ABSTRACT
In this issue, we cover an exceptional topic in Vertebrate Paleobiology that has been an enjoyable challenge for scientists and the popular media alike: the life and death of the Pleistocene cave bear (Ursus spelaeus). As an icon of the ice-age, the cave bear inhabited the glacial ecosystems of Eurasia, and it was the inspiration of a popular book written in 1976 by Björn Kurtén, entitled The cave bear story: life and death of a vanished animal. Although ‘The life and death’ was a summary of the knowledge acquired on cave bear biology at that time, four decades later, many aspects of its palaeoecology, extinction and evolution are still a matter of debate. With this volume, we aim to bring together the most recent research on cave bear biology in order to provide an update on the palaeoecology, biogeography, systematics, and phylogeny of this recently extinct ursine bear. We thus organised a symposium on the 1st of August 2017 as part of the three-day Annual Meeting of the European Association of Vertebrate Palaeontologists (EAVP) in Munich, Germany, that was an additional opportunity to announce the volume and to discuss this exciting subject face-to-face among specialists.
KEYWORDS:
Cave bear research since the publication of ‘The Life and Death’
In this section, we give a panoramic view of the studies performed on cave bear biology since the publication of ‘The Life and Death’ to date, which help us to identify the state-of-the-art and the gaps that require further research in cave bear paleobiology.
To do this, we searched in Scopus for articles including in their title, abstract, or keywords ‘cave bear’ including original peer-review articles, articles in press, conference papers, book chapters and letters. Unfortunately, our search does not cover all the research done on cave bears during the last decades because non-indexed journals and most conference proceedings are not indexed in Scopus. For example, this is the case of the contributions presented by researchers at the International Cave Bear Symposium (ICBS), which is held annually, and where researchers present their recent discoveries and advances on the cave bear fossil record and evolution. Although some of the proceedings of the ICBS are indexed in Scopus, the majority of them are not, which introduce a bias in our analysis concerning the research activity on cave bear research.
Our search retrieved 347 publications, of which only 243 documents were selected using the criterion of treating the cave bear biology as the main topic of the paper or using the cave bear as a case study for developing new analytic tools. For each entry in the dataset, we collected the year of publication, the subject area as provided by Scopus, and we classified each paper within the following topics: (i) Diet, (ii) Phylogeny, (iii) Extinction, (iv) Sexual dimorphism, (v) Taxonomy and Anatomy, (vi) Taphonomy, (vii) Population ecology, (viii) Pathology, (ix) Faunal interaction with coeval mammals including hominins, and (x) Physiology, including metabolism, encephalization, body mass and growth.
The results of this search highlight that empirical research on the cave bear biology has grown since the publication of Kurtén’s book ()). Of course, this result is probably biased by the difficulty of finding indexed papers before the nineties in Scopus, and this is probably the reason why there are not retrieved papers during the late seventies. Despite this, within our analysed time interval, four clear peaks in the number of publications are observed in 2001, 2004, 2011, and 2014, among which 2001 and 2014 being the most extreme, with 23 and 25 publications published for each year, respectively. The first peak corresponds to an increase in the use of stable isotopes as an ecophysiological tool to decipher different aspects of the cave bear biology such as metabolism or diet. This year also coincides with the publication of the extended works presented in the proceedings of VI ICBS, edited by Dr Aurora Grandal-d’Anglade in the Cadernos do Laboratorio Xeoloxico de Laxe. The second peak coincides with the publication of a special issue in Quaternary International co-edited by Professor Hervé Bocherens and Dr. Martina Pacher on Late Quaternary mammal ecology, taking advantage of the refinement of methods based on stable isotopes, different dating procedures, and ancient DNA.
Figure 1. Bibliographic summary on cave bear biology since the publication of the ‘Life and Death’ (1976) up to date performed in Scopus. (a) number of total publications per year (black line) and the moving average (dotted line); (b) percentage of publications published in each subject area; (c) institutions that have contributed substantially to the knowledge of the cave bear biology; (d) percentage of publications in each topic of research.
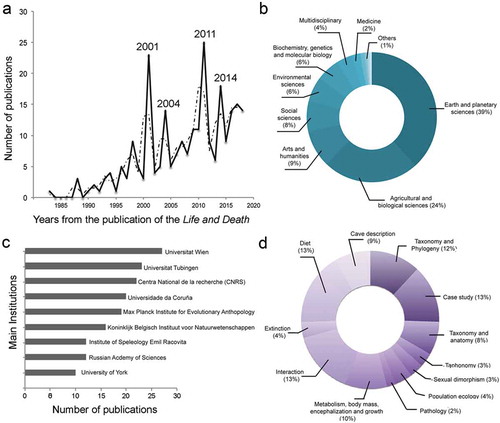
Although most of the cave bear articles have been published in journals within the areas of ‘Earth and planetary sciences’ or ‘Agricultural and biological sciences’, research on cave bear biology has also been published in journals belonging to very different areas such as the ‘Arts and humanities’ or ‘Biochemistry, genetics, and molecular biology’ ()). This is clear evidence of the multidisciplinary nature of the research on the cave bear, as it represents a model system to study different topics covered from different scientific disciplines. All this research has been performed across different institutions from different countries, and our research survey highlights the University of Vienna, the University of Tubingen, and the French funding agency Centre National de la Recherche Scientifique (CNRS) as the main centres, where most of the research on cave bears has been performed ()).
The main topics of most publications are diet (e.g. Bocherens et al. Citation1994, Citation1999, Citation2011; Bocherens Citation2018; Figueirido et al. Citation2009; Grandal-d’Anglade Citation2010; Mattson Citation1998; Pacher and Stuart Citation2009; Peigné et al. Citation2009; Pinto Llona et al. Citation2005; Richards et al. Citation2008; Van Heteren et al. Citation2009, Citation2014, Citation2016), interaction with other coeval mammals including hominins (e.g. Stiner Citation1999; MüNzel and Conard Citation2004; Bocherens et al. Citation2006; Torres et al. Citation2007; Abrams et al. Citation2014), as well as publications that use the cave bear as study case for developing or testing new analytical tools such as ancient DNA, isotopic biochemistry, or paleohistology (e.g. Debeljak Citation1996; Torres et al. Citation2003; Stiller et al. Citation2009; Stiller Citation2012; Bocherens et al. Citation2014; Leiss-Holzinger et al. Citation2015), each topic with 13% of the retrieved papers, respectively ()). Similarly, a high number of publications address taxonomy and phylogeny (e.g. Loreille et al. Citation2001; Rabeder and Nagel Citation2001; Hofreiter et al. Citation2002, Citation2004; Orlando et al. Citation2002; Rabeder and Hofreiter Citation2004; Rabeder et al. Citation2004a, Citation2004b; Stiller et al. Citation2014) with 12% of the retrieved papers. In contrast, other less studied topics were less studied, such as body mass, encephalization, growth patterns and metabolism, representing only 10% of the papers (e.g. Viranta Citation1994; Nelson et al. Citation1998; Vila-Taboada et al. Citation1999; Fernández-Mosquera et al. Citation2001; Pérez-Rama et al. Citation2011; Fuchs et al. Citation2015; Veitschegger Citation2017). Strikingly, there is a rising interest for this topic during recent years, most probably due to the advantages of new three-dimensional analytical tools.
Studies on descriptive anatomy with taxonomic purposes, which represent 8% of the total (e.g. McFarlane et al. Citation2011; Goubel et al. Citation2012), as well as studies on cave description with new cave bear remains (e.g. Pacher Citation2001; Martini et al. Citation2014; Rabal-Garcés and Sauqué Citation2015), representing 9% of the retrieved papers ()), reflect that new caves are constantly being discovered and the fossil remains within them need taxonomic assignment. However, most of such articles are published in local journals or ICBS conference proceedings, both not recorded by Scopus. Least studied topics include the cave bear extinction (4%), sexual dimorphism (3%), population ecology (4%) or pathology (2%), among others.
Our analysis of the literature evidences that some areas of cave bear biology are less explored than others, revealing the need to develop more research on these neglected topics. However, it is worth mentioning that our results could be ambiguous as several papers classified within Diet or Interaction with other coeval mammals treat also the extinction of the cave bear (e.g. Stiller et al. Citation2014).
Continuing the cave bear story: recent advances on its life and death
In this issue, a collection of innovative papers presents state of the art research about the taxonomy and phylogenetics of the ‘cave bear group’ as revealed by molecular and morphological evidence, through other papers about metabolism and life history, to more controversial aspects related to its palaeoecology and extinction chronology is presented. During the last decade various authors have found genomic, isotopic and morphological evidence to split the traditional cave bear (Ursus spelaeus sensu lato) into two species Ursus ingressus and Ursus spelaeus, the later including three subspecies: U. spelaeus speleaeus, U. spelaeus ladinicus, and U. spelaeus eremus.
The majority of the invited contributors use cutting-edge research techniques such as ancient DNA, 3D geometric morphometrics, biogeochemical analysis of stable isotopes, or radiocarbon methods for chronology, which ensure a cross-disciplinary nature of the special issue. Both mini-reviews on the specific evidence, as well as significant contributions covering new data, results, and interpretations with interest for a large multidisciplinary audience are covered by the collection.
Bocherens (Citation2018) reviews the isotopic variation in more than 300 cave bear bones and discusses the factors influencing this variability according to trophic levels and variations among plants, as well as specific physiological aspects. Comparing isotopic data of cave bears with coeval Late Pleistocene large mammals, Bocherens (Citation2018) concludes that cave bears were almost exclusively herbivorous in all analysed populations, arguing that the consumption of plants with high δ15N values, such as graminoids, forbs and possibly fungi, may explain the observed isotopic pattern.
Döppes et al. (Citation2018) provide new radiocarbon dates on specific populations of Alpine cave bears and accomplish a thorough revision of the ‘Height Dependent Extinction Line’ (HDEL), which is a compilation of the geologically most recent radiocarbon dates per altitude level. The authors conclude that a general cooling at the beginning of the Last Glacial Maximum (LGM) lead to the extinction of the cave bear in the middle of the cold stadial GS-3 (Baca et al. Citation2016). Moreover, the populations of lower altitudes went extinct earlier than those populations of higher altitudes (around 1,500 m a.s.l.), most probably because increasing aridity and subsequent expansion of steppes at the Alpine foothills.
Grandal-d’Anglade et al. (Citation2018) tackle the cave bear’s hibernation from isotopic biogeochemistry to derive important conclusions on its physiology and behaviour. Analysing isotopic values of cave bears in different ontogenetic stages, the authors show that perinatal values reflect the values for mothers during hibernation, while juveniles show differences in maternal investment. This new data together with previous evidence that each cave housed a single lineage of mitochondrial DNA haplotypes, suggest extreme fidelity to the birth site (i.e. homing behaviour; Fortes et al. Citation2016) and that cave bears formed stable maternal social groups for hibernation.
Knapp (Citation2018) presents a review on the conclusions reached by different authors about cave bear ecology, phylogeography and its possible extinction causes from a genetic perspective. Moreover, Knapp (Citation2018) discusses the directions of future genomic research to provide further insights on the biology of the cave bear.
Peigné and Merceron (Citation2017) review and compare the investigation of dental wear as a tool for inferring the diet of extinct mammals, and specially the cave bear. The authors also analyse several specimens of cave bears from Goyet cave (Belgium) using dental microwear texture analysis. Peigné and Merceron (Citation2017) provide evidence of dietary flexibility of cave bears during the predormancy period.
Pérez-Ramos et al. (Citation2018) present a 3D analysis of tooth-root morphology of all living bear species to make dietary inferences on the cave bear group. The authors conclude that the cave bears exhibit a great dietary flexibility, but most probably, they were more herbivorous than any living bear belonging to Ursus. Pérez-Ramos et al. (Citation2018) also discuss the influence of the altitude were the remains of cave bears were found with their dietary differences.
Terlato et al. (Citation2018) present chronometric, isotopic and taphonomic evidence from two Palaeolithic cave bear sites in northeastern Italy and revisit the debate about the extinction and paleoecology of the cave bear in the light of this new evidence. The authors show two new radiocarbon dates on well-preserved collagen (24,200–23,500 cal yr BP). Strikingly, these are the latest known representatives of the cave bear in Europe, several cave bear bones are carrying human-made butchering marks, and their isotopic values (δ13C and δ15N) of their bone collagen are similar to those of older cave bears from the Swabian Jura and France, suggesting that feeding behaviour of cave bears experienced few changes until their extinction in Europe.
Van Heteren et al. (Citation2018) present an innovative paper in which they analyse cranium and mandible shape of Ursus deningeri, a middle Pleistocene ‘speloid’ bear, in comparison with the late Pleistocene Ursus spelaeus, as well as with all living bear species. The authors show that U. deningeri and U. spelaeus mandibles are very similar, concluding that masticatory adaptations to an herbivorous diet were already present in the Middle Pleistocene.
Van Heteren and Figueirido (Citation2018) tackle an ecomorphological approach, using 3D geometric morphometrics of the cave bear mandible as well as of all living bear species, to assess for feeding adaptations in cave bears. The authors conclude that the mandible of the cave bear have specific traits indicative of a highly-herbivorous diet, or at least, more herbivorous than their closest living relative, the brown bear (Ursus arctos). Moreover, they propose new avenues for future research to make more refined inferences on its feeding behaviour that could be key to understand the ‘life and death’ of this vanished animal.
Veitschegger et al. (Citation2018) summarise the literature on age estimation and mortality patterns in cave bears. They also present novel data on longevity from several European localities retrieved from incremental lines of tooth cementum showing that the typical duration of the life of cave bears was ninety years, and they present twenty nine years as the oldest known age for the Middle Pleistocene cave bear, Ursus deningeri. Additionally, Veitschegger et al. (Citation2018) investigate the relative tooth emergence pattern of the permanent dentition under the Schultz’s rule framework, revealing that cave bears exhibis a heterochronic shift, which is interpreted in terms of a slightly faster life history than closely related species.
Acknowledgments
We acknowledge all the contributors to this special issue for being willing to participate in this update on cave bear biology. We also thank several people that helped us during the organization of the cave bear symposium within the annual conference of the European Association of Vertebrate Palaeontology (EAVP) held in Münich in August 2017. We are also especially grateful to Aurora Grandal d’Anglade and Hervé Bocherens for reviewing this Preface. We are also very grateful to the editor in chief of Historical Biology, Dr. Gareth Dyke, for his advice and support during the editorial work of this special issue.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abrams G, Bello SM, Di Modica K, Pirson S, Bonjean D. 2014. When Neanderthals used cave bear (Ursus spelaeus) remains: bone retouchers from unit 5 of Scladina Cave (Belgium). Quat Int. 326-327:274–287.
- Baca M, Popović D, Stefaniak K, Marciszak A, Urbanowski M, Nadachowski A, Mackiewicz P. 2016. Retreat and extinction of the Late Pleistocene cave bear (Ursus spelaeus sensu lato). Sci Nature. 103:92.
- Bocherens H. 2018. Isotopic insights on cave bear palaeodiet. Hist Bio. doi:10.1080/08912963.2018.1465419
- Bocherens H, Billiou D, Patou-Mathis M, Otte M, Bonjean D, Toussaint M, Mariotti A. 1999. Palaeoenvironmental and Palaeodietary implications of isotopic biogeochemistry of late interglacial Neandertal and mammal bones in Scladina Cave (Belgium). J Archaeol Sci. 26:599–607.
- Bocherens H, Drucker DG, Billiou D, Geneste J-M, van der Plicht J. 2006. Bears and humans in Chauvet Cave (Vallon-Pont-d’Arc. Ardèche France): insights stable isotopes radiocarbon dating bone collagen. J Hum Evol. 50:370–376.
- Bocherens H, Fizet M, Mariotti A. 1994. Diet, physiology and ecology of fossil mammals as inferred by stable carbon and nitrogen isotopes biogeochemistry: implications for Pleistocene bears. Palaeogeogr Palaeocl. 107:213–225.
- Bocherens H, Grandal-d’Anglade A, Hobson KA. 2014. Pitfalls in comparing modern hair and fossil bone collagen C and N isotopic data to reconstruct ancient diets: a case study with cave bears (Ursus spelaeus). Isotopes Environ Health Stud. 50:291–299.
- Bocherens H, Stiller M, Hobson KA, Pacher M, Rabeder G, Burns JA, Tütken T, Hofreiter M. 2011. Niche partitioning between two sympatric genetically distinct cave bears (Ursus spelaeus and Ursus ingressus) and brown bear (Ursus arctos) from Austria: isotopic evidence from fossil bones. Quatern Int. 245(2):238–248.
- Debeljak I. 1996. A simple preparation technique of cave bear teeth for age determination by cementum increments. Rev Paléobiol. 15:105–108.
- Döppes D, Rabeder G, Frischauf C, Kavcik-Graumann N, Kromer B, Lindauer S, Friedrich R, Rosendahl W. 2018. Extinction pattern of Alpine cave bears - new data and climatological interpretation. Hist Biol. doi:10.1080/08912963.2018.1487422
- Fernández-Mosquera D, Vila-Taboada M, Grandal-d’Anglade A. 2001. Stable isotopes data (δ13C, δ15N) from the cave bear (Ursus spelaeus): a new approach to its palaeoenvironment and dormancy. Proc R Soc Lond B Biol Sci. 268:1159–1164.
- Figueirido B, Palmqvist P, Pérez-Claros JA. 2009. Ecomorphological correlates of craniodental variation in bears and paleobiological implications for extinct taxa: an approach based on geometric morphometrics. J Zool. 277:70–80.
- Fortes GG, Grandal-d’Anglade A, Kolbe B, Fernandes D, Meleg IN, García-Vázquez A, Frischauf C. 2016. Ancient DNA reveals differences in behaviour and sociality between brown bears and extinct cave bears. Mol Ecol. 25(19):4907–4918.
- Fuchs M, Geiger M, Stange M, Sánchez-Villagra MR. 2015. Growth trajectories in the cave bear and its extant relatives: an examination of ontogenetic patterns in phylogeny. BMC Evol Biol. 15:239.
- Goubel H, Auguste P, Crônier C, Germonpré M. 2012. Intra-specific morphological variability in the cave bear Ursus spelaeus (Mammalia, Carnivora, Ursidae) from the Trou du Sureau (Montaigle caves, Belgium) using an outline analysis. Geodiversitas. 34:961–975.
- Grandal-d’Anglade A. 2010. Bite force of the extinct Pleistocene cave bear Ursus spelaeus Rosenmüller from Europe. CR Palevol. 9:31–37.
- Grandal-d’Anglade A, Pérez-Rama M, García-Vázquez A, González-Fortes GM. 2018. The cave bear’s hibernation: reconstructing the physiology and behaviour of an extinct animal. Hist Biol. doi:10.1080/08912963.2018.146844
- Hofreiter M, Capelli C, Krings M, Waits L, Conard N, Münzel S, Weiss G, Meyer S, Paunovic M, Jambrĕsić G, et al. 2002. Ancient DNA analyses reveal high mitochondrial DNA sequence diversity and parallel morphological evolution of late pleistocene cave bears. Mol Biol Evol. 19:1244–1250.
- Hofreiter M, Rabeder G, Jaenicke V, Withalm G, Nagel D, Paunovic M, Jambrsi G, Pääbo S. 2004. Evidence for reproductive isolation between cave bear populations. Curr Biol. 14:40–43.
- Knapp M. 2018. From a molecules’ perspective – contributions of ancient DNA research to understanding cave bear biology. Hist Bio. doi: 10.1080/08912963.2018.1434168
- Leiss-Holzinger E, Wiesauer K, Stephani H, Heise B, Stifter D, Kriechbaumer B, Spachinger SJ, Gusenbauer C, Withalm G. 2015. Imaging of the inner structure of cave bear teeth by novel non-destructive techniques. Palaeontol Electron. 18:1–15.
- Loreille O, Orlando L, Patou-Mathis M, Philippe M, Taberlet P, Hänni C. 2001. Ancient DNA analysis reveals divergence of the cave bear, Ursus spelaeus, and brown bear, Ursus arctos, lineages. Curr Biol. 11(3):200–203.
- Martini I, Coltorti M, Mazza PP, Rustioni M, Sandrelli F. 2014. The latest Ursus spelaeus in Italy, a new contribution to the extinction chronology of the cave bear. Quat Res. 81:117–124.
- Mattson DJ. 1998. Diet and morphology of extant and recently extinct northern bears. Ursus. 10:479–496.
- McFarlane DA, Sabol M, Lundberg J. 2011. A unique population of cave bears (Carnivora: ursidae) from the Middle Pleistocene of Kents Cavern, England, based on dental morphometrics. Hist Bio. 23:131–137.
- MüNzel S, Conard NJ. 2004. Cave bear hunting in Hohle Fels Cave in the Ach Valley of the Swabian Jura. Rev Paléobiol. 23(2):877–885.
- Nelson DE, Angerbjörn A, Lidén K, Turk I. 1998. Stable isotopes and the metabolism of the European cave bear. Oecologia. 116:177–181.
- Orlando L, Bonjean D, Bocherens H, Thenot A, Argant A, Otte M, Hänni C. 2002. Ancient DNA and the population genetics of cave bears (Ursus spelaeus) through space and time. Mol Biol Evol. 19:1920–1933.
- Pacher M. 2001. New excavation campaigns in the Upper Pleistocene cave bear site Potocka zijalka, Slovenia-state of investigation. Cadernos Lab. Xeolóxico de Laxe 26: 301–310.
- Pacher M, Stuart AJ. 2009. Extinction chronology and palaeobiology of the cave bear (Ursus spelaeus). Boreas. 38:189–206.
- Peigné S, Goillot C, Germonpré M, Blondel C, Bignon O, Merceron G. 2009. Predormancy omnivory in European cave bears evidenced by adental microwear analysis of Ursus spelaeus from Goyet, Belgium. PNatl Acad Sci. 106(36):15390–15393.
- Peigné S, Merceron G. 2017. Palaeoecology of cave bears as evidenced by dental wear analysis: a review of methods and recent findings. Hist Biol. 1–13.
- Pérez-Rama M, Fernández-Mosquera D, Grandal-d’Anglade A. 2011. Effects of hibernation on the stable isotope signatures of adult and neonate cave bears. Quaternaire. 4:79–88.
- Pérez-Ramos A, Kupczik K, van Heteren AH, Rabeder G, Grandal-D’ Anglade A, Pastor FJ, Serrano FJ, Figueirido B. 2018. A three-dimensional analysis of tooth-root morphology in living bears and implications for feeding behaviour in the extinct cave bear. Hist Bio. doi:10.1080/08912963.2018.1525366
- Pinto Llona AC, Andrews PJ, Etxebarría F. 2005. Taphonomy and palaeoecology of quaternary bears from Cantabrian Spain (Grafinsa. In: Oviedo. Spain). Oviedo: Fundación Oso de Asturias.
- Rabal-Garcés R, Sauqué V. 2015. A new Pleistocene cave bear site in the high mountains of the Spanish Pyrenees: la Brecha del Rincón (Huesca, Spain). Comptes Rendus Palevol. 14:311–320.
- Rabeder G, Hofreiter M. 2004. Der neue Stammbaum der alpinen Höhlenbären. Die Höhle. 55(1–4):58–77.
- Rabeder G, Hofreiter M, Nagel D, Withalm G. 2004a. New taxa of alpine cave bears (Ursidae, Carnivora). Cahiers scientifiques Hors série. 2:49–67.
- Rabeder G, Hofreiter M, Withalm G. 2004b. The systematic position of the Cave Bear from Potočka zijalka (Slovenia). Mitt Komm Quartärforsch Österr Akad Wiss. 13:197–200.
- Rabeder G, Nagel D. 2001. Problemas filogenéticos de los osos de las cavernas alpinos. Cadernos do Laboratorio Xeolóxico de Laxe: Revista de xeoloxía galega e do hercínico peninsular. 26:359–364.
- Richards MP, Pacher M, Stiller M, Quilès J, Hofreiter M, Constantin S, Zilhão J, Trinkaus E. 2008. Isotopic evidence for omnivory among European cave bears: late Pleistocene Ursus spelaeus from the Peştera cu Oase, Romania. Proc Natl Acad Sci USA. 105(2):600–604.
- Stiller M. 2012. Case study: targeted high-throughput sequencing of mitochondrial genomes from extinct cave bears via direct multiplex PCR sequencing (DMPS). Methods Mol Biol. 840:171–176.
- Stiller M, Knapp M, Stenzel U, Hofreiter M, Meyer M. 2009. Direct multiplex sequencing (DMPS)—a novel method for targeted high-throughput sequencing of ancient and highly degraded DNA. Genome Res. 19:1843–1848.
- Stiller M, Molak M, Prost S, Rabeder G, Baryshnikov G, Rosendahl W, Münzel S, Bocherens H, Grandal-d’Anglade A, Hilpert B, et al. 2014. Mitochondrial DNA diversity and evolution of the Pleistocene cave bear complex. Quatern Int. 339:224–231.
- Stiner M. 1999. Cave Bear Ecology and Interactions with Pleistocene Humans. Ursus. 11:41–58.
- Terlato G, Bocherens H, Romandini M, Nannini N, Hobson KA, Peresani M. 2018. Chronological and Isotopic data support a revision for the timing of cave bear extinction in Mediterranean Europe. Hist Bio. doi:10.1080/08912963.2018.1448395
- Torres T, Ortiz JE, Cobo R. 2003. Features of deep cave sediments: their influence on fossil preservation. Estudios geológicos. 59:195–204.
- Torres T, Ortiz JE, Cobo R, de Hoz P, García-Redondo A, Grün R. 2007. Hominid exploitation of the environment and cave bear populations. The case of Ursus spelaeus Rosenmüller-Heinroth in Amutxate cave (Aralar, Navarra-Spain). J Hum Evol. 52:1–15.
- Van Heteren AH, Arlegi M, Santos E, Arsuaga JL, Gómez-Olivencia A. 2018. Cranial and mandibular morphology of Middle Pleistocene cave bears (Ursus deningeri): implications for diet and evolution. Hist Biol. doi:10.1080/08912963.2018.1487965
- Van Heteren AH, Figueirido B. 2018. Diet reconstruction in cave bears from craniodental morphology: past evidences, new results and future directions. Hist Biol. doi:10.1080/08912963.2018.1547901
- Van Heteren AH, MacLarnon A, Rae TC, Soligo C. 2009. Cave bears and their closest living relatives: a 3D geometric morphometrical approach to the functional morphology of the cave bear Ursus spelaeus. Slovensky´ Kras Acta Carsologica Slovaca. 47(supplement 1):33–46.
- Van Heteren AH, MacLarnon AM, Soligo C, Rae TC. 2014. Functional morphology of the cave bear (Ursus spelaeus) cranium: a threedimensional geometric morphometric analysis. Quatern Int. 339–340:209–216.
- Van Heteren AH, MacLarnon AM, Soligo C, Rae TC. 2016. Functional morphology of the cave bear (Ursus spelaeus) mandible: a 3D geometric morphometric analysis. Org Divers Evol. 16:299–314.
- Veitschegger K. 2017. The effect of body size evolution and ecology on encephalization in cave bears and extant relatives. BMC Evol Biol. 17:124.
- Veitschegger K, Kolb C, Amson E, Sánchez-Villagra MR. 2018. Longevity and life history of cave bears – a review and novel data from tooth cementum and relative emergence of permanent dentition. Hist Bio. doi:10.1080/08912963.2018.1441293
- Vila-Taboada M, Fernández Mosquera D, López González F, Grandal D’Anglade A, Vidal Romaní JR. 1999. Paleoecological implications inferred from stable isotopic signatures (δ13C, δ15N) in bone collagen of Ursus spelaus ROS.-HEIN. Cadernos Do Laboratori Xeolxic De Laxe. 24:73–87.
- Viranta S. 1994. Limb bone proportions and body mass of the cave bear (Ursus spelaeus). Hist Bio. 7:239–250.
