Abstract
This study investigated the effect of consumption of miso soup containing natto (natto miso soup) over 14 days on the bacterial flora and metabolic activity of the faeces of healthy volunteers (age range 21–59 years). No marked change in the number of total bacteria was observed throughout the experimental period. During intake, the numbers of Bacillus and Bifidobacterium were increased (p<0.05), whereas numbers of Enterobacteriaceae were decreased (p<0.05) and numbers of lecithinase-positive clostridia (including Clostridium perfringens) tended to decrease. Faecal acetic and propionic acids were increased (p<0.05) while faecal indole, p-cresol, ammonia and sulfide were lowered (p<0.05) during the intake period. Faecal moisture was increased and faecal pH was decreased during the intake period, although they were not significantly different. These results indicate that consumption of natto miso soup improved the intestinal environment of the volunteers.
Introduction
Natto is an original and traditional Japanese food, and consists of soybean products fermented by Bacillus subtilis (‘Bacillus natto’). Various nutrients are present in the soybean, such as saponins, proteins, oligosaccharides including raffinose and stachyose, vitamin B1, vitamin E, vitamin K2 and dietary fibres. Natto also contains nattokinase. It has been reported that oral administration of this enzyme produces a mild and frequent enhancement of the fibrinolytic activity in the plasma and the production of tissue plasminogen activator Citation[1]. Thus, various beneficial effects of natto as a functional food are known and many medical benefits are reported. A previous study Citation[2] has indicated that natto consumption contributes to improvement of both the composition and the metabolites of the intestinal flora, and the odour of the faeces was slightly reduced during the consumption. Natto is mostly prepared raw and is usually eaten with rice, but there are various ways of cooking natto, and many dishes can be prepared. One of them is miso soup containing natto (natto miso soup). Miso soup is also a traditional Japanese food, and soybean is a main ingredient. Miso is made from soybean paste fermented with yeast, mould and bacteria and then combined with salt and water. Natto miso soup is a popular traditional dish in Japan (natto-jiru, a special miso soup containing diced natto, several kinds of vegetables, etc.). Epidemiological studies show that a high level of isoflavonoids, particularly in soybean products, was associated with a low risk of colon cancer Citation[3] and consumption of miso soup was also associated with a reduction of gastric cancer mortality Citation[4]. It has been reported that soybeans contain a number of anticarcinogens Citation[5]. Colon cancer was rare in Japan; however, it has been increasing recently among Japanese people, and the increase is due to the growing consumption of fat in the modern Japanese diet Citation[6]. A traditional Japanese diet not only contains little animal fat but also employs many soy foods, such as miso, natto, shouyu (soy sauce) and tofu (soy curd). There are no reports of investigations into the effects of intake of miso on faecal metabolic activities and the composition of faecal microflora, although the influence of intake of natto on the faecal metabolic activity and composition of faecal microflora has been investigated Citation[2].
In the present study, the effect of natto miso soup, which is a traditional Japanese food, on the bacterial flora and metabolic activity of human faeces was investigated.
Materials and methods
Preparation of natto miso soup
The natto miso soup was made as follows: 50 g of commercially available natto was added to 200 ml of miso soup and boiled for 1 min. Each volunteer consumed the natto miso soup every day during the intake period. The nutritional composition of 50 g of natto is: energy 100.0 kcal, protein 8.3 g, fat 5.0 g, carbohydrate 6.1 g and natrium 1.0 mg. The viable count of Bacillus in the natto used was 9×109/g.
Subjects and diet
The subjects were five male and three female healthy volunteers (age range 21–59 years). The experimental design is shown in . The volunteers consumed a normal free-choice diet for 1 week before the natto miso soup intake period, during which natto miso soup was consumed once a day in addition to the normal diet for two consecutive weeks. The normal free-choice diet was then resumed for 1 further week. None of the subjects received antibiotic treatment, other therapies or foods with an abundant viable culture for 1 month before or during the experiment.
Table I. Experimental design.
Collection of specimens
Faecal samples collected from each subject were immediately refrigerated and the faecal flora, pH and moisture content were analysed within 3 h. The remainder of the samples were frozen at −80°C for later analysis of bacterial metabolites.
The faecal pH, moisture, ammonia content, sulfide content, putrefactive products, short-chain fatty acids (SCFAs) and faecal flora were measured in eight subjects on day 0 before the intake period, days 7 and 14 during the intake period and day 7 after the intake period. This work was carried out in accordance with the Helsinki Declaration as updated in Tokyo, 1975.
Analysis of specimens
The methods of Mitsuoka et al. Citation[7], Citation[8] and Hara et al. Citation[9] were used for faecal microbial analysis. After thorough mixing, a series of 10-fold dilutions (10−1–10−8) was made in anaerobic diluents Citation[7]. From appropriate diluents, 0.05 ml aliquots were spread onto three non-selective agars, i.e. modified Eggerth-Gagnon (EG) agar for anaerobes Citation[7], glucose liver blood (BL) agar for anaerobes Citation[7], trypticase soy blood (TS) agar (BBL Mocrobiology System, Cockeysville, MD, USA) for aerobes Citation[8], and onto 11 selective agars, i.e. bifidobacteria selective (BS) agar for bifidobacteria Citation[7], eubacteria selective (ES) agar for eubacteria Citation[8], neomycin-brilliant green-taurocholate-blood (NBGT) agar for bacteroides Citation[7], neomycin-Nagler (NN) agar for lecithinase-positive clostridia Citation[7], modified veillonella selective (VS) agar for veillonellae and megasphaerae Citation[7], modified lactobacilli selective (LBS) agar for lactobacilli Citation[7], triphenyltetrazolium chloride-acridine orange-thallous sulfate-aesculin-crystal violet (TATAC) agar for enterococci and streptococci Citation[7], phenylethyl alcohol egg-yolk suspension (PEES) agar for staphylococci and micrococci Citation[7], potato dextrose (P) agar (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) for yeasts and molds Citation[7], deoxycholate hydrogen sulfide lactose (DHL) agar (Nissui) for enterobacteria Citation[7] and NAC agar (Nissui) for Pseudomonas aeruginosa. Eight agars (EG, BL, NBGT, BS, ES, NN, VS and LBS) were incubated at 37°C for 3 days in an anaerobic steel wool jar filled with an atmosphere of oxygen-free CO2. In addition, diluents (10−1, 10−3, 10−5) of the faecal specimens were heated at 80°C for 10 min to select for clostridial spores and a portion of 0.05 ml of each dilution was inoculated onto CW agar (Nissui) containing egg-yolk emulsion, and was incubated at 37°C for 3 days anaerobically using an AnaeroPack–Kenki (Mitsubishi Gas, Tokyo, Japan). Four agars (TATAC, PEES, NAC and P) were incubated aerobically at 37°C for 48 h, and TS and DHL agars were incubated at 37°C for 24 h. After incubation, each plate was examined for bacterial colonies. The identification of bacterial groups, yeasts and moulds was performed with Gram reaction, colonial and cellular morphologies, spore formation, aerobic growth and selected biochemical characteristics. The results were expressed as the log10 of the number of bacteria (CFU) per gram wet weight of faecal material.
Faecal content of putrefactive products was analysed as described by Yoshihara Citation[10]. Faecal content of SCFAs (succinic, lactic, formic, acetic, propionic, iso-butyric, butyric, iso-varelic and varelic) were determined by the high-performance liquid chromatography organic acid analysis system (HPLCOA, Shimazu Co. Ltd, Tokyo, Japan) using the method of Hara et al. Citation[9]. Faecal moisture was measured using approximately 1 g of sample weighed before and after drying in a vacuum oven at 105°C by an infrared moisture gauge, FD-240 type (Ketto Science Lab., Tokyo, Japan). Faecal pH values were determined with a pH meter, model D-25 (Horiba Ltd, Kyoto, Japan). Faecal concentrations of ammonia and sulfide were measured by an ion meter, IM-55G (DKK, TOA Corporation, Tokyo, Japan) with an ammonia ion selective electrode, AE-2041 type (DKK, TOA) and with a sulfide electrode, S-2021 type (DKK, TOA) by the methods of Terada et al. Citation[11].
Statistical analysis of data
The paired t test was used for analysis of pH value, moisture content, metabolic products and bacterial counts. Statistical differences were calculated as compared with the value before the intake of natto miso soup and p values <0.05 were considered to be statistically significant.
Results
Faecal microbiota analysis
All subjects remained healthy throughout the experimental period. The results of the faecal bacteria tests are shown in . No marked change in the number of total bacteria (total of anaerobes and aerobes) was observed throughout the experimental period. The numbers of bacilli and bifidobacteria were significantly increased during the intake period, whereas numbers of lecithinase-positive clostridia (including Clostridium perfringens) tended to decrease, and numbers of Enterobacteriaceae were significantly decreased during the intake period.
Table II. Effect of natto miso soup intake on faecal flora of eight human volunteersa,b.
Faecal SCFAs
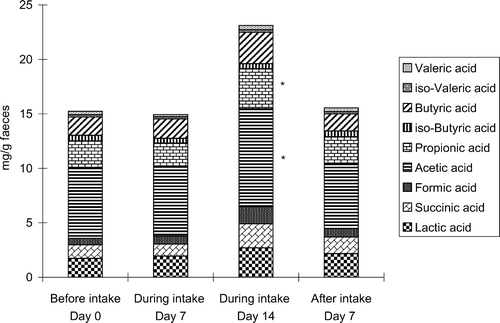
Faecal SCFAs were increased with natto miso soup consumption, this increase being especially noted on day 14 of the intake period. Acetic acid and propionic acid were increased significantly on day 14 of the intake period. No significant changes were shown in other faecal SCFAs during this period. Succinic acid and butyric acid were not influenced ().
Faecal putrefactive products, water content and pH value
Changes in the concentrations of faecal putrefactive products, pH, moisture, ammonia and sulfide are shown in and . Faecal putrefactive products were decreased with natto miso soup consumption, this decrease was especially noted on day 14 of the intake period. Faecal putrefactive products such as indole and p-cresol decreased significantly on day 14 of the intake period (). Furthermore, faecal content of ammonia and sulfide was significantly reduced during the natto miso soup consumption (). Faecal moisture was increased and faecal pH was decreased during the intake period, although they were not significantly different.
Figure 2. Change in the concentrations of faecal metabolites during intake of natto miso soup. Graphs show the mean for eight human volunteers (n=8). *Significantly different from the values before intake (p<0.05).
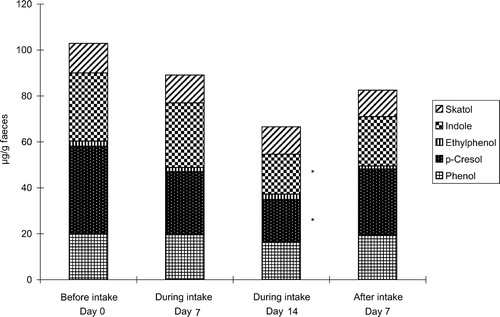
Table III. Effect of natto miso soup intake on faecal pH, concentrations of ammonia and sulfide, and water content of eight human volunteers.
Discussion
It is known that intestinal bacteria play an important role in the host's health Citation[12], Citation[13]. It has been observed that an increase of Bifidobacterium in the intestinal flora brings about beneficial effects for the host Citation[12], Citation[13]. In a previous study Citation[2], it was shown that the consumption of raw natto led to an improvement in the intestinal environment; for example, a decrease in the content of ammonia, sulfide, pH, putrefactive products and the counts of clostridia and Enterobacteriaceae, while an increase in the count of bifidobacteria, lactobacilli, faecal moisture and faecal SCFAs was observed. Similar beneficial effects were found with the intake of natto miso soup. In the present study, although no marked change in the number of total bacteria was observed throughout the experimental period, the numbers of Bacillus and Bifidobacterium were increased and numbers of Enterobacteriaceae were decreased and lecithinase-positive clostridia including Clostridium perfringens tended to decrease during intake. Futhermore, faecal acetic and propionic acids were increased, while faecal indole, p-cresol, ammonia and sulfide were lowered during the intake period. It has been reported that phenolic compounds produced by intestinal bacteria – mainly p-cresol with some phenol and 4-ethylphenol – possess tumour-promoting activity, and high concentrations of ammonia may be associated with certain toxic events in vivo Citation[14]. Concentration of faecal putrefactive products was also decreased in humans fed lactosucrose (4G-β-D-galactosylsucrose) Citation[9], chitosan Citation[11] and yoghurt Citation[15]. Faecal moisture was increased and faecal pH was decreased during the intake period, although they were not significantly different. These results indicate that consumption of natto miso soup improved the intestinal environment of the volunteers, as has been noted in humans fed raw natto Citation[2].
It is supposed that the decrease in the numbers of Enterobacteriaceae and clostridia is related to a decrease in faecal ammonia, sulfide, indole and p-cresol. Moreover, the increase of bifidobacteria may bring about a lowering of faecal pH and an increase of faecal acetic acid.
It is known that the spores of Bacillus subtilis are resistant to heat. During the cooking of natto miso soup in this study, bacterial viable counts in natto were not affected by heating. Live bacteria are important as probiotics Citation[12]. However, it is possible that the beneficial enzyme found in natto, nattokinase, is destroyed by boiling.
It has been reported that natto has various beneficial effects. In terms of bacteriology, ‘B. natto’ (B. subtilis) has antibacterial activity against Escherichia coli O157 Citation[16]. Growth of E. coli O157 on culture with ‘B. natto’ (B. subtilis) was suppressed Citation[16]. Watanabe et al. Citation[17] showed that natto consumption enhances the growth of Bacillus, Streptococcus and Lactobacillus, and reduces E. coli in rat caeca. It has been reported that raffinose and soybean oligosaccharides selectively enhance the growth of Bifidobacterium species other than Bifidobacterium bifidum in vitro Citation[9]. It has also been reported that the administration of raffinose Citation[18] and soybean oligosaccharides Citation[19] brings about an increase of bifidobacteria in human faeces. Enterobacteriaceae were decreased and bifidobacteria were increased by the intake of natto miso soup in this study. As the main ingredient of miso is soybean, miso may have beneficial effects. It has been reported that miso could act as a chemopreventive agent for colon carcinogenesis Citation[20], Citation[21]. Miso prevented N-nitroso-N-methylurea-induced mammary cancer in rats Citation[22]. It has been shown that soybean curd refuse Citation[23] and soy protein Citation[24] protected against azoxymethane-induced colon cancer in animal models. Furthermore, the inhibitory effects of soy protein fermented with lactic acid bacteria and yeasts on 1,2-dimethylhydrazine-induced colon cancer and aberrant crypt foci in mice have been reported Citation[25]. In epidemiological studies, high levels of isoflavonoids occurring particularly in soybean products were associated with a low risk of colon cancer Citation[3]. Foods made from soybeans other than traditional foods may also indicate beneficial effects such as improvement of the composition of faecal bacterial flora. Non-Japanese residents generally consume less natto than other people in Japan. Moreover, there are some people who cannot eat natto in Japan. Natto's fermented odour and sticky consistency apparently detract from its popularity. Recently, a new type of natto with very little odour has been developed.
In the present study, it is suggested that consumption of natto miso soup, which is one of the traditional Japanese foods, improved the intestinal environment of the volunteers, and inhibited the metabolic activity of the intestinal flora, resulting in reduction of the effects of aging and causation of lifestyle-related diseases. These beneficial effects were more remarkable on day 14 of the intake period than on day 7 of the intake period. Further investigations are necessary to determine the mechanism as to how natto, the B. subtilis strain contained in natto, and miso work to enhance the growth of beneficial bacteria in the intestine using in vitro experiments, a large number of human volunteers, and molecular techniques for bacterial analysis of faecal microflora at species level. The influence of foods other than natto and miso made from soybeans on human faecal properties is also of interest.
References
- Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 1990; 84: 139–43
- Terada A, Yamamoto M, Yoshimura E. Effect of the fermented soybean product “Natto” on the composition and metabolic activity of the human fecal flora. Jpn J Food Microbiol 1999; 16: 221–30
- Adlercreutz H. Epidemiology of phytoestrogens. Baillière's Clin Endocrinol Metab 1998; 12: 605–23
- Hirayama T. Relationship of soybean paste soup intake of gastric cancer risk. Nutr Cancer 1982; 3: 223–33
- Messina MJ, Persky V, Setchell KDR, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 1994; 21: 113–31
- Tsuji K, Harashima E, Nakagawa Y, Urata G, Shirataka M. Time-lag effect of dietary fiber and fat intake ratio on Japanese colon cancer mortality. Biomed Environ Sci 1996; 9: 223–8
- Mitsuoka T, Sega T, Yamamoto S. Eine verbesserte Methodik der qualitativen und quantitativen Analyse der Darmflora von Menschen und Tieren. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg I Abt Orig 1965; A195: 455–69
- Mitsuoka T, Ohno K, Benno Y, Suzuki K, Namba K. Die Faekalflora bei Menschen. IV. Mitteilung: Vergleich des neu entwickelten Verfahrens mit dem bisherigen üblichen Verfahren zur Darmfloraanalyse. Zentralbl Bakteriol Hyg I Abt Orig 1976; A234: 219–33
- Hara H, Li ST, Sasaki M, Maruyama T, Terada A, Ogata Y, et al. Effective dose of lactosucrose on fecal flora and fecal metabolites of humans. Bifidobacteria Microflora 1994; 13: 51–63
- Yoshihara I. Isothermal gas chromatographic analysis of putrefactive products in gastrointestinal contents and urine using the same dual column system. Agric Biol Chem 1981; 45: 1873–5
- Terada A, Hara H, Sato D, Higashi T, Nakayama S, Tsuji K, et al. Effect of dietary chitosan on faecal microbiota and faecal metabolites of humans. Microb Ecol Health Dis 1995; 8: 15–21
- Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989; 66: 365–78
- Mitsuoka T. Significance of dietary modulation of intestinal flora and intestinal environment. Biosci Microflora 2000; 19: 15–25
- Rowland IR, Mallett AK, Wise A. The effect of diet on mammalian gut flora and its metabolic activities. CRC Crit Rev Toxicol 1985; 16: 31–103
- Morisaki N, Saitoh Y, Terada A, Hara H, Osabe K, Muraishi K, et al Effects of yoghurt-administration on fecal flora and putrefactive metabolites of senile volunteers. Bifidus 1993;6:161–8 (in Japanese with English summary).
- Sumi H. Antibacterial activity of Bacillus natto. Growth inhibition against Escherichia coli O-157. Bio Ind 1997;14:47–50 (in Japanese).
- Watanabe T, Tsuchihashi N, Kanno A, Takai Y. Effects of natto and steamed soybeans on growth and cecal bacterial flora of rats. J Jpn Soc Nutr Food Sci 1995;48:283–9 (in Japanese with English summary).
- Benno Y, Endo K, Shiragami N, Sayama K, Mitsuoka T. Effects of raffinose intake on human fecal microflora. Bifidobacteria Microflora 1987; 6: 59–63
- Hayakawa K, Mizutani J, Wada K, Masai T, Yoshihara I, Mitsuoka T. Effects of soybean oligosaccharides on human faecal flora. Microb Ecol Health Dis 1990; 3: 293–303
- Masaoka Y, Watanabe H, Katoh O, Ito A, Dohi K. Effects of miso and NaCl on the development of colonic aberrant cryptofoci induced by azoxymethane in F344 rats. Nutr Cancer 1998; 32: 25–8
- Ohara M, Lu H, Shiraki K, Ishimura Y, Uesaki T, Katoh O, et al. Prevention by long-term fermented miso of induction of colonic aberrant crypt foci by azoxymethane in F344 rats. Oncol Rep 2002; 9: 69–73
- Gotoh T, Yamada K, Hong Y, Ito A, Kataoka T, Dohi K. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn J Cancer Res 1998; 89: 137–42
- Azuma N, Suda H, Iwasaki H, Kanamoto R, Iwakmi K. Soybean curd refuse alleviates experimental tumorigenesis in rat colon. Biosci Biotechnol Biochem 1999; 63: 2256–8
- Hakkak R, Korourian S, Ronis MJJ, Johnston JM, Badger TM. Soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats. Cancer Lett 2001; 166: 27–32
- Nakayama M, Kitajyo T, Kasuga H, Kanabayashi T, Nakamura Y. Inhibitory effects of the extract of soy protein fermented with lactic acid bacteria and yeasts on 1,2-dimethylhydrazine-induced colon cancer and aberrant crypt foci of mice. Biosci Microflora 2002; 21: 163–70