Abstract
The nature and role of human milk microbiota in the early colonization and protection of infants from infection is the subject of increasing research. This study investigated the occurrence of Lactobacillus reuteri in milk of nursing mothers living in rural or urban areas in different geographical locations. Breast milk samples were collected from 220 mothers, 6–32 days after delivery, and analysed for the presence of total lactobacilli and L. reuteri. In all, 50% of mothers from rural areas in Japan and Sweden were L. reuteri-positive, whereas mothers from urban areas in South Africa, Israel and Denmark had very low or non-detectable levels. Overall, 15% of mothers had detectable L. reuteri in their milk. There were no significant differences in the prevalence of total Lactobacillus or L. reuteri in women from rural and urban habitats in the participating countries.
Introduction
Human breast milk is considered to be of primary importance for the optimal health, growth and development of the infant Citation[1]. Aside from its nutritional value, it also contains several bioactive factors that may enhance the infant's defences Citation[1], Citation[2] and protect it against colonizing pathogens Citation[3], Citation[4]. It has been suggested that commensal and potential probiotic bacterial components present in breast milk may also be involved in the development of the infant's gastrointestinal mucosal tissues and its acquisition of a healthy gut microbiota Citation[2], Citation[5]. Mother's milk is a consistent source of microorganisms for the neonatal gut during several weeks after birth, and it is only after the introduction of solid food that the gut microbiota becomes more diverse and begins to resemble that of adults Citation[5]. The bacterial species generally found in healthy human breast milk belong to the genera Staphylococcus, Streptococcus, Lactobacillus, Micrococcus, Enterococcus and Bifidobacterium Citation[4–6]. Some of these genera originate from the surface of the nipple and the surrounding skin and may be adapted to survival in the milk ducts in the breast Citation[1], Citation[5].
Lactobacilli make up an important part of the healthy human intestinal microbiota and are thought to be involved in the control and maintenance of the microbiota Citation[7]. L. reuteri is an indigenous colonizer of the gastrointestinal (GI) tract of humans and animals Citation[7], Citation[8]. L. reuteri has been demonstrated to have probiotic properties Citation[7–12] and has previously been found in breast milk of Finnish women Citation[8], Citation[13]. Growing evidence that lactobacilli colonization at a very early age may protect the infant from developing atopic allergy Citation[14] points to a possible role for a natural supply of lactobacilli in the breast milk as a beneficial factor for the suckling infant.
Little information is available in the literature concerning the presence of lactobacilli, including L. reuteri, in human breast milk. The present study was undertaken to investigate (i) the occurrence of L. reuteri in human breast milk, and (ii) the possible links between the presence of L. reuteri and the mothers’ geographic areas of residence.
Subjects and methods
Breast milk samples were collected from women living in urban or rural areas in Sweden, Denmark, Israel, South Africa, Japan, South Korea and Peru ().
Table I. Breast milk samples obtained from urban and rural areas in seven countries.
The research plan was to receive and analyse 20 samples from rural areas and 20 samples from urban areas in each of these countries for a total of 280 samples. From urban areas we received 90% of expected samples (126 of 140), and from rural areas we received 69% of the expected samples (94 of 140 samples). Only 20% and 25% of expected samples were obtained, respectively, from Japan and Denmark. Only healthy women who had not used antibiotics within the previous 2 weeks were included in the study. The participating mothers were asked to avoid intake of any food supplements containing added lactic acid bacteria for 2 weeks before providing the samples. No other restriction to their normal dietary intake with natural components of lactic acid bacteria and bacteria was made.
The mothers were between 16 and 42 years of age (mean 28±5 years), and samples were collected 6–32 days after delivery (mean 19±7 days). They were asked to refrain from breastfeeding for 1–2 h before giving the sample, which was taken in the morning. The milk was collected by the hospital staff, with the breast and nipple surfaces cleansed without disinfectants before collection. In each case, breast milk was collected aseptically using a breast milk pump, and the first 10–15 ml of milk were collected and transferred aseptically into sterile cryo-vials. The samples were then immediately frozen and stored at −20°C for a maximum of 1 month. The samples were packed with dry ice in insulated boxes (Nexpack AB, Malmö, Sweden) and sent to a laboratory in Sweden where they were kept at −80°C for a maximum of 1 month until analysis. The frozen samples were thawed overnight in a refrigerator and then maintained at room temperature for 30 min before being diluted and plated.
Two microbiological analyses were conducted on these samples. First, the total viable lactobacilli present as cfu/ml sample was determined as follows. Each sample was 10-fold serially diluted in 0.15 M NaCl. Aliquots (100 µl) from each dilution were directly plated on de Man Rogosa Sharpe agar plates (MRS; Acumedia, Ljusne, Sweden) and lactobacilli-selective agar plates (LBS; Becton Dickinson AB, Stockholm, Sweden). Plates were incubated for 48–72 h at 37°C under anaerobic conditions (BBL® GasPak anaerobic system, Becton Dickinson AB, Stockholm, Sweden).
Secondly, the following enrichment procedure was used to amplify the number of L. reuteri cells present in these samples, and thereby qualitatively assess their presence or absence in the breast milk samples. One ml of thoroughly mixed breast milk was added to 10 ml of freshly prepared Lactobacillus Carrying Medium, containing 20 mM arabinose, 20 mM ribose, 1 mM MgSO4, 0.1 mM MnSO4, 5 µM FeSO4 (Merck, Lund, Sweden). The enrichment mixture was incubated at 37°C for 6–8 h. Thus enriched, the cultures were plated onto modified MRS agar containing 0.05 mg/ml vancomycin (Sigma Chemical Co, St Louis, MO, USA) to determine their L. reuteri content. Plates were incubated for 48–72 h at 37°C under anaerobic conditions (BBL® GasPak anaerobic system). L. reuteri colonies were identified and enumerated using a method based on the L. reuteri-specific production of reuterin from glycerol under anaerobic conditions at 37°C and pH 6–8 Citation[15]. Seventy reuterin-positive isolates were randomly selected to further identify them as L. reuteri using PCR analysis.
PCR analysis
To confirm the identity of L. reuteri among the reuterin-positive colonies, isolates were randomly selected from the MRS medium, purified by streak plating and subjected to sequence analysis of 16S rDNA, performed as described by Magnusson et al. Citation[16]. Bacterial DNA was isolated from bacteria grown in MRS broth using the DNeasy™ Tissue Kit (Qiagen). 16S rDNA was amplified by PCR (94°C for 30 s, 54°C for 30 s, 72°C for 80 s, step 1–3 30 cycles, 72°C for 10 min, 4°C for ∞) using primers 16S.S (5′-AGAGTTTGATCCTGGCTC-3′; position 8–25 in Escherichia coli 16S rRNA) and 16S.R (5′-CGGGAACGTATTCACCG-3′; position 1385–1369 in E. coli 16S rRNA). The resulting PCR product (appr. 1377 bp) was purified using the Qiagen PCR purification kit. The purified fragments were sequenced according to standard methods. The first part (about 500 bp) of the 16S rDNA sequences obtained was searched for manually and levels of similarity were analysed with the GeneBank database (http://www.ncbi.nlm.nih.gov/BLAST).
Statistical analysis
The χ2 test was used to evaluate the significant difference between geographical locations (urban and rural) and the prevalence of total Lactobacillus and L. reuteri, respectively. The Mann-Whitney U test was used to explore if significant differences existed regarding Lactobacillus counts for the above-mentioned locations. A p value of < 0.05 was considered statistically significant.
Ethical aspects
Mothers participating in the study gave informed consent before giving breast milk samples. Ethical approval was obtained by the regional committee at the Karolinska Institute, Stockholm, Sweden and in those countries where it was deemed necessary.
Results
Estimates of the total lactobacilli and specifically L. reuteri present in the samples obtained from mothers in each country and in different locations are shown in a and b. L. reuteri was found in the breast milk of mothers from around the world, and of the 220 samples collected from as many mothers, 32 were found to be positive for L. reuteri, giving an overall prevalence of 15%. Breast milk from Japanese mothers showed the highest frequency of colonization with L. reuteri (11 of 20 giving positive samples). Women from other locations showed a lower prevalence of L. reuteri. Total lactobacilli were found in higher numbers in most samples examined, with 93 of 220 samples being positive (42% prevalence) in this regard. The highest prevalence was seen in South Korea and Japan. Women living in rural areas exhibited 14% of L. reuteri and 43% of total Lactobacillus in their breast milk; women living in urban areas had comparable levels of 15% and 42%, respectively.
Figure 1. (a) Prevalence of L. reuteri and lactobacilli in human breast milk from (a) rural areas and (b) urban areas in each country (%).
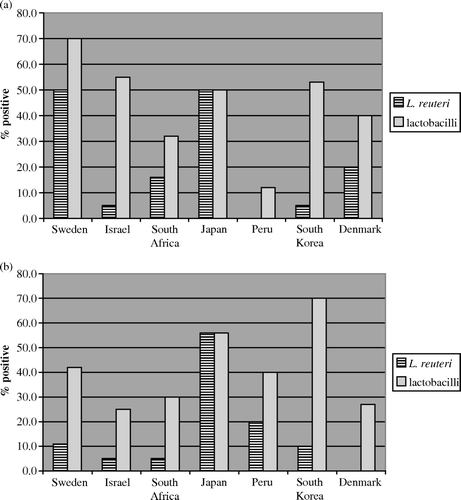
No significant differences were observed among women from rural or urban habitats (). However, women living in rural areas tended to have higher median counts of total lactobacilli in the breast milk compared with those living in urban areas (1.3×103 vs 3.0×102 cfu/ml) (see ). The highest counts were found in the breast milk from mothers in Israel, South Africa, Japan and South Korea.
Table II. Prevalence (%) of Lactobacillus and L. reuteri in rural and urban areas.
Table III. Total Lactobacillus counts in breast milk samples obtained from urban and rural areas in seven countries expressed as median values in colony-forming units (cfu) per ml breast milk for those subjects that had detectable values.
Discussion
This study is a first attempt to determine the presence of L. reuteri in human breast milk on a worldwide basis. Previous studies have shown that the initial flow of breast milk contains the most microbes, and that this is true even if the nipples have been washed before the milk is expressed Citation[17]. It is also known that the microbes most frequently isolated from breast milk are inhabitants of normal skin Citation[1]. Thus, it is not surprising that commensal staphylococci, micrococci and streptococci are among the predominant bacterial species in human milk Citation[4]. Staphylococcal species are thought to originate from the maternal skin during breastfeeding, and oral streptococci (e.g. Streptococcus salivarius) are hypothesized to be transferred from the infant mouth to the breast and from there to the milk Citation[4].
Human lactobacilli and enterococci colonize mucous membranes and can be readily isolated from the gastrointestinal and the urogenital tracts Citation[7]. The infant, however, becomes colonized during the birth process during contact with the mother's vaginal microbiota, the normal microbiota of the parents, and from the ambient environment transmitted to the mother's breast and hands during nursing. An endogenous route has also been described, namely, via dendritic cells that penetrate the gut epithelium through opening the tight junctions between intestinal epithelial cells to sample bacteria directly from the gut lumen Citation[18]. Once internalized or attached to dendritic cells (or other types of lymphocytes), bacteria may spread to other locations via the bloodstream and circulation of lymphocytes within the mucosal-associated lymphoid tissue system Citation[18]. A study of pregnant mice that were orally inoculated with a genetically labelled Enterococcus faecium strain revealed that this strain could be isolated from the amniotic fluid of the inoculated animals Citation[19]. The authors suggested that this bacterium entered the uterine environment through the bloodstream Citation[19]. Others believe this cannot be the case since the mesenteric lymph nodes act as a firewall to prevent live commensals from penetrating the systemic immune compartment Citation[20]. The fact that L. reuteri and other lactobacilli are found in the milk indicates that these bacteria have the ability to colonize the mammary ducts, in agreement with previous findings Citation[5], Citation[21]. The presence of lactic acid bacteria in the breast milk of healthy mothers may be a significant source of lactic acid bacteria for colonization of the infant gut. Specific lactic acid bacteria have been reported to have effects on the symptoms of atopic eczema and allergy Citation[14], Citation[22], Citation[23]. Based on the present study, it was determined that approximately one in seven of the mothers had L. reuteri present in their breast milk. In an earlier follow-up study with Finnish mothers (from delivery to 3 months after giving birth), it was shown that lactobacilli were present in 40% of their breast milk samples, and L. reuteri was present in 20% Citation[13]. This finding is consistent with the present study. Together, they indicate that L. reuteri is a natural microbiotic component of human milk.
The levels of lactobacilli observed in breast milk from urban and rural habitats ranged from 2.0×101 to 5.0×104 and 1.0×101 to 6.0×104 cfu/ml, respectively. Information in the literature enumerating lactic acid bacteria in breast milk is limited. Martin et al. Citation[21] have shown counts of lactic acid bacteria in breast milk to vary between 2.0×104 and 1.0×105 cfu/ml. That these numbers may be relatively low does not preclude their importance in the development of the infant's microbiome. Given the likelihood that limited numbers of L. reuteri would be found in the breast milk samples provided (i.e. below the detection limit of < 1.0×101 cfu/ml), an enrichment procedure was employed to ascertain only their presence or absence in these samples. Using this procedure it was determined that L. reuteri is present in human breast milk, and that this source may lead to colonization of the infants and subsequent growth in the developing infant gut.
The overall prevalence of lactobacilli and L. reuteri does not show any significant difference between rural and urban areas. However, there were considerable differences observed between countries and rural and urban areas, as illustrated in . Japanese women seem to have a higher prevalence of L. reuteri colonization than women from the other countries in the study. This may be related to the wide use of functional foods, probiotics and various fermented foods as an important part of the Japanese diet Citation[24]. Dietary habits and environmental conditions are known to influence the microbial composition of the gut microbiota.
It is agreed that breastfeeding is the best way to ensure that an infant receives beneficial microbiota and optimal substrates for the growth of these bacteria. These benefits are associated with a lower risk of gastrointestinal, respiratory and other infections Citation[2]. Breast milk may be considered as a natural synbiotic Citation[21], Citation[25], and evidence from this study suggests that L. reuteri is one of the beneficial components in this regard.
Conclusions
L. reuteri is found in the initial flow of human milk. It is found in a minority of nursing mothers but in countries geographically widely apart. Further studies are needed to provide a better understanding of the significance of this and other lactic acid bacteria in breast milk and their relevance to the health of the infant and mother.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Picciano MF. Nutrient composition of human milk. Pediatr Clin North Am 2001; 48: 53–67
- Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 1999; 69: 1052S–1057S
- Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr 2000; 130: 426S–431S
- Heikkilä MP, Saris PEJ. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol 2003; 95: 471–8
- Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 1999; 69: 1035S–1045S
- Martín R, Olivares M, Marín ML, Fernández L, Xaus J, Rodríguez JM. Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J Hum Lact 2005; 21: 8–17
- Reuter G. The Lactobacillus and Bifidobacterium microbiota of the human intestine: composition and succession. Curr Issues Intest Microbiol 2001; 2: 43–53
- Casas IA, Dobrogosz WJ. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 2000; 12: 247–85
- Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 2004; 70: 1176–81
- Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 2007; 119: 124–30
- Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhoea in young children. J Pediatr Gastroenterol Nutr 1997; 24: 399–404
- Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005; 115: 5–9
- Karvonen AV, Vesikari T, Casas IA. Lactobacillus and Lactobacillus reuteri: mother-child vinculum at birth and during early age of infants. In: 2nd World Congress on Anaerobic Bacteria and Infections, Nice, France, 3–6 October 1998, p183 (abstract).
- Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in the primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001; 357: 1076–9
- Chung TC, Axelsson L, Lindgren SE, Dobrogosz WJ. In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Ecol Health Dis 1989; 2: 137–44
- Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett 2003; 219: 129–35
- West PA, Hewith JH, Murphy OM. The influence of methods of collection and storage on the bacteriology of human milk. J Appl Bacteriol 1979; 46: 269–77
- Rescigno M, Urbano M, Valsazina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001; 2: 361–7
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 2005; 51: 270–4
- Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med 2006; 203: 497–500
- Martín R, Langa S, Reviriego C, Jiménez E, Marín ML, Xaus J, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003; 143: 754–8
- Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning?. Gut 2002; 51: 51–5
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Velerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003; 111: 389–95
- Arai S, Osawa T, Ohigashi H, Yoshikawa M, Kaminogawa S, Watanabe M, et al. A mainstay of functional food science in Japan – history, present status, and future outlook. Biosci Biotechnol Biochem 2001; 65: 1–13
- Gueimonde M, Laitinen K, Salminen S, Isolauri E. Breast milk: a source of Bifidobacteria for infant gut development and maturation?. Neonatology 2007; 92: 64–6