Abstract
The Mediterranean diet, rich in fruits, vegetables, and grains as well as olive oil and olives, has been associated with a lower risk of coronary heart disease and cancer. The beneficial role of olive oil has been related to its fatty acid composition and the presence of phenolic compounds. The present research aimed to study the biological activity of some olive oil iridoids, such as hydroxytyrosol and tyrosol, as microbial inhibitors and their in vitro effects on modulation of human intestinal microflora. Among the tested strains, Bifidobacterium longum, Lactobacillus salivarius, and Bacteroides vulgatus showed the highest sensitivity to the tested compounds; however, the inhibitory effect of iridoids seemed to be more effective when a mix of olive oil iridoids, instead of pure hydroxytyrosol, was used. The fermentation experiments showed that olive oil iridoids modulate the intestinal microflora, leading to a higher production of total short chain fatty acids and in particular of butyrate. These are important conditions well known to be associated with a protective effect against colon cancer development.
Introduction
Olive oil is one of the principal sources of fat in the Mediterranean diet, which is normally associated with a lower incidence of important pathologies, e.g. coronary heart disease and breast and colon cancer, all generally associated with a high consumption of animal fats and proteins.
Virgin olive oil contains an abundance of phenolic compounds, including simple phenols (hydroxytyrosol, tyrosol), aldehydic secoiridoids (oleuropein), flavonoids, and lignans – responsible for its peculiar pungent taste and its stability – that have been demonstrated, both in vitro and in vivo, to have antioxidant properties Citation[1–4].
Despite their proven health-promoting properties, the extent of degradation and absorption of phenol compounds within the intestinal tract, a critical point in the understanding of further systemic effects, is almost unknown Citation[5–7], especially in humans Citation[8], Citation[9].
A study by Visioli et al. Citation[8] showed that olive oil phenolics, namely tyrosol and hydroxytyrosol, are dose-dependently absorbed in humans after ingestion and that they are excreted in urine during the next 24 h, as glucuronide conjugates in a percentage that is about 20–60% of the ingested dose. However, Visioli et al. Citation[8] calculated the recovery of tyrosol and hydroxytyrosol in urine as the percentage of tyrosol and hydroxytyrosol intake but did not take into account the possible hydrolysis of oleuropein aglycone in the body. Vissers et al. Citation[7] estimated that the apparent absorption of olive oil phenols was at least 55–66% of the ingested dose, and 5–16 mol/100 mol were re-excreted as tyrosol and hydroxytyrosol in urine, and it is a significantly lower amount than was reported by Visioli et al. Citation[8]. The same authors found similar or lower levels of tyrosol and hydroxytyrosol in urine of subjects with a colon than in subjects without a colon, suggesting that olive oil phenols are absorbed mainly in the small intestine, rather than in the colon Citation[7]. It has been demonstrated that a fraction of dietary olive oil phenols can escape from digestion in the upper gastrointestinal tract, reach the colon and be degraded by colonic microflora Citation[5], Citation[6].
There is also evidence for the antimicrobial activity of olive oil phenols against a variety of microorganisms, also pathogens for humans or plants Citation[10–12]. Antimicrobial activity potentially alters the composition of the gut microflora and its metabolic activities Citation[13]. The biological significance of changes in the gut microflora might be expressed either by suppression of harmful microorganisms or stimulation of organisms such as lactic acid-producing bacteria (LAB), which contribute in a positive way to the nutrition and health of human beings – e.g. the prevention of genotoxicity in colon cells Citation[14], Citation[15]. Short chain fatty acid (SCFA) concentration could be considered as an indicator of microbiota alterations induced by olive oil phenols. Primarily acetic, propionic and butyric acid may directly influence the colonic mucosa, in particular butyric acid, resulting in changes in cell proliferation rates, apoptosis, and differentiation Citation[16]. In addition, SCFA production can lead to a decrease in pH values, resulting in an inhibition of enzymatic transformation of primary into secondary bile acids (BAs), which are thought to possess tumor-promoting activity Citation[17].
The aim of this work was to test several iridoids from virgin olive oil, such as hydroxytyrosol and tyrosol, as microbial inhibitors and also to study their effects on the modulation of human colonic microflora and its metabolic activities, relating to human health, utilizing a semi-continuous culture system of human fecal bacteria.
Materials and methods
Antimicrobial properties of iridoid compounds
A raw olive oil phenolic extract () and pure hydroxytyrosol, were tested. Hydroxytyrosol was synthesized using the procedure of Montedoro et al. Citation[18] and stored at −25°C under nitrogen. Stock solutions (20 mg ml−1) in dimethylsulfoxide (DMSO), were diluted 1:1 (v/v) in sterile dilution blanks Citation[19]. The procedures for extraction, separation, and quantitative evaluation of phenolic compounds from olive oil were reported previously Citation[18].
Table I. Olive oils phenolic composition and raw olive oil phenolic extract composition characterized by HPLC analysis.
The sensitivity to iridoids of several human fecal bacterial strains was tested. The choice of bacteria was based on the most representative species reported by Moore and Moore Citation[20] and Rowland Citation[17] from human fecal samples. Six bacterial strains (Bacteroides vulgatus, Bifidobacterium adolescentis, Clostridium clostridiiforme, Clostridium perfringens, Enterococcus faecalis, Lactobacillus salivarius) identified by conventional procedures (confirmed by the API system, bioMérieux, Marcy-l'Etoile, France), were used.
Each bacterial strain was inoculated in 3 ml of tryptone soya broth (TSB), and incubated overnight at 37°C. Before use, the overnight cultures were diluted 1:10 (v/v) with nutrient broth (NB). Serial dilution plate counts in nutrient agar (NA) were performed for each culture to ensure that the densities of diluted cultures were all within the range of 107−108 CFU ml−1.
All culture media utilized were obtained from Oxoid Unipath Ltd (Basingstoke, UK).
Susceptibility to iridoids
The substances were tested by the broth-microdilution method Citation[21] to determine minimal inhibitory concentration (MIC) values, using brain heart infusion broth (BHI). Final concentrations from 0.005 to 5000 µg ml−1 were tested for each substance, using a standard 5×105 CFU ml−1 inoculum. The tubes were incubated at 37°C for 24 h. MIC was defined as the lowest concentration of substance that inhibited visible bacterial growth after incubation for 24 h. All determinations were performed in triplicate.
Semi-continuous fermentation of a human complex fecal microflora
The interactions between human gut microflora and olive oil iridoids were evaluated, using a semi-continuous culture system (bioreactor), optimized in our laboratory Citation[22]. Anaerobic semi-continuous culture fermentation was conducted as reported by Zampa et al. Citation[22]. The medium used in this fermenter system was complex and designed to mimic either ileostomy fluid or ileal chyme, and was based on that formulated by Duncan and Henderson Citation[23] using additional trace elements and vitamin solution, largely derived from Balch et al. Citation[24] and Rumney et al. Citation[25]. In addition, a source of nitrogen (urea) is supplied and fecal fluid is also included to supply the bacteria with any other unknown components that may not otherwise be provided. The carbohydrate supplied (10% w/v) was cornstarch (Laboratori Dottori Piccioni, Gessate, MI, Italy) Citation[26].
Two types of olive oil were tested: one was designated as ‘impoverished’ (low level of phenols) and one as ‘original’ (). The fermentation process was designed to mimic first, the olive oil transit in the stomach, where oil is challenged by acids, then in the colon, where bacteria can degrade it.
Olive oil samples
The olive oil samples, the phenolic extract, and the pure hydroxytyrosol were characterized and provided by Prof. Servili's team, University of Perugia, Italy. The extra virgin olive oil samples were from olives of the Moraiolo cultivar. One part of the sample contained a reduced quantity of iridoids, impoverished, the other one was unmodified, original. The olive oil's phenolic content was characterized by HPLC analysis () Citation[18].
Impoverished olive oil
The procedure for the selective removal of iridoids was optimized after some evaluations regarding the types of solvent and the number of extractions to perform. The obtained results, evaluating both efficiency and selectivity of extraction, were used to define the following procedure. Distilled water (100 ml) was added to the virgin olive oil (100 g). The mix was stirred by Ultraturrax at 15 000 rpm for 60 s and then centrifuged at 3500 rpm. Extraction was repeated four times. The remaining oil was then filtered by ammonium sulfate to remove the residual water, after separation by centrifugation.
Olive oil addition
The total amount of daily addition of olive oil in the fermentation broth was 20 g/day. Addition of olive oil was programmed to simulate the two main, daily meals of a typical Mediterranean diet, immediately after the morning feeding and the evening one, at a flow rate of 1 ml min−1 (at this flow rate the addition of oil does not disturb the system). The concentration of oil in the fermenter under constant conditions was 0.03 g/ml. The first addition of oil was 8 h after bacterial inoculation.
Before addition to the fermentation broth, the oil was treated to mimic acid challenge in the stomach by addition of HCl (stock solution of 36%) to pH 2 followed by incubation at 37°C for 35 min.
Experimental design
Three experiments were performed: (i) a fermentation with daily additions of unmodified olive oil (original) to the broth, (ii) a fermentation with daily additions of impoverished olive oil (impoverished) to the broth, and (iii) a fermentation with no addition of olive oil (control). Each fermentation experiment was conducted in duplicate.
The sampling was carried out twice daily, just before fermenter feeding, at 8 h intervals. Each sample was used for bacteriological analysis, and for SCFA analysis.
Bacteriological analysis
A total viable bacteria count (aerobes and anaerobes) was routinely estimated by spreading 10-fold dilutions of the original sample (10−1−10−9) onto nonselective medium – Columbia agar + 5% sheep blood (bioMérieux). The same dilutions were spread onto selective media: Rogosa agar (Oxoid) for Lactobacillus counts, Beerens agar Citation[27] for Bifidobacterium counts, MacConkey agar (bioMérieux) for enterobacteria counts and Clostrisel agar (BBL Microbiology Systems, Cockeysville, MD, USA) for Clostridium counts. The bacteria were identified by various study parameters, such as Gram's stain, colony and cell morphology, and biochemical tests using different enzymatic kits from the API strip system (bioMérieux). The bacteriological analysis was performed at different times of fermentation: immediately after inoculation (0 h), at 24 h, at 144 h, and at 288 h after the inoculation. The agar plates, all spread in duplicate, were incubated in aerobic (aerobic total count) and anaerobic conditions (all the other counts) at 37°C for 3 days.
SCFA analysis
The production of SCFAs, their types, and proportions were studied. To detect SCFAs, the daily samples of the culture contents were analyzed by gas chromatography, as described by Cresci et al. Citation[28]. Each sample was analysed in duplicate.
Results
Antimicrobial properties of iridoid compounds
shows the results of MICs carried out with hydroxytyrosol and with raw phenolic extract. The tested strains of Lactobacillus salivarius, Bifidobacterium adolescentis, and Bacteroides vulgatus, were highly sensitive to both hydroxytyrosol (MIC value between 0.05 µg ml−1 and 0.5 µg ml−1) and raw phenolic extract (MIC value 0.5 µg ml−1), while the strain of Clostridium perfringens showed moderate sensitivity only to phenolic extract (50 µg ml−1). The tested strains of Clostridium clostridiiforme and Enterococcus faecalis did not demonstrate any susceptibility to hydroxytyrosol and raw phenolic extract (MIC ≥ 500 µg ml−1).
Table II. Minimal inhibitory concentration (MIC) of hydroxytyrosol and raw phenolic extract from extra virgin olive oil, cultivar Moraiolo, against tested bacterial strains.
Semi-continuous fermentation of a human complex fecal microflora
presents the results obtained from bacterial counts (log CFU ml−1) at 24, 144, and 288 h of fermentation. The data indicate that the addition of olive oil to the fermentation broth led to a modulation of the intestinal microflora, promoting the growth of total anaerobes, enterobacteria, bifidobacteria, and clostridia, whose counts, both for the original and for the impoverished olive oil, were always higher than for the control. On the contrary, lactobacilli were inhibited by addition of olive oil (both impoverished and original) to the fermentation broth, always giving lower counts with respect to the control. Significantly higher counts (p<0.05), with respect to the control, were obtained for enterobacteria and clostridia at 24, 144, and 288 h of fermentation, and for bifidobacteria at 144 and 288 h (for both the original and the impoverished olive oil).
Table III. Bacterial counts at 0, 24, 144, and 288 h of fermentation with daily addition of unmodified olive oil (original), with daily addition of the same olive oil impoverished of iridoids (impoverished), and with no addition of olive oil (control).
Significantly higher counts (p<0.05) were obtained for clostridia, at 144 and 288 h, during the fermentation with addition of original olive oil with respect to the fermentation with impoverished olive oil. In this case original olive oil iridoids also promoted the growth of enterobacteria and inhibited the growth of bifidobacteria. Furthermore, in vitro growth of lactobacilli did not seem to be affected by the presence of isolated iridoids in the original olive oil.
compares SCFAs (acetic, propionic, butyric acid, and total SCFA, respectively) produced at different times during the fermentation of a fecal microflora which has had daily additions of olive oil to the fermentation broth – original or impoverished. The concentrations of iso-butyrate, valerate, and iso-valerate were very low (<2 µmol ml−1) and were therefore not shown.
Figure 1. Concentration of acetic acid (A), propionic acid (B), butyric acid (C), and total SCFA (D) at 24, 72, 144, 192, and 288 h of fermentation with daily addition of unmodified extra virgin olive oil (original), with daily addition of the same olive oil impoverished of iridoids (impoverished), and with no addition of olive oil (control). (a) Significantly different from the impoverished olive oil (p<0.05, t test); (b) significantly different from the control (p<0.05, t test).
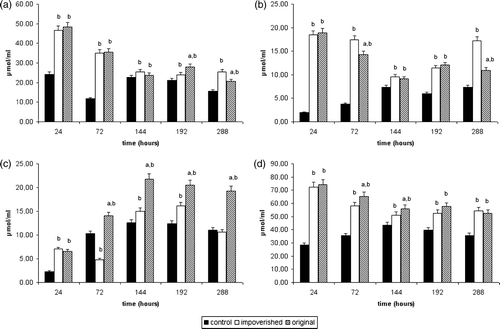
When olive oil (original or impoverished) was added to the fermentation broth, total SCFA production was always significantly higher (mean value about 54 µmol ml−1 for the original olive oil, and 52 µmol ml−1 for the impoverished olive oil) with respect to the control (mean value about 38 µmol ml−1) (D). Acetic acid and propionic acid production, for both the original and the impoverished olive oil (A and B), were also significantly higher than the control (p<0.05).
An increase of total SCFA production was induced by original olive oil iridoids (D) as compared to the impoverished olive oil fermentation. It is important to note a significantly higher production of butyric acid (p<0.05) shown during fermentation with original olive oil (C).
Discussion
Interestingly, as would be expected from previous studies Citation[29], Citation[30] our current findings show the high antimicrobial activity of olive oil catechol derivatives (hydroxytyrosol, tyrosol, and oleuropein), against some gram-positive and one gram-negative bacteria. With regard to the mechanism of action of the antimicrobial activity of olive oil iridoids, it is known that they penetrate the structurally different cell membranes of both gram-negative and gram-positive bacteria. Phenolic compounds are known to cause destruction of cell peptidoglycans or damage the cell membrane, or both Citation[29]. However, although these biophenols (hydroxytyrosol and oleuropein) have an o-diphenolic system (responsible for the antibacterial activity of olive polyphenols) on their backbone structure Citation[11], we reported a different inhibitory effect between hydroxytyrosol and raw phenolic extract against the tested strains, in particular Cl. clostridiiforme and Ent. faecalis (normally considered not beneficial for host health). One hypothesis is that a synergistic action of raw phenolic iridoids extract takes place against these two strains. On the other hand, the stronger inhibitory effect of raw phenolic extract against these two species could be due to a different phenolic compound, oleuropein (3,4-DHPEA-EDA) or another catechol derivative, present in the extract itself.
The antimicrobial activity of iridoids potentially alters the composition of the microflora and its metabolic activities Citation[13]. The addition of olive oil iridoids, promoting the growth of bifidobacteria by two or three orders of magnitude, revealed a property of virgin olive oil as bifidogenic substance. Several important health benefits may be associated with bifidogenesis Citation[31]. At the same time as this bifidobacterial predominance, a stable number of Lactobacillus was present that did not seem to be affected by the olive oil iridoids. However, olive oil iridoids increased enterobacteria and clostridia by one order of magnitude (log CFU ml−1). The increased number of clostridia was considered to be involved in the high production of butyrate detected in the fermentation system. The significant increase of SCFA production, particularly n-butyrate, induced by the addition of original olive oil to the fermentation broth, compared with the control, is indicative of a further possible benefit of olive oil iridoids to the host.
SCFAs are rapidly and efficiently taken up by the epithelial cells which line the colonic lumen. SCFAs, with their presence, provide a powerful driving force for movement of water out of the colonic lumen and consequently, may constitute an important protection against diarrhea Citation[32]. Butyrate serves as an energy-yielding substrate in the colonocytes and, additionally, affects several cellular functions (proliferation, membrane synthesis, apoptosis, differentiation). Propionate and acetate are released by the basolateral membrane to the portal circulation and may have effects far from their production site Citation[32].
Butyrate is known to play a key role in the energy metabolism of colonic epithelial cells and is thought to be important in the maintenance of colonic health in humans. In particular, its ability to modify nuclear architecture (hyperacetylation of histones), to inhibit growth of human colon cancer cell lines, and to induce death by apoptosis in colon cancer cells, is of great interest Citation[31], Citation[33], Citation[34].
Furthermore, the higher production of total SCFAs, induced in this study by original olive oil iridoids (D) may lead, if found in vivo, to a decrease in pH values, resulting in an inhibition of the enzymatic transformation of primary into secondary bile acids, which are thought to posses tumor-promoting activity Citation[17].
In conclusion, the data indicate that olive oil might be considered a potential source of promising antibacterial agents for treatment of intestinal infections in man and one may speculate that dietary intake of polyphenols contained in olive oil reduces the risk of bacterial infection in the intestinal tract Citation[11]. Additionally this study showed that polyphenols contained in the virgin olive oil modulate human intestinal microflora toward a health-promoting condition for the host. A similar effect has also been found in polyphenol-treated animals Citation[35]. At last the health-promoting condition is confirmed by SCFA composition, which describes the metabolic activity resulting from all the species growing in the fermenter.
This work was supported by CNR AGENZIA 2000 (code: CNRC008A28_003).
References
- Benavente-Garcia O, Castello J, Morente J, Ortuno A, Del Rio JA. Antioxidant activity of phenolics extracted from Olea europea L. leaves. Food Chem 1999; 68: 457–62
- Owen RW, Giocosa A, Hull WE, Haubner R, Wurtele G, Spiegelhander B, et al. Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncology 2000; 1: 107–12
- Manna C, Galletti P, Misto G, Cucciola V, D'Angelo S, Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEMS Lett 2000; 470: 341–4
- Visioli F, Poli A, Galli C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 2002; 22: 65–75
- Bravo L, Abia R, Eastwood MA, Saura-Calixto F. Degradation of polyphenols (catechin and tannic acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output. Br J Nutr 1994; 71: 933–46
- Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 1997; 51: 305–10
- Vissers MN, Zock PL, Roodenburg AJ, Leenen R, Kata MB. Olive oil phenols are absorbed in humans. J Nutr 2002; 132: 409–17
- Visioli F, Galli C, Bornet F, Mattei A, Patelli R, Galli G, et al. Olive oil phenolics are dose-dependently absorbed in humans. FEMS Lett 2000; 468: 159–60
- Caruso D, Visioli F, Patelli R, Galli C, Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001; 50: 1–3
- Capasso R, Evidente A, Schivo L, Orru G, Marcialis MA, Cristinzio G. Antibacterial polyphenols from olive oil mill waste waters. J Appl Bacteriol 1995; 79: 393–8
- Bisognano G, Tomaino A, Lo Cascio R, Crisafi G, Uccella N, Saija A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm Pharmacol 1999; 51: 971–74
- Ciaffardini G, Zullo BA. Microbiological activity in stored olive oil. Int J Food Microbiol 2001; 75: 111–18
- Malkki, Y, Cummings, JH. Dietary fibre and fermentation in the colon. Y Malkki, Cummings, JH, eds. Proceedings of COST Action 92 workshop. Espoo, Finland, 1995.
- Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, et al. Lactobacillus- and Bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer 1996; 26: 365–80
- Fuller R, Gibson GR. Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol Suppl 1997; 222: 28–31
- Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 1991; 70: 443–59
- Rowland IR. Gut microflora and cancer. Gut microflora and health – past, present and future, AR Leeds, IR Rowland. Royal Society of Medicine Press, London 1996
- Montedoro GF, Servili M, Baldioli M, Miniati E. Simple and hydrolysable compounds in virgin olive oil. 1. Their extraction, separation and quantitative and semi-quantitative evaluation by HPLC. J Agric Food Chem 1992; 40: 1571–6
- Holdeman VL, Cato EP, Moore WEC. Anaerobe laboratory manual4th edn. Virginia Polytechnic Institute and State University, Blacksburg, VA 1977
- Moore WEC, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol 1995; 61: 3202–7
- Sahm DF, Washington JA. Susceptibility tests: microdilution and macrodilution broth procedures. Manual of clinical microbiology5th edn, W Balows, J Hauster, KL Herrman, HJ. Shadomy. Am Soc Microbiol, Washington, DC 1991; 1105–16
- Zampa A, Silvi S, Fagiani R, Morozzi G, Orpianesi C, Cresci A. Effects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut microflora grown in an in vitro semi-continuous culture. Anaerobe 2004; 10: 19–26
- Duncan AJ, Henderson C. A study of the fermentation of dietary fibre by human colonic bacteria grown in in vitro semi-continuous culture system. Appl Environ Microbiol 1981; 42: 400–7
- Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS. Methanogens: re-evaluation of unique biological group. Microbiol Rev 1979; 43: 260
- Rumney CJ, Rowland IR. In vivo and in vitro models of the human colonic flora. Crit Rev Food Sci Nutr 1992; 31: 299–331
- Bearne CA, Mallet AK, Rowland IR, Brennan-Craddok WE. Continuous culture of human faecal bacteria as in vitro model for the colonic microflora. Toxicol In Vitro 1990; 4: 522–5
- Silvi S, Rumney CJ, Rowland IR. An assessment of three selective media for bifidobacteria in faeces. J Appl Bacteriol 1996; 81: 561–4
- Cresci A, Orpianesi C, Silvi S, Mastrandrea V, Dolora P. The effect of sucrose or starch-based diet on short-chain fatty acids and faecal microflora in rats. J Appl Microbiol 1999; 86: 245–50
- Tranter M, Tassou C, Nychas GJ. The effect of olive oil phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J Appl Bacteriol 1993; 74: 253–9
- Marsillo V, Lanza B, Pozzi N. Progress in table olive dedittering: degradation in vitro of oleuropein and its derivatives by Lactobacillus plantarum. J Am Oil Chem Soc 1996; 73: 593–7
- Sheppach W, Luehrs H, Menzel T. Beneficial health effects of low-digestible carbohydrate consumption. Br J Nutr 2001; 85: S23–S30
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81: 1031–64
- Sheppach W, Bartram HP, Richter F. Role of short chain fatty acids in the prevention of colorectal cancer. Eur J Cancer 1995; 31A: 1077–80
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002; 217: 133–9
- Dolara P, Luceri C, De Filippo C, Femia AP, Giovannelli L, Caderni G, et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res 2005; 591: 237–46