ABSTRACT
Objective
To compare the effects of dry needling and upper cervical spinal manipulation with interocclusal splint therapy, diclofenac, and temporomandibular joint (TMJ) mobilization in patients with temporomandibular disorder (TMD).
Methods
One hundred-twenty patients with TMD were randomized to receive six treatment sessions of dry needling plus upper cervical spinal manipulation (n = 62) or interocclusal splint therapy, diclofenac, and joint mobilization to the TMJ (n = 58).
Results
Patients receiving dry needling and upper cervical spinal manipulation experienced significantly greater reductions in jaw pain intensity over the last 7 days (VAS: F = 23.696; p < 0.001) and active pain-free mouth opening (F = 29.902; p < 0.001) than those receiving interocclusal splint therapy, diclofenac, and TMJ mobilization at the 3-month follow-up.
Conclusion
Dry needling and upper cervical spinal manipulation was more effective than interocclusal splint therapy, diclofenac, and TMJ mobilization in patients with TMD.
Introduction
Temporomandibular disorder (TMD) is considered the third most prominent pain condition world-wide [Citation1]. While only 5% of adults with TMD from the general population seek clinical treatment [Citation2], 16–59% and 33–86% of the worldwide population suffer from TMD symptoms and clinical signs, respectively [Citation3]. TMD is a multifactorial condition [Citation4] that appears to be associated with age, systemic illness, hormonal factors, habitual activity, and occlusal variation, with a strong psychosocial component [Citation5]. Headaches [Citation6] and neck pain [Citation7] also seem to be associated with TMD.
Clinical manifestations of TMD include pain in the joint and or/muscles of mastication, limited mandibular range of motion, crepitus, and functional limitation or deviation of the jaw [Citation8]. An inter-professional consortium recently updated and validated diagnostic criteria for classifying TMD according to three main groups: muscle disorders, disc displacements, and joint dysfunction [Citation9]. The clinical diagnostic criteria for all three groups has been shown to be both sensitive and specific, with excellent inter-rater reliability [Citation10]. While nonsteroidal anti-inflammatory drugs (NSAIDs) and muscle relaxants may improve symptoms associated with TMD when used as a first-line treatment [Citation11,Citation12], these drugs have significant side effects, and long-term use is not recommended [Citation13]. In addition, there is little evidence to support the long-term efficacy of surgery in patients with TMD [Citation14].
Many patients with TMD often seek conservative interventions [Citation15]; however, the evidence for using electrophysical modalities such as laser therapy, ultrasound, TENS, iontophoresis [Citation16], and also the application of isolated exercise is limited [Citation17]. Although a 2004 Cochrane review found insufficient evidence to advocate splint therapy for TMD [Citation18], a posterior meta-analysis of 538 patients found improved range of motion and decreased intensity and frequency of jaw pain following interocclusal splint therapy [Citation19]. A large-scale systematic review found inconclusive evidence for the use of temporomandibular joint (TMJ) mobilization alone for TMD [Citation20]; however, the use of joint mobilization in combination with other conservative treatments, such as exercise, is supported by current literature [Citation21–24]. Notably, mobilization or manipulation [Citation15], when used alone and directed to the upper cervical spine [Citation25] or in conjunction with a multi-modal treatment (e.g., exercise, mandibular mobilization, myofascial release, muscle energy, and/or tender-trigger point therapy) for the craniomandibular system [Citation21,Citation24] has demonstrated a large effect on mouth opening and jaw pain reduction when compared to other active interventions.
Patients with TMD have been shown to exhibit both peripheral and central pain; therefore, needling therapies may provide an additional treatment option [Citation26]. Dry needling (DN) refers to the insertion of monofilament needles without injectate into muscles, ligaments, tendons, connective tissue, scar tissue, and peri-neural tissue for the management of neuromusculoskeletal conditions [Citation27,Citation28]. While the terminology and theoretical constructs of acupuncture and DN are different [Citation29], both have been shown to elicit biochemical, biomechanical, endocrinological, and neurovascular changes associated with reductions in pain and disability [Citation30].
In a recent systematic review, DN outperformed procaine, methocarbamol and paracetamol for improving TMD pain intensity, and it resulted in significant improvements in pressure pain thresholds compared with sham DN [Citation31]. Another systematic review of 28 clinical trials concluded that both wet needling (i.e., botulinum toxin, platelet-rich plasma, or collagen) and dry needling are effective for decreasing pain and improving mouth opening in patients with TMD [Citation32].
When given separately, needling therapy and upper cervical spinal manipulation have each been found to be moderately effective for TMD. However, to date, no studies have attempted to combine these two treatments and compare their additive effects in patients with TMD. Therefore, the purpose of this multi-center, randomized clinical trial was to compare the combined effects of DN and upper cervical spinal manipulation to interocclusal splint therapy, NSAIDs, and TMJ mobilization in patients with TMD. The authors hypothesized that patients in the DN and upper cervical spinal manipulation group would experience greater improvements in jaw pain and mouth opening than those receiving splint therapy, NSAIDs, and TMJ mobilization group.
Materials and methods
Study design
This randomized, single-blinded, multi-center, parallel-group clinical trial was conducted following the Consolidated Standards of Reporting Trials (CONSORT) extension for pragmatic clinical trials [Citation33]. The trial was approved by the ethics committee at Universidad Rey Juan Carlos, Madrid, Spain (URJC-DPTO 36–2017), and the trial was prospectively registered (ClinicalTrials.gov: NCT03409874). All subjects provided and signed informed consent before their enrollment in the study.
Participants
Consecutive individuals with TMD from 10 outpatient physical therapy clinics in 10 different states (Alabama, Arizona, Florida, Georgia, Louisiana, Maryland, Michigan, Montana, North Carolina, and Virginia) were screened for eligibility criteria and recruited over a 26-month period (from February 1 2018 to March 31 2020). To be eligible, patients had to be at least 18 years old and meet the following criteria: (1) a clinical diagnosis of temporomandibular disorder consistent with the Revised TMD group 1 Muscle Disorders Diagnostic Algorithm [Citation34]; (2) having experienced TMD symptoms for at least 3 months; and (3) an intensity of TMD symptoms of at least 30 mm on the VAS (0–100 mm) [Citation35,Citation36]. The exclusion criteria are described in .
Table 1. Exclusion Criteria
Table 2. Baseline characteristics by treatment assignment
Treating therapists
Ten physical therapists (mean age, 37.3 years, SD 9.1) delivered interventions in this trial. They had an average of 10.1 (SD 7.7) years of clinical experience, had completed a 54-hour post-graduate certification program that included practical training in DN for TMD, and were current students in a 60-hour post graduate certificate program that included practical training in non-thrust joint mobilization to the TMJ and high-velocity low-amplitude thrust manipulation to the upper cervical spine. All treating therapists were Fellows-in-Training within an APTA-accredited Fellowship program in Orthopedic Manual Physical Therapy, had heterogeneous backgrounds in terms of prior manual therapy/orthopedic training, and worked in private outpatient physical therapy practice. All participating therapists were required to study a manual of standard operating procedures and participate in a 6-hr training session with a principal investigator to ensure the standardization of the protocol and treatment.
Examination procedure
All patients provided demographic information and completed self-report measures followed by a standardized history and physical examination at baseline. Participants received a standardized physical examination, during which the affected TMJ was examined, so as to confirm that the patient fell within the revised group 1 muscle disorders diagnostic algorithm; i.e., patients who presented with group II or group III TMD were ruled out [Citation34]. The physical examination included, but was not limited to, palpation of muscles of mastication with a minimum of two lbs of pressure and maximum assisted and unassisted opening. Active, pain-free mouth opening was also measured, as follows: the patient was asked to open their mouth as wide as possible without causing pain, from a supine position. At the end position, the distance between the upper and lower central incisors was measured in mm, and the average was taken over three attempts. The intra-tester reliability of this procedure has been found to be high (ICC = 0.9–0.98) [Citation37].
Randomization and blinding
Following baseline examination, patients were randomly assigned to receive dry needling and upper cervical spinal manipulation or interocclusal splint therapy, NSAIDs, and non-thrust mobilization to the TMJ. Randomization was conducted using a computer-generated randomized table of numbers created by a statistician not otherwise involved in the trial. Individual and sequentially numbered index cards with the random assignment were prepared, folded, and placed in sealed opaque envelopes for each of the 10 data collection sites. The clinicians administering the self-report outcome questionnaires were blinded to the patient’s treatment group assignment. It was not possible to blind patients or treating therapists.
Interventions
All participants received up to eight treatment sessions at a frequency of once or twice per week over a 4-week period. In either group, fewer treatment sessions could be completed if symptom resolution occurred sooner.
The active comparison group received an interocclusal appliance, NSAIDs (diclofenac), and non-thrust joint mobilization to the TMJ. The interocclusal appliances were prepared by general dentists based on the TMJ impairments of each patient. Patients were instructed to wear the device each night for 4 consecutive weeks. During the course of the study, patients were permitted to visit their healthcare provider to have their appliance adjusted, as needed. Patients in the active comparison group were also prescribed diclofenac (Voltaren) 3X50mg per day for 4 weeks. If symptoms improved, the patient was allowed to reduce the dosage to 2X50mg per day. Topical and oral diclofenac have been shown to be effective for patients with TMD [Citation38]. All patients within the comparison group were required to maintain a diary so as to ensure compliance with the nightly use of the interocclusal appliance and the diclofenac dosage. Diaries were reviewed during follow-up appointments at 2 and 4 weeks to ensure compliance.
Patients in the active comparison group also received 10 mins of impairment-based non-thrust joint mobilization per the recommendations of Shaffer et al. [Citation15]. Notably, two studies found mobilization directed to the TMJ to improve joint restriction [Citation15,Citation20]. Moreover, the use of TMJ mobilization in conjunction with other conservative strategies has been linked to improvements in pain and mandibular range of motion [Citation35,Citation39].
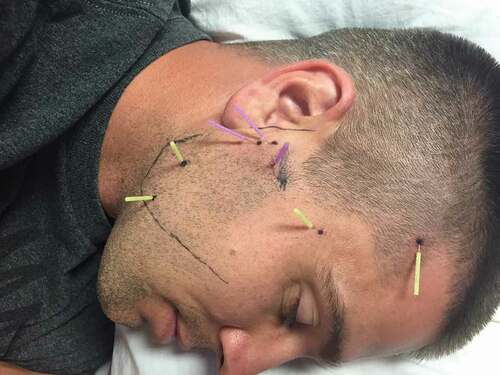
Patients allocated to the experimental group received up to eight sessions of DN at a frequency of 1–2 times per week for 4 weeks using a standardized protocol of 7 points () for 20 min, as described in Appendix 1 [Citation16]. While the exact etiology of TMD is still unknown, the condition seems to be associated with a disruption of the TMJ capsule, the articular disc, and the muscles of mastication [Citation40]. Therefore, needles targeted pathoanatomical structures of TMD, including the inferior head of the lateral pterygoid muscle, the superficial masseter muscle, the temporalis muscle, and the peri-articular capsule of the posterior TMJ [Citation41,Citation42]. Clinicians were also permitted to insert needles into the superior head of the lateral pterygoid and the medial pterygoid based on the sensitivity of the patient and/or the presence of symptoms in that region.
Sterilized disposable stainless steel acupuncture needles were used with three sizes: 0.18 mm x 15 mm, 0.25 mm x 30 mm, and 0.30 mm x 40 mm. The lateral aspect of the patient’s face and forehead were cleaned with alcohol. The depth of needle insertion ranged from 10 mm to 35 mm, depending on the anatomical structure being targeted (e.g., inferior head of the lateral pterygoid muscle, superficial masseter muscle, peri-articular capsule of the posterior TMJ, anterosuperior or anteroinferior aspect of the temporalis muscle) and the patient’s constitution (i.e., size and muscle thickness). Following insertion, needles were manipulated bi-directionally to elicit a sensation of aching, tingling, deep pressure, heaviness, or warmth. Needle manipulation has been linked to tissue mechano-transduction [Citation43,Citation44], vasodilation [Citation45,Citation46], and peripheral [Citation47,Citation48] and central analgesia [Citation49–51]. The needles were then left in situ for 15–30 mins [Citation52,Citation53], depending on the sensitivity of the patient and their response to the treatment. Clinicians were permitted to manipulate needles bi-directionally every 4–5 mins, as needed, to achieve an appropriate treatment dosage. In cases of bilateral TMD, both sides were treated, but only the most painful side at baseline was recorded and analyzed throughout the study to satisfy the assumption of independent data [Citation54].
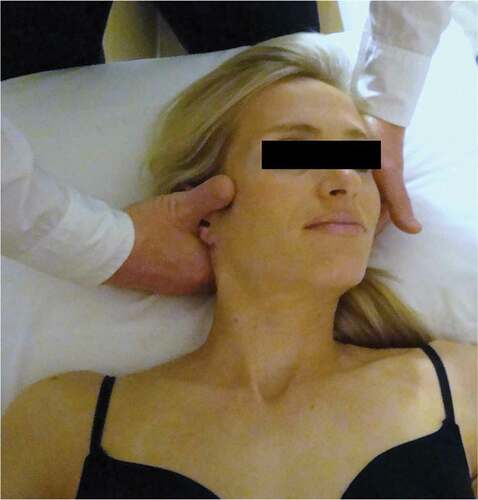
Patients in the experimental group also received at least one treatment that included high-velocity, low-amplitude thrust manipulation to the upper cervical spine () targeting C0-C1, C1-C2, or C2-C3, as described in previously published studies [Citation55,Citation56] and Appendix 1. The selection of the spinal segment to target was left to the discretion of the treating therapist and was based on a combination of patient report and manual examination findings. Clinicians were told to expect multiple audible cavitation sounds as a result of the manipulation to the upper cervical spine [Citation56–60].
The current study did not include exercise therapy as part of the experimental or comparison groups because a recent meta-analysis concluded that exercise therapy approaches used for patients with TMD did not significantly improve functional outcomes; furthermore, the most appropriate dosage parameters (frequency, intensity, and duration) remain unknown [Citation17].
Outcome measures
The primary outcome was average jaw pain intensity over the last 7 days, as measured by the Visual Analog Scale (VAS). VAS ratings were collected at baseline, 2 weeks, 6 weeks, and 3 months. The VAS consists of a 100 mm line, whereby the left side represents “no pain,” and the right side represents “the worst pain imaginable.” Patients were asked to make a mark on the line at the position that best represented their average pain intensity over the last 7 days. The VAS is an efficient, reliable, and valid method of measuring subjective pain intensity in various patient populations, including TMD [Citation61–64]. The minimal clinically important difference (MCID) for the VAS has been shown to be 9–11 mm [Citation65,Citation66], and the minimal detectable change (MDC) for pain related to TMD is 10–14 mm on the VAS [Citation67].
Secondary outcomes included jaw pain intensity over the past 24 hrs (VAS), active pain-free mouth opening (mm), and the Global Rating of Change (GROC). VAS ratings and active mouth opening outcomes were collected at baseline, 2 weeks, 6 weeks, and 3 months after the initial treatment session. Active pain-free mouth opening is a common variable used to measure functional improvements in patients with TMD [Citation35,Citation68,Citation69]. In addition, at 2 weeks, 6 weeks, and 3 months following the initial treatment session, patients completed a 15-point GROC question based on a scale described by Jaeschke et al. [Citation70]. The scale ranges from −7 (a very great deal worse) to zero (about the same) to +7 (a very great deal better). Intermittent descriptors of worsening or improving are assigned values from −1 to −6 and +1 to +6, respectively. Scores of +4 and +5 have typically been indicative of moderate changes in patient status [Citation70].
Treatment side effects
Patients were asked to report any adverse events. Adverse events were defined as a sequelae of one-week duration with any symptom perceived as distressing and unacceptable to the patient, requiring further treatment [Citation71]. The treating therapists and patients in the group that received DN as part of their treatment were instructed to pay particular attention to the presence of ecchymosis and post-needling soreness.
Sample size determination
The sample size calculations were based on detecting a between-group moderate effect size of 0.55 on the main outcome (average jaw pain intensity over the last 7 days) at 3 months, using a 1-tailed test, an alpha level (α) of 0.05, and a desired power (β) of 90%. The estimated desired sample size was calculated to be at least 58 participants per group.
Statistical analysis
Statistical analysis was performed using SPSS software, version 28.0 (Chicago, IL, USA), according to the intention-to-treat principle. Means, standard deviations, and/or 95% confidence intervals were calculated for each variable. The Kolmogorov-Smirnov test revealed a normal distribution of the variables (p > 0.05). Baseline demographic and clinical variables were compared between groups using independent Student’s t-tests for continuous data and χ2 tests of independence for categorical data.
The effects of treatment on jaw pain intensity (VAS) and active pain-free mouth opening (mm) were each examined with a 2-by-4 mixed model analysis of covariance (ANCOVA) with the treatment group as the between-subjects factor and time (baseline, 2 weeks, 6 weeks, and 3 months) as the within-subjects factor. Separate ANCOVAs were performed with VAS (average jaw pain rating over the past 7 days), VAS (average jaw pain rating over the last 24 hrs), and active pain-free mouth opening (mm) as the dependent variable. Age and duration of symptoms were entered as covariates.
For each ANCOVA, the main hypothesis of interest was the 2-way interaction (group by time) with a Bonferroni-corrected alpha of 0.0125 (four time points). The authors used χ2 tests to compare self-perceived improvement on the GROC. To enable comparison of between-group effect sizes, standardized mean differences (SMDs) in score were calculated by dividing mean score differences between groups by the pooled standard deviation. Number needed to treat (NNT) was calculated using each definition for a successful outcome (a GROC score of 5 or greater [Citation70] at 3 months and a 50% improvement from baseline to 3 months on the VAS [Citation62,Citation63,Citation65]).
Results
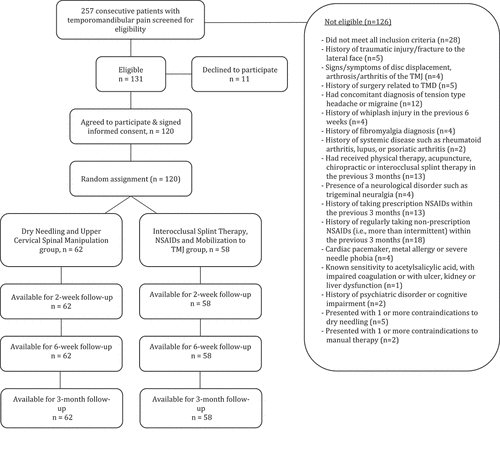
Between February 2018 and March 2020, 257 consecutive patients with TMD were screened for eligibility (). One hundred-twenty patients (46.7%) satisfied all the inclusion criteria, agreed to participate, and were randomly allocated into the DN and upper cervical spinal manipulation (n = 62) group or the interocclusal splint therapy, NSAIDs, and non-thrust joint mobilization to the TMJ (n = 58) group. Randomization resulted in similar baseline characteristics for all variables (). The reasons for ineligibility are found in , which provides a flow diagram of patient recruitment and retention. There was no significant difference (p = 0.427) between the mean number of completed treatment sessions for the DN and upper cervical spinal manipulation group (mean: 6.29) and the interocclusal splint therapy, NSAIDs, and non-thrust mobilization group (mean: 6.55). In the experimental group, the mean number of treatment sessions that included high-velocity low-amplitude thrust manipulation to the upper cervical spine was 5.23 (SD 2.02). No patients were lost at any of the follow-up periods in either group. None of the participants in any group reported receiving other interventions during the study.
Thirty-four patients assigned to the DN and upper cervical spinal manipulation group (54.8%) experienced post-needling muscle soreness, and 12 (19.4%) experienced mild bruising (ecchymosis), which most commonly resolved spontaneously within 48 hrs and 2–4 days, respectively. Three patients (4.8%) in the DN and upper cervical manipulation group experienced bruising that lasted 5–7 days before spontaneously resolving. Five patients (8.1%) in the DN and upper cervical spinal manipulation group experienced drowsiness, headache, or nausea, which spontaneously resolved within several hours. No major adverse events were reported in the dry needling and upper cervical spinal manipulation group.
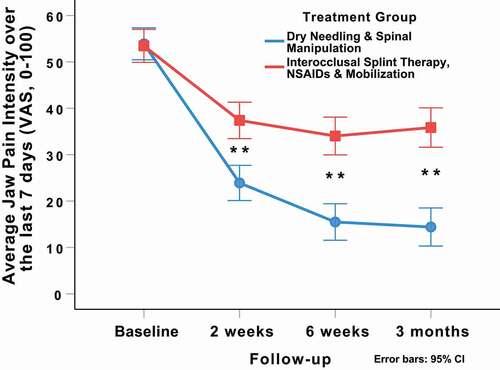
Adjusting for baseline outcomes, the mixed-model ANCOVA revealed a significant group-by-time interaction for the primary outcome of average jaw pain intensity over the last 7 days (VAS: F = 23.696; p < 0.001, ). Patients in the DN and spinal manipulation group experienced greater reductions in average jaw pain intensity at 2 weeks (Δ −13.9; 95%CI: −20.1, −7.7; p < 0.001), 6 weeks (Δ −19.0; 95%CI: −25.4, −12.6; p < 0.001), and 3 months (Δ −21.9; 95%CI: −29.1, −14.7; p < 0.001) than those in the interocclusal splint therapy, NSAIDs, and non-thrust TMJ mobilization group (). For the primary outcome (average jaw pain intensity over the last 7 days), between-group effect sizes for the VAS were large (SMD: 0.81; 95%CI: 0.44, 1.19) at 2 weeks, 6 weeks (SMD: 1.07; 95%CI: 0.69, 1.45), and 3 months (SMD: 1.10; 95%CI: 0.72, 1.48) after the first treatment session in favor of the DN and spinal manipulation group.
Table 3. Within-group and between-group mean scores by randomized treatment assignment
Figure 4. Evolution of average jaw pain intensity over the last 7 days (VAS) throughout the course of the study, stratified by randomized treatment assignment. Values are mean and 95% confidence interval.
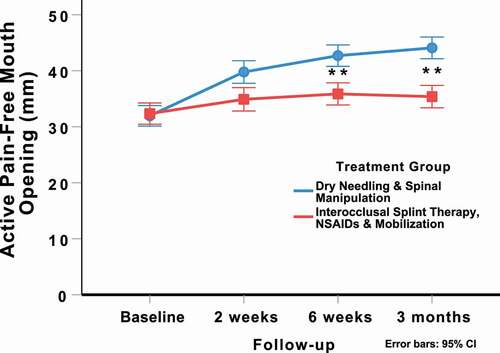
The intention-to-treat analysis also revealed a significant group-by-time interaction for active pain-free mouth opening (mm: F = 29.902; p < 0.001, ) in favor of the DN and spinal manipulation group (). For active pain-free mouth opening (mm), between-group effect sizes were large at 2 weeks (SMD: 0.96; 95%CI: 0.58, 1.34), 6 weeks (SMD: 1.21; 95%CI: 0.82, 1.60), and 3 months (SMD: 1.61; 95%CI: 1.19, 2.02) after the first treatment session in favor of the DN and spinal manipulation group.
Figure 5. Evolution of active pain-free mouth opening (mm) throughout the course of the study, stratified by randomized treatment assignment. Values are mean and 95% confidence interval.
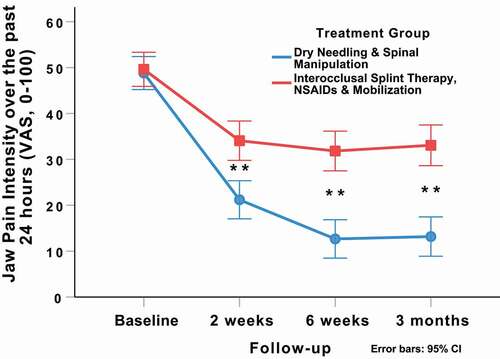
There was a significant group-by-time interaction for jaw pain intensity over the past 24 hrs (VAS: F = 22.432; p < 0.001, ) in favor of the DN and spinal manipulation group (). Between-group effect sizes for jaw pain intensity over the past 24 hrs (VAS) were moderate (SMD: 0.71; 95%CI: 0.34, 1.07) at 2 weeks, large (SMD:1.14; 95%CI: 0.75, 1.52) at 6 weeks, and large (SMD: 1.05; 95%CI: 0.66, 1.43) at 3 months after the first treatment session in favor of the DN and spinal manipulation group.
Figure 6. Evolution of jaw pain intensity over the past 24 hrs (VAS) throughout the course of the study, stratified by randomized treatment assignment. Values are mean and 95% confidence interval.
Based on the cutoff score of ≥ +5 on the GROC [Citation70], significantly (X2 = 22.558; p < 0.001) more patients (n = 44, 71%) within the DN and spinal manipulation group achieved a successful outcome compared to the interocclusal splint therapy, NSAIDs, and TMJ mobilization group (n = 16, 28%) at 3 months follow-up (). Therefore, based on the cut-off score of ≥ +5 on the GROC, the NNT was 2.3 (95%CI 1.7, 3.7) in favor of the DN and spinal manipulation group at 3-month follow-up. Likewise, based on a 50% improvement from baseline to 3 months in average jaw pain intensity over the last 7 days on the VAS, the NNT was 1.8 (95%CI 1.4, 2.5) in favor of the DN and spinal manipulation group at 3-month follow-up.
Table 4. Self-perceived improvement measured with the Global Rating of Change (GROC) in both groups [n (%)]
Discussion
A mean of 6 sessions of DN primarily targeting the inferior head of the lateral pterygoid muscle, the superficial masseter muscle, the anterosuperior and anteroinferior aspects of the temporalis muscle, and the peri-articular capsule of the posterior TMJ combined with upper cervical spinal manipulation resulted in greater improvements in average jaw pain intensity over the last 7 days (Δ −21.9; 95%CI: −29.1, −14.7; p < 0.001), jaw pain intensity over the past 24 hrs (Δ −19.1; 95%CI: −25.7, −12.5; p < 0.001), and active pain-free mouth opening (Δ 9.1 mm; 95%CI: 7.1, 11.1; p < 0.001), in comparison to interocclusal splint therapy, NSAIDs, and non-thrust joint mobilization to the TMJ at the 3-month follow-up.
For average jaw pain intensity over the last 7 days (VAS), between-group effect sizes were large at 6 weeks and 3 months, respectively, in favor of the DN and spinal manipulation group. The between-group difference for change in the primary outcome (average jaw pain intensity over the last 7 days) at 3 months, as measured by the VAS, was large and exceeded the MCID (9–11 mm) [Citation65,Citation66] and the MDC (10–14 mm) for pain [Citation67]. For active pain-free mouth opening (mm), the point estimate for the between-group difference at 3 months also demonstrated a large between-group effect size in favor of the DN and spinal manipulation group. The NNT suggests for every two patients treated with the combination of DN and upper cervical spinal manipulation rather than interocclusal splint therapy, NSAIDs and non-thrust joint mobilization, one additional patient with TMD achieves clinically important reductions in jaw pain intensity and “moderate” to “large” changes in self-perceived improvement ratings at 3 months.
In a review of seven trials, Jung et al. [Citation72] concluded there is limited evidence for the use of acupuncture for TMD. However, only one trial [Citation73] in the Jung et al. [Citation72] review utilized manual needle manipulation, and 60 of the 91 needle locations were inserted into distal points (i.e., primarily in the hands and feet) far removed from the region of pain and dysfunction instead of the local muscles of mastication and/or peri-articular tissue associated with the TMJ capsule. Notably, acupuncture [Citation74–76] and DN [Citation69,Citation77] trials that have directed needling to the local muscles of mastication (i.e., the lateral pterygoids, masseter, and temporalis) with manual and/or electric stimulation have reported statistically significant improvements in pain and function, which is consistent with the findings of the present study. While a number of studies further recommend acupuncture [Citation78] and DN [Citation79] for joint osteoarthritis, the present study is one of the first to additionally insert needles in structures anatomically related to the posterior capsule of the TMJ itself, a primary anatomical structure that is seemingly associated with the pathophysiology of TMD [Citation40,Citation42,Citation80]. This approach may be advantageous, as it may facilitate mechano-transduction of peri-articular connective tissue [Citation43,Citation44], improved vasodilation and, hence, blood flow to the affected area [Citation81,Citation82], opioid recruitment [Citation81,Citation83,Citation84], and joint lubrication [Citation85,Citation86].
Similar to the findings of the present study, the use of spinal manipulation directed to the upper cervical spine has previously been found to improve jaw pain, mouth opening, pressure pain sensitivity, and mandibular kinematics (i.e., amplitude and velocity) in patients with TMD and/or neck pain [Citation22,Citation87–89], which may be due to the concomitant movement of the occipito-atlantal (C0-C1) joint and the C1-C3 facet joints and their neurophysiological association in the activation of the muscles of mastication [Citation90]. There is also a significant overlap of the C1-C3 dorsal horns that receive nociceptive afferent input from the upper neck and the trigeminocervical nucleus [Citation91,Citation92]. Given that the trigeminal nerve provides motor innervation to the muscles of mastication and sensory innervation to the TMJ via the auriculotemporal branch of the mandibular branch of the trigeminal nerve [Citation93], there is a neurophysiological relationship between the upper cervical spine and TMD.
Limitations
There are three important limitations to the current trial. First, the present study did not use a placebo-needling or control group. Although the authors recognize the use of a placebo-needling group as an ideal situation [Citation94], the goal of the current study was to compare an experimental intervention (DN and upper cervical spinal manipulation) to a common conventional intervention (interocclusal splint therapy, NSAIDs, and mobilization to the TMJ) to more accurately determine the new treatment’s effect size [Citation95,Citation96] without the potential for an inflated between-group effect size [Citation96,Citation97]. Trials measure relative efficacy of a treatment compared to a control, placebo, or usual care [Citation94]. The authors believe the question of whether the experimental intervention (DN and upper cervical spinal manipulation) works any better or provides any different outcome than a common conventional intervention (interocclusal splint therapy, NSAIDs, and mobilization to the TMJ) is meaningful to clinicians and to patients with TMD. In addition, a recent secondary analysis of an individual patient data meta-analysis of 29 trials (n = 19,827) of acupuncture for chronic pain concluded that real acupuncture was superior to sham needling irrespective of the subtype of control or sham procedure (penetrating or non-penetrating) [Citation98]. Moreover, a PRISMA-compliant meta-analysis of nine trials and 231 patients found real acupuncture to be more effective than nonpenetrating and laser sham acupuncture for reducing TMD pain [Citation99]. Second, there is a risk of treatment bias secondary to all treating therapists being associated with the same post-graduate fellowship program in orthopedic manual physical therapy. However, treatment bias is not uncommon in manual therapy trials that require a very specific and advanced skill set. Future studies could compare the effectiveness of direct manual therapy procedures (e.g., high-velocity low-amplitude thrust manipulation) with indirect manual therapy approaches (e.g., muscle energy techniques) in patients with TMD.
Third, the interocclusal appliances in the comparison group were prepared by general dentists based on the needs of each individual patient. As such, different types of appliances may have been used. Moreover, some appliances may have required more frequent and/or involved adjustments for some patients than others, which may have caused some variability within the comparison group.
Conclusion
The results of the current randomized clinical trial demonstrated that patients with TMD who received dry needling and upper cervical spinal manipulation experienced significantly greater improvements in jaw pain intensity and active pain-free mouth opening compared to the group that received interocclusal splint therapy, NSAIDs, and non-thrust joint mobilization to the TMJ. Future studies should examine the effectiveness of different types and dosages of dry needling and spinal manipulation and include a long-term follow-up.
Author contributions, data sharing, and patient involvement
JD, RB, KV, and CFdlP participated in the conception, design, data acquisition, statistical analyses, data interpretation, drafting and revision of the manuscript. PB and IY were involved in the data interpretation, drafting and revision of the manuscript. GS, CL, and NE were involved in data collection and revision of the manuscript. All authors read and approved the final version of the manuscript. All data relevant to the study are included in the article or are available as supplementary files. Although the study was approved by the ethics committee at Universidad Rey Juan Carlos, Madrid, Spain (URJC-DPTO 36-2017) and the trial was prospectively registered (ClinicalTrials.gov: NCT03409874), there was no additional patient and/or public involvement in the design, conduct, interpretation, and/or translation of the research.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgments
The authors wish to thank all the participants of the study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Disclosure Statement
Dr. Dunning is the President of the American Academy of Manipulative Therapy (AAMT) and the Director of the AAMT Fellowship in Orthopaedic Manual Physical Therapy. AAMT provides postgraduate training programs in spinal manipulation, spinal mobilization, dry needling, extremity manipulation, extremity mobilization, instrument-assisted soft tissue mobilization, therapeutic exercise, and differential diagnosis to licensed physical therapists, osteopaths, and medical doctors. Drs. James Dunning, Raymond Butts, Paul Bliton, and Ian Young are senior instructors for AAMT. The other authors declare that they have no potential competing interests. None of the authors received any funding for this study.
Additional information
Funding
References
- Prasad SR, Kumar NR, Shruthi HR, et al. Temporomandibular pain. J Oral Maxillofac Pathol. 2016;20(2):272–275. DOI:https://doi.org/10.4103/0973-029X.185902.
- Gauer RL, Semidey MJ. Diagnosis and treatment of temporomandibular disorder. Am Fam Phys. 2015;91(6):378–388.
- Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307.
- Mujakperuo HR, Watson M, Morrison R, et al. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database Syst Rev. 2010(10):CD004715
- Svensson P, Kumar A. Assessment of risk factors for oro-facial pain and recent developments in classification: implications for management. J Oral Rehabil. 2016;43(12):977–989.
- Speciali JG, Dach F. Temporomandibular dysfunction and headache disorder. Headache. 2015;55(1):72–83.
- Bragatto MM, Bevilaqua-Grossi D, Regalo SC, et al. Associations among temporomandibular disorders, chronic neck pain and neck pain disability in computer office workers: a pilot study. J Oral Rehabil. 2016;43(5):321–332. DOI:https://doi.org/10.1111/joor.12377.
- Gil-Martinez A, Paris-Alemany A, Lopez-de-Uralde-Villanueva I, et al. Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions. J Pain Res. 2018;45:571–587.
- Harrison AL, Thorp JN, Ritzline PD. A proposed diagnostic classification of patients with temporomandibular disorders: implications for physical therapists. J Orthop Sports Phys Ther. 2014;44(3):182–197.
- Schiffman EL, Ohrbach R, Truelove EL, et al. The research diagnostic criteria for temporomandibular disorders. V: methods used to establish and validate revised axis I diagnostic algorithms. J Orofac Pain. 2010;24(1):63–78.
- Kurita Varoli F, Sucena Pita M, Sato S, et al. Analgesia evaluation of 2 NSAID drugs as adjuvant in management of chronic temporomandibular disorders. Sci World J. 2015;359152. 2015.
- Mejersjo C, Wenneberg B. Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: a randomized controlled trial. J Oral Rehabil. 2008;35(10):729–738.
- Swift JQ, Roszkowski MT, Alton T, et al. Effect of intra-articular versus systemic anti-inflammatory drugs in a rabbit model of temporomandibular joint inflammation. J Oral Maxillofac Surg. 1998;56(11):1288–1295. discussion 1295-1286. DOI:https://doi.org/10.1016/S0278-2391(98)90611-5.
- Jerjes W, Upile T, Abbas S, et al. Muscle disorders and dentition-related aspects in temporomandibular disorders: controversies in the most commonly used treatment modalities. Int Arch Med. 2008;1(1):23. DOI:https://doi.org/10.1186/1755-7682-1-23.
- Shaffer SM, Brismee JM, Sizer PS, et al. Temporomandibular disorders. Part 2: conservative management. J Man Manip Ther. 2014;22(1): 13–23.
- Butts R, Dunning J, Pavkovich R, et al. Conservative management of temporomandibular dysfunction: a literature review with implications for clinical practice guidelines (narrative review part 2). J Bodyw Mov Ther. 2017;21(3):541–548. DOI:https://doi.org/10.1016/j.jbmt.2017.05.021.
- Dickerson SM, Weaver JM, Boyson AN, et al. The effectiveness of exercise therapy for temporomandibular dysfunction: a systematic review and meta-analysis. Clin Rehabil. 2017;31(8):1039–1048. DOI:https://doi.org/10.1177/0269215516672275.
- Al-Ani MZ, Davies SJ, Gray RJ, et al. Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. Cochrane Database Syst Rev. 2004(1):CD002778
- Zhang C, Wu JY, Deng DL, et al. Efficacy of splint therapy for the management of temporomandibular disorders: a meta-analysis. Oncotarget. 2016;7(51):84043–84053. DOI:https://doi.org/10.18632/oncotarget.13059.
- Bronfort G, Haas M, Evans R, et al. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18:3.
- Armijo-Olivo S, Pitance L, Singh V, et al. Effectiveness of manual therapy and therapeutic exercise for temporomandibular disorders: systematic review and meta-analysis. Phys Ther. 2016;96(1):9–25. DOI:https://doi.org/10.2522/ptj.20140548.
- Calixtre LB, Moreira RF, Franchini GH, et al. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trials. J Oral Rehabil. 2015;42(11):847–861. DOI:https://doi.org/10.1111/joor.12321.
- Herrera-Valencia A R-MM, Martin-Martin J, Cuesta-Vargas A, et al. Efficacy of manual therapy in temporomandibular joint disorders and its medium-and long-term effects on pain and maximum mouth opening: a systematic review and meta-analysis. J Clin Med. 2020;9(3404):1–13.
- Martins WR, Blasczyk JC, Aparecida Furlan de Oliveira M, et al. Efficacy of musculoskeletal manual approach in the treatment of temporomandibular joint disorder: a systematic review with meta-analysis. Man Ther. 2016;21:10–17.
- La Touche R, Martinez Garcia S, Serrano Garcia B, et al. Effect of manual therapy and therapeutic exercise applied to the cervical region on pain and pressure pain sensitivity in patients with temporomandibular disorders: a systematic review and meta-analysis. Pain Med. 2020;21(10):2373–2384. DOI:https://doi.org/10.1093/pm/pnaa021.
- Harper DE, Schrepf A, Clauw DJ. Pain mechanisms and centralized pain in temporomandibular disorders. J Dent Res. 2016;95(10):1102–1108.
- Dunning J, Butts R, Mourad F, et al. Dry needling: a literature review with implications for clinical practice guidelines. Phys Ther Rev. 2014;19(4):252–265. DOI:https://doi.org/10.1179/108331913X13844245102034.
- Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6(1):83–90.
- Zhou K, Ma Y, Brogan MS. Dry needling versus acupuncture: the ongoing debate. Acupunct Med. 2015;33(6):485–490.
- Butts R, Dunning J, Perreault T, et al. Peripheral and spinal mechanisms of pain and dry needling mediated analgesia: a clinical resource guide for health care professionals. Int J Phys Med Rehabil. 2016;216(4):2.
- Vier C, Almeida MB, Neves ML, et al. The effectiveness of dry needling for patients with orofacial pain associated with temporomandibular dysfunction: a systematic review and meta-analysis. Braz J Phys Ther. 2019;23(1):3–11. DOI:https://doi.org/10.1016/j.bjpt.2018.08.008.
- Nowak Z, Checinski M, Mitecka-Buchta A, et al. Intramuscular injections and dry needling within masticatory muscles in management of myofascial pain. Systematic Review of Clinical Trials. Int J Environ Res Public Health. 2021;18(18):9552 .
- Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390.
- Look JO, Schiffman EL, Truelove EL, et al. Reliability and validity of axis I of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) with proposed revisions. J Oral Rehabil. 2010;37(10):744–759. DOI:https://doi.org/10.1111/j.1365-2842.2010.02121.x.
- La Touche R, Fernández‐De‐Las‐Peñas C, Fernández‐Carnero K, et al. The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J Oral Rehabil. 2009;36(September 9):644–652. DOI:https://doi.org/10.1111/j.1365-2842.2009.01980.x.
- Gonzalez-Iglesias J, Cleland JA, Neto F, et al. Mobilization with movement, thoracic spine manipulation, and dry needling for the management of temporomandibular disorder: a prospective case series. Physiother Theory Pract. 2013;29(8):586–595. DOI:https://doi.org/10.3109/09593985.2013.783895.
- Goulet JP, Clark GT, Flack VF, et al. The reproducibility of muscle and joint tenderness detection methods and maximum mandibular movement measurement for the temporomandibular system. J Orofac Pain. 1998;12(1):17–26.
- Di Rienzo Businco L, Di Rienzo Businco A, D’Emilia M, et al. Topical versus systemic diclofenac in the treatment of temporo-mandibular joint dysfunction symptoms. Acta Otorhinolaryngol Ital. 2004;24(5):279–283.
- Carmeli ESS, Bloomenfeld I, Bloomenfeld I. Comparative study of repositioning splint therapy and passive manual range of motion techniques for anterior displaced temporomandibular discs with unstable excursive reduction. Physiotherapy. 2001;87(1):26–36.
- Butts R, Dunning J, Perreault T, et al. Pathoanatomical characteristics of temporomandibular dysfunction: where do we stand? (Narrative review part 1). J Bodyw Mov Ther. 2017;21(3):534–540. DOI:https://doi.org/10.1016/j.jbmt.2017.05.017.
- Scully C. Oral and maxillofacial medicine: the basis of diagnosis and treatment. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2008.
- Scully C. Oral and maxillofacial medicine: the basis of diagnosis and treatment. 3rd ed. Edinburgh, UK: Churchill Livingstone; 2013.
- Goldman N, Chandler-Militello D, Langevin HM, et al. Purine receptor mediated actin cytoskeleton remodeling of human fibroblasts. Cell Calcium. 2013;53(4):297–301. DOI:https://doi.org/10.1016/j.ceca.2013.01.004.
- Langevin HM, Bouffard NA, Fox JR, et al. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol. 2011;226(5):1166–1175. DOI:https://doi.org/10.1002/jcp.22442.
- Sandberg M, Lundeberg T, Lindberg LG, et al. Effects of acupuncture on skin and muscle blood flow in healthy subjects. Eur J Appl Physiol. 2003;90(1–2):114–119. DOI:https://doi.org/10.1007/s00421-003-0825-3.
- Yim YK, Lee H, Hong KE, et al. Electro-acupuncture at acupoint ST36 reduces inflammation and regulates immune activity in collagen-induced arthritic mice. Evid Based Complement Alternat Med. 2007;4(1):51–57. DOI:https://doi.org/10.1093/ecam/nel054.
- Choi YJ, Lee JE, Moon WK, et al. Does the effect of acupuncture depend on needling sensation and manipulation? Complement Ther Med. 2013;21(3):207–214. DOI:https://doi.org/10.1016/j.ctim.2012.12.009.
- Takano T, Chen X, Luo F, et al. Traditional acupuncture triggers a local increase in adenosine in human subjects. J Pain. 2012;13(12):1215–1223. DOI:https://doi.org/10.1016/j.jpain.2012.09.012.
- Lundeberg T, Lund I. Are reviews based on sham acupuncture procedures in fibromyalgia syndrome (FMS) valid? Acupunct Med. 2007;25(3):100–106.
- Kong J, Gollub R, Huang T, et al. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13(10):1059–1070. DOI:https://doi.org/10.1089/acm.2007.0524.
- Zhou W, Benharash P. Significance of “Deqi” response in acupuncture treatment: myth or reality. J Acupunct Meridian Stud. 2014;7(4):186–189.
- MacPherson H, Maschino AC, Lewith G, et al. Characteristics of acupuncture treatment associated with outcome: an individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLoS One. 2013;8(10):e77438. DOI:https://doi.org/10.1371/journal.pone.0077438.
- Vickers AJ, Vertosick EA, Lewith G, et al. Acupuncture trialists c: acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. 2018;19(5):455–474. DOI:https://doi.org/10.1016/j.jpain.2017.11.005.
- Menz HB. Analysis of paired data in physical therapy research: time to stop double-dipping? J Orthop Sports Phys Ther. 2005;35(8):477–478.
- Dunning JR, Butts R, Mourad F, et al. Upper cervical and upper thoracic manipulation versus mobilization and exercise in patients with cervicogenic headache: a multi-center randomized clinical trial. BMC Musculoskelet Disord. 2016;17:64.
- Dunning J, Mourad F, Barbero M, et al. Bilateral and multiple cavitation sounds during upper cervical thrust manipulation. BMC Musculoskelet Disord. 2013;14:24.
- Beffa R, Mathews R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J Manipulative Physiol Ther. 2004;27(2):e2.
- Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004;29(13):1452–1457. (Phila Pa (Phila Pa. DOI:https://doi.org/10.1097/01.brs.0000129024.95630.57.
- Reggars JW. The manipulative crack. Frequency analysis. Australas Chiropr Osteopathy. 1996;5(2):39–44.
- Evans DW, Lucas N. What is ‘manipulation’? A reappraisal. Man Ther. 2010;15(3):286–291.
- Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157.
- Le Resche L, Burgess J, Dworkin SF. Reliability of visual analog and verbal descriptor scales for “objective” measurement of temporomandibular disorder pain. J Dent Res. 1988;67(1):33–36.
- Conti PC, de Azevedo LR, de Souza NV, et al. Pain measurement in TMD patients: evaluation of precision and sensitivity of different scales. J Oral Rehabil. 2001;28(6):534–539. DOI:https://doi.org/10.1046/j.1365-2842.2001.00727.x.
- Stuginski-Barbosa J, Silva R, Cunha C, et al. Pressure pain threshold and pain perception in temporomandibular disorder patients: is there any correlation? Rev Dor. 2015;16(1). DOI:https://doi.org/10.5935/1806-0013.20150005.
- Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med. 2001;38(6):639–643.
- Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–638.
- Stoustrup P, Kristensen KD, Verna C, et al. Orofacial symptoms related to temporomandibular joint arthritis in juvenile idiopathic arthritis: smallest detectable difference in self-reported pain intensity. J Rheumatol. 2012;39(12):2352–2358. DOI:https://doi.org/10.3899/jrheum.120437.
- Naeije M, Te Veldhuis AH, Te Veldhuis EC, et al. Disc displacement within the human temporomandibular joint: a systematic review of a ‘noisy annoyance’. J Oral Rehabil. 2013;40(2):139–158. DOI:https://doi.org/10.1111/joor.12016.
- Fernandez-Carnero J, La Touche R, Ortega-Santiago R, et al. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofac Pain. 2010;24(1):106–112.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. https://doi.org/10.1016/0197-2456(89)90005-6.
- Carlesso LC, Macdermid JC, Santaguida LP. Standardization of adverse event terminology and reporting in orthopaedic physical therapy: application to the cervical spine. J Orthop Sports Phys Ther. 2010;40(8):455–463.
- Jung A, Shin BC, Lee MS, et al. Acupuncture for treating temporomandibular joint disorders: a systematic review and meta-analysis of randomized, sham-controlled trials. J Dent. 2011;39(5):341–350.
- Schmid-Schwap M, Simma-Kletschka I, Stockner A, et al. Oral acupuncture in the therapy of craniomandibular dysfunction syndrome – a randomized controlled trial (RCT). Mid-Euro J Med. 2006;118(42): 36–42
- List T, Helkimo M. Acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. II. A 1-year follow-up study. Acta Odontol Scand. 1992;50:375–385.
- Johansson H, Sojka P, Jung A, et al. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses. 1991;35(3):196–203.
- Zhu X. Electro acupuncture on 90 cases with temporo-mandibular joint disorder. Fujian J Trad Chinese Med. 2007; 38(13):26–32.
- Gonzalez-Perez LM, Infante-Cossio P, Granados-Nunez M, et al. Deep dry needling of trigger points located in the lateral pterygoid muscle: efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Med Oral Patol Oral Cir Bucal. 2015;20(3):e326–333. DOI:https://doi.org/10.4317/medoral.20384.
- Chen N, Wang J, Mucelli A, et al. Electro-acupuncture is beneficial for knee osteoarthritis: the evidence from meta-analysis of randomized controlled trials. Am J Chin Med. 2017;45(5):965–985. DOI:https://doi.org/10.1142/S0192415X17500513.
- Dunning J, Butts R, Young I, et al. Periosteal electrical dry needling as an adjunct to exercise and manual therapy for knee osteoarthritis: a multicenter randomized clinical trial. Clin J Pain. 2018;34(12):1149–1158. DOI:https://doi.org/10.1097/AJP.0000000000000634.
- Manfredini D. Etiopathogenesis of disk displacement of the temporomandibular joint: a review of the mechanisms. Indian J Dent Res. 2009;20(2):212–221.
- Ahsin S, Saleem S, Bhatti AM, et al. Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. Pain. 2009;147(1–3):60–66. DOI:https://doi.org/10.1016/j.pain.2009.08.004.
- Loaiza LA, Yamaguchi S, Ito M, et al. Electro-acupuncture stimulation to muscle afferents in anesthetized rats modulates the blood flow to the knee joint through autonomic reflexes and nitric oxide. Auton Neurosci. 2002;97(2):103–109. DOI:https://doi.org/10.1016/S1566-0702(02)00051-6.
- Huang J, Zhuo L-S, Wang -Y-Y, et al. [Effects of electroacupuncture on synovia IL-1beta and TNF-alpha contents in the rabbit with knee osteoarthritis]. Zhen Ci Yan Jiu = Acupuncture Research. 2007;32(2):115–118.
- Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503.
- Li Z-D, Cao L-H, Wang S-C. [Effect of moxibustion in treating knee joint osteoarthritis and its relation with contents of hyaluronic acid in serum and synovial fluid]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29(10):883–885.
- Wu MX, Li XH, Lin MN, et al. Clinical study on the treatment of knee osteoarthritis of shen-sui insufficiency syndrome type by electroacupuncture. Chin J Integr Med. 2010;16(4):291–297. DOI:https://doi.org/10.1007/s11655-010-0513-1.
- Monaco A, Cozzolino V, Cattaneo R, et al. Osteopathic manipulative treatment (OMT) effects on mandibular kinetics: kinesiographic study. Eur J Paediatr Dent. 2008;9(1):37–42.
- Oliveira-Campelo NM, Rubens-Rebelatto J, Marti NVFJ, et al. The immediate effects of atlanto-occipital joint manipulation and suboccipital muscle inhibition technique on active mouth opening and pressure pain sensitivity over latent myofascial trigger points in the masticatory muscles. J Orthop Sports Phys Ther. 2010;40(5):310–317. DOI:https://doi.org/10.2519/jospt.2010.3257.
- Mansilla-Ferragut P, Fernandez-de-las Penas C, Alburquerque-Sendin F, et al. Immediate effects of atlanto-occipital joint manipulation on active mouth opening and pressure pain sensitivity in women with mechanical neck pain. J Manipulative Physiol Ther. 2009;32(2):101–106. DOI:https://doi.org/10.1016/j.jmpt.2008.12.003.
- Braun de Castro M, Valentim da Silva R, Basilio F. Effects of manual therapy in the treatment of temporomandibular dysfunction - a review of the literature. Man Ther Posturol Rehabil J. 2017;15(520):1–7.
- Spadaro A, Ciarrocchi I, Masci C, et al. Effects of intervertebral disc disorders of low back on the mandibular kinematic: kinesiographic study. BMC Res Notes. 2014;7:569.
- Lin CS. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9(4):e94300.
- Fernandez-de-Las-Penas C, Galan-Del-Rio F, Alonso-Blanco C, et al. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disoders. J Pain. 2010;11(12):1295–1304. DOI:https://doi.org/10.1016/j.jpain.2010.03.005.
- Kamper SJ. Control groups: linking evidence to practice. J Orthop Sports Phys Ther. 2018;48(11):905–906.
- Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34(9):917–928.
- Faraone SV. Interpreting estimates of treatment effects: implications for managed care. PT. 2008;33(12):700–711.
- Kamper SJ. Interpreting outcomes 2-statistical significance and clinical meaningfulness: linking evidence to practice. J Orthop Sports Phys Ther. 2019;49(7):559–560.
- MacPherson H, Vertosick E, Lewith G, et al. Influence of control group on effect size in trials of acupuncture for chronic pain: a secondary analysis of an individual patient data meta-analysis. PLoS One. 2014;9(4):e93739. DOI:https://doi.org/10.1371/journal.pone.0093739.
- Wu JY, Zhang C, Xu YP, et al. Acupuncture therapy in the management of the clinical outcomes for temporomandibular disorders: a PRISMA-compliant meta-analysis. Medicine (Baltimore). 2017;96(9):e6064.