Abstract
Volatile sulphur compounds (VSCs) produced by anaerobic microorganisms on the tongue are major contributors to oral malodour. Antimicrobial agents such as zinc salts may therefore indirectly reduce the production of VSCs. The aim of this study was to evaluate the effect of four zinc-containing sorbitol lozenge formulations (0.1–0.5% zinc gluconate) on oral malodour (‘morning breath’) by breath and tongue flora analysis on 24 healthy volunteers. Chlorhexidine (0.2% chlorhexidine gluconate) mouthwash was used as positive control and sorbitol lozenges as negative control. Breath odour was evaluated by a Halimeter® and organoleptic readings, and tongue flora by culture of oral microorganisms before, and at intervals over a 60 min period after, treatment. All treatments were effective in reducing sulphides in breath odour but chlorhexidine and 0.5% zinc lozenges produced the greatest reduction. All treatments produced a significant decrease (p<0.001) in bacterial counts 15 min post treatment, with chlorhexidine being most effective (p<0.05). Counts on fastidious anaerobic agar containing vancomycin correlated positively with Halimeter® readings. The combination of methods used provided a broader approach to the assessment of breath odour.
Introduction
Previous studies have shown that zinc salts have an antimicrobial effect and that they reduce production of volatile sulphur compounds (VSCs) Citation[1], Citation[2]. Zinc ions have a high affinity for sulphur and are known to inhibit formation of sulphur compounds Citation[3–5]. Thus, zinc has been incorporated into lozenges to aid in the antimicrobial control of oral malodour, because of its low toxicity and other favourable properties such as non-staining of dentition Citation[2]. A zinc-containing mouthwash reduced mouth air VSCs by 80–90% up to 3 h post rinsing Citation[2], Citation[6]. Chlorhexidine (0.2%) mouthwash has been shown to reduce bad breath for 8 h Citation[7] and is thus often used as a positive control in clinical trials. It has a wide spectrum of antimicrobial activity but is not recommended for long-term usage because of tooth staining.
The most simple and widely accepted approach to assess the degree of oral malodour is to directly ‘sniff’ expelled mouth air Citation[8], Citation[9]. This is referred to as an organoleptic assessment and closely simulates everyday situations in which bad breath is detected. However, organoleptic measurement is inevitably subjective Citation[10] and therefore susceptible to inter- and intra-examiner variability. Other confounding factors influencing measurement relate to the psycho-physiological state of individual judges influencing their sense of smell. These factors may include hunger, menstrual cycle, head position, and the degree of attentiveness and expectation Citation[9]. To reduce variations a panel of judges is often employed in clinical studies so that a mean score may be used. However, this approach also has drawbacks, since in sequential measurements, concentration and composition of gases in an individual's breath will vary.
The tongue is highly papillated and has a large surface area that supports a high bacterial density in relation to other oral mucosal surfaces Citation[11]. Odour-producing organisms, mainly Gram-negative anaerobes that produce volatile sulphur compounds via the metabolism of glycoproteins and other proteinaceous compounds, form part of the tongue population, but this biofilm population is very difficult to study or model. A major problem is obtaining a representative and reproducible sample that relates to a given surface area of the tongue Citation[12]. One simple method for evaluating the effect of mouth rinses on the oral microflora involved determination of colony counts in saliva Citation[13]. However, the use of a sterile toothbrush to obtain a sample of a particular area of the tongue increased sample reliability of a given area, compared with tongue scrapings or saliva samples, and also improved access to the back of the tongue (posterior third) Citation[14–16].
A wide range of genera is responsible for oral malodour, and the complexity of the tongue flora poses problems in determining and isolating key species. However, if the overall ecology of the tongue environment is considered, then focus on more general properties of key populations is possible Citation[17]. The ecological plaque hypothesis provides a physiological approach to the control of of biofilms associated with caries, periodontal disease, denture stomatitis and oral malodour Citation[18]. A postulated dynamic relationship between environmental change and ecological shifts implies that disease might be prevented either through a direct inhibition of the putative pathogens, or by interfering with the key environmental/physiological factors that drive the ecological shift. For oral malodour, it is the obligate anaerobic, usually Gram-negative and often black-pigmented species that are of concern. For ecological control of oral malodour, there are two potential approaches: the use of a general antimicrobial agent that can reduce the overall counts of tongue species, or a more specific alteration in the tongue ecology, providing a reduction in the odour-causing organisms. In order to monitor change in the tongue microflora related to oral malodour, a culture medium that selects for, and indicates the presence of, key populations (i.e. obligate anaerobic, usually Gram-negative and often black-pigmented) may be employed. Analogous media have been successfully used to indicate acidogenic or alkaligenic properties of dental and denture plaque in cross-sectional and longitudinal clinical studies Citation[19].
This paper describes studies on oral malodour that incorporate organoleptic scoring, Halimeter® readings and tongue sampling for bacterial counts and focuses on the use of an antimicrobial agent for breath odour reduction.
Materials and methods
Culture media
Four media were used for the culture of anaerobic species associated with malodour: fastidious anaerobe agar (FAA), Lab M, Lab 90 (Bury, Manchester, UK) with 6% sterile horse blood for total anaerobes; FAA & vancomycin (VAN) for total obligate anaerobes (vancomycin [Sigma], 2.5 mg L−1 was added to FAA); Veillonella medium (VEILL; Difco Laboratories, Detroit, USA), and a medium for fusobacteria (FUSO), comprising trypticase soy agar (40 g), crystal violet (8 ml of 500 ppm [0.05 g 100−1 ml]), with vancomycin (0.75 ml L−1 from stock 10 000 mg ml−1), pH 8.0.
Ten ml aliquots of reduced transport fluid (yeast extract 5.0 g L−1, bacteriological peptone 1.0 g L−1, sodium chloride 8.5 g L−1, L-cysteine 0.5 g L−1, glycerol 150 ml, sodium dihydrogen orthophosphate 0.868 g L−1, potassium dihydrogen orthophosphate 0.528 g L−1, Tween 80, 1.0 g L−1, made up to 1 L with distilled water, pH 6.9–7.1) were autoclaved in screw-capped bottles and cooled in an anaerobic cabinet, after which caps were tightened and bottles were stored at 4°C
Lozenge treatments ()
Lozenges were kindly provided by Nabisco Inc., New Jersey, USA. Treatment A was the positive control (chlorhexidine mouthwash, 0.2%), treatment B was the negative control and treatments C, D, E and F were sorbitol lozenges containing an increasing amount of zinc gluconate (0.1%, 0.2%, 0.5%). The high concentration of zinc and the resultant effect on taste necessitated the addition of aspartame (E) or vitamin C (F).
Table I. Summary of lozenge formulations, assigned code and abbreviations used in the clinical trial.
Methods
Twenty-four healthy volunteers (11 male, 13 female), aged 25–50 years agreed to a preliminary screen and the completion of a health questionnaire. The exclusion criteria were: (i) medical history of infectious disease (e.g. hepatitis C, HIV, tuberculosis); (ii) rampant caries, severe gingivitis, advanced periodontitis, oral thrush; (iii) antibiotic medication within 1 month before the start of the trial or during the trial period; (iv) consumption of medicated sweets containing antimicrobial agents; (v) changes in oral hygiene during the trial; (vi) consumption of foods associated with oral malodour (e.g. garlic, onion, chilli, curry) on the day before sampling and the sampling day, and wearing strong perfumed cosmetics on the sampling day; (vii) substantial false dentition; (viii) smokers.
All procedures were in accordance with the Helsinki Agreement. Ethical approval was given by the School Research and Ethics Committee.
Subjects were assigned a program of lozenge consumption according to the Williams Latin square design. All participants were asked to refrain from any oral hygiene, food or drink for 12 h before sampling. They were also asked to avoid spicy foods and not to wear any perfumed products on the day of the study, as the odours may interfere with organoleptic assessment. Each subject visited the laboratory once a week for 6 weeks. At a designated time between 8 am and 9 am, they consumed one of the six treatments labelled A to F for 1 min.
Breath and microbial analysis occurred at intervals over a 60 min period via (i) Halimeter®; (ii) organoleptic measurements (scale 0–5) by trained judges and (iii) counts of tongue flora sampled using a sterile child-size toothbrush (head area = 1.9 cm2) according to the previously described method Citation[13].
Halimeter® measurements
Halimeter® readings were taken before organoleptic assessment and any treatment (time 0), then at 60 min after treatment and organoleptic assessment. A flexible plastic drinking straw was attached to the Halimeter® inlet (model RH-17, Interscan Corp., Chatsworth, CA, USA) through which the oral air was drawn and a measurement was made of sulphide levels in parts per billion (ppb). In order to standardize the end of a 1.5 ml Eppendorf tube was cut off, enabling a straw to be fed through the tube. The placing of the tubing on the straw was made so as to ensure that the distance the straw was inserted into the mouth was the same for each subject (approximately 2.5 cm). The subject closed their mouth for 2 min before closing their mouth slightly over the straw, with their teeth resting behind the ridge of the Eppendorf tube, while the Halimeter® recorded the level of suphide. Two peak measurements were recorded for each individual.
Organoleptic scoring
Organoleptic assessment of breath in this study was carried out according to Rosenberg's (1991) scale for the assessment of breath odour. The degree of odour was scored on a 1–5 scale where 0 = no odour, 1 = barely noticeable odour, 2 = slight odour, 3 = moderate odour, 4 = strong odour and 5 = extremely foul odour. Both odour judges attended a training course prior to the study.
Organoleptic assessment was carried out by two trained judges before and 5, 15, 30 and 60 min after lozenge consumption/mouthwash. Each subject was seated in a small room, away from other laboratory activities, and asked to keep their mouth closed, with their lips sealed, for 2 min, breathing through the nose. Subsequently, the subject opened their mouth, without exhaling, and a trained examiner sniffed as closely as possible to the open mouth to assess odour level.
Tongue sampling and microbial counts
Tongue samples were taken using a sterile child-size toothbrush. Clumps of bristles at the tip of a sterile medium-size soft bristle toothbrush (Wisdom Suregrip, Wisdom Toothbrush Co., Evanston, IL, USA) were removed with a hot wire to produce a rectangular area (1.9 cm2). Four cm was removed from the handle using a hot wire, so that the entire brush and handle would fit into a 1.8 mm thick Pyrex boiling tube, enabling sterilization in the autoclave. The tip of the handle was covered in foil to prevent contamination via brush holding during sampling. The top of the boiling tube was also covered with foil to prevent contamination after removal from the autoclave. Brushes could be re-used several times post cleaning and autoclaving.
During sampling, the brush was firmly rocked on the dorsum region of the tongue for 5 s before (right-hand side of tongue) and 5 (right-hand side of tongue), 15 (centre of tongue) and 30 min (left-hand side of tongue) after lozenge consumption/mouthwash. Four toothbrushes were therefore prepared for each subject on each sampling day.
After each sampling, the brush was then placed back into the boiling tube, the RTF (10 ml) was decanted from the screw-topped bottles into each boiling tube, the foil was removed from the brush handle, and the tube was vortex mixed for 20 s. The brush was removed using sterile forceps (to be cleaned, disinfected and autoclaved for re-use), and the resultant suspension and 10−2 and 10−3 dilutions were inoculated onto media using a spiral plater. All plates were incubated anaerobically at 37°C for 5 days.
Statistical analyses
A one-way analysis of variance (ANOVA) was used to determine significance of percentage decrease in Halimeter® readings and paired t tests were carried out to assess significant differences between treatments.
Results
Halimeter® measurements
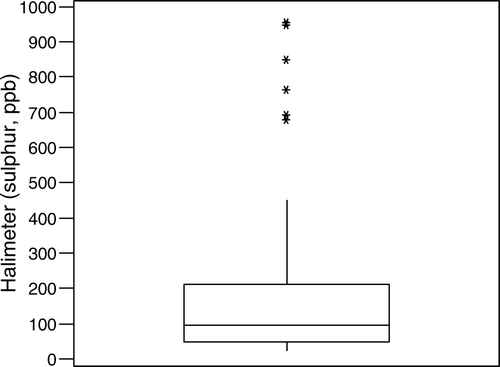
The mean baseline score (t = 0) for all 24 patients visiting the laboratory on six occasions (one visit for each treatment) was 154.7±14.4 sulphur (ppb). The data (n=144) were slightly skewed ().
Figure 1. Box and whisker plot for baseline sulphur readings for all subjects collated from each visit. The median is represented by the middle line in the box (94 ppb sulphur) and the lower quartile (48 ppb sulphur) and the upper quartile (210 ppb sulphur) are the bottom and top lines, respectively. The extreme points at the ends of the error bars show that Halimeter® scores for patients ranged from a minimum of 23 to a maximum of 954.
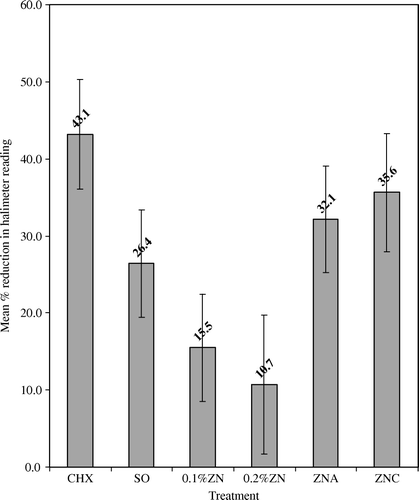
To evaluate the effectiveness of each treatment, the percentage reduction in Halimeter® scores obtained 60 min after use was calculated for each subject. Data were pooled to establish a mean percentage reduction (). All treatments reduced Halimeter® scores (p<0.05). The chlorhexidine mouthwash and 0.5% zinc lozenges were most effective (p<0.05).
Organoleptic measurements
Results of organoleptic readings showed a good correlation between the two judges throughout the trial (R = 0.723, p<0.05). Thus, a mean score was used to indicate results for each treatment. A good correlation (R = 0.683, p<0.05) was observed between organoleptic and Halimeter® scores at time 0 for all 24 individuals.
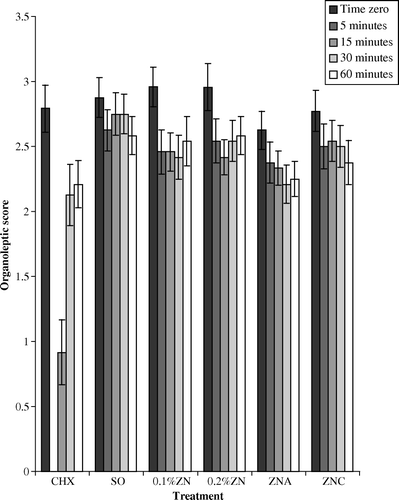
The mean organoleptic score for the group at baseline was 2.8±0.06. The distribution of data was fairly symmetrical, unlike that for the Halimeter® readings. Baseline scores were reduced significantly (p<0.05) after 5 min for all treatments, with the greatest reduction after the chlorhexidine mouthwash. Breath odour scores also remained low for chlorhexidine compared with other treatments after 15 min (). The judges gave scores of 0 when no odour could be detected due to masking by the odour of the chlorhexidine. The effect of the chlorhexidine mouthwash was always significantly greater than that of the negative control (p<0.05). After 15, 30 and 60 min odour levels for all agents were still significantly lower than original values, except for the negative control at 15 and 30 min.
Microbiological analysis
Despite considerable variation in data, all treatments produced a significant decrease (p < 0.001) in counts at 15 min from time zero on all media. After 30 min counts began to rise. The effect of a chlorhexidine mouthwash was significant after 15 min (p < 0.05), but not after 30 min. A significant positive relationship was observed between Halimeter® readings and VAN counts (R = 0.386, p < 0.05), with negative correlations observed between Halimeter® readings and counts on the other media
Counts on FAA and VAN media were high (log 7) compared with counts on FUSO and VEILL (log 5.5 and 6.2). A positive correlation was also observed between the detection of sulphur by organoleptic methods and the number of black-pigmented anaerobes on FAA and VAN media.
Discussion
The present clinical trial utilized a range of protocols to monitor breath levels before and after treatment for oral malodour. In order to attain maximum odour levels for the subject group, morning breath was assessed. It has been noted that morning breath may be related to reduced salivary flow and stagnation rather than directly to oral malodour, but from our results it appears that higher baseline breath levels in a normal population enables better discrimination between factors affecting odour.
The Halimeter® was reliable in monitoring any decrease in VSC levels. Results from the Halimeter® also correlated well with organoleptic scores. These latter gave a more ‘rounded’ result in that not only was the level of VSC ‘measured’, but the odour that emanates as perceived by the human nose – perhaps more discriminately – was also included.
Organoleptic assessment
Two trained judges gave organoleptic scores for all individuals throughout the study and a mean score was used to analyse the results. It has been observed that odour training gives rise to reproducible odour scores and that it is the most reliable method Citation[7]. A correlation coefficient of 0.723 between odour judges in this study is very similar to that of other breath trials Citation[19]. However, it has also been stated that 0.7 ‘was not an impressive result for a technique that purports to be the gold standard for this field’ Citation[19].
When individuals rinsed with chlorhexidine mouthwash the organoleptic result after 5 min was 0 in all cases. This was because any odour previously detected was no longer evident, because the mouthwash completely masked any odour. The subjects in this trial disliked the chlorhexidine mouthwash and commented on subsequent numbness of the tongue. The odour-masking effect was not observed with the zinc lozenges or sorbitol control lozenge. Chlorhexidine is highly effective in reducing oral malodour and counts of oral microorganisms but it cannot be used routinely because of tooth staining, loss of taste and burning sensation. Clearly zinc-containing mouthwashes are preferable.
At 60 min after rinsing all treatments showed a reduction in odour compared with scores given at time 0. This substantivity was not apparent using microbiological culture media, but was noted using the Halimeter®. Breath odour assessment generates one reading for the whole mouth, whereas microbiological sampling of one part of the tongue comprises large numbers of microorganisms. The variability of microbiological counts might preclude observation of any minor effects. It has been stated that any oral activity may also cause a decrease in odour, e.g. salivary stimulation caused by talking or jaw movement. An additional negative control might be useful in this context.
A percentage reduction in VSC levels for each subject was calculated to compare effectiveness of treatment. Extreme readings for several individuals sometimes skewed the data, although this was not representative for the bulk of the sample group. Skewness was not observed using organoleptic assessment data, perhaps because of the additional ‘bouquet’ included in the organoleptic assessment, or due to the use of fewer discriminators for severe odour in the subjective assessment scale. In some studies, high odour and low odour groups have been used Citation[16], but in this study, the majority of the group had low odour levels. Variations were hopefully minimized in this study by providing guidelines, which included restrictions on diet and oral hygiene for 12 h before testing. All individuals were compliant.
All treatments had positive effects, but chlorhexidine and the 0.5% zinc formulations were most effective in reducing sulphides in breath odour, as might be expected. A zinc-containing mouthwash (0.2%) has been demonstrated to reduce mouth air VSC by 80–90% 3 h afterwards Citation[1], Citation[3], Citation[5]. Results from this study are much lower, with the greatest effect at 35.6%±7.67 by 0.5% zinc. The differences are attributable to the increasing zinc concentration (range from 0.2% to 0.84%), the delivery system (i.e. frequent mouth rinsing or lozenge consumption) and sample population. The presence of aspartame or vitamin C in the 0.5% zinc lozenges did not significantly affect activity. Subjects commented on the pleasant flavour of lozenge F (0.5% zinc with vitamin C), and this improved flavour might result in increased sucking action, and the lozenge remaining in the mouth longer. In addition, the presence of vitamin C, namely ascorbate, could have enhanced the antimicrobial effect of zinc to encourage reactive oxygen species if H2O2 and/or oxygen is present.
It is now accepted that the origin of breath odour is mostly bacteria residing on the tongue, and it is therefore appropriate to sample the tongue when assessing potential treatments for breath odour. In preference to sampling saliva or tongue samples (via swab) or scraping, the impressed toothbrush method was used. It is possible that upon repeat sampling of the same site on the tongue, more anaerobes deeper in the biofilm might have been isolated, causing some variability. Thus different sites on the tongue were sampled during the study. However, in a separate study in our laboratory, seven sequential samplings from the same site on the same tongue did not reveal any major differences in key microbial populations (results not presented).
There is considerable variation in the microbial population at different sites on the tongue Citation[16]. A 10-fold increase in bacteria at the back of the tongue is seen compared to the front. Post-nasal drip, which falls directly at the back of the tongue, may contribute to this difference. Again, in a separate study in our laboratory, we failed to note any significant differences on the amount or composition of the flora of the left, right and central regions of the tongue (results not presented). To date few studies have described the healthy tongue flora Citation[15]. Most studies have only sampled the tongue flora to assess odour potential. An improved knowledge of the nature of the healthy tongue biofilm is required, but as with other biofilms on host surfaces, it is difficult to sample, model in vitro, or investigate in situ without using biopsy material.
The culture of tongue flora on fastidious anaerobe agar containing vancomycin provides a useful additional indicator of malodour in an individual, with counts obtained relating to data obtained from the Halimeter®. An association was also observed with the detection of sulphur by organoleptic methods and the number of black-pigmented anaerobes on FAA and VAN. Media for Gram-negative anaerobes or sulphide-producing microorganisms Citation[20] and counts from a less selective medium also correlated with breath odour, but not with Halimeter® data Citation[21]. No previous studies could be found that demonstrate a relationship between a selective culture medium and Halimeter® reading.
There are several considerations involved in the choice of culture media used in clinical studies, where a large amount of samples and tests generate large amounts of data. If media selected are not sufficiently specific, then key groups may be obscured. However, if media are too selective, then numbers of key groups are too low for significant changes in counts to be observed. Similarly, if groups of microorganisms are subsequently isolated and characterized, and if characterization is not performed at a sufficient level, then relationships again may not be seen. The vancomycin-containing medium used in these clinical studies was sufficiently selective to allow the growth of a broad population of microorganisms associated with and indicative of oral malodour. In agreement with other studies, the reduction of mouth odour and counts on vancomycin isolating strict anaerobes supports the theory that this group of organisms are major contributors to oral malodour production.
Conclusion
A chlorhexidine mouthwash was effective in reducing oral malodour and microbial counts in a group of healthy individuals over a 60 min period post-consumption. Lozenges containing 0.5% zinc gluconate were also effective, although to a lesser extent. Effects were more apparent using Halimeter® and organoleptic assessment of breath odour than using microbiological counts, but counts on fastidious anaerobe agar containing vancomycin generally agreed with other assessment methods.
Acknowledgements
The authors are grateful to Nabisco Inc. for funding this research, and to Anne Leahy-Gilmartin, Colin McKenzie, Paul Spencer, Mohammed El-Maaytah, Maaike Pietsche and Lindsay Smith for their assistance in the clinical trials.
References
- Yaegaki K, Suetaka T. The effect of zinc chloride mouthwash on the production of oral malodour, the degradations of salivary cellular elements and proteins. Journal of Dental Health 1989; 39: 377–86
- Rölla G, Jonski G, Young A. The significance of the source of zinc and its anti-VSC effect. Int Dent J 2002; 52: 233–5
- Ng W, Tonzetich J. Effect of hydrogen sulphide and methyl mercaptan on the permeability of oral mucosa. J Dent Res 1984; 63: 994–7
- Pratten J, Pasu M, Jackson G, Flanagan A, Wilson M. Modelling oral malodour in a longitudinal study. Arch Oral Biol 2003; 48: 737–43
- Kleinberg I, Codipilly M. Modelling of the oral malodour system and methods of analysis. Quintessence Int 1999; 30: 357–69
- Rosenberg M, Gelernter I, Barki M, Bar-Ness R. Day long reduction of oral malodour by a two phase oil:water mouthrinse as compared to chlorhexidine and placebo rinses. J Periodontol 1992; 63: 39–43
- Rosenberg M, McCulloch CA. Measurement of oral malodour: current methods and future prospects. J Periodontol 1992; 63: 776–82
- El-Maaytah MA, Hartley MG, Greenman J, Porter SR, Scully CM. Relationship of the salivary incubation test with oral malodour levels. Bad breath: a multidisciplinary approach, D van Steenberghe, M Rosenberg. Leuven University Press, Leuven 1996; 135–42
- Murata T, Yamaga T, Iida T, Miyazaki H, Yaegaki K. Classification and examination of halitosis. Int Dent J 2002; 52: 181–6
- Rosenberg M, Kulkarni GV, Bosy A. Reproducibility and sensitivity of oral malodour measurements with a portable sulphide monitor. J Dent Res 1991; 70: 1436–40
- Marsh P, Martin M. Oral microbiology3rd edn. Chapman and Hall, London 1992
- Greenman J. Microbial aetiology of halitosis. Dental plaque revisited; oral biofilms in health disease, HN Newman, M Wilson. Bioline, Cardiff 1999; 419–42
- Hartley G, McKenzie C, Greenman J, El-Maaytah MA, Scully C, Porter S. Tongue microbiota and malodour; effects of metronidazole mouthrinse on tongue microbiota and breath odour. Microb Ecol Health Dis 1999; 11: 226–33
- Addy M, Jenkins S, Newcombe RG. The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts. J Clin Periodontol 1991; 18: 90–3
- Jenkins S, Addy M, Wade W, Newcombe RG. The magnitude and duration of some mouthrinse products on salivary bacterial accounts. J Clin Periodont 1994; 21: 397–401
- Hartley MG, El-Maytaah MA, McKenzie C, Greenman J. The tongue microbiota of low odorous and malodorous individuals. Microb Ecol Health Dis 1996; 9: 215–23
- Coykendall AL. A method to quantitate the tongue flora. J Dent Res 1995;74:127 ( abstract).
- Verran J, Doran AL, Smith C. Denture stomatitis, oral malodour and oral biofilm ecology. Biofilm community interactions: chance or necessity, P Gilbert, D Alison, M Brading, J Verran, J Walker. BioLine, Cardiff 2001; 131–40
- Brunette DM. Introduction to the proceedings of the fifth international conference on breath odour research. Int Dent J 2002; 52: 177–80
- Awano S, Gohara K, Kurihara E, Ansai T, Takehara T. The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int Dent J 2002; 52(Suppl 3)212–16
- De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodour. J Am Dent Assoc 1995; 126: 1384–93