAN and MCD remained stable and that of FSGS showed a decline. In the SGN category, which represented 21.54% of the total cases, the leading conditions were LN and HSN. The proportion of BNS in biopsy-proven SGN exhibited an upward trend, whereas the proportion of diabetic nephropathy (DN) did not display a similar increase.
The spectrum of PGN exhibits regional variations and shifts in prevalence over time. It has been reported that IgAN was the most common type of PGN in east Asian and European countries as well as Australia [Citation6,Citation14–16], but less than 5% of PGN in Africa [Citation17]. In the renal biopsy database of our study, IgAN consistently represented the most common histopathologic diagnosis of PGN, with its proportion fluctuating between 40.06% and 48.40% throughout the eleven-year study period. Research have revealed that the consistent pattern of allele frequency differences among populations across all Genome Wide Association Studies (GWAS) loci mirrors the prevalence of the disease and is associated with the diversity of local pathogens, indicating that multi-locus adaptive processes may have sculpted the contemporary epidemiological profile of IgAN [Citation18]. MN was the second leading type of biopsy-proven PGN in this study, exhibiting a significant surge throughout the eleven-year study period, which was consistent with several previous reports in China [Citation19–21]. The increasing frequency of membranous nephropathy has prompted the need for additional research to delve into the factors driving this upward trend. The prominent rising trend of MN could be possibly associated with air pollution. Xu et al. [Citation22] showed that each 10 μg/m3 increase in PM2.5 concentration associated with 14% higher odds for MN in regions with high PM2.5 concentration. In the northern and northeastern China, where air pollution was much more severe than the southern, the proportion of idiopathic MN exceeded that of IgAN since 2014 and became the predominant pathological type of PGN [Citation23]. It has been reported that the prevalence of FSGS in Asian population was relatively lower than other races [Citation24]. In China, Zhou et al. [Citation21] and Li et al. [Citation2] from two independent centers in China reported that the proportion of FSGS in PGN was 2.5% and 6.0% respectively, significantly lower than the countries in America. In the USA and Brazil, FSGS was the most prevalent PGN with percentage as 37.8% and 24.6% respectively [Citation7,Citation25]. In our center, the proportion of FSGS in biopsy-proven PGN was less than 10%, which was consistent with other centers in China. In addition, it is noteworthy that the diagnosis of MsPGN nearly vanished in the latter half of the study’s duration at our center. This could be attributed to advancements in pathological diagnostic techniques. Similar observations have been reported in both national and international studies [Citation21,Citation26,Citation27].
The most prevalent type of biopsy-proven SGN in the present study was LN, consistent with data from other centers in China [Citation21,Citation28]. It has been reported that kidney involvement in SLE was more frequent in several races including Asians with unclear mechanisms [Citation29]. A study from Korea consistently revealed that LN was the most common type of SGN [Citation30]. There was a statistically significant decrease in its proportion between 2008 and 2013 compared to 2014 and 2018 in our study. Another study from China illustrated the similar trend [Citation31]. The second leading type of SGN in this study was HSN. Compared to adults, HSN is significantly more prevalent among children, with approximately 30% experiencing renal involvement [Citation32]. It is estimated that 5–15% of children with HSN may progress to chronic renal failure as they transition into adulthood [Citation33]. Hu et al. [Citation34] found that HSN accounted for more than 70% of SGN in children under the age of 14 while less than 15% in adult patients. We observed that the proportion of HSN in SGN had a declining trend during the eleven-year period, consistent with another previous report from China [Citation28]. It is noteworthy that the frequency of LN and HSN in our study were higher than those reported in other centers. This could be explained by the prominent and leading status of the Dermatology Department in our hospital, attracting more patients with dermatological conditions.
Type 2 diabetes (T2D) has become a globally threatening health issue and shown a dramatic rising prevalence. More than 40% of the individuals with type 2 diabetes could develop DN [Citation35]. DN has become the leading cause of CKD in the United States, and nearly half of CKD patients could be attributed to diabetes [Citation12]. An increased trend of CKD caused by diabetes has been reported in both highly developed and less developed countries [Citation12,Citation36]. Zhang et al. [Citation14] found that the percentage of hospital discharges due to diabetic kidney disease had surpassed that due to glomerulonephritis since 2011 based on a national hospitalized patient database. However, we failed to observe an obvious rising trend of DN during the eleven-year period. The discrepancy could be attributed to the indication of renal biopsy in patients with diabetic kidney disease. Patients with diabetes who develop proteinuria in the presence of other microvascular complications such as retinopathy are highly suggestive of diabetic nephropathy and under these circumstances, renal biopsy is not strongly indicated. It is worth noting that patients with diabetes mellitus and kidney disease could exhibit other types of kidney lesions than DN. In our study, the detailed medical history regarding diabetes mellitus was not shown in the results, prohibiting a comprehensive representation of the histological spectrum among patients with diabetes mellitus. Similar with diabetes mellitus, the prevalence of hypertension has increased over the recent decade and about one-third of adults have hypertension according to population surveys in China [Citation37]. The trend of BNS showed a rising trend in our study, consistent with the increasing prevalence of hypertension in China. This is due to the fact that the diagnosis of hypertension-related nephropathy is more dependent on pathological findings than DN.
We revealed that paraprotein GN was much more common in individuals aged 45 years and above, compared to those below the age of 45. Renal amyloidosis was the predominant type of paraprotein-related kidney disease. Our findings highlight the critical need for renal amyloidosis screening in individuals over 45 presenting with proteinuria.
There are several limitations in our study. First, our study has concentrated on the pathological spectrum, with diagnoses established based on the findings from renal biopsy and the clinical data available at that time point. The clinical details during follow-up periods have been not analyzed in the study, limiting the establishment of more comprehensive diagnoses. Second, this is a retrospective study demonstrating the frequency of biopsy-proven renal diseases among patients with indications for renal biopsy, not the entire spectrum of renal diseases. Third, our study’s single-center focus may limit the representativeness of our patient sample for the entire Chinese population, given that local practices and indications for renal biopsy could vary across different centers and influence the observed frequency of histological patterns of kidney diseases. Fourth, there were some mixture of pathological pattern of injuries and etiology-based diagnoses in classification system. Fifth, the reasons for prescribing kidney biopsy for each individual were not analyzed in our study.
Conclusion
The eleven-year retrospective study from the single center in China revealed that PGN was the major histologic diagnosis among patients undergoing renal biopsy. The most frequent type of PGN remained to be IgAN, followed by MN which increased dramatically. The top two etiology of SGN were LN and HSN. BNS presented a rising trend while DN did not, which could be possibly attributed to indications of renal biopsy. We showed the changing spectrum of biopsy-proven renal diseases, which could possibly serve as a valuable reference for the diagnosis, treatment, and prevention of kidney diseases.
Ethical approval
The study was performed in accordance with the declaration of Helsinki and the study protocol was approved by the ethics committee of Huashan hospital, Fudan University. All of the subjects gave their written informed consent.
Figure2024 v2.pptx
Download MS Power Point (1.5 MB)Supplementary_Table_v2 new.docx
Download MS Word (16.7 KB)Disclosure statement
The authors report no conflict of interest.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Stratta P, Segoloni GP, Canavese C, et al. Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis. 1996;27(5):631–639. doi:10.1016/s0272-6386(96)90096-7.
- Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–923. doi:10.1111/j.1523-1755.2004.00837.x.
- Rivera F, López-Gómez JM, Pérez-García R, Spsnish Registry of Glomerulonephritis. Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant. 2002;17(9):1594–1602. doi:10.1093/ndt/17.9.1594.
- Rychlík I, Jancová E, Tesar V, et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19(12):3040–3049. doi:10.1093/ndt/gfh521.
- Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Research Group on Progressive Chronic Renal Disease. Nephron. 1999;82(3):205–213. doi:10.1159/000045404.
- Chang JH, Kim DK, Kim HW, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009;24(8):2406–2410. doi:10.1093/ndt/gfp091.
- Polito MG, de Moura LA, Kirsztajn GM. An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25(2):490–496. doi:10.1093/ndt/gfp355.
- Braden GL, Mulhern JG, O’Shea MH, et al. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;35(5):878–883. doi:10.1016/s0272-6386(00)70258-7.
- Haas M, Spargo BH, Coventry S. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis. 1995;26(5):740–750. doi:10.1016/0272-6386(95)90437-9.
- Mubarak M, Kazi JI, Naqvi R, et al. Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology (Carlton). 2011;16(1):87–92. doi:10.1111/j.1440-1797.2010.01410.x.
- Zhang X, Liu S, Tang L, et al. Analysis of pathological data of renal biopsy at one single center in China from 1987 to 2012. Chin Med J (Engl). 2014;127(9):1715–1720. doi:10.3760/cma.j.issn.0366-6999.20132765.
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi:10.1016/S0140-6736(13)60687-X.
- Yang C, Wang H, Zhao X, et al. CKD in China: evolving Spectrum and Public Health Implications. Am J Kidney Dis. 2020;76(2):258–264. doi:10.1053/j.ajkd.2019.05.032.
- Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–906. doi:10.1056/NEJMc1602469.
- Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16(7):1364–1367. doi:10.1093/ndt/16.7.1364.
- Hanko JB, Mullan RN, O’Rourke DM, et al. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24(10):3050–3054. doi:10.1093/ndt/gfp254.
- Okpechi IG, Ameh OI, Bello AK, et al. Epidemiology of Histologically Proven Glomerulonephritis in Africa: a Systematic Review and Meta-Analysis. PLoS One. 2016;11(3):e0152203. doi:10.1371/journal.pone.0152203.
- Magistroni R, D’Agati VD, Appel GB, et al. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 2015;88(5):974–989. doi:10.1038/ki.2015.252.
- Pan X, Xu J, Ren H, et al. Changing spectrum of biopsy-proven primary glomerular diseases over the past 15 years: a single-center study in China. Contrib Nephrol. 2013;181:22–30.
- Zhou FD, Zhao MH, Zou WZ, et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant. 2009;24(3):870–876. doi:10.1093/ndt/gfn554.
- Zhou Q, Yang X, Wang M, et al. Changes in the diagnosis of glomerular diseases in east China: a 15-year renal biopsy study. Ren Fail. 2018;40(1):657–664. doi:10.1080/0886022X.2018.1537930.
- Xu X, Wang G, Chen N, et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27(12):3739–3746. doi:10.1681/ASN.2016010093.
- Li J, Cui Z, Long J, et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrol Dial Transplant. 2018;33(12):2173–2181.
- Sim JJ, Batech M, Hever A, et al. Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis. 2016;68(4):533–544. doi:10.1053/j.ajkd.2016.03.416.
- Dragovic D, Rosenstock JL, Wahl SJ, et al. Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol. 2005;63(1):1–7. doi:10.5414/cnp63001.
- Chen L, Luodelete M, Dong C, et al. Pathological spectrum of glomerular disease in patients with renal insufficiency: a single-center study in Northeastern China. Ren Fail. 2019;41(1):473–480. doi:10.1080/0886022X.2019.1620774.
- Shin HS, Cho DH, Kang SK, et al. Patterns of renal disease in South Korea: a 20-year review of a single-center renal biopsy database. Ren Fail. 2017;39(1):540–546. doi:10.1080/0886022X.2017.1348955.
- Hou J-H, Zhu H-X, Zhou M-L, et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis (Basel). 2018;4(1):10–19. doi:10.1159/000484717.
- Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Epidemiology of systemic lupus erythematosus. Expert Rev Clin Immunol. 2017;13(8):799–814. doi:10.1080/1744666X.2017.1327352.
- Lim BJ. The spectrum of biopsy-proven renal diseases in Korea. Kidney Res Clin Pract. 2020;39(1):1–3. doi:10.23876/j.krcp.20.019.
- Chen X-J, Huang Y, Yuan S, et al. Changes in spectrum of biopsy-proven kidney diseases within decade: an analysis based on 10 199 cases from South China. Postgrad Med J. 2023;100(1179):20–27. doi:10.1093/postmj/qgad094.
- Davin JC, Coppo R. Henoch-Schonlein purpura nephritis in children. Nat Rev Nephrol. 2014;10(10):563–573. doi:10.1038/nrneph.2014.126.
- Schärer K, Krmar R, Querfeld U, et al. Clinical outcome of Schonlein-Henoch purpura nephritis in children. Pediatr Nephrol. 1999;13(9):816–823. doi:10.1007/s004670050707.
- Hu R, Quan S, Wang Y, et al. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10(1):10994. doi:10.1038/s41598-020-67910-w.
- Bailey RA, Wang Y, Zhu V, et al. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7(1):415. doi:10.1186/1756-0500-7-415.
- Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. doi:10.1038/nrneph.2015.173.
- Lewington S, Lacey B, Clarke R, et al. The Burden of Hypertension and Associated Risk for Cardiovascular Mortality in China. JAMA Intern Med. 2016;176(4):524–532. doi:10.1001/jamainternmed.2016.0190.