Abstract
Bacterial vaginosis (BV) is a condition whose prevalence has been reported at 29% and whose adverse effects are becoming more and more recognized. Current antibiotic treatment often does not cure the infection, nor prevent relapses. The recent clinical finding that probiotic lactobacilli can augment antibiotic efficacy was investigated in a series of in vitro studies. Gardnerella vaginalis ATCC 14018, an organism associated with BV, and probiotic Lactobacillus rhamnosus GR-1 as well as freshly isolated Lactobacillus plantarum KCA were dispensed into microplates containing different concentrations of metronidazole ranging from 0 µg/ml as control to 8000 µg/ml. Growth of the strains was markedly inhibited at high antibiotic concentrations, but a significant (p=0.005) stimulatory effect occurred for L. rhamnosus GR-1 at 64 µg/ml and 256 µg/ml (p=0.02). After the organisms entered stationary phase, the stimulatory effect declined. At concentrations ≥4000 µg/ml, there was marked growth inhibition of more than 71% on the two lactobacilli strains, while Gardnerella vaginalis was inhibited over 70% at ≥3000 µg/ml. The surprising ability of probiotic lactobacilli to survive and grow in metronidazole may have implications for complimentary probiotic and antibiotic therapy of BV.
Introduction
Bacterial vaginosis (BV) is a condition in which lactobacilli-dominated microbiota is replaced by multiple potentially pathogenic bacteria in biofilms attached to the vaginal epithelium Citation[1]. The primary etiological agents are Atopobium, Prevotella, Mobiluncus, and Gardernella vaginalis Citation[2], and their colonization leads to elevation of vaginal pH, and in many cases production of amines (fishy odor) and an inflammatory response that can result in discharge. Until recently the condition was not widely diagnosed due to reliance on the culture, which has failed to detect many of the anaerobes, detection of amines, which are not always present in sufficiently high amounts, and microscopic analysis, which is not available in many physicians’ practices. In addition, many women have self-diagnosed symptoms and signs of vaginal problems as being caused by yeast, and have treated themselves with over-the-counter anti-fungals, not realizing that they have BV Citation[3]. The recent finding of a 29% prevalence of BV among women in North America Citation[4] emphasizes the significance of this condition, particularly as it has been associated with an increased risk of sexually transmitted infections and preterm labour Citation[5–8].
Treatment of BV comprises oral or vaginal use of metronidazole or clindamycin, but relatively high failure rates, increased drug resistance, side effects, and common relapses make it important that alternative management strategies be found Citation[9–13]. In a study by Thomas et al. Citation[14], either oral or vaginal metronidazole did not effectively restore Lactobacillus morphotypes or lower vaginal pH, thus not meeting Federal Drug Administration (FDA) criteria for cure. In terms of chemotherapeutic effect, metronidazole is known to be active against anaerobes, but not significantly active against Gardnerella vaginalis and facultative anaerobes Citation[15]. One suggestion has been to use long-term, low dose metronidazole suppressive therapy Citation[16], similar to the way recurrent urinary tract infections are managed. Although such an approach raises concerns over inducing drug resistance, it may be the only current solution for a condition that adversely affects the quality of life of so many women. The addition of probiotic lactobacilli might be worthy of consideration given a previous study showing that this augments the effective cure of BV Citation[17]and a study showing that it can cure BV Citation[18]. To explore this possibility and examine some potential mechanisms involved in how these two regimens work together, a series of in vitro studies were undertaken to examine the effect of metronidazole on the growth of lactobacilli.
Materials and methods
Test strains
Growth inhibition in metronidazole was assessed using probiotic Lactobacillus rhamnosus GR-1, freshly isolated Lactobacillus plantarum KCA, and control strain Gardnerella vaginalis ATCC 14018.
Vaginally defined medium + peptone (VDMP)
The composition of the vaginally defined medium simulating female genital tract secretions for the growth of vaginal microbiota was described by Geshnizgani and Onderdonk Citation[19]. This was slightly modified with the addition of 0.5% proteose peptone. Briefly, stock solutions () in part 1 of the VDM consisting of NaCl (3.5 g/L), KCl (1.5 g/L), K2HPO4 (1.7 g/L), dextrose (10.8 g/L), cysteine HCl (0.5 g/L), and 0.5 g/L of proteose peptone were dissolved in 800 ml of deionized water and the volume was brought up to 877.80 ml. After adjustment of pH to 7.2 with NaOH, the solution was autoclaved for 15 min at 121°C and then cooled to room temperature. The components of part II of the VDM were added to the vessel. Hemin and vitamin K solutions were prepared separately in 1 N NaOH. Solutions 1–6 were autoclaved for 15 min at 121°C. Solutions 7–9 were sterilized by passage through a membrane filter (pore size 0.22 µM).
Table I. Vaginally defined medium + 0.5% proteose peptone (VDMP).
Growth kinetics of the strains in VDMP
The growth curves of G. vaginalis, L. rhamnosus GR-1, and L. plantarum KCA were determined by a microdilution method in VDMP. Briefly, fresh cultures of the lactobacilli strains were obtained after incubation for 24 h on MRS broth, while G. vaginalis was grown in Columbia broth and 10% of the cultures were prepared with sterile phosphate-buffered saline (PBS). From these preparations, 10 µl was added in duplicates to 240 µl of freshly prepared VDMP in a Falcon® microplate (MicrotestTM, Becton Dickinson Labware, NJ, USA). The microplate was covered with an adhesive lid and loaded in the Multiscan microreader set at 37°C for continuous kinetic optical density measured at 600 nm for 24 h.
Inhibition test
The broth microdilution technique, recommended by the National Committee for Clinical Laboratory Standards (NCCLS), was used to test bacterial susceptibility to metronidazole (SIGMAULTRA, Sigma-Aldrich, CA, USA). Fresh cultures were diluted to obtain 5×105 CFU/ml. The organisms were dispensed into microplates in triplicate with VDMP containing different concentrations of metronidazole ranging from 0 µg/ml to 8000 µg/ml. The plates were covered with a sterile adhesive lid and incubated at 37°C in a Multiscan microplate reader set for continuous kinetic optical density measured at 600 nm for 24 h. The growth kinetics for the OD at 1 h intervals were read automatically.
The growth inhibition was calculated as follows: growth inhibition (%)=[(ODmcont–ODmmet)/ODmcont]×100; where ODmcont = mean optical density of the control and ODmmet = mean optical density of the different concentrations of metronidazole.
Statistics
Growth inhibitions were calculated as percentages with respect to the total mean optical density after incubation for 24 h and different metronidazole concentrations. The optical density values at 0 µg/ml of metronidazole served as control. Comparisons between the effect on G. vaginalis, L. rhamnosus GR-1, and L. plantarum KCA were made by use of the Student's t test. Differences were regarded as significant with p value < 0.05.
Results
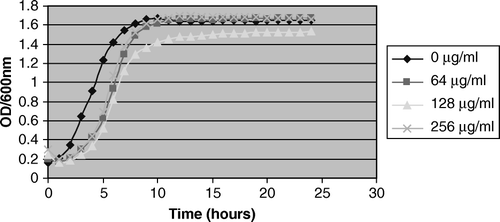
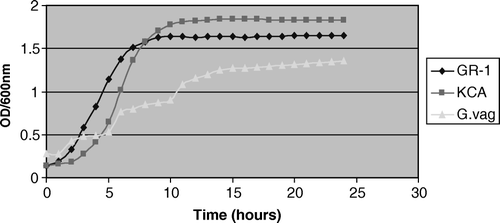
There was no significant difference in the growth kinetics of the L. rhamnosus GR-1 and L. plantarum KCA in VDMP up to 15 h. The strains attained a maximum optical density after 8–10 h of incubation (), and thereafter reached stationary phase. In contrast, there was a significant difference (p=0.004) between the growth kinetics of the tested lactobacilli strains and G. vaginalis, with the latter reaching maximum growth phase at 10% preparation within 15 h.
Figure 1. Growth curve kinetics of 10% overnight broth culture of G. vaginalis, L. rhamnosus GR-1, and L. plantarum KCA in VDMP.
As expected, the bacterial growth was markedly inhibited at high concentrations (≥4000 µg/ml) of metronidazole. However, a significant (p=0.005) stimulatory effect occurred with L. rhamnosus GR-1 at 64 µg/ml and 256 µg/ml when compared with the control before attainment of stationary phase (, ). In contrast, there was no growth stimulatory effect of metronidazole at all the tested concentrations on L. plantarum KCA and G. vaginalis ( and ). In , there was a consistent result in all the replicates as L. plantarum KCA grew at a faster rate and to a higher OD600nm in the presence of 256 µg/ml than in 128 µg/ml of metronidazole. At 1000 µg/ml of metronidazole, G. vaginalis was markedly inhibited (49.3%) compared with the L. rhamnosus GR-1 and L. plantarum KCA strains, which had growth inhibition of 7.4% and 13.3%, respectively.
Figure 2. Growth stimulation of L. rhamnosus GR-1 in 64 µg/ml and 256 µg/ml metronidazole. Note the growth stimulation of L. rhamnosus GR-1 between 0 and 10 h of incubation at 64 µg/ml of metronidazole compared with 0 µg/ml.
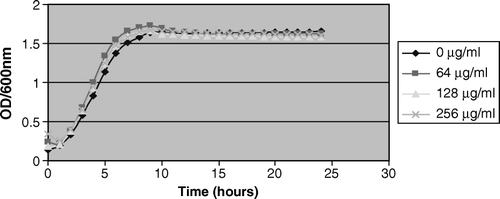
Figure 4. Comparative growth inhibition of G. vaginalis, L. rhamnosus GR-1, and L. plantarum KCA in metronidazole.
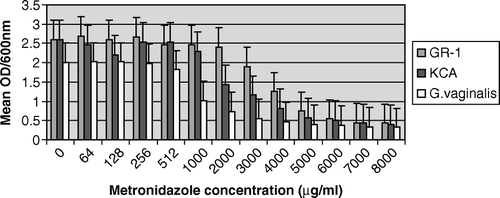
Table II. Percentage of growth inhibition of lactobacilli in different concentrations of metronidazole in the presence of vaginally defined medium with peptone.
Discussion
This is only the second study to show that a Lactobacillus strain can grow in the presence of metronidazole, following the report on eight clinical isolates growing in concentrations of ≥128 and ≤256 µg/ml Citation[20]. This raises interesting possibilities regarding the management of BV. The tendency with a standard therapeutic approach is to use high concentrations to kill the offending pathogen. In the case of BV, two problems arise: first, there are several ‘pathogens’ and it would take a broad-spectrum agent to attack each one; and second, these organisms evolve in biofilm structures that are not well penetrated by antibiotics Citation[21], perhaps explaining the poor clinical outcomes and high recurrences following metronidazole use. Furthermore, although most strains of lactobacilli can resist metronidazole Citation[22], in BV the lactobacilli are either not present or are present in depleted numbers. Thus, during therapy the chance of vaginal lactobacilli being able to grow in metronidazole is remote. Unless indigenous lactobacillus strains recolonize from anal transfer, the patient is at high risk of redeveloping the condition. This consistent disruption of the microbiota has been known for some time Citation[23], and has been confirmed in clinical trials, such as by Nyirjesy et al. Citation[24] who found no improved restoration of lactobacilli after 21–30 days of intravaginal metronidazole.
The advantage of administering probiotic lactobacilli with lower concentrations of antibiotics could improve management of BV through disruption of the biofilms, as found for L. rhamnosus GR-1 and L. iners AB-1 against G. vaginalis Citation[25]. This has been shown through inhibition of the growth and indeed killing the pathogens through hydrogen peroxide production and other mechanisms Citation[26], Citation[27], plus restoration of a lactobacilli-dominated microbiota upon completion of therapy. Such a strategy is more likely to prevent relapses and allow the healthy indigenous microbiota to redevelop, whether it comprises lactobacilli or other commensal strains Citation[28].
The finding that metronidazole concentrations >1000 µg/ml resulted in partial growth inhibition against the Gardnerella and two lactobacilli strains suggested that the recommended dose of 500 mg orally or 0.75% (7.5 mg/g) intravaginally, twice daily for 7 days would be expected to eradicate both pathogen and non-pathogen. However, an oral dose of 2 g has been found to only give a vaginal concentration of 26 µg/ml after 6 h Citation[29], raising the possibility that not only does it not adversely affect any lactobacilli that might be present in BV, but it would also not inhibit the Gardnerella. In an effort to enhance the efficacy of metronidazole, one study added lactic acid vaginal gel, with improved results and promotion of restoration of indigenous lactobacilli Citation[30].
The stimulatory effect observed with L. rhamnosus GR-1 but not L. plantarum KCA at 64 µg/ml metronidazole is interesting. It could be that at this concentration, the L. rhamnosus GR-1 was using the drug as a carbon source, but the L. plantarum strain was unable to do so. This does not mean that the L. plantarum KCA strain is any less a candidate for probiotic applications and since it was recovered from a healthy woman, and since no L. plantarum strains have been tested as vaginal probiotics, it remains worthy of further investigation. The stimulatory effect of metronidazole on Lactobacillus rhamnosus GR-1 at 64 µg/ml and 256 µg/ml but not at 128 µg/ml is surprising as the result was consistent after three repeated experiments. However, further studies are warranted to decipher whether this effect could be observed in most probiotic lactobacilli.
In summary, this study provides a rationale for the conjoint use of probiotic lactobacilli, with low dose metronidazole to better manage a condition that is highly prevalent in women.
Acknowledgements
This study was supported by grants from the CIHR and Ontario Neurotrauma Foundation. Dr Reid declares that he owns patents which include L. rhamnosus GR-1 and this strain has been licensed by Chr Hansen.
References
- Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005; 353: 1899–911
- Burton JP, Devillard E, Cadieux PA, Hammond JA, Reid G. Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. J Clin Microbiol 2004; 42: 1829–31
- Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002; 99: 419–25
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109: 114–20
- Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997; 350: 546–50
- Koumans EH, Kendrick JS. CDC Bacterial Vaginosis Working Group. Preventing adverse sequelae of bacterial vaginosis: a public health program and research agenda. Sex Transm Dis 2001; 28: 292–7
- Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 2003; 37: 319–25
- Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2007; 21: 375–90
- McGregor JA, Larson PG. King Holmes, bacterial vaginosis and women and babies. Int J Gynecol Obstet 1999; 67(Suppl 1)S9–S11
- Austin MN, Beigi RH, Meyn LA, Hillier SL. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol 2005; 43: 4492–7
- Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193: 1478–86
- Eschenbach DA. Bacterial vaginosis: resistance, recurrence, and/or reinfection?. Clin Infect Dis 2007; 44: 220–1
- Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis 2007; 44: 213–19
- Thomas KK, Sanchez S, Garcia PJ, Holmes KK. Why do different criteria for ‘cure’ yield different conclusions in comparing two treatments for bacterial vaginosis?. Sex Transm Dis 2005; 32: 526–30
- Hillier SL, Holmes KK. Bacterial vaginosis. Sexually transmitted diseases3rd edn, KK Holmes, PF Sparling, P-M Mardh, SM Lemon, WE Stamm, P Piot, et al. McGraw Hill, New York 1999; 563–86
- Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194: 1283–9
- Anukam KC, Osazuwa EO, Ahonkhai I, Ngwu M, Osemene G, Bruce AW. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 2006; 8: 1450–4
- Anukam KC, Osazuwa E. Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect 2006; 8: 2772–6
- Geshnizgani AM, Onderdonk AB. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol 1992;1323–6.
- Simoes JA, Aroutcheva AA, Shott S, Faro S. Effect of metronidazole on the growth of vaginal lactobacilli in vitro. Infect Dis Obstet Gynecol 2001; 9: 41–5
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005; 106(5 Pt 1)1013–23
- Austin MN, Meyn LA, Hillier SL. Susceptibility of vaginal bacteria to metronidazole and tinidazole. Anaerobe 2006; 12: 227–30
- Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS 1997; 8: 489–94
- Nyirjesy P, McIntosh MJ, Gattermeir DJ, Schumacher RJ, Steinmetz JI, Joffrion JL. The effects of intravaginal clindamycin and metronidazole therapy on vaginal lactobacilli in patients with bacterial vaginosis. Am J Obstet Gynecol 2006; 194: 1277–82
- Saunders S, Bocking A, Challis J, Reid G. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf B Biointerfaces 2007; 55: 138–42
- Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol 2006; 48: 424–32
- Sethi S, Das A, Sharma M. Inhibition of Gardnerella vaginalis by lactobacilli. Int J Gynaecol Obstet 2006; 93: 158–9
- Forney LJ, Foster JA, Ledger W. The vaginal flora of healthy women is not always dominated by Lactobacillus species. J Infect Dis 2006; 194: 1468–9
- Davis B, Glover DD, Larsen B. Analysis of metronidazole penetration into vaginal fluid by reverse-phase high-performance liquid chromatography. Am J Obstet Gynecol 1984; 149: 802–3
- Decena DC, Co JT, Manalastas RM, Jr, Palaypayon EP, Padolina CS, Sison JM, et al. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J Obstet Gynaecol Res 2006; 32: 243–51