Abstract
Objective: Moderate food restriction, such as a 20–40% reduction of the ad libitum intake without causing malnutrition, delays or prevents morphological and functional changes related to aging and overnutrition. This study was performed to evaluate the effects of moderate to severe food restriction (20%, 40%, and 60% of ad libitum intake) for 28 days on some bacterial groups in the cecum and certain biochemical variables in male Sprague Dawley rats. Materials and methods: One group was allowed free access to food (control), while the others were given food restricted to 20%, 40%, and 60% of the ad libitum consumption for 28 days. At the end of the experiment control and food-restricted animals were sacrificed and cecum weight, pH of cecal contents, and counts of total anaerobes, total aerobes, lactobacilli, and coliform bacteria in cecal contents were determined. Metabolic effects of food restriction were evaluated by analyzing some biochemical parameters in plasma and serum. Results: The effect of 60% restriction was severe; three animals died before the 28th day of experiment in this group. In general, biochemical variables did not change in 20% and 40% food-restricted groups. Although lactobacilli counts of the 20% and 40% restricted groups were significantly increased when compared with the 60% restricted group, none of the three groups were differed from the control group. Furthermore, the total aerobe bacteria counts of the 20% restricted group were significantly lower than those of the 60% restricted group. Conclusion: The results of this study suggest that restriction of food by 20% and 40% may affect cecal microbiota beneficially without causing considerable changes in the metabolism of the host, while 60% restriction causes dietary stress.
Introduction
It is well established that nutritional deficiencies, imbalances, and overnutrition are very common problems affecting the health and welfare of man and animals Citation[1–3]. Nutritional deficiencies may occur in several ways, one of which is inadequate dietary intake. Increased metabolic demands and nutrient loss due to diseases and the poor quality of protein are other common reasons Citation[4]. Overnutrition has many adverse effects such as chronic degenerative diseases, tumors in early life, and decreased lifespan in both humans and animals. Prevention and treatment of adverse effects of overnutrition require the control and management of food intake Citation[3], Citation[5].
Moderate food restriction, such as a 20–40% reduction of the ad libitum intake without causing malnutrition, delays or prevents morphological and functional changes related to aging and overnutrition, and so improves survival in laboratory animals Citation[2], Citation[3], Citation[5–8]. Possible mechanisms of these beneficial effects of food restriction are reduced oxidative stress, reduced glycosylation and glucooxidation, and changes in the neuroendocrine system Citation[7], Citation[9–11].
The gastrointestinal microbiota is influenced by the quality and quantity of food consumed and has different effects on many physiological processes of the host. Gut microbiota aids digestion, synthesizes volatile fatty acids, vitamin K and B complex vitamins, stimulates the immune system, converts dietary precarcinogens to non-carcinogens, and forms a barrier to pathogenic microorganisms. Gut bacteria can also transform certain dietary substances to precarcinogens or carcinogens under different conditions Citation[12], Citation[13]. Evidence suggests a strong link between dietary factors, the metabolic activities of the gastrointestinal microbiota, and bowel cancer Citation[14], Citation[15]. Although there are many studies related to the effect of starvation or malnutrition on gastrointestinal microbiota Citation[16–19], the number of studies related to the effects of food restriction on gastrointestinal microbiota is limited and the results are conflicting Citation[20], Citation[21]. It has been reported that 20–40% food restriction affects the cecal microbiota in rats beneficially and it may influence aging-related physiological processes through alterations in gut microbiota and its metabolic products Citation[20]. However, the results of one study suggest that food restriction has little effect on intestinal microbiota of rats Citation[21].
This study was performed to evaluate the influences of moderate to severe food restriction (20%, 40%, and 60% of ad libitum intake) on cecal microbiota and certain biochemical variables in rats.
Materials and methods
Animals and experimental procedures
This study was approved by the Animal Ethics Committee of Istanbul University (27.05.2002, registration number: 42). Thirty-six male Sprague Dawley rats (approximately 3 months old) were entered from Adnan Menderes University Veterinary Faculty Laboratory Animal Unit, which was reared as a closed colony for 5 years. The animals entering the study had not received any treatment and had not been submitted to any other stress effect. All animals were housed individually and the experiment was started after an adaptation period of 10 days. Eight of the 36 animals were only used to determine the amount of ad libitum food consumption (AL group). The amount of the food consumption was determined weekly for 4 weeks and started 1 week before the study, and the data gathered were used to calculate the mean food consumed as per gram of body weight per day. Twenty-eight of the 36 animals were divided into 4 groups and each group consisted of 7 animals. One of the groups was allowed free access to food and water (control group), while the other groups were given food restricted 20%, 40%, or 60% of the ad libitum consumption of the AL group for 28 days, respectively (i.e. 20%, 40%, or 60% reduction of the total amount of mean daily feed consumed per gram body weight by the AL group during the previous week). The temperature was maintained at 22±2°C during the experiment. Lighting was on a 12-hour light-dark cycle. All the rats were fed on rodent diet (Best Yem Gebze-Turkey) as shown in . The food consumption and body weights were recorded weekly.
Table I. Nutrient content of rodent diet.
Biochemical analysis
At the end of the experiment, blood was withdrawn from each rat's tail into heparinized and nonheparinized tubes under ether anesthesia. The effects of food restriction on the metabolism of protein, carbohydrate, and lipid, and liver function were assessed by measuring the concentrations of total protein, albumin, urea, creatinine, ammonia, glucose, triglyceride, cholesterol, ALT, and AST in plasma. Serum electrolyte concentrations were measured with an ion selective electrode (EasyLyte, Medica). Plasma analyses were performed in a photometer (Microlab 200®, Merck) by commercial kits according to the manufacturers’ instructions. Total protein, albumin, urea, creatinine, glucose, triglyceride, cholesterol, ALT, and AST reagents were purchased from Biolabo® France and ammonia reagent (171-C) from Sigma Diagnostic®.
Preparation of cecal specimens and microbiological analysis
At the end of the experiment, animals were euthanased by cervical dislocation after blood withdrawal, and cecal contents were collected aseptically. Each cecum was washed with water, dried, and weighed. Cecal contents were diluted to 10–1 in buffered peptone water (Oxoid-CM509) with 0.5 g/L L-cysteine hydrochloride (Calbiochem-2430) and then mixed gently with a pipette. Aliquots of 1 ml were diluted in reduced physiologic peptone water [1 g/L bacteriological peptone (Oxoid-L37), 8 g/L NaCl (Calbiochem-567440), and 0.5 g/L L-cysteine hydrochloride] in decimal steps and vortex mixed Citation[22]. The cecal pH in 10–1 dilution solution was measured by an electronic pH meter. From each of the dilutions, 0.05 ml was plated onto selective media. Nutrient agar was used to determine total aerobes (Oxoid CM3) with dilutions of 10−2–10−7. Total anaerobes were counted on Columbia blood agar (Oxoid CM331) with dilutions of 10−3–10−8. For lactobacilli, MRS agar (Oxoid CM361) was used with dilutions 10−2–10−7. Coliform bacteria were counted on violet red bile lactose agar (Oxoid CM107) with dilutions 10−1–10−6. Nutrient agar was incubated aerobically at 37°C for 1 day. Columbia blood agar was incubated at 37°C in an anaerobic jar (Merck anaeorocult) with a gas generating kit (Oxoid BR 038) for 4 days. Violet red bile agar and MRS agar were incubated microaerobically at 37°C, for 1 and 3 days, respectively. After incubation, colonies were counted according to the colony morphology.
Statistical analysis
Bacterial counts were calculated and recorded as log10 colony forming unit (cfu) per gram of cecal content. SPSS 10.0 for Windows statistical package program was used for statistical analysis. The data were analyzed by one-way analysis of variance (ANOVA). Body weight data were analyzed with the analysis of variance for repeated measures. When differences between groups occurred, Duncan test was used to find out which group the difference(s) originated from. All data were expressed as the mean±standard error of mean (SEM). Statistical significance was set at an α level of p<0.05.
Results
Food consumption and body weight
Mean body weights and food consumption of the AL group and mean food consumption of the restricted groups are given in and , respectively. Three rats died on the 24th and 26th days of the experiment in the 60% restricted groups
Table II. Mean body weight (g) and food consumption values of ad libitum (AL) group (g/day).
Table III. Mean food consumption of food-restricted rats (g/day) (n = 7).
The mean body weight of control and 20% feed-restricted groups increased, while it decreased in the other two groups during the experiment (). When mean body weights in the pre-experimental period were compared with final weights, they increased 18% in the control and 5% in the 20% restricted groups (p < 0.001 and p < 0.01) and decreased 16% and 40% in the 40% and 60% food-restricted groups, respectively (p < 0.001). At the end of the experiment, the mean body weight of control rats was higher than that of the food-restricted groups (p<0.001). The mean body weights of 20% and 40% restricted groups were also higher than that of the 60% restricted group from the second week until the end of the experiment (p < 0.001). The mean body weights of 20% and 40% restricted groups were not different from each other during the experimental period ().
Table IV. Mean body weights of rats fed ad libitum (control) and food-restricted rats (g) (n=7).
Biochemical variables
The concentrations of total protein, albumin, glucose, and cholesterol were significantly decreased (p<0.05, p<0.005, p<0.001, and p<0.01 respectively), while the concentration of urea (p<0.01), ALT, and AST were increased (p<0.001) in the 60% food-restricted group compared with the other groups (). In general, 20% and 40% food restrictions did not influence the biochemical variables, except in the 40% food-restricted group the plasma concentration of glucose was significantly lower than those of the 20% restricted and the control groups (p<0.001). Furthermore, the mean plasma urea concentration of rats with 40% food restriction was higher than that of the 20% restricted group (p<0.01).
Table V. Some biochemical variables in rats fed ad libitum (control) and food- restricted rats (n=7).
Weight, pH, and microbiota of cecum
The mean values of these parameters are given in . Mean cecum weights were proportionally decreased with the restriction rate. In the 60% food-restricted group, mean cecum weight was significantly lower than in the other groups (p<0.01). Mean cecum weight of the 40% restricted group was also lower than that of the control group (p<0.01). However, the difference in mean cecum weight between the control and 20% restricted groups was not statistically confirmed. In general, the mean pH of cecal contents did not change. Only in the 20% restricted group, the mean pH of the cecal content was lower than the other groups but the difference was not significant. There were no significant correlations between pH and the bacterial groups ().
Table VI. Weight (g), pH, and microbiota of cecum in rats fed ad libitum (control) and food-restricted rats (log10 cfu/g) (n=7).
Table VII. Correlations between pH values and bacteria.
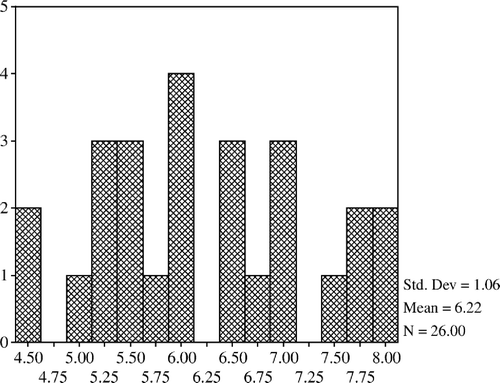
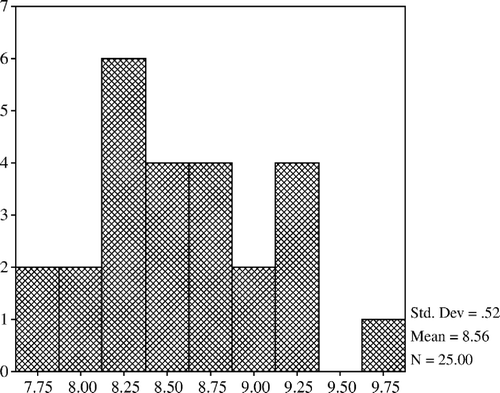
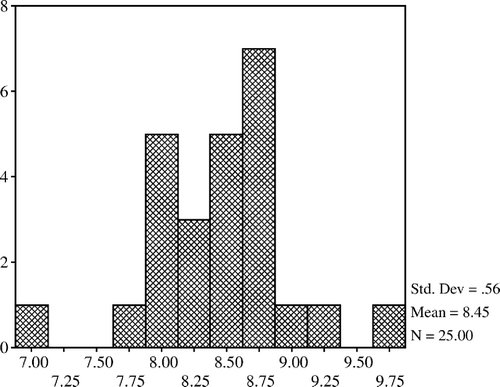
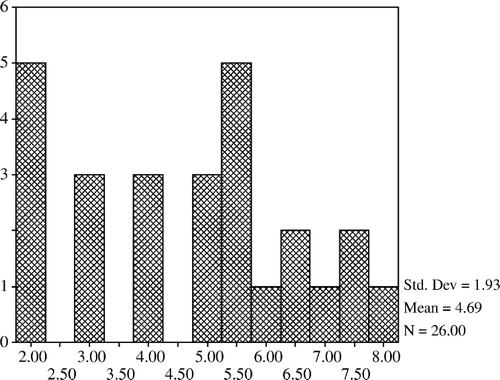
The mean count of lactobacilli was higher in the 20% and 40% restricted group compared with the controls but it was lower in the 60% restricted group. Lactobacilli counts of the 20% and 40% restricted groups were not different from that of the control; however, they were significantly increased when compared with the 60% restricted group (p<0.05). The mean count of total aerobic bacteria in the 20% restricted group was significantly lower than that of the 60% restricted group (p<0.05). The mean counts of total aerobes and coliform bacteria were lower in the 20% and 40% restricted groups compared with the controls but the differences were not confirmed (). In addition, total aerobe bacteria have a positive correlation with coliform bacteria (p<0.001) and negative correlations with lactobacilli (p<0.01) (). The mean count of total anaerobic bacteria was not significantly different between the groups but a positive correlation was determined between the total anaerobic bacteria and lactobacilli (p<0.01) (). Histograms of total aerobes, total anaerobes, lactobacilli, and coliform bacteria are shown in , and , respectively.
Discussion
Changes in quality and quantity of food intake may alter the gastrointestinal microbiota Citation[17], Citation[18], Citation[20], Citation[23], Citation[24]. This study revealed that quantitative changes in food intake such as 20%, 40%, and 60% restriction of ad libitum intake altered the cecal microbiota. In comparison with the other restricted groups, the effects of 60% food restriction were severe. The mean body weight decreased by 40% and three rats died during the experiment in this group. This situation may be explained by the malnourishment of these rats and their increased catabolism resulting from decreased total protein and albumin concentrations and increased urea concentration. In these animals an increase in ALT and AST activities indicates that liver degeneration occurred. In the 20% restricted group, body weight increased slightly and biochemical variables did not show any considerable change. Although body weight decreased in the 40% restricted group, metabolic activity did not differ from the control group except for the decrease in plasma glucose concentration. It has been reported that 40% restriction of food decreases plasma insulin and glucose concentration Citation[25], Citation[26].
When compared with the control group, the mean count of lactobacilli was slightly higher in the 20% and 40% restricted groups but it was lower in the 60% restricted group. So, lactobacilli counts of the 20% and 40% restricted groups were significantly different from those of the 60% restricted group, but insignificantly different from the control group. Furthermore, in the 20% restricted group total aerobic bacteria were significantly lower than in the 60% restricted group. In the 20% and 40% restricted groups, the counts of coliform bacteria were also lower than those of other groups, but differences among groups were not significant.
The effects of moderate food restriction (20–40%) on intestinal microbiota have been reported in only two studies in the literature. Morishita Citation[20] reported that 20% and 40% restriction of food resulted in increased numbers of bifidobacteria, Bacteroidaceae, and lactobacilli in proportion to the restriction rate. The latter study also reported a non-significant decrease in the number of enterobacteria in the 40% restricted group. In contrast to the report by Morishita, Henderson et al. Citation[21] found no significant effect of 40% food restriction on the predominant anaerobic intestinal microbiota. Our results partly agree with the findings of Morishita Citation[20].
Generally, stressfull regimens such as starvation or protein malnutrition decrease the levels of total anaerobic bacteria and/or lactobacilli but increase the levels of coliform bacteria in the large bowel Citation[16–19], Citation[27].
In this study, severe food restriction (60%) acts as an acute unpredictable stressor for the animal. It is acceptable that immediately after an animal is subjected to an important stress, the adrenal cortex in response to signal from the hypothalamus, via the pituitary, rapidly produces quantities of corticosterone Citation[28]. Secondary manifestations resulting from the increased corticosterone include lymphocytopenia, thymus involution in the cell-mediated immune defenses, and related loss of tissue mass of the spleen and peripheral lymph nodes together with loss of corporal tissue mass Citation[29], Citation[30]. On the other hand, moderate food restriction or caloric restriction have also resulted in adrenal hypertrophy and increased plasma corticosterone concentrations. However, their effects become different from those of classic stress and generally have beneficial effects for the host. Hypercorticism (rhythmic hypercorticism in the absence of elevated corticotropin releasing factor (CRF) and adrenocorticotropin (ACTH) levels) is one the fundamental mechanisms by which caloric restriction produces its beneficial effects on disease and longevity Citation[31]. Moderate food restriction also activates immune parameters. T-cell function augments in food-restricted mice Citation[32], and food-restricted mice have a strong antititumor immunity Citation[33].
Moreover, acute stress causes mucin release from rat colon via activated neurons and mast cells by CRF Citation[34]. In several stressed conditions increased mucin secretion is a substrate for some proteolytic bacteria such as clostridia Citation[30], Citation[35] or enteropathogenic Escherichia coli, Salmonella, and Helicobacter pylori Citation[36]. Severe food restriction generates stres for both host and microorganism in the gastrointesinal tract. First, in order to stay alive and to maintain food intake, both of them should decrease the rate of proliferation Citation[36]. The theoretical paradigm described by Alverdy et al Citation[36] stated that under conditions of extreme stress, nutrient deprivation, and host signals that indicate severe and prolonged catabolism and inflammation, bacteria express virulence as a survival mechanism. The consequences of enhanced bacterial virulence expression under such circumstances result in a disturbance in host barrier and immune function Citation[36].
Diet and environment are reported as important factors for the establishment of the gastrointestinal microflora Citation[37], Citation[38]. In the present study, the high iron level in the diet consumed by animals (300 mg/kg) could affect bacterial changes. Iron uptake plays a role in bacterial establishment, as growth of some bacterial species is dependent on iron availability Citation[39]. For example, in countries where chronic malnutrition is prevalent, iron deficiency anemia protects against invasion by colonizing organisms Citation[36]. Iron uptake involves iron-repressible outer-membrane proteins which act as receptors for iron-binding host glucoproteins and allow iron to be internalized in the bacteria Citation[40]. Some pathogenic microorganisms also compete with host lactoferrin and transferrin via iron-binding protein known as siderophore Citation[41].
Iron intake is also associated with pH (colon acidification). At low pH (colon acidification), iron uptake – especially by enteropathogens – is reduced and their population is maintained at a low level Citation[42], Citation[43]. In this study cecum pH was lower in the 20% restricted group than in the other groups but this difference was not significant. No correlations between the pH values and bacterial groups were observed.
Conclusions
The metabolism of the host did not show considerable changes in the 20% and 40% restricted groups but 60% restriction could affect the host metabolism detrimentally. Cecal lactobacilli count increased in the 20% and 40% restricted groups and the total aerobe bacteria count decreased in the 20% restricted group compared with the 60% restricted group. So, determination of bacterial metabolic activity and bacterial enzymes in moderate food restrictions may provide important information on these aspects. Further detailed studies are required for understanding of the interactions between host and microbiota in moderate food restrictions.
This work was part of the doctorate thesis and supported by the Research Fund of the University of Istanbul; project number: T-975/19022001.
References
- Dwyer JT. Dietary change: convergence of prevention and treatment measures. Top Clin Nutr 1991; 6: 42–9
- Weindruch RH, Kristie JA, Cheney KE, Walford RL. Influence of controlled dietary restriction on immunologic function and aging. Fed Proc 1979; 38: 2007–16
- Keenan KP, Ballam GC, Dixit R, Soper KA, Laroque P, Mattson BA, et al. The effects of diet, overfeeding and moderate dietary restriction on Sprague-Dawley rat survival, disease and toxicology. J Nutr 1997; 127: 851–6
- Senoo H. Physiology of stress and starvation-like conditions. The laboratory rat, GJ Krinke. Academic Press, London 2000; 447–54
- Keenan KP, Ballam GC, Haught DG, Laroque P. Nutrition. The laboratory rat, GJ Krinke. Academic Press, London 2000; 57–72
- Masoro EJ. Retardation of aging process by food restriction: an experimental tool. Am J Clin Nutr 1992; 55: 1250–2
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol 2000; 35: 299–305
- Weindruch R. Caloric restriction: life span extension and retardation of brain aging. Clin Neurosci Res 2003; 2: 279–84
- Weindruch R, Kayo T, Lee C, Lee CK, Prolla TA. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J Nutr 2001; 131: 918S–923S
- Weindruch R, Keenan KP, Carney JM, Fernandes G, Feuers RJ, Floyd RA, et al. Caloric restriction mimetics: metabolic interventions. J Gerentol 2001; 56: 20–33
- Yu PB. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 1996; 21: 651–68
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4: 430–5
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31: 107–33
- Gorbach SL, Goldin BR. Nutrition and the gastrointestinal microflora. Nutr Rev 1992; 50: 378–81
- Hill MJ. Bile, bacteria and bowel cancer. Gut 1983; 24: 871–5
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun 1974; 9: 591–8
- Deitch EA, Winterton J, Berg R. Effect of starvation, malnutrition and trauma on the gastrointestinal tract flora and bacterial translocation. Arch Surg 1987; 122: 1019–24
- Deitch EA, Ma WJ, Ma L, Berg RD, Specian RD. Protein malnutrition predisposes to inflammatory-induced gut-origin septic states. Ann Surg 1990; 211: 560–7
- Allori C, Aguero G, de Ruiz Holgado AP, de Nader OM, Perdigon G. Gut mucosa morphology and microflora changes in malnourished mice after renutrition with milk and administration of Lactobacillus casei. J Food Protect 2000; 63: 83–90
- Morishita Y. Effect of food restriction on caecal microbiota and short-chain fatty acids concentrations in rats. Microb Ecol Health Dis 1995; 8: 35–9
- Henderson AL, Cao WW, Wang RF, Lu MH, Cerniglia CE. The effect of food restriction on the composition of intestinal microflora in rats. Exp Gerontol 1998; 33: 239–47
- Hartemink R, Rombouts FM. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. J Microbiol Methods 1999; 36: 181–92
- Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and short-chain fatty acids, pH and microflora in rats. J Nutr 1997; 127: 130–6
- Drasar BS, Crowther JS, Goddard P, Hawksworth G, Hill MJ, Peach S, et al. The relation between diet and the gut microflora in man. Proc Nutr Soc 1973; 32: 49–52
- Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev 1988; 46: 89–104
- Masoro EJ, McCarter RJM, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. J Gerentol 1992; 47: 202–8
- Tannock GW. Modification of the normal microbiota by diet, stress, antimicrobial agents, and probiotics. Gastrointestinal microbiology, Vol 2. Gastrointestinal microbes and host interactions, RI Mackie, BA White, RE Isaacson. Chapman Hall, New York 1997; 434–66
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992; 267l: 1244–52
- Bezirtzoglou E, Konstadi M, Voidarou C, Kostakis D, Marselos M. Influence of psychological stress on the fecal carriage of indicator bacteria. Microecol Ther 1999; 28: 49–53
- Tsiotsias A, Voidarou C, Skoufos J, Simopoulos C, Konstadi M, Kostakis D, et al. Stress-induced alterations in intestinal microflora. Microb Ecol Health Dis 2004; 16: 28–31
- Leakey JEA, Seng JE, Barnas CR, Baker VM, Hart RW. A mechanistic basis for the beneficial effects of caloric restriction on longevity and disease: consequences for the interpretation of rodent toxicity studies. Int J Toxicol 1998; 17(Suppl 2)5–56
- Hishinuma K, Nishimura T, Konno A, Hashimoto Y, Kimura S. Augmentation of mouse immune functions by dietary restriction: an investigation up to 1 year of age. Ann Nutr Metab 1990; 34: 76–84
- Matsuzaki J, Yamaji R, Kiyomiya K, Kurebe M, Inui H, Nakano Y. Implanted tumor growth is suppressed and survival is prolonged in sixty percent of food-restricted mice. J Nutr 2000; 130: 111–15
- Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, et al. Acute stress causes mucin release from rat colon. Am J Physiol 1996; 271: G884–92
- Mullie C, Romond MB, Yazourh A, Libersa C, Bezirtzoglou E, Romond C. Influence of stress on faecal carriage of C. perfringens. Microb Ecol Health Dis 2002; 14: 118–21
- Alverdy J, Zaborina O, Wu L. The impact of stress and nutrition on bacterial–host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care 2005; 8: 205–9
- Bezirtzoglou E, Romond MB, Romond C. Modulation of C. perfringens, intestinal colonization in infants delivered by caesarian section. Infection 1989; 17: 232–6
- Bezirtzoglou E, Romond C. Effect of the feeding practice on the establishment of bacterial interactions in the intestine of the newborn delivered by cesarian section. J Perinat Med 1989; 17: 139–45
- Singer E, Yazourh A, Romond MB, Odou MF, Bezirtzoglou E, Dubreuil L, et al. In vivo relationship between intestinal Bifidobacteria overgrowth and B. fragilis repression induced by consumption of bifidobacterial cell-free whey. Anaerobe 1999; 5: 505–8
- Otto BR, Verweij van Vught AM, Van Doorn J, MacLaren DM. Outer membrane proteins of B. fragilis and B. vulgatus in relation to iron uptake and virulence. Microb Pathog 1988; 4: 279–87
- Dale SE, Doherty-Kirby A, Lajoie G, Heinrichs DE. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect Immun 2004; 72: 29–37
- Bezkerovainy A. Iron transport and utilization by Bifidobacteria. Biochemistry and physiology of Bifidobacteria. CRC Press, Boca Raton, FL 1989
- Mullie C, Romond MB, Yazourh A, Bezirtzoglou E, Romond C. Modification to intestinal glycosidase activities following Bifidobacterium breve C50 oral challenge in C3H mice. Anaerobe 1999; 5: 499–504