ABSTRACT
Background
Chondromyxoid fibroma (CMF) is a rare cartilaginous tumor, accounting for < 1% of benign bone tumors. We report a case of temporomandibular joint (TMJ)-CMF, involving the pterygopalatine space and skull base and discuss its epidemiology, clinical characteristics, and management.
Case presentation
A 56-year-old woman presented with facial asymmetry and progressive mouth opening restriction due to a mass expanding upwardly to the auriculotemporal region. Using digital techniques to determine the lesion’s boundary and reconstruct the normal glenoid fossa, the temporalis myofascial flap was transplanted between the titanium mesh and condyle to reconstruct the disc after tumor resection.
Conclusion
This case highlights the importance of identifying patients with TMJ-CMF.
Introduction
Chondromyxoid fibroma (CMF) is an exceedingly rare, benign bone neoplasm, accounting for less than 1% of primary bone tumors and 2% of benign bone tumors. CMF is a distinctive tumor of cartilaginous origin that is prevalent among adolescents and young adults [Citation1]. Although a wide skeletal distribution has been observed, the lesion frequently affects the metaphyses of the major long bones, particularly the tibia [Citation1,Citation2]. However, craniomaxillofacial involvement is rare.
Information on the incidence of CMF in the temporomandibular joint (TMJ) is limited; approximately 0.06% of reported cases have CMF in the zygomatic bone, nasal septum, maxillary sinus, and mandible [Citation3,Citation4]. The clinical management of TMJ-CMF is highly challenging because of its scarcity. Herein, we describe a case of a patient with primary TMJ-CMF and share our experience of treating it using digital surgery.
Case description
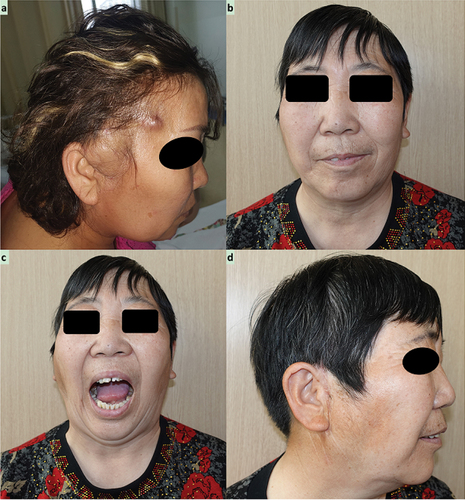
A 56 year-old Chinese Han woman was admitted to our institute in October 2020, presenting a 1-year history of a gradually enlarging mass in the preauricular region and a 2-week history of increasing intumescence with occasional tinnitus. Her chief complaint was progressive limitation of mouth opening and facial asymmetry due to the upward of this mass into the auriculotemporal region. The part of this mass protruding from the preauricular region was moderately hard and semi-mobile with slight tenderness in the auriculotemporal region (). Mandibular deflection concomitant with TMJ clicking was also observed during mouth opening.
Figure 1. Preoperative extraoral view reveals a prominent bulge coated with normal skin color and temperature. Postoperative photographs reveals a basically symmetrical facial profile (b) frontal view; d. right side view), and (c) normal mouth opening of 45 mm.
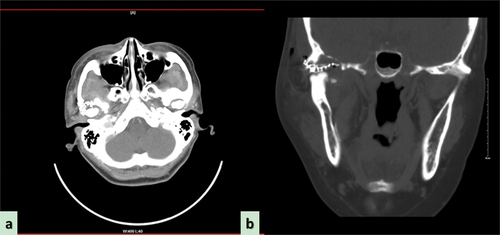
Craniofacial plain and enhanced CT scans confirmed hyperostosis and sclerosis of the right condyle, along with the presence of a mass involving the pterygopalatine space and invading the skull base (). Magnetic resonance imaging of the craniofacial area revealed a large and irregular soft tissue mass shadow in the right TMJ, with long T1 and mixed short T2 signals that appeared annular after enhancement, with multiple small septa around the periphery. The right TMJ was occupied by the mass, which had slightly pushed the right parotid gland inward, consistent with a bone origin (). To further confirm the diagnosis, we performed a fine-needle aspiration of the mass as a preoperative biopsy. Taking a scientifically rigorous attitude into consideration, a presumptive diagnosis of a chronic inflammatory reaction and a suspected CMF-like lesion in the TMJ was made (), emphasizing the necessity for further routine pathological examination of surgical specimens.
Figure 2. Preoperative computed tomography scan reveals the wormlike osteolysis of the right condylar head presenting with swelling, cloudy flocculent ground-glass opacity, relatively clear boundary, thin bone in the middle of the cranial fossa, low continuity, and involving the temporomandibular joint. (a) Computed tomography (CT) sagittal plane, (b) CT axial plane, (c) CT coronal plane. (d) Preoperative magnetic resonance imaging reveals the lesion exhibiting hypointensity, presenting as an oval soft tissue shadow on the sagittal T1-weighted image. (e) Heterogeneous hyperintensity on the axial T2-weighted image and (f) T1-enhanced weighted image in the coronal position shows ring enhancement.
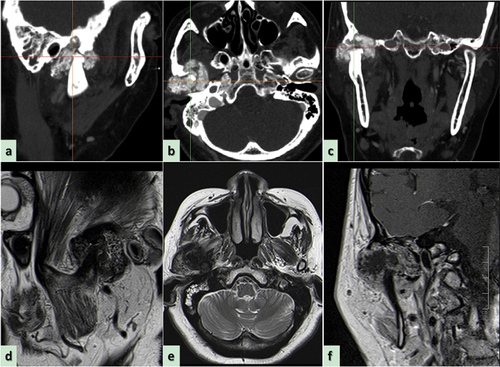
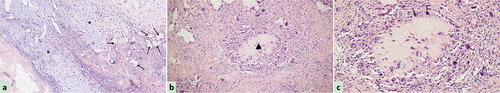
Figure 3. Morphological characteristics of preoperative biopsy using fine needle aspiration revealed through hematoxylin-eosin staining. (a) Abundant mucoid and loosely distributed spindle-shaped or stellate cells (black asterisk, original magnification × 100), and rich fibrous septal lobes in cartilage matrix (black arrow, original magnification × 100); (b) an atypical lesion of fibrous septal lobe containing circularly close-packed cells surrounding clear mucus and extracellular matrix (black triangle, original magnification × 200); (c) a large amount of mononuclear spindle-like stromal cells mixed with occasional multinucleated giant cells (black arrow, original magnification × 400).
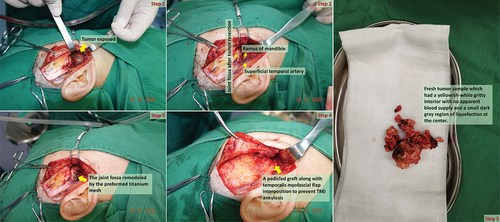
In addition, imaging examination results indicated that the right superficial temporal artery and maxillary vein were intertwined with the tumor, which may have caused massive intraoperative hemorrhage, increasing the difficulty of the surgery. Therefore, an interventional radiologist was consulted to perform intravascular embolization. The imaging files (DICOM format) were imported into Mimics software (version 21.0; Materialise Inc., Leuven, Belgium) for 3D plane reorientation and reconstruction [Citation5–8]. Based on the 3D printing model, an individualized preformed titanium mesh (Titanium Cranioplasty Mesh System, Bidoia Co. Ltd., Padua, Italy) modified with CAD (computer-aided design)/CAM (computer-aided manufacturing) techniques to closely match to the TMJ glenoid fossa to facilitate the recovery of the destructed fossa ().
Figure 4. Digital design and fabrication of simulation model for reconstructive surgery. (a,b) the close relationship between the tumor and blood vessels; (c) image registration technique and inverse design for restoring the anatomical shape of the destruted fossa [Citation8]; (d,e) 3D printing model displaying the exact location of the tumor and the nature of its relationship with the peripheral blood vessels, nerves, and other tissues; (f) placement of titanium mesh preoperatively.
![Figure 4. Digital design and fabrication of simulation model for reconstructive surgery. (a,b) the close relationship between the tumor and blood vessels; (c) image registration technique and inverse design for restoring the anatomical shape of the destruted fossa [Citation8]; (d,e) 3D printing model displaying the exact location of the tumor and the nature of its relationship with the peripheral blood vessels, nerves, and other tissues; (f) placement of titanium mesh preoperatively.](/cms/asset/273608fa-61be-4387-a2a1-80dfceafd0bb/ycra_a_2357988_f0004_oc.jpg)
After excluding the contraindications of surgery, under general anesthesia, the TMJ was approached through a preauricular incision with a 1.5 ~ 2.0 cm extension into the temporal hairline to completely resect the tumor [Citation9]. Intraoperatively, the root of the posterior zygomatic arch was damaged by the tumor, which was bulbous, expanded, and protruding forward into the posterior, internal, and lateral TMJ. After thorough tumor resection, an approximately 1.0 × 1.5 cm defect was created in the articular fossa, while the endocranium was intact. Resection and volume reduction were performed at the middle of the tumor. A 1.5-cm wide pedicled temporalis myofascial flap was obtained and rotated beneath the zygomatic arch with the fascia facing the titanium mesh and the muscle toward the reshaped and polished condylar surfaces; the flap was secured with 3–0 resorbable suture material to reconstruct the disc after complete tumor resection [Citation9]. Layer-wise closure was performed with resorbable sutures; the skin at the preauricular region was sutured with prolene 6–0 sub-cutaneous sutures, whereas the scalp and abdominal skin were sutured with 3–0 silk sutures. The isolated specimen appeared as yellowish-white gravel-like bone inside the tumor, with a small dark gray liquefaction region in the center, without an apparent blood supply (). Vacuum suction drains were placed, and pressure dressings were applied. The suction drain was removed after 48 h, the sutures from the pre-auricular area were removed on the 5th postoperative day, and those from the temporal region on the 10th day.
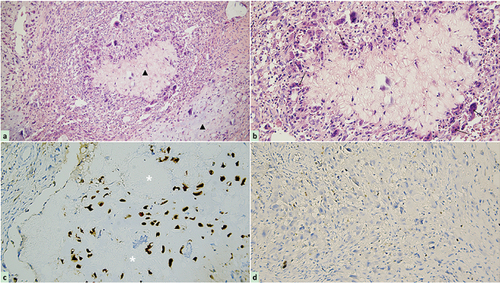
Postoperative histopathological analysis of paraffin sections confirmed diffuse proliferation of mononuclear spindle cells admixed with occasional multinucleated giant cells, with variable myxoid to chondroid stroma, representing various stages of cartilaginous development . Immunohistochemistry results showed positive staining for S-100 (cartilage+) and Ki-67 (≤1%+) indicating low proliferation capacity. Based on these findings, the tumor was considered benign .
Figure 6. Photomicrograph of the tumor following hematoxylin-eosin staining and immunohistochemistry. (a) The cellular components are less prominent in cartilage matrix and mucoid (black triangle, original magnification × 200), but myofibroblastic-like spindle cell proliferation with loosely arranged myxoid stroma, minimal cellular atypia, and rare mitoses, along with storiform or fascicular pattern can be observed in the remaining areas; (b) a small amount of multinucleated giant cells (black arrow, original magnification × 400) admixed with plentiful mononuclear spindle cells surrounding clear mucus and cartilage matrix forming a pathologic lobular structure; (c) immunohistochemical staining is positive for S-100 in the chondrocytes (white asterisk, original magnification × 200) (d) but negative for Ki-67 in all kinds of cells (less than 1% positive).
Distinguishing between benign and malignant bone tumors is a primary concern. Although this disease is considered a benign tumor and is not difficult to diagnose, there is some complexity in distinguishing between tissue types. The differential diagnoses included chondrosarcoma, chondroblastoma, and fibrous dysplasia with mucoid transformation. A chondrosarcoma with a highly mucinous transformation may have a lobulated appearance and astrocytes, similar to CMF. It is important to remember that CMFs are always benign manifestations, whereas chondrosarcomas have invasive imaging features. The lobulation of chondrosarcomas is generally not as prominent as that of CMF, where the central cells are sparse and the surrounding cells are abundant. Fibrous septal lobes with peripheral intramucinous infiltration are common in CMFs, whereas chondroblastomas do not show a tendency for lobular growth. A highly mucinous fibrous dysplasia may sometimes be misdiagnosed as CMF; however fibrous dysplasia does not exhibit lobular growth patterns. Despite the lack of consensus on specific biomarkers for diagnosing CMF, the final diagnosis was confirmed as TMJ-CMF based on a combination of morphology, immunohistochemistry, and radiology.
The patient was discharged on the 10th postoperative day without complications, and no recurrence was detected on physical examination or CT during the 1-year follow-up (). In addition, the maximum postoperative mouth opening was 45 mm and the pain visual analog scale score was two points .
Discussion
CMF is a remarkably uncommon benign bone tumor that was first described in 1948 by Jaffe and Lichtenstein as a separate entity from benign chondroblastoma or chondrosarcoma [Citation10]. It arises from cartilage-forming mesenchymal tissue, which can differentiate into cartilage and fibrous tissue, and is histologically characterized by varying proportions of lobular, spindle-shaped, or stellate cells in a myxoid background [Citation1,Citation4]. According to Chinese studies, CMF accounts for 1.1 ~ 1.75% of bone tumors and 2.1% of benign bone tumors [Citation3]. Nevertheless, according to non-Chinese studies, CMF comprises < 1% of bone tumors [Citation1,Citation2,Citation4]. The age of onset and sex distribution of CMF vary considerably, but it is typically seen in patients in the second to third decades of life, with a slight female predominance [Citation11]. Wu et al. [Citation12] reported that almost half of the CMF cases occur in the long bones (46.9%), although the flat bones (30.3%) and the bones of the hands and feet (17.3%) are also commonly affected. Only 5.4% of the tumors involve the skull and facial bones, including the maxilla, mandible, frontal bone, orbital floor, zygoma, and temporal bone.
Pain is observed in most patients often being mild, local, intermittent, and present for months or years prior to consultation. Occasionally, cases are asymptomatic, and the lesion is discovered on routine roentgenography or due to trauma. Most patients are aware of a mass, which may be tender. Occasionally, symptoms are pronounced. In two series of nine cases (age 5 ~ 10 years), pain, swelling, and restriction of function developed rapidly, within 3 ~ 6 weeks, leading to speculation that the lesion may be more aggressive in childhood [Citation12,Citation13].
CMF involving the TMJ may be accompanied by facial swelling, pain, and limitations in mandibular movement. Upon reviewing the patient’s long medical history, the leading symptoms were limited mouth opening and pain localized to the TMJ area at rest and during function. At present, only one case of TMJ-CMF has been documented, underscoring the need for a complete radiological investigation of this joint in patients with clinical symptoms localized in this region [Citation14]. The CT findings of CMF lack specificity, although they typically exhibit characteristics consistent with benign tumors [Citation15]. This include partially lobulated outline, clear bone septa, and some degree of mineralization varying from slight to marked, sharp margins and sclerotic rims of the tumor. In the present case, no significantly sclerotic scalloped margin demarcated the boundary of the lesion; however, the cortex appeared thinned and expanded, associated with the tumor, and indicated by cloudy flocculent ground-glass opacities.
Distinguishing CMF from chondrosarcoma poses a significant challenge in morphologic consideration. In this respect, gross appearance may be helpful [Citation16]. CMFs are generally pale, flesh-colored, and unexpectedly firm, reflecting the presence of fibrous component. In addition, CMFs are incompletely lobulated and, as a rule, confined by an intact periosteum. This is true even in areas where the cortical shell cannot be roentgenographically delineated. From the perspective of tumor genetics, direct targeting of the glutamate receptor gene GRM1 is a necessary and highly specific driver event for CMF development, which is rare-to-absent in other cartilaginous tumors [Citation17].
Despite the benign behavior of CMFs, the recurrence rate has been reported to be as high as 11%, mainly during the first year following the initial surgery [Citation12]. Nevertheless, the rate increases to 80% when surgical excision is incomplete [Citation18]. The administration of radiotherapy is controversial due to its potential association with an increased risk of malignant transformation despite the absence of confirmed cases involving the TMJ [Citation13]. CMFs are mainly treated with simple curettage and wide resection, with or without allografting. Some clinicians prefer curettage, especially because of its cosmetic and functional aspects, although the postoperative recurrence rate is as high as 25% [Citation19]. We recommend surgical resection based on the digitalized technique as the primary treatment for TMJ-CMF to ensure accurate reconstruction of the defect. Driven in part by its good long-term outcomes, total alloplastic TMJ replacement surgery has become an increasingly common procedure for the treatment of end-stage TMJ disease [Citation7,Citation20]. An important goal of TMJ replacement surgery is the accurate placement of implant system components. Significant malpositioning risks damaging vital structures or causing malocclusions, leading to increased surgical morbidity and the need for further surgery. Complications, including facial nerve damage, alveolar nerve injuries, neuropathic pain, loss of sensation in the skin, hearing problems, and infection are rare occurring in less than 5% of patients [Citation21]. The Techmedica CAD/CAM patient-fitted TMJ Total Joint Replacement device reported by Wolford et al. [Citation22] continues to function well based on a 21-year follow-up data analysis of a prospective cohort study. With biomaterials tailored to patient specificity, lower functional loading forces applied to the TMJ complex than to the knee and hip, and the relative ease and speed of implantation, these devices should have a lifespan greater than their orthopedic joint counterparts. Patients in this study showed sustained improvements in TMJ pain, jaw function, ability to eat solid food, and quality of life, suggesting that this prosthesis is unlikely to fail due to material wear. Successful outcomes of managing end-stage joint disease with alloplastic joint replacement devices in orthopedic and maxillofacial surgery have been well documented for decades. However, evidence supporting the use of TMJ replacement to reconstruct mandibular defects created by removing benign mandibular lesions is scarce [Citation23].
Conclusion
We would like to highlight the rarity of bony tumor at the temporomandibular joint. A bony or cartilaginous tumor in this region should raise the possibility of chondromyxoid fibroma in the differential diagnosis. The importance lies in both treatment and prognosis. Treatment of this tumor does not require aggressive bone removal, similar to chondrosarcoma, and thus has less morbidity. Prognostically, the tumors grow slowly; if recurrence is anticipated, they will occur after a longer period. Most importantly, adjuvant radiation is not indicated for this type of tumor. Although the CT features are suggestive of this tumor, the rarity of the TMJ location underscores the importance of histological evaluation for definitive diagnosis. Therefore, in case of questions regarding the diagnosis, an experienced bone pathologist should review the slides.
Acknowledgments
We would like to express our cordial appreciation for the support of Prof. Hui Liu, and Dr. Zhi-qiang Song for professional assistance in our work, and Prof. Wen-li Cui for pathological consultation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol. 2021;28(3):119–138. doi: 10.1097/PAP.0000000000000293
- Waldman S, Shimonov M, Yang N, et al. Benign bony tumors of the paranasal sinuses, orbit, and skull base. Am J Otolaryngol. 2022;43(3):103404. doi: 10.1016/j.amjoto.2022.103404
- Ying Z, Zhihui Z, Xiaojuan S. [Chondromyxoid fibroma of the mandible: a case report]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2016;34(6):654–656. Chinese. doi: 10.7518/hxkq.2016.06.020
- Vered M, Wright JM. Update from the 5th edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022;16(1):63–75. doi: 10.1007/s12105-021-01404-7
- Li CX, Liu X, Gong ZC, et al. Morphologic analysis of condyle among different disc status in the temporomandibular joints by three-dimensional reconstructive imaging: A preliminary study. BMC Oral Health. 2022;22(1):395. doi: 10.1186/s12903-022-02438-1
- Li CX, Liu H, Gong ZC, et al. Effects of osseous structure based on three-dimensional reconstructive imaging evaluation in the assessment of temporomandibular joint disc position. Clin Oral Investig. 2023;27(4):1449–1463. doi: 10.1007/s00784-023-04936-0
- Li C, Shi W, Gong Z, et al. Anterolateral thigh perforator flap made by customized 3D-printing fabrication of fixed positioning guide for oromaxillofacial reconstruction: a preliminary study. Med Oral Patol Oral Cir Bucal. 2023;28(1):e41–e47. doi: 10.4317/medoral.25558
- Li CX, Xie X, Li M, et al. A pilot investigation of condylar position and asymmetry in patients with unilateral posterior scissors-bite malocclusion based on three-dimensional reconstructive imaging technique. BMC Musculoskelet Disord. 2023;24(1):253. doi: 10.1186/s12891-023-06384-z
- Li CX, Yu P, Gong ZC, et al. Modified minimally invasive surgery in reconstructing the temporomandibular joint disk by transplantation of the temporalis myofascial flap. BMC Musculoskelet Disord. 2023;24(1):7. doi: 10.1186/s12891-023-06128-z
- Jaffe HL, Lichtenstein L. Chondromyxoid fibroma of bone; a distinctive benign tumor likely to be mistaken especially for chondrosarcoma. Arch Pathol (Chic). 1948;45(4):541–551.
- Fomete B, Adeosun OO, Awelimobor DI, et al. Chondromyxoid fibroma of the mandible: Case report and review of the literature. Ann Maxillofac Surg. 2014;4(1):78–80. doi: 10.4103/2231-0746.133072
- Wu CT, Inwards CY, O’Laughlin S, et al. Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol. 1998;29(5):438–446. doi: 10.1016/s0046-8177(98)90058-2
- Lersundi A, Mankin HJ, Mourikis A, et al. Chondromyxoid fibroma: a rarely encountered and puzzling tumor. Clin Orthop Relat Res. 2005;439:171–175. doi: 10.1097/01.blo.0000174685.62379.6a
- Sellami M, Doyon D, Deboise A, et al. Fibrome chondromyxoïde de l’articulation temporo-mandibulaire. A propos d’une observation [Chondromyxoid fibroma of the temporomandibular joint. Apropos of a case report]. J Radiol. 1984;65(2):97–100. French.
- Cappelle S, Pans S, Sciot R. Imaging features of chondromyxoid fibroma: report of 15 cases and literature review. Br J Radiol. 2016;89(1064):20160088. doi: 10.1259/bjr.20160088
- Zhong J, Si L, Geng J, et al. Chondromyxoid fibroma-like osteosarcoma: a case series and literature review. BMC Musculoskelet Disord. 2020;21(1):53. doi: 10.1186/s12891-020-3063-5
- Nord KH, Lilljebjörn H, Vezzi F, et al. GRM1 is upregulated through gene fusion and promoter swapping in chondromyxoid fibroma. Nat Genet. 2014;46(5):474–477. doi: 10.1038/ng.2927
- Gherlinzoni F, Rock M, Picci PCF. Chondromyxoid fibroma. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1983;65(2):198–204. doi: 10.2106/00004623-198365020-00008
- Khosla RK, Nguyen C, Messner AH, et al. Chondromyxoid fibroma of the mandible in an adolescent: case report and microsurgical reconstructive option. Cleft Palate Craniofac J. 2015;52(2):223–228. doi: 10.1597/13-243
- Mian M, Ackland D, Fink S, et al. Accuracy of custom temporomandibular joint replacement surgery using a virtual surgical planning protocol. Oral Maxillofac Surg. 2021;25(3):367–371. doi: 10.1007/s10006-020-00928-6
- Vorrasi J, Harris H, Karras M, et al. Prosthetic temporomandibular joint replacement (TJR): stock or custom? A single institution pilot comparison. Oral Surg Oral Med Oral Pathol Oral Radiol. 2023;135(2):185–191. doi: 10.1016/j.oooo.2022.06.005
- Wolford LM, Mercuri LG, Schneiderman ED, et al. Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ concepts device. J Oral Maxillofac Surg. 2015;73(5):952–960. doi: 10.1016/j.joms.2014.10.032
- Teschke M, Naujokat H. Indications for alloplastic temporomandibular joint replacement in maxillofacial surgery for benign lesions: A review of the literature and clinical cases. Front Oral Maxillofac Med. 2023;5:39. doi: 10.21037/fomm-22-41