Abstract
Objective:Aspergillus niger is one of the fungi exploited industrially for the production of various metabolites including the enzyme phytase. Intensive sporulation is a serious crisis in using this fungus, hence, in the present study an attempt was made to obtain an asporulating strain. Materials and methods:A. niger CFR 335, a local phytase-producing isolate, was induced to physical mutation by ultraviolet (UV) radiation and chemical mutation using different concentrations of ethyl methane sulphonate (EMS) and N-methyl N′-nitro N-nitrosoguanidine (NTG). Results: UV exposure for 30 min, 10 mM EMS and 25 µg ml−1 of NTG were found to be optimum for maximum killing effect of 26.3%, 95% and 80% of the spores, respectively. In this experiment, a stable auxotrophic mutant (ala−) of A. niger CFR 335 with suppressed sporulation was obtained. The auxotrophic mutant M-1 (A. niger CFR 335 ala−) showed an improved specific activity of 1.26 U mg−1 as compared with the parent strain (0.85 U mg−1). Conclusion: Sporulation was drastically reduced in the mutant M-1 without affecting phytase yield, whereas the production rate was proportional to the rate of sporulation in the parent strain (A. niger CFR 335).
Introduction
Phytic acid (myo-inositol hexakisphosphate) is a storage form of phosphorus in plants Citation[1], legumes Citation[2] and oil seeds Citation[3], Citation[4]. The most striking chemical impact of phytic acid is its strong chelating ability with multivalent cations, to form cation–phytic acid complexes Citation[5]. Minerals of concern in this regard include phosphorus, calcium, zinc, iron, copper and magnesium. In the context of human and animal nutrition, the following two aspects of phytic acid are critically important Citation[6], Citation[7]. (i) Monogastric animals have only low levels of phytate-degrading enzymes in their digestive tracts, and since phytic acid itself is not resorbed, feed for pigs and poultry is commonly supplemented with inorganic phosphate to meet the phosphorus requirements of these animals. (ii) Phytic acid is an antinutrient factor, since it complexes with proteins and a variety of metal ions and therefore decreases the dietary availability of these nutrients. The antinutritive properties, its value as a phosphorus source and its role in causing eutrophication have prompted researchers to develop a method to remove phytic acid completely from the feed or to hydrolyse it economically using phytase enzyme.
The phytases (myo-inositol hexakisphosphate 3- and 6-phosphohydrolases; EC 3.1.3.8 and 3.1.3.26) are a subfamily of the histidine acid phosphatases Citation[8] and are found naturally in plants and microorganisms, particularly fungi. With the growth of the biotechnology industries, filamentous fungi, a principal source of enzymes and metabolites, have been widely employed in traditional food fermentation processes, such as the koji process Citation[9]. Features such as low cost and high productivity have attracted many research efforts in both molecular genetic techniques and bioprocess improvements Citation[10]. Exploitation of aspergilli began early with the use of Aspergillus niger and Aspergillus oryzae for the production of citric acid and taka-amylase through fermentation. Although several other fungi are currently in use, A. niger remains a favourite for industries. This may be attributed to its GRAS status, great secretory potential and the wealth of information accrued on its fermentation. Of all the organisms surveyed, A. niger was found to produce the most active extracellular phytase Citation[11]. Various strains of A. niger have been shown to produce three extracellular acid phosphatases, a meso-inositol hexaphosphate phosphohydrolase (E.C. 3.1.3.8) (Phyt A phytase) with two pH optima (2.5 and 5.0) and a non-specific phosphomonoesterase (E.C.3.1.3.2) (Phyt B) with a pH optimum of 2.0–2.5 that hydrolyses phytin Citation[12–15].
Apart from good yield of phytase enzyme, the fungus poses a serious threat to the researchers in rendering potential risk for the handlers. The fungus produces large amounts of dark conidiospores that hamper the extraction of enzyme and cause health risks such as allergic bronchopulmonary aspergillosis if not handled properly Citation[16], Citation[17]. A strain with phytase overproduction and lower sporulation rate is of high value from an industrial point of view. With this aim, the present study focused on developing a strain of A. niger CFR 335 with asporogenicity or delayed sporulation through induced mutations without any adverse effect on phytase production.
Materials and methods
Strain
A. niger CFR 335, a native isolate from soil samples obtained from poultry livestock areas, was used in the present study (). About 50 different soil samples were screened for the isolate. The culture was grown for 4–5 days at 30°C on complete medium (CM) slants in triplicate. Completely grown slants were maintained in the refrigerator until further use.
Media
Complete medium (CM) comprised (g/l): yeast extract 2.5, malt extract 5.0, glucose 10.0 and agar 20.0; pH 5.5±0.2. Minimal medium (MM) comprised (g/l): yeast nitrogen base without amino acid and ammonium sulphate 1.7, succinic acid 10.0, sodium hydroxide 6.0, glucose 2.0 and agar 20.0; pH 5.8±0.2. Phytase screening medium (PSM) was as described by Howson and Davis Citation[18].
A CAMAG UV chamber was used for physical mutation. Ethyl methane sulphonate (EMS) and N-methyl N-nitro N-nitrosoguanidine (NTG) and all the amino acids were obtained from Sigma Co., USA. The chemicals used for the phytase assay were obtained from Sigma and from Qualigen Chemicals, India Ltd.
Mutagenesis by UV radiation
Young spores, 16–18 h old, were harvested using 0.1% Tween-80. Then 1 ml samples of the spore suspension containing 2.4×106 cells ml−1 were subjected to UV irradiation for different time exposures (10, 20 and 30 min) in a sterile petri dish using a CAMAG UV chamber, which emits radiations of 254 nm at a distance of 25 cm Citation[19]. The experiment was carried out in triplicate. The spore suspensions were intermittently agitated during the course of irradiation. The treated spore suspensions were then incubated at 4°C in the dark overnight for any DNA repair to take place. Suspensions were suitably diluted and appropriate dilutions were plated on CM and incubated for 4–5 days at 30°C. Spore-killing percentage was plotted against UV exposure.
Mutagenesis by EMS
Aliquots of 1 ml of the spore suspensions harvested as described above were treated with different concentrations of EMS (2, 4, 6, 8 and 10 mM) in sterile distilled water Citation[19]. The suspensions were incubated for 1 h at 30°C with a brief agitation. This experiment was carried out in triplicate. After incubation, the mutagen was completely removed by centrifugation at 2000 rpm. The spore suspensions were thoroughly washed twice with sterile distilled water, resuspended in 0.01 M sterile buffered saline (pH 7.0) and then refrigerated overnight in the dark before plating for any DNA repair to take place. Suitably diluted spore suspensions were plated on CM and incubated for 4–5 days at 30°C. The percentage of spore killing was plotted against the concentration of EMS.
Mutagenesis by NTG
Aliquots of 1 ml of the spore suspensions were treated with different concentrations of NTG (5, 10, 15, 20 and 25 µg ml−1). The suspensions were incubated for 1 h at 30°C with a brief agitation. The experiment was carried out in triplicate. After incubation, the mutagen was completely removed by centrifugation at 2000 rpm. The spore suspensions were thoroughly washed twice with sterile distilled water, resuspended in 0.01 M sterile buffered saline (pH 7.0) and then refrigerated overnight in the dark before plating for any DNA repair to take place. Suitably diluted spore suspensions were plated on CM and incubated for 4–5 days at 30°C. The percentage of spore killing was plotted against the concentration of NTG.
Characterization of putative mutants for auxotrophy
The UV, NTG and EMS mutagenized colonies grown on CM were replica plated using sterile velvette cloth on minimal medium (MM) plates and incubated for 24–36 h at 30°C Citation[20]. The colonies that did not grow on MM plates were picked up from the master plate (CM) and individually tested for their growth factor requirement by inoculating into a liquid minimal medium containing appropriate concentrations of individual amino acids Citation[21]. About 2000 colonies were screened for auxotrophic strains with amino acid markers. Growth of the culture in tubes supplemented with a particular amino acid indicated its amino acid requirement and was marked as the respective auxotroph. The experiment was carried out in triplicate and repeated to confirm the growth and stability of the mutant.
Characterization of putative mutants for reduced sporulation
About 500 putative mutants with morphological variations were screened for spore suppression. Spores were harvested from all the mutants after cultivating on CM plates for up to 10 days. The spores were counted using a haemocytometer (Feinoptic Bad Blankenburg, Germany) and were expressed as number of spores ml−1. This was compared with the parent strain (A. niger CFR 335) after confirmation by repeated experiments and the average consistent result of the percentage reduction in sporulation was reported.
Characterization of putative mutants for phytase production
About 62 putative mutants with reduced sporulation were qualitatively screened for phytase enzyme by plating them on PSM containing 0.5% calcium phytate as substrate. About 10 positive mutants were selected based on the size of the zone of hydrolysis around the colony and quantitatively screened for phytase enzyme production by cultivating them in a wheat bran medium. Fresh wheat bran (50 g) was placed in a 1 L Erlenmeyer flask and autoclaved for 40 min at 121°C. The total moisture was maintained at 60% level with sterile distilled water. The medium was inoculated with 2.4×106 cells ml−1 of each of the mutants and incubated at room temperature for 5 days in well-aerated conditions. The crude enzyme was extracted from each of the mutant flasks using 1:5 w/v sodium acetate (0.2 M) buffer, pH 4.5.
Phytase assay
The crude enzyme preparation was quantitatively assayed for phytase enzyme by the method of Heinonen and Lahti Citation[22]. The assay was initiated by mixing 1 ml of diluted (1:10) crude enzyme with 0.5 ml of sodium acetate (0.2 M) buffer, pH 4.5, and 0.5 ml of sodium phytate (15 mM) (Sigma). The reaction mixture was incubated at 40°C in a water bath for 45 min. The reaction was terminated by adding 2 ml of 15% trichloroacetic acid. Assay mixture (0.5 ml) was then mixed with 4 ml of 2:1:1 v/v of acetone, 10 mM ammonium molybdate and 5 N sulphuric acid (AAM solution) and 0.4 ml of citric acid (1 M). The amount of free phosphate released was determined spectrophotometrically at 355 nm. A standard graph was plotted using potassium dihydrogen phosphate with working concentration ranging from 30 to 360 µM. Protein quantifications were made by the method of Bradford Citation[23] and compared with the standard prepared using bovine serum albumin.
The phytase production by different mutants obtained through induced mutation of A. niger CFR 335 was presented as the standard error means (±SEM). The linear and quadratic contrasts were determined by SAS Citation[24] for different concentrations of EMS and NTG used for spore-killing percentage.
Results and discussion
Non-ionizing radiations such as UV at the wavelength of 254 nm is absorbed by DNA leading to thymine dimers that cause mispairing during DNA replication, which leads to permanent mutation if not repaired. Thus mutation is frequently employed in the field of microbiology for improving industrially important microorganisms for product development. Young spores of A. niger CFR 335 are often sensitive to induced mutation by UV radiation, as observed in the present study.
The results obtained in the present study indicated the highest spore-killing rate of 26.3% when the spores were exposed to UV for 30 min, which was 16% higher than the minimum killing rate of 10.2% when exposed for 10 min. With increase in the exposure time, killing rate increased and an inhibitory effect on growth was observed in CM. An improved catalytic efficiency and thermostability of Escherichia coli, pH 2.5, acid phosphatase/phytase expressed in Pichia pastoris was achieved by site-directed mutagenesis Citation[25]. A similar kind of expression of gene of A. fumigatus wild-type phytase and the mutant genes were expressed in P. pastoris GS115 by site-directed mutagenesis with PCR Citation[26], and the results revealed an increased specific activity of the Q23L from 0.51 U mg−1 of the wild type to 1.09 U mg−1 at pH 5.5. Similarly, in the present study an improvement in the specific activity of the parent strain from 0.85 U mg−1 to 1.26 U mg−1 of the mutant M-1 was observed (). However, the exact mechanism and site of mutation are not known. An increase in phytase activity of A. niger NRRL 3135 phytase A (phy A) has been shown at intermediate pH levels (3.0–3.5) by site-directed mutagenesis of its gene at amino acid residue 300 Citation[27]. It was shown that a single mutation, K300E, improved phytic acid hydrolysis at 37°C by 56% and 19% at pH 4.0 and 5.0, respectively.
Table I. Phytase production of A. niger CFR 335 and its 10 mutants.
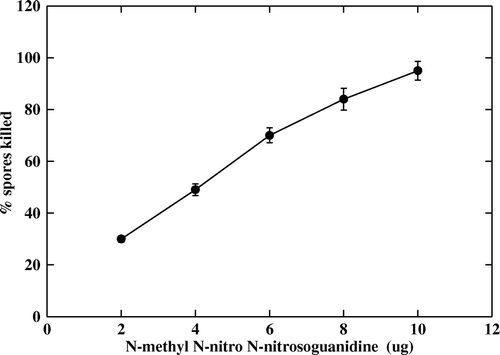
The results showed a highest spore killing rate of 95% when 10 mM ml−1 of EMS was used and a lowest killing rate of 32% when treated with 2 mM ml−1 of EMS. The killing percentage increased with the increase in the concentration of EMS (). A maximum killing rate of 80% was observed when the spores were treated with 25 µg ml−1 of NTG, while a minimum killing rate of ≥15% was observed with 5 µg ml−1 of NTG. Beyond 25 µg ml−1 concentration of NTG there was 100% killing of the spores (). Thus, from the results it was observed that the killing percentage of the spores was proportional to the concentration of both EMS and NTG.
Figure 2. Effect of ethyl methane sulphonate (EMS) on A. niger CFR 335 spores. Linear effect of EMS on spore killing was significant (p < 0.001).
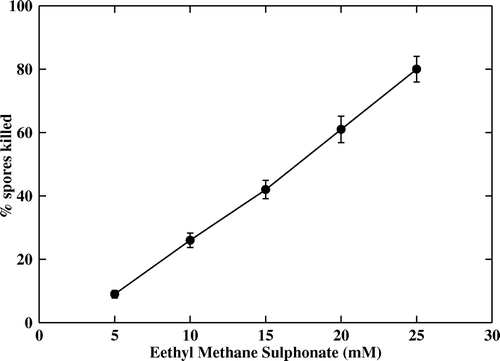
Figure 3. Effect of N-methyl N-nitro N-nitrosoguanidine (NTG) on A. niger CFR 335 spores. Linear effect of NTG on spore killing was significant (p < 0.001).
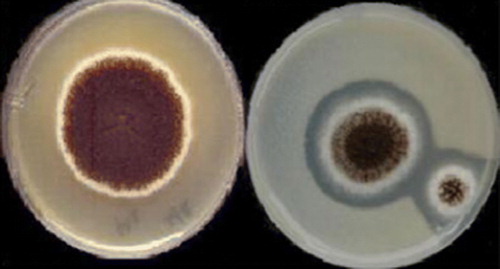
The parent strain (A. niger CFR 335), auxotrophic mutant (A. niger CFR 335 ala−) and other unstable auxotrophic mutants are shown in . Even though the relationships between amino acid auxotrophy and colony morphology variations were concisely proved, the exact mechanism of occurrence of such a phenotype is not known. Amino acid auxotrophy might play a vital role in suppressing the spore formation, which is supported by the present observation. Of the 2000 colonies screened for auxotrophy, 2 colonies were found to be auxotrophic for alanine, valine and isoleucine. The mutant that had val− and isoleu− markers reverted back to its original form after repeated subculturing, whereas the ala− auxotroph remained stable even after several subcultures.
Figure 4. Centre plate shows parent strain (A. niger CFR 335) and the surrounding plates show its mutants.
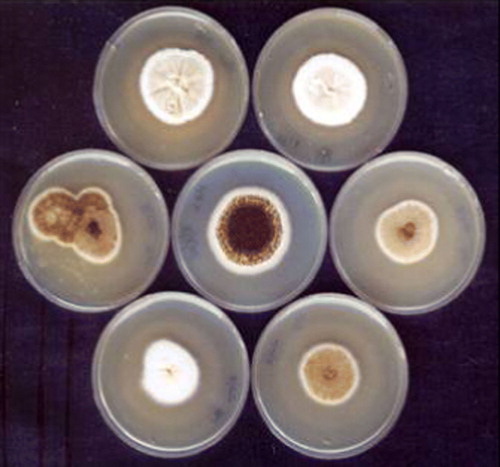
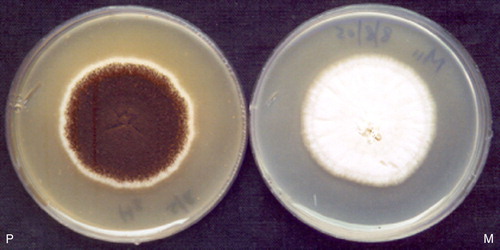
A. niger CFR 335 ala− auxotrophic mutant () showed about 88% reduction in the sporulation compared with the wild strain counterpart, which was confirmed by repeated cultivation in nutrient medium. The sporulation rate of the parent strain was found to be 3.2×106 ml−1 and the auxotrophic mutant had a rate of 3.84×105 ml−1. This mutant also showed morphological variations with white vegetative mycelium, unlike its parent counterpart, which is pale yellow in colour with black conidiospores (). Quantitatively, the production of phytase by the auxotroph did not differ much with respect to the parent strain. A similar kind of experiment was carried out and an improved strain of A. niger showing 3.2-fold higher extracellular phytase activity was developed where hygromycin resistance was used as the mutant selection marker Citation[28]. It was shown that the mutation of phiA, a gene from A. nidulans, resulted in reduced growth and severely reduced sporulation. The abnormality is traced to the phialides, which divided several times instead of forming a single flask-shaped cell. The importance of phiA for phialide and conidium development was supported by immunohistochemistry experiments that showed the protein to be mainly present in these two cell types. The possibility that changed expression of phiA is correlated with growth arrest, which is caused by inhibited V-ATPases, was suggested Citation[29]. It was also revealed that the mutation of PkcA allele of calC2 strain of A. nidulans showed an alteration in hyphal growth and conidiogenesis Citation[30].
In conclusion, the results showed that 30 min of UV exposure, 10 mM EMS and 25 µg ml−1 of NTG were effective in producing maximum killing effect of A. niger CFR 335 spores. An auxotrophic mutant of A. niger CFR 335 with a trait of delayed sporulation was obtained in the present study that may reduce the risk of allergic bronchopulmonary aspergillosis caused by long-term handling of the spores. The mutant also retained its characteristic of phytase yield, which is beneficial from the industrial point of view. Asporulation is an important genotypic change that bears relationship with the alteration of a particular gene responsible for the phialide and conidia formation. The possibility of the involvement of membrane diffusion caused by mutagens cannot be ruled out for delayed sporulation. Studies of this sort of investigation on fungal asporulation, particularly A. niger, are very sparse. Thus, such studies could open a new area in the field of microbiology and biotechnology, wherein numerous health risks related to fungal spores could be evaded. A study investigating the exact site of mutation leading to delayed sporulation is in progress.
Acknowledgements
The authors are grateful to the University of Mysore for a Research Fellowship and they are also thankful to the Director, Central Food Technological Research Institute, Mysore, India for permitting them to work at the Food Microbiology Department of the Institute. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Suzuki M, Tanaka K, Kuwano M, Yoshida KT. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): implications for the phytic acid biosynthetic pathway. Gene 2007; 405: 55–64
- Piddington CS, Houston CS, Paloheimo M, Cantrell M, Miettinen-Oinonen A, Nevalainen H, et al. The cloning and sequencing of the genes encoding phytase (phy) and pH 2.5-optimum acid phosphatase (aph) from Aspergillus niger var. awamori. Gene 1993; 133: 55–62
- Haddad J, Greiner R, Allaf K. Effect of instantaneous controlled pressure drop on the phytate content of lupin. LWT Food Sci Technol 2007; 40: 448–53
- Grases F, Perello J, Prieto RM, Simonet BM, Torres JJ. Dietary myo-inositol hexaphosphate prevents dystrophic calcifications in soft tissues: a pilot study in Wistar rats. Life Sci. 2004; 75: 11–19
- Pagano AR, Yasuda K, Roneker KR, Crenshaw TD, Lei XG. Supplemental Escherichia coli phytase and strontium enhance bone strength of young pigs fed a phosphorus- adequate diet. J Nutr 2007; 137: 1795–801
- Reddy NR, Sathe SK, Salunkhe DK. Phytates in legumes and cereals. Adv Food Res 1982; 28: 1–92
- Wang C, Eufemi M, Turano C, Giartosio A. Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry 1996; 35: 7299–307
- Mitchell DB, Vogel K, Weimann B, Pasamontes L, van Loon APGM. The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 1997; 143: 245–52
- Hara S, Kitamoto K, Gomi K. New developments in fermented beverages and foods with Aspergillus. Aspergillus: biology and industrial applications, JW Bennett, MA Klich. Butterworth-Heinemann, Boston 1992; 133
- Banerjee AC, Kundu A, Ghosh SK. Genetic manipulation of filamentous fungi. New horizons in biotechnology, S Roussos. Kluwer Academic Publishers, Dordrecht, The Netherlands 2003; 193–8
- Gunashree BS, Venkateswaran G. Effect of different cultural conditions for phytase production by Aspergillus niger CFR 335 in submerged and solid-state fermentations. J Ind Microbiol Biotechnol 2008; 35: 1587–96
- Omogbenigun FO, Nyachoti CM, Slominski BA. The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. J Anim Sci 2003; 81: 1806–13
- Sariyska MV, Gargova SA, Koleva LA, Angelov AI. Aspergillus niger phytase: purification and characterization. Biotechnol Biotechnol Eq 2005; 19: 98–105
- Ha NC, Kim YO, Oh TK, Oh BH. Preliminary X-ray crystallographic analysis of a novel phytase from a Bacillus amyloliquefaciens strain. Acta Crystallogr D Biol Crystallogr 1999; 55: 691–3
- Ehrlich KC, Montalbano BG, Mullaney EJ, Dischinger HC, Ullah AHJ. Identification and cloning of a second phytase gene (Phyt B) from Aspergillus niger (ficuum). Biochem Biophys Res Commun 1993; 195: 53–6
- Hideaki H, Shigeru T, Hayato K, Takashi S, Hirotsugu T, Akihisa F, et al. Allergic bronchopulmonary aspergillosis due to Aspergillus niger without bronchial asthma. Respiration 1999; 66: 369–72
- Müller A, Lehmann I, Seiffart A, Diez U, Wetzig H, Bort M, et al. Increased incidence of allergic sensitisation and respiratory diseases due to mould exposure: results of the Leipzig Allergy Risk children Study (LARS). Int J Hyg Environ Health 2002; 204: 363–5
- Howson SJ, Davis RP. Production of phytate-hydrolyzing enzyme by some fungi. Enzyme Microbiol Technol 1983; 5: 377–82
- Usha T, Venkateshwaran G, Sarada R, Ravishankar GA. Studies on Haematococcus pluvialis for improved production of astaxanthin by mutagenesis. World J Microbiol Biotechnol 2001; 17: 143–8
- Venkateswaran, G. Lipid modification in Rhodotorula gracilus: biochemical and genetic studies. PhD thesis, University of Mysore, India, 1997.
- Holliday RA. New method for the identification of biochemical mutants of microorganisms. Nature 1956; 178: 987
- Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatases. Anal Biochem 1981; 113: 313–17
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein dye binding. Anal Biochem 1976; 72: 248–54
- SAS Institute. SAS/STAT guide or personal computers. Ver. 6.03. SAS Inst., Cary, NC, 1988.
- Rodriguez E, Wood ZA, Karpus PA, Lie XG. Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris. Arch Biochem Biophys 2000; 382: 105–12
- Gu WN, Yang PL, Wang YR, Luo HY, Meng K, Wu NF, et al. Mutation research on Q23L and Q23LG272E in phytase derivated from Aspergillus fumigatus. Sheng Wu Gong Cheng Xue Bao 2007; 23: 273–7
- Mullaney EJ, Daly CB, Kim T, Porres JM, Lei XG, Sethumadhavan K. Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biochem Biophys Res Commun 2002; 297: 1016–20
- Chelius MK, Wodzinski RJ. Strain improvement of Aspergillus niger for phytase production. Appl Microbiol Biotechnol 1994; 41: 78–83
- Petter M, Schnürer J, Gerhart E, Wagner H. Characterization of phiA, a gene essential for phialide development in Aspergillus nidulans. Fungal Genet Biol 2003; 40: 234–41
- Teepe AG, Loprete DM, He Z, Hoggard TA, Hill TW. The protein kinase C orthologue PkcA plays a role in cell wall integrity and polarized growth in Aspergillus nidulans. Fungal Genet Biol 2007; 44: 554–62