Abstract
Background
In patients with gamma-hydroxybutyrate (GHB) use disorder (GUD), withdrawal can have a fulminant course with rapid progression of severe, potentially life-threatening complications. Case: We present a 45-year old man with severe GHB withdrawal, resistant to conventional treatment with pharmaceutical GHB, high doses of benzodiazepines and baclofen. GHB withdrawal finally responded to thiopental-induced coma therapy, with burst suppression pattern on electroencephalography (EEG). The patient fully recovered, without withdrawal or residual neuropsychiatric symptoms. Discussion: To our knowledge, this is the first case report in which barbiturates were used to induce a coma to treat severe, treatment resistant GHB withdrawal. This case suggests barbiturate coma therapy might be considered in severe GHB withdrawal which does not respond to conventional treatment.
Background
Gamma-hydroxybutyrate (GHB) is a drug of abuse, particularly popular in the party scene.Citation1–3 Prolonged regular use (1 week to 6 months) can lead to dependence and use “around the clock” (every 1–3 h).Citation4 GHB dependence frequently results in cycles of intoxication and withdrawal as GHB has a narrow therapeutic window and short plasma half-life (30–50 min).Citation4 Despite the relatively low prevalence of GHB use in the European Union (0.1–0.4%), over 10% of drug-related hospital admissions are linked to GHB or its precursor gamma-butyrolactone (GBL).Citation2
Although the GHB-related mortality risk is not well-known, acute GHB-related hospital presentations are often severe as most patients are not communicative due to an altered state of consciousness, commonly combined with low body temperature, hypotension, and bradycardia.Citation1,Citation5–7 Acute hospital presentations may be especially severe in cases involving other substance use, which is related to a higher need of treatment, higher need for admission to the Intensive Care Unit (ICU) and longer hospital stay.Citation1,Citation5–8 In the Netherlands, 61% of GHB abusers report polydrug use, mainly alcohol, cocaine, and amphetamines.Citation3
A major risk in GHB dependence is the potentially life-threatening GHB withdrawal syndrome, which can be aggravated in case of polydrug abuse.Citation8 Withdrawal symptoms develop within the first hours after the last ingestion. Clinical features may be agitation, confusion, hallucinations, delusions, seizures, autonomous dysregulation (tachycardia, tachypnea, hypertension), kidney failure, and cardiac arrest.Citation4,Citation9 GHB withdrawal can be counteracted by benzodiazepines and pharmaceutical GHB as first-line treatment.Citation4,Citation9–11 In the Netherlands, over the past years tapering with pharmaceutical GHB has become the dominant strategy to treat GHB withdrawal.Citation12 Studies have shown that titration and tapering of pharmaceutical GHB is a safe strategy to assist GHB detoxification.Citation13
Several case reports suggest GHB withdrawal can be resistant to benzodiazepines.Citation4,Citation9,Citation14,Citation15 This might be explained by pharmacodynamic differences between GHB and benzodiazepines. GHB binds to GHB receptors and mainly GABA-B receptors, benzodiazepines exert their sedative effects through the GABA-A receptor.Citation4,Citation9,Citation14 We present a 45-year old man with severe GHB withdrawal, resistant to benzodiazepines, pharmaceutical GHB, and baclofen. Only after an induced barbiturate coma withdrawal subsided.
Clinical scenario
A 45-year-old man was admitted to our psychiatric ward for GHB detoxification. He was dependent on GHB, using 10–15 ml every hour. However, during the 48 h before admission, he had used 40 ml of GHB of unknown concentration every hour. In addition, he used 4–6 l of beer, diazepam 30 mg, and cannabis 3 joints daily in the months prior to hospital admission. Occasionally, he used amphetamines (frequency unknown), but not in the weeks before admission. He had no other relevant medical or psychiatric history. It was his first detoxification of GHB or other substances, including alcohol and benzodiazepines.
Unexpectedly, he was brought in after intoxication with 300 mg diazepam. On admission, he was deeply sedated. Physical examination revealed no peculiarities, except a Glasgow Coma Scale (GCS) of 3. Due to intoxication, no medications were started. Within 1.5 h after admission his condition changed into severe agitation, physical aggression, and confusion. GHB withdrawal was presumed. He received pharmaceutical GHB 6 g (sodium oxybate; 500 mg GHB/ml) and diazepam 15 mg. Because he remained severely agitated and could not take his medication orally, monitoring of vital signs was required and he was transferred to the ICU.
At the ICU, administration of pharmaceutical GHB 6 g was continued every 2 h and diazepam was replaced by midazolam intravenously (dosage up to 30 mg/h). Pharmaceutical GHB was administered via nasogastric tube (NG-tube). Thiamin 250 mg was administered intravenously to prevent a potential Wernicke encephalopathy. Over the next two days, he remained unconscious (GCS: 3) and showed mild motor restlessness. Restlessness increased acutely during several attempts to taper midazolam in order to regain consciousness. He developed severe tachypnea (respiratory rate up to 70 per minute), tachycardia (heart rate up to 170 per minute), and hypertension (systolic rate up to 230 mmHg) when lowering of the midazolam dose was attempted.
On the second day, the patient had to be intubated due to respiratory insufficiency. The dose of pharmaceutical GHB was increased to 10 g every 2 h. Clonidine intravenously 1 mcg/kg/h was added to counteract withdrawal symptoms.Citation16
On the third day, midazolam was gradually cross-tapered with pharmaceutical GHB, resulting in doses of 10 mg/h and 20 g every 2 h, respectively. Subsequently, his agitation decreased, while he remained unconscious (GCS: 3). However, he developed hypernatremia (164 mmol/l), probably due to the high sodium load in sodium oxybate (20 g sodium oxybate contains 3.64 g sodium). Consequently, the dosage of pharmaceutical GHB had to be lowered to 10 g every 2 h, which resulted in recurrence of severe agitation. Propofol intravenously 300 mg/h and baclofen (via NG-tube) 20 mg 3 times a day were administered to counteract agitation and motor restlessness. Midazolam intravenously 10 mg/h was continued.
Over the next 16 days, the patient was treated with midazolam intravenously (10 mg/h), pharmaceutical GHB (10 g every 2 h via NG-tube), propofol intravenously (300 mg/h), and baclofen 20 mg 3 times daily (via NG-tube). He remained unconscious. Repeated attempts to taper sedatives resulted immediately in agitation and autonomous dysregulation.
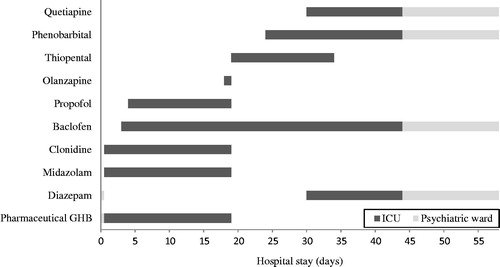
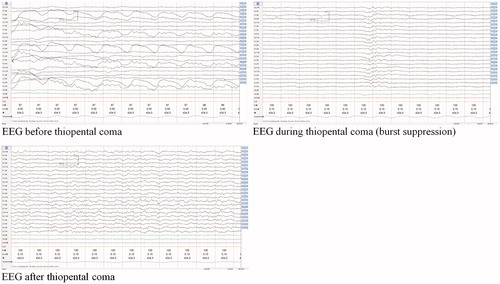
Facing over 2 weeks of inability to taper sedatives, due to agitation and autonomous dysregulation, it was decided to replace all sedatives (except baclofen 20 mg 3 times a day) with barbiturates (see ). Before starting barbiturates, intracerebral pathology was ruled out by a neurological examination and MRI of the cerebrum. Subsequently, a thiopental coma was induced targeting a burst suppression pattern on electroencephalography (EEG) (see ), using thiopental intravenously 25 mg/ml. The patient received two bolus infusions of 10 mg/kg in 30 min, followed by a continuous infusion of 3 mg/kg/h, with continuous EEG monitoring.
During the next 4 days, he was treated with a thiopental-induced burst suppression coma. He was hemodynamically stable and needed controlled mechanical ventilation. Daily echocardiography ruled out the development of cardiac depression. During the coma, the patient received osmotic laxatives twice a day and magnesium sulfate 15 g daily to prevent constipation.
Then, in 3 days, thiopental was switched to phenobarbital (via NG-tube) 125 mg twice a day. Subsequently, thiopental was stopped. The patient gradually woke up. Quetiapine (up to 150 mg per day) and diazepam (up to 30 mg per day) were prescribed to support sleep and suppress psychomotor restlessness. The patient slowly recovered, without neurological or psychiatric symptoms, and showed no confusion on the CAM-ICUCitation17 during his further stay at the ICU.
Two weeks later, the patient was transferred to the psychiatric ward for a 2-week observation period. During the observation period, he showed no signs of neurological damage, cognitive impairment, or withdrawal in psychiatric and neurological examinations.
After a hospital stay of 58 days, he was discharged in good mental and physical health under a drug regimen of phenobarbital (125 mg twice a day), baclofen (20 mg 3 times a day), diazepam (20 mg a day), and quetiapine (150 mg a day). He was referred to the outpatient addiction care center where he was treated prior to hospital admission. It was recommended to taper phenobarbital and diazepam after discharge. Baclofen was continued as part of GHB relapse prevention.Citation18 However, the patient relapsed into GHB abuse in the subsequent months.
Discussion
To our knowledge, this is the first case report on barbiturate-induced coma to treat severe, treatment-resistant GHB withdrawal. Despite 19 days using conventional treatments (high doses of pharmaceutical GHB, benzodiazepines and baclofen), the patient only recovered after thiopental-induced burst suppression coma. This case suggests barbiturate coma therapy might be considered in case of severe GHB withdrawal, unresponsive to conventional treatment.
According to most international guidelines, benzodiazepines are the first step in treatment of GHB withdrawal. Pharmaceutical GHB or barbiturates may be added in case of unresponsiveness to benzodiazepines.Citation4,Citation9,Citation10 However, in the Netherlands the dominant strategy to assist GHB detoxification is tapering with pharmaceutical GHB.Citation12 This has repeatedly been shown to be a safe and successful treatment option, with potentially fewer complications, such as delirium, compared to benzodiazepines.Citation11,Citation13 Moreover, pharmaceutical GHB has been suggested for the treatment of alcohol and benzodiazepine withdrawal, which is common practice in several countries.Citation19 Therefore, we started with pharmaceutical GHB directly, when first withdrawal symptoms were observed.
Though the recommended starting dose of pharmaceutical GHB is 3 g when patients use over 6 ml GHB every 2 h, with subsequent up-titration with a maximum of 1.5 g every 2 h,Citation11 our patient received 6 g pharmaceutical GHB every 2 h when withdrawal symptoms emerged. As a result, he might have switched between severe intoxication, withdrawal, and re-intoxication. However, multiple attempts to taper sedatives, including pharmaceutical GHB, did not improve his condition.
The initial presentation of our patient was further complicated by intoxication with diazepam and alcohol. Although the patient did not use other benzodiazepines, no quantitative bio-assessment of benzodiazepines was performed, and therefore additional effects of other benzodiazepines cannot be fully ruled out. Symptoms of GHB intoxication are more severe in the case of co-use of other substances.Citation5–7 Moreover, diazepam and alcohol might have dampened GHB withdrawal symptoms initially. As the diazepam and alcohol plasma concentrations gradually lowered, the patient might have gone into more severe withdrawal of GHB, as well as into synergistic withdrawal of alcohol and benzodiazepines. Alcohol withdrawal symptoms may appear 6–24 h after discontinuation and diazepam withdrawal symptoms 4–8 h after the last dose.Citation16,Citation19 These combined withdrawal syndromes might have had synergistic effects, complicating the clinical picture during the first hours of admission.
Given the anticipated involvement of GHB and GABA-A and -B receptors, we aimed at targeting these receptors by the administration of pharmaceutical GHB, benzodiazepines, and baclofen respectively. Despite these medications, the patient continued to have unstable vital signs and agitation, even after the typical duration of GHB (2–15 days) and alcohol withdrawal (5–7 days).Citation4,Citation16 Diazepam withdrawal generally lasts up to 3–4 weeks,Citation20 however, during the first 19 days administration of benzodiazepines exceeded his daily use of diazepam 30 mg prior to hospital admission. It is therefore unlikely that the clinical withdrawal picture can be fully explained by benzodiazepine withdrawal.
Although this was the patient’s first detoxification, any previous episodes of unsupervised withdrawal might have occurred. In alcohol use disorder it has been suggested that the severity of withdrawal symptoms may worsen after repeated episodes of withdrawal due to increased neuronal excitability, referred to as kindling.Citation21 It is thought that kindling may also occur in the repetitive withdrawal of other sedatives, like benzodiazepines.Citation22 It might be possible that kindling also occurs in repetitive GHB withdrawal, but to the best of our knowledge kindling has not been described in GHB use disorder to date. Yet, there are some suggestions that the number of GHB-induced comas is related to cerebral damage and consequent cognitive impairment.Citation23,Citation24
Based on several case reports in which GHB withdrawal responded well to barbiturates, we decided to switch all sedatives except baclofen to the barbiturate thiopental. To our knowledge, 9 cases have been published in which barbiturates showed a positive effect on GHB withdrawal syndrome (see ).Citation14,Citation15,Citation25–27 However, none of these cases reported comorbid alcohol or benzodiazepine dependence.
Table 1. Case reports concerning treatment of GHB or GBL withdrawal with barbiturates.
Moreover, we used a thiopental-induced coma with EEG confirmed burst suppression. Burst suppression is an EEG pattern observed in inactivated brain states, such as deep sedation or coma.Citation28 Drug-induced burst suppression is used for the treatment of refractory status epilepticus.Citation28 After several unsuccessful attempts to taper sedatives, it was hypothesized that thiopental-induced coma therapy with burst suppression pattern on EEG might be effective in our patient. During the thiopental-induced coma, his EEG was continuously monitored to maintain burst suppression, and for early identification of toxicity (including isoelectric EEG).Citation29
Thiopental-induced coma treatment is complex since thiopental has a narrow therapeutic window, which potentially overlaps toxicity: the therapeutic window is reported between 25 and 50 mg/L, and toxicity starts from 30 to 70 mg/L.Citation29 Cardiac depression and severe constipation can be serious adverse effects of thiopental.Citation29 Therefore, an echocardiogram was performed daily during the coma, and laxatives were started from the beginning of thiopental administration.
It is poorly understood how barbiturates can be effective when pharmaceutical GHB, benzodiazepines, and baclofen fail to counteract GHB withdrawal. Barbiturates also act on GABA-A and -B receptors, but in addition antagonize glutamatergic AMPA and kainate receptors.Citation30 It has been suggested that antagonizing glutamatergic receptors might explain the effectiveness of barbiturates in the treatment of resistant GHB withdrawal, particularly in case of manifest glutamatergic overactivity.Citation14 Indeed, GHB has dose-dependent effects on glutamate and dopamine release,Citation4,Citation9 and GHB withdrawal has been associated with glutamatergic hyperactivation, resulting in agitation and motor restlessness.Citation8,Citation14 Similar effectiveness of barbiturates in case of treatment-resistant alcohol withdrawal further supports the idea that barbiturate-induced coma might be a last resort option if withdrawal symptoms are treatment-resistant.Citation31,Citation32
Taken together the case presented here shows that barbiturate-induced coma therapy might be effective for patients with severe GHB withdrawal resistant to conventional treatment with pharmaceutical GHB, benzodiazepines, and baclofen. Burst suppression, observed on EEG, might be a good way to monitor whether the coma is deep enough to fully suppress neuronal hyperexcitation. Future studies are needed to compare different treatment strategies for GHB detoxification (e.g., pharmaceutical GHB versus benzodiazepines), and to further explore the potential role of barbiturates for GHB withdrawal in case of treatment resistance.
References
- Van Laar MW, Van Gestel B, Cruts P. 2019. Netherlands national drug monitor 2018. Utrecht, The Netherlands: Trimbos Institute.
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2019: Trends and Developments. https://data.europa.eu/doi/10.2810/191370. Published 2019. Accessed November 21, 2019.
- Wisselink DJ, Kuipers WG, Mol A. Key figures addiction care 2015 National Alcohol and Drugs Information System. Houten: LADIS (National Alcohol and Drugs Information System); 2016. ISBN/EAN: 9780-90-5726-059-9
- Kamal RM, van Noorden MS, Wannet W, Beurmanjer H, Dijkstra BAG, Schellekens A. Pharmacological treatment in γ-hydroxybutyrate (GHB) and γ-butyrolactone (GBL) dependence: detoxification and relapse prevention. CNS Drugs. 2017;31(1):51–64.
- Galicia M, Nogue S, Miro O. Liquid ecstasy intoxication: clinical features of 505 consecutive emergency department patients. Emerg Med J. 2011;28(6):462–466.
- Galicia M, Dargan PI, Dines AM, et al. Clinical relevance of ethanol coingestion in patients with GHB/GBL intoxication. Toxicol Lett. 2019;314:37–42.
- Corkery JM, Loi B, Claridge H, et al. Gamma hydroxybutyrate (GHB), gamma butyrolactone (GBL) and 1,4-butanediol (1,4-BD; BDO): a literature review with a focus on UK fatalities related to non-medical use. Neurosci Biobehav Rev. 2015;53:52–78.
- Kamal RM, Dijkstra BAG, Loonen AJ, De Jong CAJ. The effect of co-occurring substance use on gamma-hydroxybutyric acid withdrawal syndrome. J Addict Med. 2016;10(4):229–235.
- McDonough M, Kennedy N, Glasper A, Bearn J. Clinical features and management of gamma-hydroxybutyrate (GHB) withdrawal: a review. Drug Alcohol Depend. 2004;75(1):3–9.
- Adis Medical Writers. Treat γ-hydroxybutyrate (GHB) and γ-butyrolactone (GBL) dependence with benzodiazepines first, then with other approaches if benzodiazepine-resistant. Drugs Ther Perspect. 2017;33(11):523–528.
- Kamal R, Dijkstra BAG, van Iwaarden JA, van Noorden MS, de Jong CAJ. Practice-Based Recommendations for the Detoxification of Patients with GHB Abuse Disorders (protocol in Dutch). Amersfoort, The Netherlands: Resultaten Scoren; 2013.
- Beurmanjer H, Verbrugge CAG, Schrijen S., Schellekens AFA, DeJong CAJ, Dijkstra BAG Behandeling van GHB afhankelijkheid na detoxificatie; Eindrapportage NISPA GHB Monitor 2.0, Nijmegen: NISPA; 2016. ISBN/EAN: 978-90-819800-6-7
- Dijkstra BAG, Kamal R, van Noorden MS, de Haan H, Loonen AJM, De Jong CAJ. Detoxification with titration and tapering in gamma-hydroxybutyrate (GHB) dependent patients: the Dutch GHB monitor project. Drug Alcohol Depend. 2017;170:164–173.
- Ghio L, Cervetti A, Respino M, Belvederi Murri M, Amore M. Management and treatment of gamma butyrolactone withdrawal syndrome: a case report and review. J Psychiatr Pract. 2014;20(4):294–300.
- Sivilotti ML, Burns MJ, Aaron CK, Greenberg MJ. Pentobarbital for severe gamma-butyrolactone withdrawal. Ann Emerg Med. 2001;38(6):660–665.
- Mirijello A, D’Angelo C, Ferrulli A, et al. Identification and management of alcohol withdrawal syndrome. Drugs. 2015;75(4):353–365.
- Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379.
- Beurmanjer H, Kamal RM, de Jong CAJ, Dijkstra BAG, Schellekens AFA. Baclofen to prevent relapse in gamma-hydroxybutyrate (GHB)-dependent patients: a multicentre, open-label, non-randomized, controlled trial. CNS Drugs. 2018;32(5):437–442.
- Mannucci C, Pichini S, Spagnolo EV, et al. Sodium oxybate therapy for alcohol withdrawal syndrome and keeping of alcohol abstinence. Curr Drug Metab. 2018;19(13):1056–1064.
- Weaver MF. Prescription sedative misuse and abuse. Yale J Biol Med. 2015;88(3):247–256.
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry. 2005;2(5):25–31.
- Allison C, Pratt JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol Ther. 2003;98(2):171–195.
- Raposo Pereira F, McMaster MTB, Polderman N, de Vries YDAT, van den Brink W, van Wingen GA. Adverse effects of GHB-induced coma on long-term memory and related brain function. Drug Alcohol Depend. 2018;190:29–36.
- Raposo Pereira F, McMaster MTB, Polderman N, de Vries YDAT, van den Brink W, van Wingen GA. Effect of GHB-use and GHB-induced comas on dorsolateral prefrontal cortex functioning in humans. Neuroimage Clin. 2018;20:923–930.
- Rosenberg MH, Deerfield LJ, Baruch EM. Two cases of severe gamma-hydroxybutyrate withdrawal delirium on a psychiatric unit: recommendations for management. Am J Drug Alcohol Abuse. 2003;29(2):487–496.
- Schneir AB, Ly BT, Clark RF. A case of withdrawal from the GHB precursors gamma-butyrolactone and 1,4-butanediol. J Emerg Med. 2001;21(1):31–33.
- Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92(1):89–96.
- Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23.
- Huynh F, Mabasa VH, Ensom MHH. A critical review: does thiopental continuous infusion warrant therapeutic drug monitoring in the critical care population? Ther Drug Monit. 2009;31(2):153–169.
- Nardou R, Yamamoto S, Bhar A, Burnashev N, Ben-Ari Y, Khalilov I. Phenobarbital but Not Diazepam Reduces AMPA/kainate Receptor Mediated Currents and Exerts Opposite Actions on Initial Seizures in the Neonatal Rat Hippocampus. Front Cell Neurosci. 2011;5:16.
- Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management - a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458–467.
- Martin K, Katz A. The role of barbiturates for alcohol withdrawal syndrome. Psychosomatics. 2016;57(4):341–347.