Abstract
The understanding of the pathogenesis of vaginitis is directly dependent upon the factors which promote the colonization by bacterial genital microflora. Disruption of the bacterial barrier function afforded by the predominant microflora can lead to colonization by pathogenic or opportunistic pathogenic subdominant species. The present study focused on the effects of stress on the bacteria of the vaginal flora. For this purpose two types of psychological stress were employed: acute and subacute mild unpredictable stress. Estimation of the vaginal flora of 22 Wistar rats was performed according to the distinct phases of the estrous cycle: PE (proestrous), E (estrous), ME (metestrous), and DE (diestrous). Bacteria isolated from the vaginal flora were identified following Bergey's Manual criteria. Escherichia coli, Clostridium, and Clostridium perfringens (vegetative and spore forms), showed increased numbers at PE-E after acute and subacute stress. Enterococcus was increased after acute stress, in all stages, while Streptococcus was increased after acute stress only at ME-DE. Staphylococcus aureus was detected at ME-DE only after stress. Staphylococcus epidermidis seemed to be predominant, but not influenced by stress at PE-E. However, it was detected in increased numbers at ME-DE after stress. Fusobacterium was detected at low levels in all phases and remained unaffected after exposure to stress. Both types of stress increased the numbers of Bacteroides at PE-E. Finally, the absence of Lactobacillus was noticed at PE-E 24 h after acute and subacute mild unpredictable stress, while Lactobacillus was decreased at ME-DE 72 h after stress. Hormonal fluctuations during the different phases of the estrous cycle may be associated with alterations in the vaginal flora. The data showed that stress acting on the vaginal flora and its immunity potential may contribute to disturbances of the natural balance in the area by altering the numbers of several bacteria that belong to the vaginal microflora. Continuous research on the factors that regulate the vaginal microflora is considered as essential, to prevent complications in infections that may be caused by indigenous microorganisms.
Introduction
The microbiological flora of the lower female genital tract provides a dynamic, complex example of microbial colonization. When an exogenous bacterial species, with its array of virulence factors, is introduced into the host, disease does not always occur. Conversely, under selected conditions, commensal endogenous bacteria can participate in disease processes Citation[1]. The critical element for the development of a disease is the inhibitory or synergistic inter-relationship among bacteria, pathogenic prerequisites, and other microbes Citation[1], which may be altered under the influence of endogenous or exogenous factors, an event that is potentially responsible for the development of a disease Citation[1–4].
A variety of endogenous factors influence the composition of the vaginal and cervical microflora in the healthy female Citation[5]. The most notable change that takes place in the female genital tract is modification of the vaginal microbial flora in response to hormonal influences exerted upon genital epithelia. Cornification of the vagina, as a result of estrogen secretion, presents a very different tissue substrate to microorganisms in females of reproductive age compared with that in prepubertal and postmenopausal women. The normal vaginal microflora also changes according to the degree of maturation of the vaginal epithelium Citation[6], Citation[7].
The aim of this study was to investigate the effect of stress on bacterial colonization of the lower genital tract of cyclic female rats. For this purpose, two different types of psychological stress were employed, acute stress and subacute mild unpredictable stress.
Materials and methods
Animals
Adult intact female Wistar (Kuo/Ioa/rr) rats (2 months old, bred at Ioannina University animal house) were housed in plastic cages under 12 h light/dark cycle with lights on at 6.00 am. Standard rodent chow and tap water were provided ad libitum. All animal use procedures conformed to international European ethical standards (86/609-EEC) and the French National Committee (dècret 87/848) for the care and use of laboratory animals.
Estrus cycle monitoring
Estrous cycle stage was monitored by analysis of cell types in vaginal lavages. Daily vaginal lavages of cycling female rats were collected in the morning at 9.00–10.00, for at least 20 consecutive days and allowed to dry on microscope slides. Slides were then fixed with methanol (3 min) and stained with Giemsa solution 20% (Merck). Identification of cell types was made microscopically according to published methods Citation[8]. Only rats that could be reliably staged for estrous cycle stage and with normal estrous cycle were used. An abnormal estrous cycle was defined as constant DE and the cycles lasted longer than 5 days. Animals with abnormal estrous cycle were excluded.
Stress paradigm
Acute stress
Before the stress task, sampling from the vaginal-cervical mucosa took place and these samples were used as control samples. Accordingly, in the morning of either proestrous (PE), estrous (E), metestrous (ME) or diestrous (DE), between 09.00 and 10.00, immediately after vaginal lavage for screening of the estrous cycle phase, rats were exposed to electric foot-shock of 1.5 mA for 30 min (one shock per 30 s; total 60 electric foot-shocks). Samples were also collected 24 h and 72 h after stress to assess the bacterial colonization in vaginal-cervical mucosa.
Subacute mild unpredictable stress
Rats were exposed to mild unpredictable stress for five consecutive days as follows. First day: exposed to food deprivation for 24 h; second day: kept in cages on a tilted floor for 24 h; third day: exposed to white noise for 2 h (Lafayette instrument, model 15800); fourth day: exposed to restraint stress for 1.5 h in small animal study units (Physiograph MKIII, Narco Bio System; 5×15 cm); fifth day: exposed to electric foot-shock 1.5 mA, for 20 min (one shock per 30 s; total 40 electric foot-shocks).
Stress tasks took place between 09.00 and 11.00 h, in a place isolated from other environmental stimuli, immediately after the vaginal lavage for the screening of the estrous cycle phase. Before the first stress task in the morning of PE, E, ME, and DE, vaginal-cervical mucosa sampling took place and these samples were used as control samples. In addition, vaginal-cervical mucosa sampling took place 24 h and 72 h after the last stress task (electric foot-shock) to assess the effect of stress on bacterial colonization in the lower genital tract.
Microbiological determinations
Serial dilutions were performed in Ringer's solution followed by spreading on MacConkey agar, Columbia blood agar, Chapman agar, and MRS agar incubated overnight at 37°C. An aliquot of the solutions was heated for 10 min at 75°C, followed by spreading in lactose-sulfite (LS) broth Citation[9].
Numbers of Clostridium perfringens were estimated by performing decimal dilutions in the LS broth incubated overnight in a water-bath at 46°C. Characteristic colonies of Escherichia coli and Enterobacteriaceae were counted and identified using the Api 20E system. Characteristic colonies of Staphylococcus spp. were counted. Catalase and coagulase determination was also performed and finally they were identified by the API Staphylococci system. Characteristic colonies of anaerobes were counted and identified by the API ANA system.
Results
Variation of the vaginal microflora within estrous cycle phases
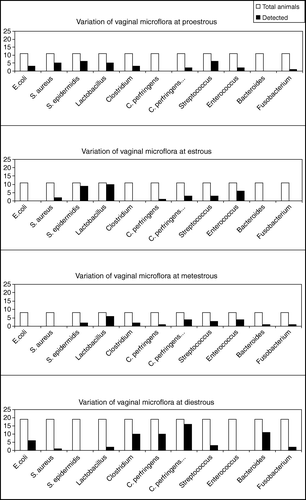
The following findings were observed. (i) There was a tendency for increased presence of E. coli at DE, while it was absent at E and ME (). (ii) Staphylococcus aureus was detected at PE and to a lesser extent at E, but it was undetectable at ME and DE (). (iii) Staphylococcus epidermidis levels were found to be increased at PE and E (p<0.003; ) and were declined at ME, while they were undetectable at DE. (iv) Lactobacillus was detected at high levels mainly at E and ME and to a lesser extent at PE, while it was almost absent at DE (p<0.05). (v) Clostridium spp. were detected at PE, ME and mainly at DE and not at E (). (vi) Clostridium perfringens vegetative and spore forms were elevated at DE and almost absent in all the other phases of the estrous cycle (p<0.05; ). (vii) Streptococcus was present in all phases of the estrous cycle (). (viii) Enterococcus were detected at high levels at E and ME (p<0.01; ), but was absolutely absent at DE. (ix) Bacteroides were present at high levels at DE (p<0.005; ), while they were almost absent in all other phases of the estrous cycle. (x) Fusobacterium was detected at low levels at PE, ME and DE, but it was undetectable at E ().
Figure 1. Fluctuations in the vaginal microflora in the four distinct phases of the rat estrous cycle. White columns show the total population of the tested animals at the specific phase of the estrous cycle. The black columns correspond to the animals where the organism was detected at the specific phase of the estrous cycle.
Assessment of vaginal microflora variation after exposure to acute stress
Proestrous (PE)
No significant changes were observed 24 h after exposure to acute stress in vaginal flora at PE. However, there was a tendency towards increased presence of vegetative forms of C. perfringens and, in particular, of the spore forms (9 of 11 after exposure to acute stress, while before stress it was 1 of 6). Other Clostridium spp. showed increased numbers after acute stress. There was a tendency for increased Bacteroides at PE, 24 h after acute stress.
Estrous (E)
There was a strong tendency for increased spore forms of C. perfringens at E, 24 h after acute stress.
Metestrous (ME)
Lactobacillus was found to be increased 24 h after acute stress at ME (p<0.05). There was also a strong tendency for increase in C. perfringens in both vegetative and spore forms.
Diestrous (DE)
Bacteroides were increased at DE 24 h after exposure to acute stress (p<0.05). A tendency for increase in Enterococcus was also observed.
PE-E and ME-DE phases of estrous cycle
When the estrous cycle was divided into two phases, the first covering PE-E and the second covering ME-DE, the following findings were observed.
There was a tendency for increased presence of the vegetative and the spore forms of C. perfringens and other Clostridium spp. at PE-E (8 of 19 versus 1 of 9 and 16 of 19 versus 2 of 9, respectively) 24 h after exposure to acute stress. In contrast, no significant differences were found in these bacteria at ME-DE after acute stress (). The presence of C. perfringens in the vaginal flora remained increased at PE-E, even 72 h after acute stress (). E. coli also showed increased numbers at PE-E after acute stress ().
Figure 2. Variation of the rat vaginal microflora throughout the estrous cycle after acute stress. The upper panel shows the alterations in the specific bacteria at PE and E, 24 h (black columns) and 72 h (grey columns) after acute stress. The lower panel shows the alterations in the specific bacteria at ME and DE, 24 h and 72 h after acute stress. The values are expressed as percentage of the animals where the bacterium was detected.
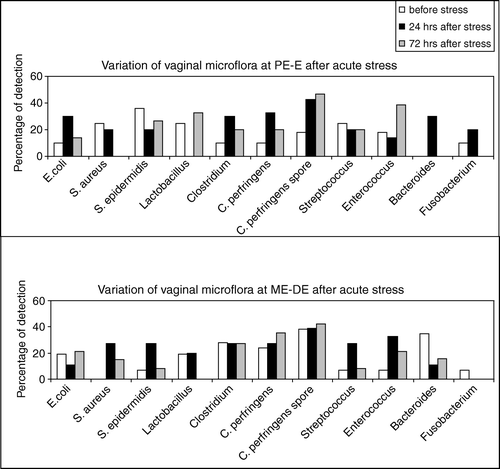
Enterococcus was increased after acute stress, in all stages, while Streptococcus was increased after acute stress only at ME-DE (). S. aureus was detected at ME-DE only after acute stress () and it was not affected by stress at PE-E (). S. epidermidis seemed to be predominant, but not influenced by stress at PE-E (). However, it was detected in increased numbers at ME-DE after both types of stress.
Fusobacterium was detected at low levels in all phases and it was not affected by acute stress (). Although Bacteroides were undetectable before stress at PE-E, their numbers were increased 24 h after acute stress. It is noteworthy that Bacteroides returned to undetectable levels 72h after acute stress (). At ME-DE, the numbers of Bacteroides were detected at high levels, but suppressed after acute stress (). Lactobacillus was absent at PE-E, 24 h after acute stress, while at ME-DE it disappeared 72 h after acute stress ().
Assessment of vaginal microflora after exposure to subacute mild unpredictable stress
Proestrous(PE)
Lower counts of Lactobacillus were found at PE 24 h after exposure to repeated mild unpredictable stress compared with non-stressed animals (1 of 13 versus 5 of 6; p<0.05).
Estrous (E)
E. coli was increased at E, 24 h after exposure to subacute mild unpredictable stress (8/10 versus 0/8; p<0.03). An increase in C. perfringens spore forms was also observed after stress (6/10 versus 2/8). In contrast, Lactobacillus disappeared after stress (0/10 versus 8/8; p<0.01).
Metestrous (ME)
There was a tendency for decreased numbers of Lactobacillus at ME 24 h after subacute mild unpredictable stress (1/11 versus 5/5).
Diestrous (DE)
There was a strong tendency for decrease in Clostridium sp. and C. perfringens in both vegetative and spore forms (0/5 versus 6/11).
PE-E and ME-DE phases
When the estrous cycle was divided into two phases, the first covering PE and E and the second covering ME and DE, the following findings were observed.
At 24 h after exposure to subacute mild unpredictable stress there was a tendency for increased presence of E. coli and C. perfringens (spore forms) at PE-E (). It is noteworthy that E. coli disappeared at ME-DE 24 h after subacute stress (). C. perfringens (spore forms) at ME-DE remained at normal levels after stress ().
Figure 3. Variation of the rat vaginal microflora throughout the estrous cycle after subacute mild unpredictable stress. The upper panel shows the alterations in the specific bacteria at PE and E, 24 h (black columns) and 72 h (grey columns) after subacute mild unpredictable stress. The lower panel shows the alterations in the specific bacteria at ME and DE, 24 h and 72 h after subacute mild unpredictable stress. The values are expressed as percentage of the animals where the organism was detected.
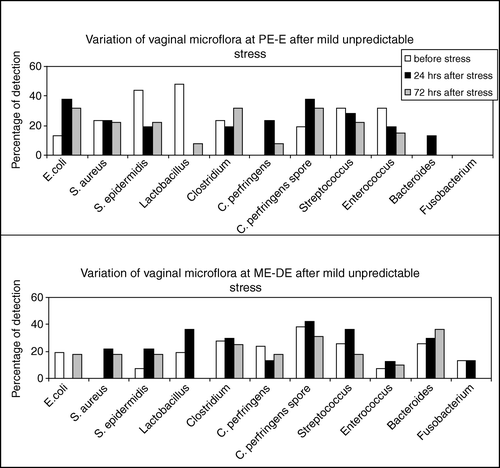
S. aureus was detected at ME-DE only after stress ( and ), while it was not affected by stress at PE-E (). S. epidermidis seemed to be predominant, but suppressed by subacute stress at PE-E. In contrast, it was detected in increased numbers at ME-DE after subacute stress ().
Although Lactobacillus sp. was detected at high levels in almost all animals at PE-E, it disappeared 24 h after subacute, mild unpredictable stress (p<0.001; ) and remained at low levels even 72 h after stress (p<0.03; ). In contrast, Lactobacillus numbers were increased 24 h after stress at ME-DE and disappeared 72 h after stress (). Streptococcus appeared to be unaffected by subacute stress in all phases ().
Fusobacterium was not detected at PE-E and subacute stress had no effect on it. Although Fusobacterium was detected at ME-DE, stress again had no effect ( and ). Bacteroides, although undetectable at PE-E before subacute stress, were detected 24 h after stress (p<0.05; ). Moreover, they were found in increased numbers at ME-DE, but stress had no effect on them ().
Discussion
In recent decades a lot of interest has been focused on the effect of stress in the regulation of various functions and the detoxifying mechanisms of the living organisms Citation[3], Citation[4], Citation[10–12]. The stressed organism is defortified and possesses diminished abilities for defending itself against infections, cancer, and other diseases Citation[2–4], Citation[13]. This may be due, among other factors, to the wide range of secondary manifestations that result in stress-induced plasma glucocorticoid increase Citation[14]. Moreover, the influence of the endogenous steroid hormones that are produced during the different phases of the estrous cycle (PE, E, ME, and DE) seem to play a key role in the control of the genital microflora.
Many studies have focused on the vaginal flora and the factors that modify it Citation[15–19]. To our knowledge, thorough studies regarding the fluctuations of the bacterial vaginal flora at the different phases of the estrous cycle under the influence of stress have never been conducted. It is known that the genital tract of mammals represents an ecosystem in which numerous interactions occur Citation[20], Citation[21] that contribute to an equilibrium that is greatly essential for the health and well-being of the organisms Citation[1], Citation[6], Citation[22].
Most of the predominant bacteria of the vaginal flora are anaerobes and classically –according to Doderlein 1892 – the normal vaginal flora of healthy women is mainly composed of Lactobacilllus sp. Disruption of this homogenous entity reflects an abnormal vaginal flora, which is usually associated with infectious complications of the female genital tract.
Drug treatments including antibiotics, immunosuppressive chemotherapies, and steroid hormone administration could influence the vaginal microflora and its associated characteristics. It is noteworthy that preoperative and postoperative microbiology in reproductive women showed differences in microbial numbers, with the microbial charge being superior postoperatively Citation[23], Citation[24].
The stress-induced neuroendocrine and biochemical alterations Citation[10], Citation[12] may result, among other effects, in disturbances in the organism's immune potential and the balance of the vaginal and intestinal flora, conditions that may have severe consequences for health Citation[3], Citation[11], Citation[12], Citation[25], Citation[26].
In the present study, the interest was focused on the cyclic variations of the vaginal flora that potentially occur after stress. For this purpose, evaluation of the vaginal microflora was performed before and after two different types of psychological stress – acute and the subacute mild unpredictable stress. The evaluation of the vaginal microflora was effected by stained smear techniques so as to appoint the estrous cycle phases (PE, E, ME, DE), together with microbiological estimation for detection of aerobes and anaerobes. In this study, we had a special interest in C. perfringens, as this bacterium and its spores are reported to show strong survival even in ecosystems submitted to stress Citation[25], Citation[26]. It has been reported that stress, such as a space flight, led to modifications in intestinal balance, mainly affecting bifidobacteria Citation[27].
Extensive research has been focused on the reproductive endocrinology and vaginal cytology of rats, which have been chosen as experimental models, because their vaginal flora has a lot of similarities with the human Citation[14], Citation[19], Citation[20]. However, some differences have also been reported. A notable low frequency of Lactobacillus spp. was observed in rat vaginal flora, compared with the classic Doderlein microflora of healthy women, where it was detected in all cases. Quantitative studies reported that the density of the bacterial population of the vagina showed the highest level at estrous, the estrogen-dominated part of the cycle Citation[1], Citation[28].
The first part of the present study estimated the vaginal bacterial flora qualitatively during the four phases of the estrous cycle. Earlier investigations that focused on the qualitative aspect of the vaginal flora Citation[6], Citation[17], Citation[20] were restricted to one specific phase of the estrous cycle. Therefore, the design of the present study gives important knowledge on the ecosystem status at the four distinct phases of the estrous cycle.
E. coli was detected at PE and DE, while it was absent at E and ME. Enterococcus was mainly detected at E and ME and was absent during DE. Total aerobe and anaerobe counts that were enumerated in this study raise the question of vaginal flora stability. Our observation that S. epidermidis is a predominant microorganism in the rat genital flora is consistent with other reports Citation[16], Citation[20], Citation[29]. The numbers of S. epidermidis appear to be increased at PE and E, compared with DE and ME. S. aureus was detected mainly at PE and to a lesser extent at E. Streptococcus was present in all phases of the estrous cycle, with higher levels at PE. Lactobacillus was also present in all phases of the estrous cycle, with increased numbers at E and ME. Regarding the anaerobic status of the microflora, C. perfringens, in both vegetative and spore forms, was present in all phases – showing an increasing capacity throughout the estrous cycle, from PE to DE. Fusobacterium was detected at limited levels in all phases. Finally, Bacteroides were absent at PE and E stages and showed increased numbers at DE.
It is noteworthy that within the female rat vaginal flora, lactobacilli showed decreasing numbers from E to DE. These decreasing numbers seem to coincide with an ascending tendency of C. perfringens from PE to DE. It is evident that when lactobacilli are detected in decreased numbers, conversely, C. perfringens is detected in increased numbers. We could assume that lactobacilli are responsible for the decrease in C. perfringens numbers and vice versa. There is evidence that a ‘factor’ may be related to this situation. A relationship between Lactobacillus and vaginal acidity has been reported previously Citation[1], Citation[5], with only acidophilic species being suitable for growth in an acidic pH environment Citation[5], Citation[6], Citation[20]. In contrast, C. perfringens is a putrefactive alkalophile bacterium. However, the vaginal pH does not exhaustively explain the balance of the vaginal flora, because an absolute correlation between pH and Lactobacillus does not exist [30], as the alkaline cervix is populated by Lactobacillus and other bacteria as well. Therefore, a question arose about the mechanism and the conditions for the reproduction of the vaginal flora pattern. It should also be stated that other factors such as ovarian activity, mucins, antibacterial substances, and endogenous hormonal status play a key role in the balance of the vaginal flora, supported by an eventual antagonism, via the pH, between Lactobacillus and C. perfringens.
These bacteriological changes seem to be associated with differences in the vaginal cytology within the four distinct phases of the estrous cycle. A previous study reported quantitative alterations in the genital microflora of female rats and important bacterial counts were observed during the phase of estrous. These numbers were 105–106 times greater than viable counts observed during metestrous and diestrous Citation[31].
In the first part of the present study, the effect of acute stress on rat vaginal flora throughout the estrous cycle was assessed. In summary, exposure to acute stress led to increased numbers of C. perfringens and the other Clostridium vegetative forms at PE-E, in comparison with control populations in a non-stressed state. The sporulated forms of C. perfringens were also found to be increased. Stress increased the numbers of E. coli, a known non-fastidious microorganism, at PE-E. The numbers of Bacteroides were also increased at this phase of the estrous cycle after stress. Streptococcus and Enterococcus showed increased numbers at ME-DE after acute stress. The anaerobic Fusobacterium was slightly increased at PE-E only after acute stress. S. aureus and S. epidermidis were also increased at ME-DE only after acute stress and finally, Lactobacillus absence was observed at PE-E, 24 h after acute stress.
In conclusion, exposure to acute stress increased numbers of putrefactive bacteria such as E. coli and C. perfringens, and this effect seems to coincide with an absence of Lactobacillus. The arguments proposed previously for this observation are always valid hereby.
Regarding the effect of subacute mild unpredictable stress, increased numbers of C. perfringens (spore and vegetative forms) and other clostridia were observed at PE-E, coinciding with an absence or decrease of Lactobacillus. E. coli was increased after subacute stress at PE-E only, while it disappeared at ME-DE. Although Bacteroides were undetectable before subacute stress at PE-E, they were detected after stress. It is noteworthy that Bacteroides were detected at high levels at ME-DE but they were not affected by stress. As a result of the effect of subacute mild unpredictable stress upon the vaginal microflora, E. coli and C. perfringens presented a dropping profile within estrous cycle from PE to DE. In contrast, Lactobacillus showed an ascending profile. A similar oppositional effect has been observed previously between Bifidobacterium bifidum and C. perfringens in the newborn's intestine, favored in this case by the type of feeding Citation[22], Citation[30].
When the cytological profile was correlated to bacterial counts, a linear regression was obtained for the majority of the cornified cells (45%), as well as for the leucocytes (35%). Similar data were also observed by other authors Citation[7], Citation[20], Citation[21]. The data that remained unexplained by the linear regression may be attributed to the biovariation, experimental error Citation[7], or the host itself.
In conclusion, the variation of the bacterial population dynamics in the rat vaginal tract seems to be closely related to the control of the bacterial flora balance. The particular rise of a putrefactive flora that is dominated by E. coli, Clostridium sp., C. perfringens, and Streptococcus is stated. All the above bacteria are potentially pathogenic and involved in serious gynecologic and obstetric complications. S. epidermidis, which belongs to the major rat genital tract flora, does not seem to be markedly influenced by stress. This last organism is also frequently involved in gynecologic infections. Lactobacillus is either absent or detected at low levels after exposure to stress. The fluctuations in the vaginal flora after exposure to stress, along with modifications in the hormonal balance and other vital functions of the body, may explain several pathophysiological disturbances witnessed under stress and act as a cornerstone in the explanation of several gynecologic disturbances.
References
- Larsen B, Monif GRG. Understanding of the bacterial flora of the female genital tract. Clin Inf Dis 2001; 32: e69–77
- Anisman H, Kokkinidis L, Sklar LS. Neurochemical consequences of stress. Contributions of adaptive processes. Stress: psychological and physiological interactions, S Burchaeld. Hemisphere Publishing, New York 1985; 67–98
- Chrousos GP, Kino T. Interactive functional specificity of the stress and immune responses and the defense against 2 major classes of bacteria. J Infect Dis 2005; 192: 551–5
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA 1992; 267: 1244–52
- Bezirtzoglou, E, Romond, C. Vaginal flora at term. In: Program and Abstracts of the 88th Annual Meeting of the American Society for Microbiology, Miami Beach, FL: American Society for Microbiology, 1988, (abstract).
- Larsen B. Vaginal flora in health and disease. Clin Obstet Gynecol 1993; 36: 107–21
- Larsen B, Markovetz AJ, Galask RP. Spatial relationship of the genital microflora to the vaginal epithelium of female rats: transmission electron microscopy. Appl Environ Microbiol 1978; 35: 444–9
- Evans HM, Long JA. The oestrous cycle in the rat and its associated phenomena. University of California Press, Berkeley, CA 1922
- Bezirtzoglou E, Romond C. Rapid identification and enumeration of C. perfringens in the human fecal flora. Microb Ecol Health Dis 1990; 3: 159–63
- Konstandi M, Johnson EO, Marselos M, Kostakis D, Fotopoulos A, Lang MA. Stress-mediated modulation of B(α)P-induced hepatic CYP1A1: role of catecholamines. Chem Biol Interact 2004; 147: 65–77
- Konstandi M, Kostakis D, Harkitis P, Marselos M, Johnson E, Adamidis K, et al. Role of adrenoceptor-linked signaling pathways in the regulation of CYP1A1 gene expression. Biochem Pharmacol 2005; 69: 277–87
- Konstandi M, Kostakis D, Harkitis P, Johnson JO, Marselos M, Adamidis K, et al. Benzo(α)pyrene-induced up-regulation of CYP1A2 gene expression: role of adrenoceptor-linked signaling pathways. Life Sci 2006; 79: 331–41
- Konstandi M, Marselos M, Camus-Radon AM, Johnson E, Lang MA. The role of stress in the regulation of drug metabolizing enzymes in mice. Eur J Drug Metab Pharmacokinet 1998; 23: 483–90
- Bezirtzoglou E, Konstadi M, Voidarou C, Kostakis D, Marselos M. Influence of psychological stress on the fecal carriage of indicator bacteria. Microecol Ther 1999; 28: 49–53
- Galask RP, Larsen B, Ohm MJ. Vaginal flora and its role in disease entities. Clin Obstet Gynecol 1976; 19: 61–81
- Larsen B, Galask RP. Vaginal microbial flora. Practical and theoretical relevance. Obstet Gynecol 1980; 55: s100–15
- Brown WJ. Variations in the vaginal bacterial flora a preliminary report. Ann Intern Med 1982; 96: 931–4
- Gorbach SL, Menda KB, Thadepalli H, Keith L. Anaerobic microflora of the cervix of healthy women. Am J Obstet Gynecol 1973; 117: 1053–5
- Barthett JG, Moon NE, Goldstein RP, Goren B, Onderdonk AB, Polk BF. Cervical and vaginal bacterial flora: ecologic niches in the female lower genital tract. Am J Obstet Gynecol 1978; 130: 658–81
- Larsen B, Markovetz AJ, Galask RP. The bacterial flora of the female rat genital tract. Proc Soc Exp Biol Med 1976; 151: 571–4
- Larsen B, Markovetz AJ, Galask KP. Scanning electron microscopy of vaginal colonization. Appl Environ Microbiol 1977; 33: 470–6
- Bezirtzoglou E. Anaerobic colonization of the large intestine in newborn infants delivered by caesarean section. G Ital Patol Clin 1996; 3: 48–53
- Ledger, WJ, Campbell, C, Wilson, Jr. Postoperative adnexal infections. Obstet Gynecol 1968;31:83–9.
- Neary MP, Allen J, Okubadejo OA, Payne DJ. Preoperative vaginal bacteria and postoperative infection in gynaecological patients. Lancet 1973; 2: 1291–4
- Tsiotsias A, Voidarou C, Skoufos J, Simopoulos C, Konstadi M, Kostakis D, et al. Stress-induced alterations in intestinal microflora. Microb Ecol Health Dis 2004; 16: 28–31
- Mullie C, Romond MB, Yazourh A, Libersa C, Bezirtzoglou E, Romond C. Influence of stress on faecal carriage of C. perfringens. Microb Ecol Health Dis 2002; 14: 118–21
- Lizko NM. The dysbacteriosis of extreme states. Antiobiot Med Biotechnol 1987; 32: 184–6
- Bartlett JG, Onderdonk AB, Drude E, Goldstein C, Anderka M, Alpert S. Quantitative bacteriology of the vaginal flora. J Infect Dis 1977; 136: 271–7
- Larsen B, Markovetz AJ, Galask RP. Role of estrogen in controlling the genital microflora of female rats. Appl Environ Microbiol 1977; 34: 534–40
- Bezirtzoglou E, Romond MB, Romond C. Modulation of C. perfringens, intestinal colonization in infants delivered by caesarian section. Infection 1989; 17: 232–5
- Larsen B, Markovetz AJ, Galask RP. Relationship of vaginal cytology to alterations of the vaginal microflora of rats during the oestrous cycle. Appl Environ Microbiol 1977; 33: 556–62