ABSTRACT
The Oligocene rodent assemblage from Gözükızıllı (Anatolia, Turkey) is described. The assemblage is relatively small, but it contains the new large and hypsodont baluchimyine Daxneria fragilis nov. gen. nov. sp. In addition, the ctenodactyline Sayimys, two new species of Eucricetodon: E. ruber nov. sp. and E. oculatus nov. sp. are present. Rare are the glirids Bransatoglis and Peridyromys/Microdyromys and a dipodid that could not be classified. The assemblage contains Asian and some European elements and is dated as early part of the late Oligocene (29–26 Ma). The small fauna from Gözükızıllı is important because only a few Paleogene rodent faunas are known from Asia Minor.
Introduction
Few Paleogene rodent faunas have been described from Asia Minor and south-eastern Europe. Recently several late Eocene and early Oligocene faunas have been reported from southern Serbia (de Bruijn et al. Citation2018a). Late Oligocene rodent assemblages from Bosnia-Herzegovina and Serbia are Banovići (de Leeuw et al. Citation2011; de Bruijn et al. Citation2013), Ugljevik and Paragovo (van de Weerd et al. Citationin prep.). Ünay et al. (Citation2003a) summarized the data on the five known Oligocene rodent faunas from Anatolia (Turkey); two of those, Gözükızıllı-1 and Yeniköy, have so far remained undescribed. Despite geographic proximity, the Oligocene faunas from the Balkans and Asia Minor show large compositional differences, underscoring the paleogeographic complexity of the region. In general these assemblages contain limited Western European elements, in contrast, the influence from Asia and the Indian sub-continent is strong. New data is for that reason welcome as we are far from understanding the paleo-biogeography.
Below we study the assemblages from Gözükızıllı-1 and nearby Güvendik, both located in the Çankiri-Çorum basin. Although large samples from the Gözükızıllı-1 site were screen-washed, the assemblages contain a low number of species. Among these are two new species of the cricetid Eucricetodon and a new baluchimyine genus. Eucricetodon is a common element in the Oligocene of Europe and fairly common in Asia. Baluchimyinae are initially described as a ctenodactyloid rodent subfamily from the Oligocene of the Indian sub-continent, it is now included in the mainly African infraorder of the Hystricognathi. Several studies (Marivaux and Boivin Citation2019 and references therein) of the Hystricognathi detail the relations between the Asian Baluchimyinae and the several African families. The cheek teeth of the new baluchimyine genus from Turkey are large and show a peculiar type of hypsodonty, this and its dental pattern are unlike any other genus of this subfamily. Several of the Paleogene faunas from Asia Minor contain baluchimyines, suggesting that the subfamily may have been fairly common in the region during that time slice. However, the subfamily is absent in the much better known latest Oligocene and early Miocene of the region.
The Gözükızıllı-1 locality
The first rodent teeth from the lower part of the Kızılırmak Formation near Gözükızıllı in the Çankiri-Çorum basin were collected by Engin Ünay formerly with the Turkish Geological Survey (MTA) and Nuredtin Kaymakcı (Middle East Technical University) in 1997. Teams of Utrecht University and the MTA made the collection that is described below during 1999 and 2000. Purpose was to date the non-marine Kızılırmak and Güvendik Formations as part of the geological mapping of the region. In total approximately 1200 kg of sediment was washed and sieved from grey silty clays of two levels at the Gözükızıllı-1 site (40° 09ʹ 9.0”N, 34° 02ʹ 5.34”E, ), the lower horizon Gözükızıllı-1a and the stratigraphically 1.5 m higher horizon −1b. Specimens from these horizons have been labelled GOZ1a and GOZ1b. Most of the washed sediment was from 1b; the differences between −1a and −1b are not significant and in the systematic descriptions below, the samples are not differentiated. The rodents found suggest a late Oligocene age (Ünay et al. Citation2003a). During a later visit by a team of the MTA and the Muséum National d’Histoire Naturelle (Paris, France) some fragmentary larger mammal fossils were found near Gözükızıllı-1, in a site named Gözükızıllı-2 and near Kızılırmak, about 21 km to the north, in the upper member of the formation (Antoine et al. Citation2008). A detailed map of the area, with Gözükızıllı-1 and −2 is in Antoine et al. (Citation2008). Métais et al. (Citation2016) describe a third site, Gözükızıllı-3, and positioned the three sites in a stratigraphic section measured near the village Gözükızıllı.
Figure 1. Satellite image of the area near Gözükızıllı village with the two sites where fossil rodents have been collected. Image from Google Earth, date 19/06/2020, copyright 2020 CNES/Airbus, 2020 Google, 2020 Basarsoft
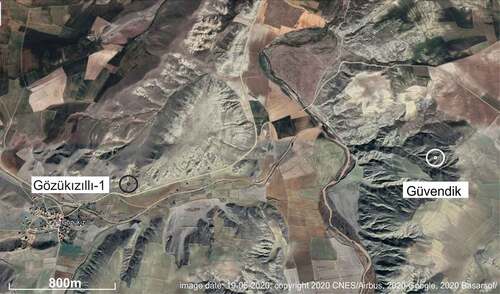
The Güvendik site is situated in the Güvendik Formation about 3 km west of Gözükızıllı village 40° 09ʹ 13.48”N and 34° 03ʹ 53.32”E (). The fossiliferous bed was detected by Engin Ünay, a clay sample of approximately 60 kg was taken in 1999 for screen-washing.
The basin-fill of the Çankiri-Çorum basin has been described by Karadenizli (Citation2011), a summary is in Métais et al. (Citation2016). The non-marine Tertiary sequence starts with about 1150 m coarse-clastics of the Incik Formation interpreted as alluvial fan, braided-river and sandy meandering-river deposits. This is overlain by the Güvendik Formation, which includes bedded gypsum, anhydrite, laminated mudstones, massive mudstones and sandstones. The unit is deposited under evaporitic lake conditions. The Kızılırmak Formation overlies and is partly lateral of the Güvendik Fm. It consists of massive mudstones, sandstones and conglomerates. Interpretations of its depositional environments range from deep-channel braided-river in its lower parts to floodplains with meandering rivers in its upper part. Kayseri-Özer et al. (Citation2017) reported on the climatic analysis of a few pollen samples from the Güvendik Formation. This analysis suggests a mixed mesophytic forest with elements indicating a dry and warm subtropical climate with low seasonality.
Methods
The material studied has been obtained by wet-screening fossiliferous sediments in the field on a set of fixed sieves, finest mesh is 0.5 mm. Concentrates have been treated with acetic acid to reduce the quantity and sorted to the 0.5 mm fraction under a microscope. Length and width of the teeth were measured with a Leitz Ortholux microscope with mechanical stage and measuring clocks. The measurements are given in millimetre units. Lower case letters refer to the lower dentition, upper case letters refer to the upper dentition. All figured specimens are shown as from the right side, if the original is from the left side the character on the plates has been underlined. Figured specimens from Gözükızıllı-1 are retouched SEM photographs. Other illustrations were prepared from photographic material made available by Laurent Marivaux (Université de Montpellier) or copied from published papers. A description of the method to prepare sections of incisors is available as electronic supplementary material with the online version of de Bruijn et al. (Citation2018b).
Systematic Paleontology
Order Rodentia Bowdich, Citation1821
Suborder Ctenohystrica Huchon, Catzeflis and Douzery, Citation2000
Infraorder Hystricognathi Tullberg, Citation1899
Subfamily Baluchimyinae Flynn et al., Citation1986
Introduction
The high-crowned cheek teeth described in the following were initially considered to represent a aberrant ctenodactylid (Ünay et al. Citation2003a). However, the reduction of the posterior side of the M3 and the presence of a large mesoconule on the DP4 and M1 and M2 point to the Baluchimyinae, a subfamily described from the early Oligocene of Pakistan (Flynn et al. Citation1986; Flynn and Cheema Citation1994) with six new genera. Marivaux et al. (Citation2002) and Marivaux and Welcomme (Citation2003) added several species and a new genus of similar age from Baluchistan (Pakistan) to this group of rodents. Marivaux et al. (Citation2000) described a new baluchimyine rodent from the late Eocene from Thailand and Sallam et al. (Citation2009) included Ottomania de Bruijn et al. (Citation2003) and Confiniummys de Bruijn et al. (Citation2003) into the baluchimyines, thus extending the geographic range of the subfamily from Thailand to Asia Minor.
The large Ottomania and the small Confiniummys from the latest Eocene of Süngülü (Eastern Anatolia) were initially included by de Bruijn et al. (Citation2003) in the family Ctenodactyli-dae de Bruijn et al. (Citation2003) noted the resemblance of Ottomania with the baluchimyines, but the genera were included in the ctenodactylids because of their supposedly non-molariform P4/p4. However, these P4/p4 were misidentified, the correctly associated P4/p4 are molariform and the two genera belong to the baluchimyines.
A single M1 or M2 from Kavakdere in the Turkish Thrace basin, classified as Ctenodactyloidea indet. by Ünay-Bayraktar (Citation1989), is with its large metaconule a typical baluchimyine. De Bruijn et al. (Citation2003) link the specimen to Ottomania, we assign it to Lindsaya Flynn et al. (Citation1986), although it is much larger than the single species included in the genus. The half molar illustrated by Gabunia and Bendukize (Citation1990, ) from Benara (Georgia) is very similar to the specimen of Ünay-Bayraktar and may be included in the same genus. The large M1 or M2 from Benara illustrated in Gabunia and Bendukidze (Citation1990, ) is possibly a baluchimyine, but it does not seem to resemble any genus known to date.
Figure 2. Nomenclature of tooth parts of Daxneria nov. gen. described in this paper (adapted after Flynn et al. Citation1986).
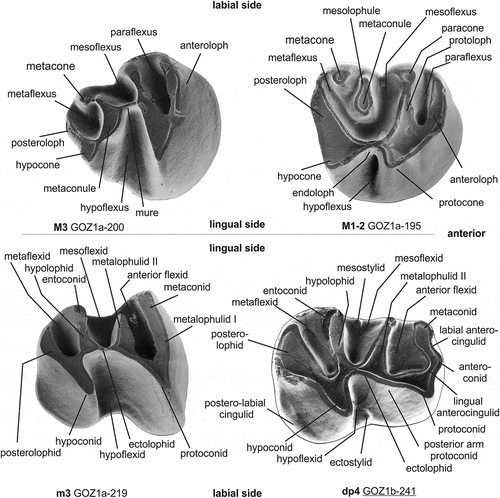
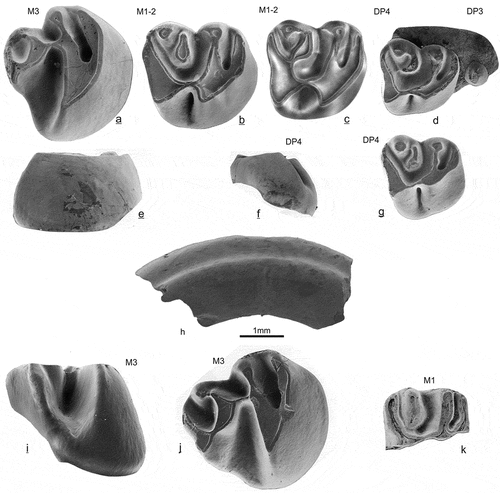
Figure 3. Upper cheek teeth of Daxneria fragilis nov. gen. nov. sp. (a-j) and Sayimys sp. (k) from Gözükızıllı-1, occlusal, lingual or antero-lingual view. Daxneria fragilis: a & e GOZ1b-237, b GOZ1a-195, c GOZ1b-233, d GOZ1b-277, f & g GOZ1b-228, h upper incisor, no number, i & j GOZ1a-199. Sayimys sp.: k GOZ1b-251
Phylogenetic studies of Asian and North African hystrico-gnathous rodents including baluchimyines are by Marivaux et al. (Citation2002), Sallam et al. (Citation2011, Citation2012), Barbière and Marivaux (Citation2015), Sallem and Seiffert (Citation2016) and Marivaux and Boivin (Citation2019). These studies focus on the phylogeny of hystricognathous rodents and the relations between Asian and North African taxa. There is general agreement that hystricognaths originated from an Asian stock of ctenodactyloids and made the Tethys Ocean crossing to Africa as early as the middle Eocene. Marivaux and Boivin (Citation2019) proposed several models of Tethys crossings (including return trips) that could explain the results of their phylogenetic research. None of the phylogenetic studies cited above formalized the taxonomic relations suggested by the various cladograms. A relevant outcome is that the Asian baluchimyines are polyphyletic, splitted since the middle Eocene. Keeping in mind that rodent cheek teeth are notorious in developing convergent and parallel dental patterns, we refrain from taking a position on the possible phylogenetic subdivisions and continue to indicate the Asian, potentially hystrico-gnathous , taxa as baluchimyines. We include the following genera in this clearly heterogeneous group:
Baluchimys Flynn et al., Citation1986; Pakistan
Lindsaya Flynn et al., Citation1986; Pakistan
Lophibaluchia Flynn et al., Citation1986; Pakistan
Hodsahibia Flynn et al., Citation1986; Pakistan
Asterattus Flynn and Cheema, Citation1994; Pakistan
Bugtimys Marivaux et al., Citation2002; Pakistan
Ottomania De Bruijn et al., Citation2003; Turkey
Confiniummys De Bruijn et al., Citation2003; Turkey
Daxneria nov. gen. Gözükızıllı-1; Turkey
The type locality of Ottomania and Confiniummys is latest Eocene, whereas the three sites in Pakistan (Y-GSP-417, Z108 and Paali nala C2) have been dated as latest-early Oligocene, slightly older than Gözükızıllı-1 (see below). Zindapiria Flynn and Cheema (Citation1994), originally included in the Baluchimyinae, is considered a ‘dipodoid rodent’ (Marivaux and Welcomme Citation2003; Marivaux et al. Citation2004). The terminology used in the description of the dental elements is shown in .
Daxneria nov. gen.
Derivatio nominis:
Named after Dr Gudrun Daxner, eminent scientist, in recognition of her studies and management of the Mongolian-Austrian project on the Oligocene of the Valley of Lakes (Mongolia) and Neogene Austrian fossil rodents.
Type species: Daxneria fragilis nov. sp.
Diagnosis:
Large baluchimyine with high-crowned molars, thin enamel and without cement. The M3 and m3 are anteriorly higher than posteriorly. DP4 and M1-2 with a large metaconule. M3 with broad anterior side (anteroloph, protocone, protoloph) and reduced narrow posterior side (hypocone-metaloph-posteroloph). Metalophulid II long and well-developed on dp4 and lower molars. The dp4 is relatively short, it has a chevron-shaped transverse anterior lophid formed by the protoconid, the metaconid and the anterocingulids.
Differential diagnosis:
Daxneria fragilis shows some superficial resemblance with high-crowned ctenodactylid species such as the early Miocene Sayimys giganteus López-Antoñanzas et al. (Citation2004), but differs from all ctenodactylids in the presence of the large and well-developed metaconule on DP4 and M1-2 and the reduced posterior side of the M3. A similar structure of the M3 and a large metaconule on M1-2 and DP4 occur in baluchimyine species from the Indian subcontinent, hence its allocation to that group. However, crown-height, size and position of the metaconule clearly differentiates Daxneria from all the known baluchimyine genera.
Daxneria fragilis nov. sp.
(, 4a-l, 5, 7 v-y, 8 t-w)
Figure 4. Lower cheek teeth Daxneria fragilis nov. gen. nov. sp. and Sayimys sp. (lower row) from Gözükızıllı-1, occlusal and labial views. Daxneria fragilis: a & e GOZ1a-219, b GOZ1b-247, c & f GOZ-1b-248, d & g GOZ1b-241, h & i GOZ1a-220, j & k GOZ1a-202, l lower incisor no number. Sayimys sp.: m & n GOZ1b-253, o & p GOZ1b-252
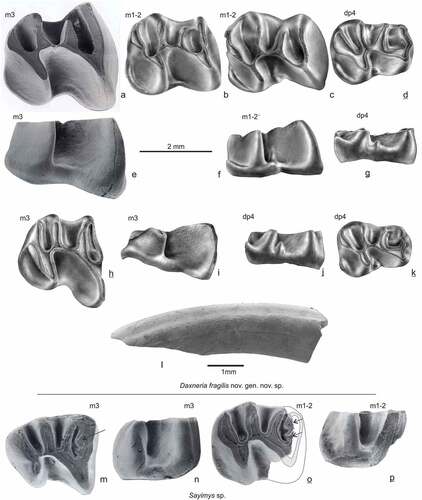
Figure 5. Sections of the lower incisor of Daxneria fragilis nov. gen. nov. sp. a-c transverse sections; b enlargement of square in a, c enlargement of square in b. d-f sagittal sections: e enlargement of square in d, f enlargement of square in e
Derivatio nominis: fragilis meaning fragile; its molars are fragile because of their thin enamel.
Synonymy: Ctenodactylidae gen. B. sp. 1 (Ünay et al. Citation2003a)
Holotype: M1 or M2 sin. GOZ1a-195 ()
Type locality: Gözükızıllı-1a, Anatolia Turkey ()
Other sites with Daxneria fragilis: Gözükızıllı-1b. The late Oligocene rodent assemblage from Ugljevik (Bosnia-Herzegovina; van de Weerd et al. Citationin prep.) contains three damaged cheek teeth that are close in morphology and size to Daxneria fragilis.
Age: late Oligocene
Paratypes: Incomplete specimens have numbers between brackets, s = sinistral, d = dextral. Gözükızıllı-1a: DP3: 191d, 192d, 198?; DP4: 193s, 194s; M1 or M2: 195s, 196d, 197d; M3: 200d; dp4: 201s, 202s, (205d), 206d, (207d), 208d; m1-2: 211d, (212s), (213d), (214d), (215d), (216d); m3: 219d, (220d).
Gözükızıllı-1b: DP3: 221d, 222d, 223d, 224d, 225d, 226d; DP3 & DP4: 227d; DP4: 228s; M1-2: (231d), 233s, (236d); M3: 237s; dp4: 241s, 242s, (243s), (244d), 245d, (246d); M1 or M2: 247d, 248d, (249s), (250s).
Measurements: see
Table 1. Measurements of Daxneria fragilis nov. gen. nov. sp. from Gözükızıllı-1a and 1b; shown are minimum, maximum, mean, number of observations and standard deviation
Diagnosis: as for the genus
Differential diagnosis: as for the genus
Description
DP3 (, maxilla fragment). The ten specimens (seven left, three right) have an oval outline and a single root. A transverse ridge is present in the middle, posteriorly a low cingulum connects to the ridge at the lingual tooth margin. A few specimens have a trace of an anterior cingulum.
DP4 (, 3f-g, 7y). is low-crowned and molariform, four complete specimens are in the sample. A broad lingual and two smaller labial roots are preserved on one specimen. The oblique anteroloph and protoloph are smoothly curved; the protoloph ends in a cuspidate paracone. The flexus between metaconule and metacone is shallow. Two specimens have a rounded metaconule without mesolophule. On two others the metaconule extends into a mesolophule, together half-moon shaped, one of these has a low connection to the metacone (). The metacone is rounded. The hypoflexus is short, the endoloph is close to the lingual border of the occlusal surface.
M1-2 (, 3b-c 7w-x). Four complete and two incomplete M1 or M2 are available, these are higher crowned than the DP4. The M1 or M2 has four roots, the lingual two roots are either closely together or fused. The anteroloph and protoloph are smoothly curved, but less oblique than on DP4. The protoloph ends in the cuspidate paracone. The paraflexus is transverse; the mesoflexus is deep and curved backwards. The posteroloph is isolated from the metacone on all four M1-2, it has a low connection to the metaconule in one specimen (). The metaconule has a short mesolophule. The metacone is rounded, it is slightly higher and separated from the lower metaconule by a shallow flexus and from the posteroloph by the deeper metaflexus. The posteroloph is very wide near the hypocone, the anterior arm of the hypocone is connected to the protocone forming a short, high and narrow endoloph on all specimens.
M3 (). Two complete specimens are in the sample. Characteristic are the high anterior sides. The anterior side is wide, the posterior side is narrow and reduced. The protocone, its anterior arm and the anteroloph form a smoothly curved anterior tooth margin; the paraflexus is long. The protoloph ends in a cuspidate paracone. The hypoflexus is very long, resulting in a sharp V-shaped and pinched protocone. The posterior side of the molar is strongly reduced; the homology of the dental elements is shown in . The hypocone is reduced and shifted labially, it is fused with the metaconule; in one specimen it is narrow (), on the other it is larger (). The hypoflexus and metaflexus are separated by a thin and high mure, a connection between metaconule and protoloph. The metacone is small and attached to the metaconule, the metaflexus forms a small basin.
dp4 (). Twelve specimens are in the two samples, of which five have the anterior part damaged. The dp4 is low-crowned. The metaconid and protoconid are connected by a transverse ridge incorporating the labial and lingual anterocingulid and the anteroconid. The anteroconid is only slightly protruding, with wear it disappears as a separate element. The protoconid is triangular. The metaconid of one specimen () has a short labially-directed spur. All specimens have a well-developed and long cristid, slightly curved forward, and reaching the lingual tooth margin, named here the metalophulid II. The connection to the posterior arm of the protoconid is broad. The metalophulid II separates a large anterior flexid from the mesoflexid, the mesoflexid is deeper than the anterior flexid. A low ectostylid is present in the hypoflexid of six out of ten teeth; in two out of twelve teeth a minor mesostylid is present in the mesoflexid. The posterolophid is broad with a relatively narrow connection to the hypoconid. An unworn specimen shows that the posterolophid is higher than the cuspids. The postero-labial cingulid is well-developed, with a well-developed stylid on one specimen ().
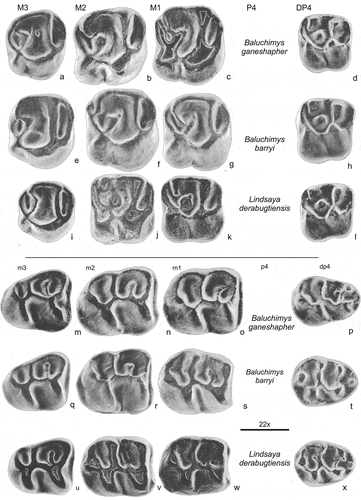
Figure 6. Upper and lower cheek teeth of Baluchimys ganeshapher (a, b, c, d, m, n, o, p), Baluchimys barryi (l, f, g, h, q, r, s, t) and Lindsaya derabugtiensis, all three from site Y-GSP417 (Flynn et al., Citation1986). The bar represents 1 mm; the approx. enlargement has been added
m1-2 (). There are two complete m1or m2, seven are incomplete with the anterolophulid broken. Metaconid and entoconid are merged into the relatively narrow lophids. Protoconid and hypoconid are strongly pinched and have an antero-labial direction. The metalophulid I is about straight and transverse, with wear it will become slightly curved. In slightly worn and worn specimens the metalophulid II is long, reaching the tooth border. The posterolophid shows an obtuse angle at its posterior side reflecting the merged hypoconulid. The posterolophid narrows toward the hypoconid, in about half of the specimens, it reaches the entoconid. A strong postero-labial cingulid is present on all ten specimens.
m3 (). There are two m3; one is complete, one has a damaged anterior side. Both have a strongly-pinched protoconid, jutting out far more in antero-labial direction than the hypoconid, resulting in a wide anterior side of the molar. Similar to the m1-2, the outline of the molar is at its base indented between protoconid and hypoconid. The metalophulid II is well developed and long, the anterior flexid is narrow and longer than the mesoflexid. The posterolophid narrows toward the hypoconid. An obtuse angle at its posterior side marks the hypoconulid. A postero-labial cingulum is present on one () but almost absent on the other specimen ()
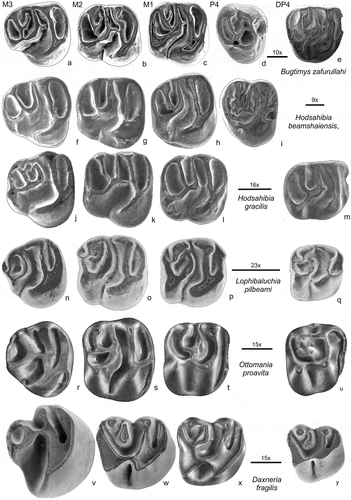
Figure 7. Comparison of upper cheek teeth of Daxneria fragilis (v-y) with Bugtimys Marivaux et al., Citation2002 site Paali nala C2 (a-e), Hodsahibia Marivaux & Welcomme Citation2003 site Paali nala C2 (f-h), H. gracilis Paali nala C2 (j-m), Lophibaluchia Flynn et al., Citation1986 site Y-GSP417 (n-q) and Ottomania de Bruijn et al., Citation2003 site Süngülü. Daxneria, Ottomania and Lophibaluchia retained their DP4 and have no P4. The bar represents 1 mm; the approx. enlargements have been added, these are not the same
Incisor enamel
The broad upper incisor has a sulcus (). The relatively slender lower incisor has a faint sulcus () and a smooth slightly crenulated surface. The enamel thickness is approximately 250 μm (). The portio externa (PE) is thin (~5% of the total), it consists of radial enamel (), with prisms bending upward and very thin IPM (inter prismatic matrix). The portio interna (PI) is very thick (~95%) consisting of transverse multiserial Hunter-Schreger bands (HSB). The bands contain ~ three to four prisms (), these make an angle with the normal on the enamel-dentine junction (EDJ) of about 35°. Thin plates of IPM are present in the bands (5f arrowed), the crystallites of the IPM plates are orthogonal to the prisms. A PLEX (prisma-less external layer) is not observed.
Comparisons
Flynn et al. (Citation1986) described four sectioned baluchimyine incisors from site Y-GSP417 (Oligocene, Pakistan); genus and species have not been identified. Martin (Citation1992) shows two details of the longitudinal sections of one of these specimens. Relative thickness of the PE is about 20%, the PI shows multiserial HSB, IPM is thick and anastomosing making a sharp angle with the prisms. Marivaux et al. (Citation2000) illustrate the structure of the Baluchimys krabiensis (late Eocene, Thailand). The PI is ~20% of total enamel thickness. The longitudinal sections show multiserial HSB with thick IPM. The PI in cross-section shows reticulate IPM in a wavy pattern. Daxneria shares the multiserial HSB pattern with the baluchimyids from Pakistan and Thailand, but differs from these in the relative thickness of the PE and in the thickness, morphology and crystallite direction of the IPM.
Although the dental morphology of Daxneria is unique, the cheek teeth suggest affinities with the Baluchimyinae. In order to facilitate comparison we reduce the array of these Asian rodents into two groups of species and illustrate and compare these with Daxneria.
The first group that may be recognized consists of the very similar Baluchimys and Lindsaya (). Like Daxneria, permanent premolars are not known for this group and we posit that these taxa retain their dp4 and DP4. Upper molars of this group are characterized by a relatively well developed metaconule without, or with a short mesolophule, the metaconule is connected to the hypocone, an endoloph is present. The upper molars of Daxneria, share the endoloph and the tendency to isolate the metaconule with Baluchimys and Lindsaya, but the metaconule of Daxneria has a much more labial position. Major structural differences between Daxneria and this group are observed in the M3. The posterior side of the M3 of Daxneria is strongly reduced, hypocone and metacone are tiny, but the metaconule is relatively large. The hypoflexus is very deep and a mure is present. The anterior side of the M3 of Daxneria is wide, considerably wider than that of the M1or M2. The M3 of Baluchimys and Lindsaya is slightly smaller than the M2, its posterior side shows some reduction. The M3 of Baluchimys and Lindsaya shows a protocone that is expanded in posterior direction, hypocone and metacone are small, a hypoflexus is nearly absent. Lower molars of Baluchimys and Lindsaya show a short or weak metalophulid II, that of Daxneria is strong, long and ends free. In Daxneria protoconid and hypoconid are long and narrow.
The second group of species is composed of Bugtimys, Hodsahibia, Lophibaluchia and Ottomania (). Confiniummys resembles Ottomania in most dental characteristics, it is neither illustrated here nor further discussed because it is poorly documented. Lophibaluchia, Ottomania and Daxneria retained their dp4 and DP4, while Bugtimys and Hodsahibia have a p4 and P4. Three genera of this group have no endoloph on M1-3 (Bugtimys, Hodsahibia, Lophibaluchia; ). On Ottomania the protoconid and hypoconid of the DP4 and most M1 are connected, but not on the M2 (). The endoloph is present on all upper cheek teeth of Daxneria (). Upper molars of the four genera in this group are more or less lophodont with three curved lophs. The third loph of the M1 and M2 connects the hypocone through the metaconule and mesolophule to the labial tooth margin (). In Hodsahibia, Bugtimys and Ottomania the metacone is connected to metaconule through the labial part of the metaloph resulting in an Y-shaped configuration of these lophs. The Y-configuration is poorly developed in Lophibaluchia because of absence of the metacone-metaconule connection (). The M3 shows a reduced posterior region, in particular in Lophibaluchia and Ottomania. The M3 of Ottomania illustrated in has a mure, metacone and labial metaloph are absent. Other specimens (de Bruijn et al. Citation2003, plate 1 ) have no mure and a tiny metacone and labial metaloph are present. Compared to this group of four genera, the tooth pattern of the posterior side of upper molars of Daxneria is rather different. There is no metaloph, metacone and metaconule are isolated or nearly isolated and the metaconule has a labial position. There is an endoloph on the M1-2 of Daxneria and the hypoflexus is sharp and short. The M3 of Daxneria is strongly reduced, it resembles that of Ottomania, but in Ottomania the third loph of the M3 incorporates hypocone, lingual part of the metaloph, metaconule and mesolophule; the metacone and its connection to the metaconule has completely disappeared on the specimen in . In Daxneria the third loph is composed of hypocone, an enlarged metaconule and a tiny metacone.
Figure 8. Comparison of lower cheek teeth of Daxneria fragilis with Bugtimys Marivaux et al. (Citation2002) site Paali nala C2, (a-e); Hodsahibia beamshaiensis Marivaux and Welcomme (Citation2003) site Paali nala C2, (f-h); H. gracilis Marivaux and Welcomme (Citation2003) site Paali nala C2, (i-k); Lophibaluchia Flynn et al. (Citation1986) site Y-GSP417, (l-o) and Ottomania de Bruijn et al. (Citation2003), site Süngülü, (p-s). Daxneria, Ottomania and Lophibaluchia retained their DP4 and have no P4. The bar represents 1 mm; the approx. enlargements have been added, these are not the same
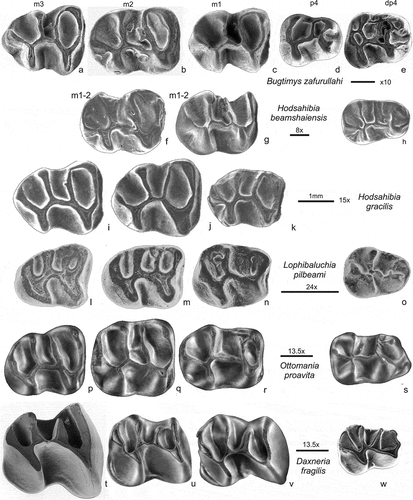
The lower molars of Baluchimys and Lindsaya () have a short metalophulid II that mostly ends free. Bugtimys, Hodsahibia, Lophibaluchia and Ottomania () have a long metalophulid II that may end free near the lingual border or is connected to the posterior side of the metaconid. The metalophulid II of Daxneria is long and ends free in all preserved specimens.
In addition to the tooth pattern, it is the partial hypsodonty that characterizes the molars of Daxneria and differentiate it from all other genera included in the Baluchimyinae. Daxneria clearly represents a separate branch within this Asian subfamily that was already supposed to be polyphyletic.
Premolars
In assemblages consisting of isolated molars the determination of dental positions can be difficult. To associate isolated molars in assemblages containing several related species is even more challenging, certainly so when collections are small. The recognition of permanent and deciduous molars is often problematic, see the discussion in Flynn and Cheema (Citation1994). Retention of the deciduous premolars is fairly common among the Ctenohystrica. In order to establish whether this is the case in the Asian baluchimyines the morphology of the deciduous and the permanent premolars has to be established. We compared first the cheek teeth of all well-illustrated Asian baluchimyine genera (Flynn et al. Citation1986; Flynn and Cheema Citation1994; Marivaux and Welcomme Citation2003) and some North African Ctenohystrica. In baluchimyines the P4, if present, is more or less triangular or heart-shaped, with an insignificant hypocone. In contrast, the hypocone of the DP4 is sturdy, resulting in a more or less square-outline of the occlusal surface, the anteroloph is well developed. This P4/DP4 morphology is illustrated in Hodsahibia and Bugtimys () and is seen as well in Dianomys (Eocene Wang Citation1984, Citation2001) and African genera included in the late Eocene “protophiomyines’’ (sensu Marivaux and Boivin Citation2019). The p4 of baluchimyines has the four main cuspids (protoconid, hypoconid, metaconid and entoconid) well-developed and is posteriorly wider than anteriorly, the anteroconid is absent (). In contrast, the dp4 is long and the anteroconid may be present. P4/p4 with this morphology have not been found in the assemblages of Daxneria, Ottomania and Lophibaluchia, so the P4 and p4 positions in the composed tooth-rows remains vacant in . In small assemblages of isolated teeth the absence of the permanent fourth premolar may be accidental, but for Ottomania, Baluchimys, Lindsaya and Lophibaluchia there is enough material to suggest that the absence of P4 and p4 is not accidental. Our working hypothesis is that within the Asian baluchimyines the genera Hodsahibia and Bugtimys have a P4 and p4, but Baluchimys, Lindsaya, Lophibaluchia, Ottomania and Daxneria have no permanent fourth premolar, but retained their deciduous cheek teeth.
Family Ctenodactylidae Gervais, Citation1853
Subfamily Ctenodactylinae Hinton, Citation1933
Introduction
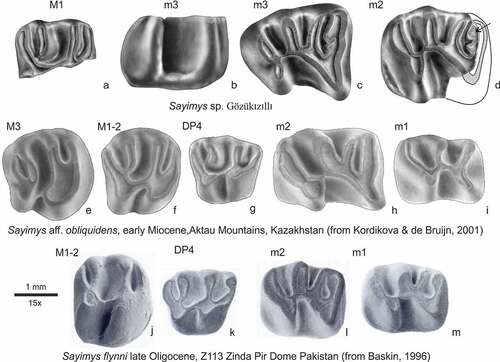
Ctenodactylidae radiated in Central Asia during the Oligocene, where some eight genera have been described from several basins (Wang Citation1997). Extensive studies of the rodents from the Valley of Lakes (Mongolia, see Oliver et al. Citation2017 and references therein) and from Ulantatal in neighbouring Chinese Inner Mongolia (see Gomes Rodrigues et al. Citation2014 and references therein) document the details of Oligocene ctenodactylid expansion. Most species become extinct during the late Oligocene, only the hypsodont Distylomys Wang (Citation1988), Prodistylomys Wang (Citation1988), Sayimys Wood (Citation1937) and a species of Yindirtemys Bohlin (Citation1946) survived into the early Miocene. The ctenodactylid Sayimys Wood (Citation1937) is present in the early and middle Miocene of China (Bohlin Citation1946), Kazakhstan (Kordikova and de Bruijn Citation2001), Anatolia (López-Antoñanzas et al. Citation2004; Hartman et al. Citation2019), Greece (López-Antoñanzas et al. Citation2005) and the early Oligocene and Miocene of the Indian subcontinent (see Hartman et al. Citation2019 and references therein). With the formation of a land connection between Turkey and Arabia during the early Miocene, Ctenodactylidae migrated into Arabia and beyond, into northern Africa (Wessels et al. Citation2003; López-Antoñanzas, Citation2015). Middle and late Miocene African genera are Sayimys (including Proafricanomys López-Antoñanzas et al. Citation2015), Metasayimys Lavocat (Citation1961), and Africanomys Lavocat (Citation1961). Giant ctenodactylids have been found on Sardinia, these are part of a Miocene insular fauna (de Bruijn and Rümke Citation1974); a Pleistocene insular fauna with the ctenodactylid Pellegrinia De Gregorio (Citation1887) is known from Sicily. Today four genera survive in northern Africa. The three specimens described below from Gözükızıllı are included in Sayimys, this means that these are the so far the oldest known record of the genus.
Sayimys Wood Citation1937
Sayimys sp. , 4m-p, 9a-dLocalities: GOZ1b
Synonymy: aff. Sayimys sp. (Ünay et al. Citation2003a)
Materials and measurements: GOZ1b-251d, damaged M1 (); length 2.04 mm); GOZ1b-252s, incomplete m1-2 (; length 2.33 mm, width 2.34 mm); GOZ1b-253s, m3 (; length 2.62 mm, width 2.38 mm).
Description
The damaged upper molar is possibly a M1 (, 9a), because it is relatively small, compared to the associated lower molars. The molar shows the four lophs typical for Sayimys, the metaloph is isolated, that is, not connected to the posteroloph.
The high-crowned m1 or m2 is incomplete. Judging by its width, it is probably a m2. The anterior side of the anterolophid is missing ( and 9d), so it was a bit longer than the measured length. The posterior side of the anterolophid shows two crenulations (arrowed in , 9d). The metalophulid II is long, bending slightly forward, reaching the lingual tooth border. A well-developed postero-labial cingulid is present.
The high-crowned m3 is complete. The posterior side of the anterolophid shows two crenulations (arrowed in ), similar to the m1 or m2. The metalophulid II is long reaching the lingual tooth border. The hypolophid is not in line with the oblique ectolophid, but these form an obtuse angle. The protoconid is long and narrow, jutting out in labial direction. The hypoconid is relatively small, a minor constriction is present between this cuspid and the posterolophid.
Comparisons
The three molars from Gözükızıllı-1 have been compared with the Oligocene ctenodactylids from the well-known basins in Mongolia and Chinese nei Mongol (Wang Citation1997; Vianey-Liaud et al. Citation2006 &, Citation2010; Gomes Rodrigues et al. Citation2014; Oliver et al. Citation2017) but they do not fit any of the Oligocene genera. Instead, the molars resemble Sayimys. shows the molars from Gözükızıllı and Sayimys specimens from the early Miocene of the Aktau Mountains (Kazakhstan; Kordikova and de Bruijn Citation2001) and from Z113 in the Zinda Pir Dome (Pakistan). The Z113 site was originally described as early Miocene, but placed in the late Oligocene by Métais et al. (Citation2009). Unfortunately, not all tooth positions are represented in the Gözükızıllı-1 material and in the compared assemblages. The well-developed and long metalophulid II in the Gözükızıllı lower molars is also present in Sayimys from Aktau and in S. flynni (Baskin, Citation1996) from Z113, but it is absent in S. minor de Bruijn et al. (Citation1981) and S. giganteus López-Antoñanzas et al. (Citation2004). The narrow protoconid protruding in labial direction is also present in Sayimys from Aktau and in S. giganteus. The Gözükızıllı-1 material seems closest to that from the Aktau mountains described as Sayimys aff. obliquidens. Sayimys obliquidens from its type area Taben Buluk is larger. Moreover, there are doubts about the homogeneity of its type assemblage and about the location and age of the type locality (Hartman et al. Citation2019); therefore, classification as Sayimys sp. is preferred.
Figure 9. Comparison of Sayimys sp. from Gözükızıllı with Sayimys aff. obliquidens from the middle member of the Chul’adyr Formation Aktau Mountains, Kazakhstan (e-i) and S. flynni from Z113, Zinda Pir, Pakistan (j-m)
Suborder Supramyomorpha D’Elia, Fabre and Lessa, 2019
Family Muridae, Illiger Citation1811Subfamily Eucricetodontinae Mein andFreudenthal, Citation1971a
Eucricetodon Thaler, Citation1966
Type species: Cricetodon collatum Schaub, Citation1925
Type locality: Küttigen Germany, age late Oligocene
Introduction
The subfamily Eucricetodontinae was originally meant to contain all European ‘Oligocene’ hamster-like rodents except the Paracricetodontinae Mein and Freudenthal, Citation1971a and the Melissiodontinae Schaub, Citation1925. Freudenthal et al. (Citation1992) restricted the subfamily basically to the genus Eucricetodon, while the genera Mirabella de Bruijn et al., Citation1987 (now Mirrabella de Bruijn et al., Citation2007) and Eumyarion Thaler, Citation1966 were added with a question mark. In the meantime a large number of new species of Eucricetodon s.l. all over Eurasia have been described.
lists the species included in Eucricetodon (including Atavocricetodon) from Europe with type locality and age using the Paleogene mammalian MP zones (Schmidt-Kitler Citation1987; Aguilar et al. Citation1997) and the Neogene MN zones (de Bruijn et al. Citation1992). The listing is approximately from old to young.
Table 2. List of species of Eucricetodon from Europe. The allocation into groups is after Freudenthal & Martin-Suarez (Citation2016)
Several of the Western European species included in Eucricetodon are based on limited type material, on holotypes from unknown locality and age, or on an incomplete and worn tooth row. Notorious in this respect are the holotypes from the Quercy in France in old museum collections. Mainly because of this Vianey-Liaud (Citation1972) considered these species ‘inutilisable’ (translation unusable). These are listed in . To declare these species ‘inutilisable’ was entirely correct. Unfortunately, Comte (Citation1985) and Freudenthal (Citation1994) revived Eucricetodon dubius while Dienemann (Citation1987) revived E. murinus and E. incertus. Freudenthal (Citation1994) rejected the holotype selected by Mayo (Citation1982) for E. dubius (Mont-de-Doury) and stated that Schaub (implicitly) designated a mandibula from the Quercy. Next Freudenthal classified a large collection of isolated molars from Vivel del Rio (Spain, early Oligocene, MP28) under that name. Earlier, Freudenthal et al. (Citation1992) considered Eucricetodon praecursor a synonym of E. dubius, a view maintained by Freudenthal and Suarez (Citation2016).
Table 3. Eucricetodon species based on limited type material from unknown type localities and of uncertain age
Eucricetodon gerandianus (Gervais, Citation1848–1852) is another problematic species. Its type locality is Langy near St-Gérand-le-Puy (France). Daams (Citation1976) reported that the holotype of Gervais is lost and that further collection at that site is impossible. He proposed a ‘“hypotypoid”’, three upper molars figured in Schaub (Citation1925; plate 3, ) from la Chaux near St. Croix in Switzerland and described a good collection from Cetina de Aragon (Spain) as E. gerandianus. The holotype selected by Daams was rejected by Hugueney (Citation1999) because a ‘hypotypoid has no nomenclatural value’ and she designated a neotype, a lower tooth row, from St-Gérand-le-Puy figured in Schaub, a site close to the original type locality. Many pages have been printed concerning uncharacteristic and incomplete holotypes without paratypes, originating from unknown localities and of imprecisely known ages. The best and easiest solution to solve these problems is to ring-fence or quarantine these species, that is, restrict these species to their holotypes and ignore these names in future studies.
Problematic, but for different reasons, are Eucricetodon infralactorensis and E. quadratus both from Estrepouy (France). The size range of Eucricetodon from Estrepouy is very large and therefore Viret et al. (Citation1930) recognized two species, the smaller E. infralactorensis and the larger E. quadratus. However, later collecting at Estrepouy suggested that E. quadratus falls within the size range of E. infralactorensis and is thus its junior synonym, while there is as yet an unnamed much smaller species in the sample (see for a discussion Hugueney and Bulot Citation2011).
Freudenthal (Citation1996) described the subgenus genus Atavocricetodon (type species A. atavoides) and included atavus, murinus, huberi, nanus, nanoides, hugueneyae, nanoides and minusculus. In this way he subdivided Eucricetodon into an early Oligocene and a late Oligocene-early Miocene group. Eucricetodon species described from Asia and Asia Minor are in . Marivaux et al. (Citation1999) described a new Atavocricetodon species from the Oligocene of Pakistan extending the geographic range of the genus to the Indian subcontinent. de Bruijn et al. (Citation2003) maintained the subgenus Atavocricetodon, but considered it a ‘morpho-subgenus’with a ‘primitive’ dental pattern and included some Asian species. Gomes Rodrigues et al. (Citation2013) showed that the outline of the M1 cannot be used to distinguish Atavocricetodon from Eucricetodon and argued that the distinction of the two genera is not meaningful. Maridet et al. (Citation2009) described a late Oligocene Eucricetodon assemblage from the Junggar basin (northern China) and noted its resemblance to the latest Oligocene E. longidens from Europe. Lopez-Guerrero et al. (Citation2017) studied Eucricetodon from the early Oligocene to early late Oligocene succession in the Valley of Lakes and linked the observed trends in dental morphology to environmental change.
Table 4. List of Asian Eucricetodon species
Freudenthal and Martin-Suarez (Citation2016) reviewed European Atavocricetodon-Eucricetodon and recognized a chronological sequence of an Atavocricetodon group and four groups in Eucricetodon.
the Atavocricetodon group occurring in MP21-23 (early part early Oligocene)
the huerzeleri group occurring in MP24-27 (‘middle’ Oligo-cene)
the dubius group occurring in MP27-29 (late Oligocene)
the ‘praecursor’group occurring in MP 29 (late Oligocene)
the collatus group occurring in MP29-MN3 (latest Oligo-cene – earliest Miocene)
The groups of Freudenthal and Martin-Suarez have been added to the species in . Confusing is the name praecursor group when the holotype of E. praecursor is a synonym of E. dubius. The differences between the five groups as defined by Freudenthal and Martín-Suárez (Citation2016) are subtle, such as size of the cusps (difficult to grasp on illustrations) and frequencies of dental characters. Further, Freudenthal and Martin-Suarez claimed presence of morphological breaks between the groups, implying that each of the groups immigrated into the European realm and that ‘ancestor-descendant relationships’ cannot be demonstrated except within the collatus group. For a discussion on late Oligocene-early Miocene Eucricetodon (the collatus group) see Hugueney (Citation1999) and Berthet et al. (Citation2005).
Eucricetodon from Gözükızıllı-1
Fairly large assemblages of isolated molars of Eucricetodon have been collected from the two horizons in Gözükızıllı-1. The size distribution of the molars, in particular of the third molars, shows the presence of two species (). The separation into two groups of the first and second molars is uncertain because the large and the small specimens have the same morphology and cluster metrically. Thus, some small specimens of the large species and large specimens of the small species may have been wrongly assigned.
Figure 10. Length-width scatter diagrams of the molars of the two species of Eucricetodon from Gözükızıllı-1 (red points) and E. margaritae (black crosses) from Pareja. The line separates the Gözükızıllı points into E. oculatus nov. sp. and E. ruber nov. sp. The measurements of E. margaritae from Pareja have been digitised from Daams et al. (Citation1989, Figure 3)
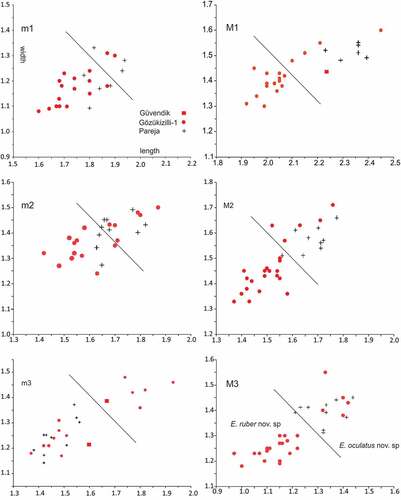
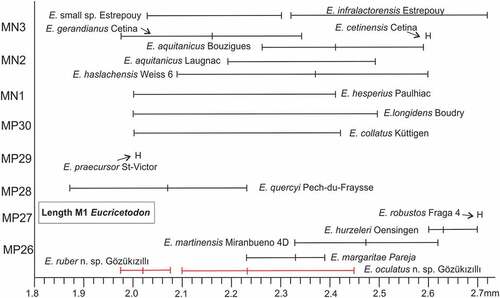
The lengths of the Gözükızıllı-1 M1 has been compared with that of the late Oligocene and early Miocene species from Europe (). Similar to Gözükızıllı several European sites have two species of Eucricetodon. shows that many sites show large ranges in the length of the M1.
Figure 11. The length of M1 (range and average) of Eucricetodon from Gözükızıllı compared with that of late Oligocene and early Miocene species of Eucricetodon from Europe. For Bouzigues see Aguilar (Citation1974); for Cetina (de Aragon) see Daams (Citation1976); for Estrepouy see Hugueney & Bulot: for Fraga 4 see Augusti & Arbiol 1989; for Laugnac see Daams (Citation1976); for Mirambueno 4D see Freudenthal (Citation1994). for Oensingen, St-Victor-la-Coste and Pech-du-Fraysse see Vianey-Liaud (Citation1972); for Paulhiac, Boudry and Küttigen see Engesser Citation1985; for Pareja see Daams et al. (Citation1989); for Ulm & Weiss-6 see Dienemann (Citation1987)
We were hesitant to name new species because in the list of formally named species of Eucricetodon that we compiled are already over 40 species, of which 12 described from sites in Asia and Asia Minor. However, the split anterocone on the M1 of large and small Eucricetodon from Gözükızıllı-1 restricted the playing field to a few late Oligocene and early Miocene species from western Europe that are included in the huerzeleri and collatus groups of Freudenthal & Suarez (Citation2016). The small species is described as Eucricetodon ruber nov. sp. and the large species as E. oculatus nov. sp. The nomenclature of parts of Eucricetodon molars is shown in .
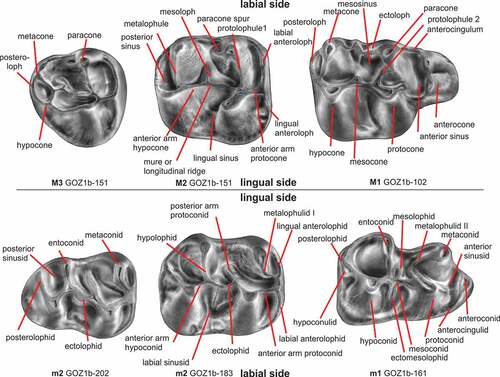
Figure 12. Nomenclature of parts of Eucricetodon molars (adapted after Mein and Freudenthal Citation1971b)
Eucricetodon ruber nov. sp.
()
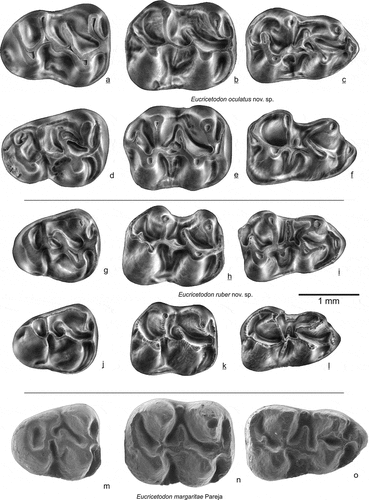
Figure 13. Upper molars of Eucricetodon oculatus, E. ruber from Gözükızıllı-1 and E. margaritae from Pareja (Spain). E. occulatus: a GOZ1b-151, b GOZ1b-131, c GOZ1b-102 holotype, d GOZ1b-141, e GOZ1b-121, f GOZ1b-111; E. ruber: g GOZ1b-156, h GOZ1b-126, i GOZ1b-106, j GOZ1b-144, k GOZ1b-128, l GOZ1b-105 holotype; E. margaritae: m-o
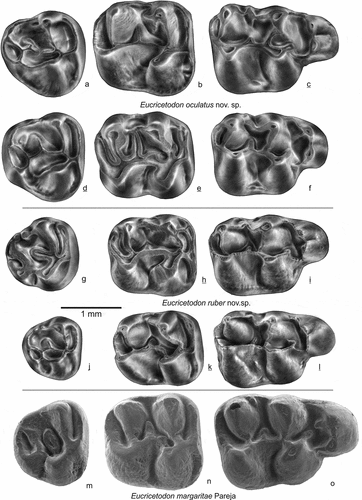
Figure 14. Lower molars of Eucricetodon oculatus,Eucricetodon oculatus, E. ruber from Gözükızıllı-1 E. ruber from Gözükızıllı-1 and E. margaritae from Pareja (Spain). E. oculatus: a GOZ1b-202, b GOZ1b-183, c GOZ1b-162, d GOZ1b-211, e GOZ1b-182, f GOZ1b-161; E. ruber: g GOZ1b-216, h GOZ1b-186, i GOZ1b-166, j GOZ1b-204, k GOZ1b-188, l GOZ1b-167; E. margaritae: m-o
Derivatio nominis: based on the name of the type locality Gözükızıllı-1 which means red eyes in Turkish. We included the word red in the name of this small species (ruber means red) and include eye (oculus) in the name of the large species.
Holotype: M1 dext. GOZ1b-105 ()
Type locality: Gözükızıllı-1b, about 21 km south of Kızılırmak, Anatolia Turkey.
Other sites with Eucricetodon ruber: Gözükızıllı-1a, Güvendik
Age: late Oligocene
Paratypes:
Gözükızıllı-1a: M1: 101s*, 102s, 103s, 104s, 105s, 113d, 115d, 116d, 117d, 118d; M2: 101s*, 121s, 123s, 124s, 133d, 135d, 136d; M3: 141s, 142s, 143s, 151d, 153d, 154d, 155d, 156d;
m1: 161s, 165s; m2: 171s, 172s, 174d, 175d, (176d), 177d, 178d; m3: 184s, 185s, 186s.
Gözükızıllı-1b: M1: 105s, 106s, 107s, 108s, 109s, (110s), (112d), 114d; M2: 123s, 124s, 125s, 126s, 127s, 128s, 129s, 134d, 135d, 136d, 137d, 140d; M3: 143s, 144s, 145s, 146s, 147s, 148s, 155d, 156d, 157d, 158d. m1: 163s, 165s, 166s, 167s, 168s, 171d, 172d, 173d, 174d, 175d, 176d, 177d, 178d; m2: 185s, 186s, 187s, 188s, 193d, (194d), (195d), 196d; m3: 204s, 205s, 214d, −215d, 216d.
The molars indicated with * are from the same individual, s = sinistral, d = dextral, incomplete specimens between brackets.
Synonymy: Eucricetodon sp. 2 (Ünay et al. Citation2003a)
Measurements: see
Table 5. Measurements of Eucricetodon ruber nov. sp. from Gözükızıllı-1 and Güvendik
Diagnosis: Small Eucricetodon with a split anterocone on M1 and without posterior spur (the anterolophule). Cusps high and well-developed, ridges low to very low. Low ectolophs on M1 and M2. The m1 with a cuspidate anteroconid and pointed anterior outline.
Comparisons: the comparisons of E. ruber with other species is given below in combination with E. oculatus.
Description: apart from the size E. ruber and E. oculatus are identical in dental morphology, therefore see below for the combined description of the species.
Eucricetodon oculatus nov. sp.
( and 14a-f)
Derivatio nominis: based on the name of the type locality Gözükızıllı which means red eyes in Turkish. We included the word eye in the name of the larger species (oculatus means with eyes) and include red (ruber) in the name of the small species.
Holotype: M1 dext. GOZ1b-102 ()
Type locality: Gözükızıllı-1b, 20 km south of Kızılırmak, Anatolia Turkey
Other sites with Eucricetodon oculatus: Gözükızıllı-1a and Güvendik-1
Age: late Oligocene
Paratypes:
Gözükızıllı-1a: M1: 111d, (112d); M2: 131d; m3: 181s.
Gözükızıllı-1b: M1: (101s), 102s, (103s), (104s), 111d, 115d, (120s); M2: 121s, 122s, 130s, 131d, 132d; M3: 141s, 142s, 149d, 150d, 151d. m1: 161s, 162s; m2: 181s, 182s, 183s, 189d, 191d; m3: 201s, 202s, 211d, 212d.
Synonymy: Eucricetodon sp. 3 (Ünay et al. Citation2003a).
Measurements: see
Table 6. Measurements of Eucricetodon oculatus nov. sp. from Gözükızıllı-1 and Güvendik
Diagnosis: Larger than Eucricetodon ruber, but with identical dental morphology. Anterocone on M1 with two cusps and without anterolophule. Cusps high and well-developed, ridges low to very low. Low ectolophs on M1 and M2 or spurs on the paracone and metacone may be present. The cusp-like anteroconid on the m1 gives it a pointed anterior outline.
Description of Eucricetodon ruber and E. oculatus
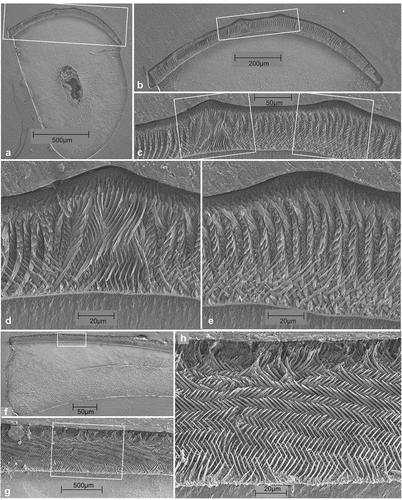
Lower Incisor (). The material consists of isolated and broken specimens; we have therefore not been able to differentiate the incisors of the two species. We assume that the enamel of the lower incisor of both species have the same microstructure. The external surface of the lower incisor is smooth with two parallel longitudinal ridges, enamel thickness is 200 ųm. Portio interna (PI) is very thick (~80%), portio externa (PE) is reduced. The PI consists of longitudinal uniserial Hunter-Schreger Bands (HSB). The intra-prismatic matrix (IPM) of the internal part of the PI makes an angle of about 45 degrees to the HSB; in the external part of the PI the IPM is parallel to the prisms. An anticline in the prisms of the PI is present below the mesial ridge. The PE consists of tangential enamel, the prisms disappear toward the outer surface into the prisma-less external layer (PLEX). Schmelzmuster is type 10 (Kalthoff Citation2000).
Figure 15. Transverse sections (a-e) and sagittal sections (f-h) of the lower incisor of Eucricetodon from Gözükızıllı-1
M1 (, f, i, l). The cusps are high and pointed, ridges are low and thin. The anterocone is subdivided into two conules and shows an anterior groove; the labial conule is distinctly higher and is better developed than the lingual one. A low anterior cingulum is present at both sides of the anterocone, connecting low to the proto- and paracone. There are no other connections between the anterocone and proto- and paracone. The anterior arm of the protocone is short, it either reaches the base of the paracone, or it ends free (). The posterior arm of the protocone continues into the mure, a protolophule-2 is attached to the mure. The mesoloph is low and variable in length, it is confluent with the anterior arm of the hypocone (). One of the molars has a short ectomesoloph. Spurs on paracone or metacone are either absent, short or are long and developed into low ectolophs (). The short metaloph is poorly developed and may be incomplete, it mostly connects to the posterior arm of the hypocone (). The posteroloph is low and connects to the base of the metacone.
Table 7. Character states in upper molars of Eucricetdon ruber nov. sp. and E. oculatus nov. sp. from Gözükızıllı-1
M2 (). The lingual anteroloph is short. The labial anteroloph is low, it connects to the base of the paracone. The lingual sinus is strongly curved in anterior direction. The protolophule-1 connects to the anterior arm of the protocone. The posterior arm of the protocone and the anterior arm of the hypocone form the curved mure. The mesoloph may be short, of medium length or long; on specimens where it is of medium length it may reach the base of the metacone ().
The curved metalophule inserts on the hypocone anterior of its apex. The posterior arm of the hypocone is confluent with the posteroloph; the low posteroloph inserts at the base of the postero-labial side of the metacone. The paracone spur may be absent, short or long and forms a complete but low ectoloph (). A few specimens of both species have a low cingulum, labial of the spurs or ectoloph. One specimen of E. oculatus () has a short spur attached to the mure anterior of the mesolophule.
M3 (, d, g, j). The metacone is the highest cusp in used specimens. All specimens tend to close the lingual sinus by means of spurs on protocone and hypocone () but the closure is incomplete on some. The lingual anteroloph is short, the labial one is long. The protolophule-1 inserts on the anterior arm of the protocone. The metacone and hypocone are incorporated into a posterior loph that connects hypocone, metacone and paracone. It is lowest and weakest between metacone and paracone. Irregular ridges possibly homologous to the metalophule, anterior arm of the hypocone and mure are present in the central basin. The mesoloph can be long and connected to the posteroloph, of medium length or absent (). The curved mure inserts on the protoloph. One small specimen has no connection between protocone and protolophule. Some molars of both species have a faint very low second mesolophule.
m1 (). The cusps are high and pointed, the ridges are low to very low. The pointed anterior side of the molar bears a rounded anteroconid. An anterior cingulid descends at both sides from the anteroconid; the distance between anteroconid and metaconid is short, resulting in a high lingual anterior cingulid. The anterior arm of the protoconid may end free or can be connected low to the anteroconid. The metalophulid I is absent, the metalophulid II and posterior arm of the protoconid form together a ‘‘<” -shaped ridge. The hypolophid connects to the anterior arm of the hypoconid or to the ectolophid. The posterolophid comes down from the hypoconid, rounds the posterior side of the molar and ascends the entoconid. Some m1 have a well-developed free-ending posterior arm of the hypoconid. On others this ridge is short or absent. All specimens have a spur on the metaconid, some on the entoconid as well, but these do not develop into a complete ridge. The mesolophid can be absent, rudimentary, of medium length or long, many specimens have a rudimentary ectomesolophid.
m2 (). The ridges of the m2 are better developed and higher than in the m1. Unworn teeth have pointed cusps. The labial anterolophid descends sharply to the antero-labial corner of the protoconid. The lingual anterolophid is short; on slightly worn specimens it is situated at the anterior side of the metaconid. A short and weak metalophulid I connects to the anterior tooth border between the lingual and labial anterolophid. It is absent in one molar of Eucricetodon ruber. The posterior arm of the protoconid ends free halfway the lingual tooth border; on some specimens it reaches the posterior side of the metaconid, in particular in the m2 of E. ruber. It is absent in one m2 of E. oculatus. The ectolophid shows a minor thickening, indicating an incipient mesoconid with lingually as well as labially short spurs. Similar to the m1, the posterolophid descends from the hypoconid, rounds the posterior side of the molar and ascends the entoconid. A hypoconulid is present on some specimens; these have a minuscule remnant of the free-ending posterior hypoconid arm. A very low cingulid is present in the labial sinusid of most m2.
m3 (). The m3 of E. oculatus is long and slender with relatively high and strong ridges. The m3 of E. ruber is more reduced. The posterolophid is sturdy, well developed, it rounds the posterior side of the m3, connects to the entoconid, and continues as a lingual ridge along the border to the metaconid. The entoconid of the smaller species, E. ruber, is small and incorporated into the posterolophid. In E. oculatus the ridge along the lingual border is less well-developed and the entoconid stands out as a separate cusp. The anterior side of the m3 is similar to that of the m2. The posterior arm of the protoconid is long, has an oblique postero-lingual orientation and ends free. On one specimen of E. oculatus it touches the lingual border; on one m3 of E. ruber it bends forward and connects low to the metaconid. The hypoconid is smaller than the protoconid. The ectolophid is well developed; a hypolophid connects to the entoconid. One m3 of E. ruber () has a low ridge at the posteroir side of the metaconid, connecting metalophulid 1 to the posterior arm of the protoconid.
Comparisons and discussion
Eucricetodon oculatus is very close in dental morphology to E. ruber, but it is slightly larger (), the differences in the frequencies of character states are not significant. The m3 of E. oculatus is long and slender with relatively high and strong ridges. The m3 of E. ruber is more reduced. The few molars from Güvendik match with those of E. ruber and E. oculatus. The size of one of the two m3 is closer to those of E. ruber (), the other is closer to E. oculatus and so is the single complete M1.
The two Gözükızıllı species have been compared with Oligocene species that have a bicuspidate anterocone on M1: Eucricetodon martinensis, E. margaritae, E. huberi and E. huerzeleri. These species form the fairly homogenous huerzeleri group of Freudenthal and Martín-Suárez (Citation2016). E. martinensis Freudenthal (Citation1994) is larger than E. ruber and E. oculatus (), its anterocone in a part of the specimens is bicuspidate. All six M1 of E. margaritae from the type locality have an anterocone divided into two cusps; this species shows a complete overlap in size with Eucricetodon from Gözükızıllı (). All molars except the m3 of E. margaritae are comparable in size to E. oculatus, but the m3 of E. margaritae is equal in size to E. ruber (). The published figures of the M1 of E. huerzeleri (Vianey-Liaud Citation1972; Dienemann Citation1987) show a bicuspidate anterocone, however, this species is much larger than the two Gözükızıllı species.
compares the dental morphology of E. margaritae with that of E. ruber and E. oculatus. Eucricetodon margaritae and the two Gözükızıllı species share the split anterocone on M1, but compared to E. margaritae, the anterocone that of E. ruber and E oculatus is narrower. E. margaritae may have a backward spur (an anterolophule) on the anterocone. The metalophule on M1 is directed backwards or absent in E. ruber and E. oculatus, but it is slightly directed forward in E. margaritae. The posterior sinus is lingually open in E. margaritae, closed in E. ruber and E. oculatus. The M1 of E. ruber. and E. oculatus has ectolophs or spurs on paracone/metacone, these are absent in E. margaritae. On M2 the lingual sinus of E. ruber and E. oculatus is much stronger curved forward than in E. margaritae. On m1 the anterolophulid is missing in E. ruber and E. oculatus, but can be present in E. margaritae. The m1 of E. margaritae always has the metalophulid I and often the metalophulid II; in E. ruber and E. oculatus. the metalophid I is always absent.
The early Miocene of Spain and France has yielded some other species that may show a bicuspidate anterocone of the M1, these species are in the collatus group of Freudenthal and Martín-Suárez (Citation2016). An assemblage from Cetina de Aragon (Spain) included in E. gerandianus by Daams (Citation1976), has a double anterocone in 13 out of 27 M1. It is of similar size as Eucricetodon from Gözükızıllı (), but differs because of its relatively broad M1 and m1. E. infralactorensis from Estrepouy also has a split anterocone, but similar to the material from Cetina de Aragon, its M1 is relatively wide. After compiling the list containing 41 formally described species of Eucricetodon species it was a surprise that only E. margaritae from the early part of the late Oligocene of Spain resembles the two sibling species from Gözükızıllı in size and morphology.
lists the eleven samples for which the microstructure of the lower incisor is available. Early Oligocene Eucricetodon samples (included in the atavus group) have a type 1 microstructure, the latest Oligocene and Miocene species (included in the collatus group) have type 4. In between is a mixed bag from the early part of the late Oligocene with various ‘groups’ and schmelzmuster types. Much more microstructure data is needed and the allocation to group of the various species needs to be reviewed. But, it seems to us that Gomes Rodrigues et al. (Citation2013) are unduly pessimistic on the merits of the enamel microstructure. The schmelzmuster data adds to our suspicion that Eucricetodon is polyphyletic.
Table 8. Schmelzmuster types of lower incisors in Eucricetodon samples. The allocation to group (grp) is after Freudenthal and Martín-Suárez (Citation2016)
Suborder Eusciurida Flynn et al., Citation2019
Family: Gliridae Muirhead, Citation1819
The three glirid teeth from Gözükızıllı are the oldest record of the family in Anatolia.
Subfamilly: Bransatoglirinae Daams and de Bruijn, Citation1995
Bransatoglis cf. sjeni Ünay-Bayraktar, Citation1989
()
Figure 16. The Dipodidae and Gliridae from Gözükızıllı: a Dipodidae gen. et sp. indet. GOZ1b-260; Bransatoglis cf. sjeni: b GOZ1b-255, c GOZ1b-258; Peridyromys or Microdyromys: d GOZ1b-257
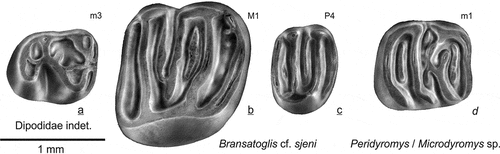
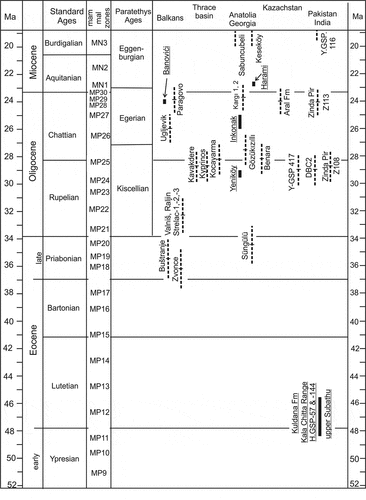
Figure 17. Chronostratigraphic scheme of Palaeogene faunas from Anatolia and the wider region of the Balkans and Southern Asia. Broken lines indicate uncertainty ranges, duration of these are arbitrarily set at 8% of the estimated age. Locations with a magneto-stratigraphic or chronostratigraphic age estimate have a solid range and names are underlined. References on the sites are: Aral Fm (Lucas et al. Citation1998; Bendukidze et al. Citation2009), Banovice (de Bruijn et al. Citation2013), Benara (Métais et al. Citation2016), Buštranje, Strelac, Valniš, Zvonce (de Bruijn et al. Citation2018ab), Harami, Inkonak, Kargi, Yeniköy, Keseköy (Krijgsman et al. Citation1996; Ünay et al. Citation2003a), Kala Chitta Range/upper Subathu Grp. (Hussain et al. Citation1978; Gingerich Citation2003), Kavakdere, Kocayarma (Ünay-Bayraktar Citation1989), Kyprinos (Doukas and Theocharopoulos Citation1999), Paragovo, Ugljevik (Marković et al. Citation2019; van de Weerd et al. Citationin prep), Sabuncubeli (de Bruijn et al. Citation2006), Süngülu (de Bruijn et al. Citation2003), Y-GSP417, DBC2, Z108 (Métais et al. Citation2009), Y-GSP116 (Hartman et al. Citation2019), Z113 (Lindsay and Flynn Citation2016)
Materials & measurements: P4 dext. GOZ1b-258, length 0.64, width 0.86 mm; M1 sin. GOZ1b-255; length 1.34 mm, width 1.45 mm.
Synonymy: Bransatoglis cf. complicatus (Ünay et al. Citation2003a)
Description and comparisons
The P4 () does not have an interdental facet on its anterior side, so this species did not have a DP3. There are narrow notches separating the lingual ends of the protoloph and posteroloph from the protocone. The long anterior centroloph is not connected to the paracone. The posterior centroloph is absent. There is an indistinct low extra ridge in the valley between the metaloph and the posteroloph.
The occlusal surface of the M1 () is narrower anteriorly than posteriorly. The endoloph is complete. The anterior centroloph is longer than the posterior one, but does not reach the endoloph. There is a well-developed extra-ridge between the protoloph and the anterior centroloph and an indistinct incipient extra-ridge between the protoloph and the anteroloph.
The two Bransatoglis cheek teeth from Gözükızıllı-1 are the same size as those of B. sjeni from Kocayarma (Thrace basin; Ünay-Bayraktar Citation1989). The P4 from Gözükızıllı-1 differs from the type material of that species in lacking the posterior centroloph. The endoloph of the M1 from Gözükızıllı-1 is complete whereas the lingual ends of the anteroloph and posteroloph are separated from the protocone by a notch on the specimens from Kocayarma. The morphology of the specimens from Gözükızıllı is interpreted as more advanced than the material from Kocayarma.
Subfamily indet.
cf. Peridyromys/Microdyromys sp. ()
Material and measurements: m1sin. GOZ1b-258; length 0.90 mm, width 0.82 mm.
Synonymy: Glirulus sp. (Ünay et al. Citation2003a).
Description and comparisons
This small m1 probably has a somewhat aberrant morphology in showing a connection between the incomplete metalophid and the centrolophid. This configuration is unusual but the tooth has the common basic dental pattern of a glirid m1 with a rather long centrolophid, an anterior extra-ridge and a posterior extra-ridge. This basic morphology and its small size are shared by a number of species of the genera Peridyromys and Microdyromys. This small m1 shows the presence of a second genus and species of Gliridae in Gözükızıllı and documents fauna exchange between Europe and Anatolia.
cf. Dipodidae gen. et sp. indet.
()
Materials and measurements: m3sin GOZ1b-259, length 0.89 mm, width 0.72 mm and m3 dex GOZ1b-260, length 0.87 mm, width 0.72 mm.
Remarks
The two m3 with a cricetid pattern do not show characteristic features to ascertain whether they represent a cricetid or a dipodid. Possible options in this size group are in the pseudocricetodontines and the dipodines. The only records of the Pseudocricetodontinae in the Palaeogene of Anatolia are from Yeniköy and Süngülü (de Bruijn et al. Citation2003). Since Dipodidae occur in several late Oligocene assemblages from the area we tentatively allocate the two m3 to cf. Dipodidae gen. et sp. indet.
Biogeography and Chronology
shows the distribution and numbers of first and second molars and the minimum numbers of individuals in the two Gözükızıllı horizons. Striking is the relatively low number of species considering the large samples that have been taken. Daxneria teeth are rather fragile and break easily during the washing and screening process. The minimum number of individuals of Daxneria is based on four right m1 in Gözükızıllı-1a and three left d4 in Gözükızıllı-1b. Daxneria and Sayimys are Asian elements, the glirids Bransatoglis and cf. Peridyromys/Microdyromys have an European origin.
Table 9. Composition of the faunas found in Gözükızıllı-1a, −1b and Güvendik. Shown are the total number of first and second molars (M1/2) and the minimum number of individuals (N) as determined from the largest number of one of the dental elements. The cross indicates that no first or second molar has been found, but that the taxon is represented by another dental element
summarises the chronostratigraphic position of Gözükızıllı with respect to the Eocene and Oligocene rodent faunas described from the wider region, including the Balkans, Thrace basin, Anatolian basins, Georgia, Kazakhstan and Pakistan. Eucricetodon is the first cricetid to arrive in Central and Western Europe, marking the ‘Grande Coupure’. It is present in Anatolia in Süngülü (latest Eocene), Yeniköy and Gözükızıllı-1. The two species of Eucricetodon from Gözükızıllı resemble E. margaritae from the late Oligocene of Spain (MP26). Krijgsman et al. (Citation1996) suggested that the sites in Anatolia with Eucricetodon are older than the faunas dominated by Meteamys and Muhsinia (Inkonak, Kargı), giving the Gözükızıllı-1 fauna a range between approx. 29 and 26 Ma (). Métais et al. (Citation2016) presented a preliminary age of approx. 28 Ma for Gözükızıllı-2 and −3 based on unpublished magneto-stratigraphic data. If correct, Gözükızıllı-1 (stratigraphically ~35 m above Gözükızıllı-2) could be about 28–27 Ma.
The faunas from Yeniköy and Gözükızıllı have a low diversity. Ünay et al. (Citation2003a) therefore suggested the possibility of insularity for Anatolia during the Oligocene. Palaeogeographic maps lend some support for this hypothesis, but the published maps covering the Oligocene differ in details. Jones (Citation1999, Figures 14.6–14.8) assumes a marine connection between the Indian Ocean and the Caspian region, thus isolating Anatolia from the east. However, Meulenkamp and Sissingh (Citation2003, ) and Métais et al. (Citation2015) showed a corridor between Anatolia and the Iranian block. Whatever the geography may have been, migration was possible, perhaps the corridor area functioned as a filter-bridge. A marine barrier existed between Anatolia and the Thrace basin during the late Eocene-early Oligocene (Meulenkamp and Sissingh Citation2003, ) explaining the large difference between the Oligocene rodent faunas from the Thrace basin and Anatolia. This barrier disappeared after 30 Ma (Okay et al. Citation2019), but the difference between the rodent faunas from the Thrace basin and Anatolian persisted for some time. During the late Oligocene high-diversity faunas with ‘balanced pan-Anatolian character’ appear in Inkonak and Kargı (Ünay et al. Citation2003a).
Only a few Eocene and early Oligocene rodent faunas are known from the great swathe of country formed by the southern rim of Asia, the Indian continental block, Asia Minor and areas in between (a palaeogeographic map is in Gomes Rodrigues et al. Citation2012). The age of most cluster near 29–28 Ma (). Baluchimyines have been found in most of these, in the larger assemblages of the late-early Oligocene of Pakistan with up to six species. They thus form an important part of the rodent faunas in this poorly sampled region. However, baluchimyines are completely absent in the late Eocene and early Oligocene faunas of the Balkans. Baluchimyines are believed to have evolved from an Eocene ancestor close to the chapattimyids found in the Kuldana Formation (Flynn Citation1986; Marivaux and Boivin Citation2019), but an enormous gap in the fossil record separate these groups ().
The three glirid teeth from Gözükızıllı are the oldest record of the family from Anatolia. The glirids (being of European origin) in Gözükızıllı show that migration from Europe to Anatolia was possible. This seems supported by the similarity of Gözükızıllı Eucricetodon to a late Oligocene Spanish species.
Antoine et al. (Citation2008) and Métais et al. (Citation2016) reported on the age of the finds of larger mammals in Gözükızıllı-2 and −3 in the lower Kızılırmak Formation. In contrast to the rodent faunas, Antoine et al. (op. cit.) and Métais et al. (op. cit.) do not see evidence for geographic isolation in the larger mammals. They indicate that the large mammals are late Oligocene, probably slightly younger than those of Benara (Georgia). Gabunia and Bendukidze (Citation1990) illustrated the cricetids Eucricetodon, Paracricetodon, Pseudocricetodon and the glirid Peridyromys from Benara with simple line drawings. Comparison with good illustrations confirm their identifications. The worn half molar of in Gabunia and Bendukidze may be included in the baluchimyine Lindsaya. The Benara rodent faunule may thus be close in age to those from Gözükızıllı and the Thrace basin (Ünay-Bayraktar Citation1989).
Conclusions
The Gözükızıllı-1 rodent faunas are of low diversity and contain Asian and European elements. Eucricetodon is dominant, two new species E. ruber and E. oculatus are described. These are morphologically very similar, but differ in size. The two Eucricetodon species are morphologically close to the late Oligocene E. margaritae from Spain. The new genus Daxneria, an Asian element in the assemblage, is well represented in Gözükızıllı-1. It is included in the Baluchimyinae, a subfamily known from the latest Eocene of Thailand, the ‘“middle”’ Oligocene of the Indian sub-continent and the latest Eocene of Süngülu (Asia Minor). Daxneria fragilis has a specialised dentition with high-crowned teeth, strong roots and thin enamel. Similar to several other baluchimyine genera Daxneria fragilis has no permanent fourth premolar, but retains its deciduous premolar. The M3 of Daxneria resembles those of Ottomania and Lophibaluchia in having a reduced posterior part. The ctenodactyl Sayimys sp. is another Asian element in Gözükızıllı-1. It is the oldest record of this genus so far. The two glirid species in Gözükızıllı-1 are European elements. The possible dipodid completes the faunal list. Our age estimate of this fauna is 29–26 Ma, that is in the early part of the late Oligocene.
Acknowledgments
We gratefully acknowledge the facilities offered by the MTA (Turkish Geological Survey) during our field parties, in particular Gerçek Saraç is thanked for his support. Pablo Peláez-Campomanes (Museo Nacional de Ciencias Naturales in Madrid) was most helpful in providing casts of Eucricetodon. The permission of Lawrence Flynn (Harvard University) and Laurent Marivaux (Université de Montpellier) to reproduce some figures and to supply photographs is greatly appreciated. The SEM pictures of the cheek teeth were made by Tilly Bouten (Utrecht University) and retouched by Jaap Luteijn (Utrecht University); the SEM photographs of incisor microstructure were made by Hans Meeldijk (Utrecht University). Discussions with Thomas Martin (Bonn University) elucidated the incisor microstructure of Daxneria. Marinus Lockefeer is thanked for his expert Latin advice. This paper benefited from the detailed reviews of Myriam Boivin (Universidad Nacional de San Salvador de Jujuy, Argentina) and an anonymous reviewer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguilar J-P. 1974. Les Rongeurs du Miocène inférieur en Bas-Languedoc et les corrélations entre échelles stratigraphiques marine et continental. Géobios. 4(7):345–398. doi:10.1016/S0016-6995(74)80015-X.
- Aguilar J-P, Legendre S, Michaux J 1997. Actes du Congrès BiochroM’97. Mémoires et Travaux de l’Institut de Montpellier de l’École Pratique des Hautes Études. 21.
- Agusti J, Arbiol S. 1989. Nouvelles espèces de rongeurs (Mammalia) dans l’Oligocène supérieur du bassin de l’Ebre (N. E. de l’Espagne). Geobios. 22(3):65–275. doi:10.1016/S0016-6995(89)80132-9.
- Antoine P-O, Karadenizli L, Saraç G, Sen S. 2008. A giant rhinocerotoid (Mammalia, Perissodactyla) from the Late Oligocene of north-central Anatolia (Turkey). Zool J Linn Soc. 152(3):581–592. doi:10.1111/j.1096-3642.2007.00366.x.
- Barbière F, Marivaux L. 2015. Phylogeny and evolutionary history of hystricognathous rodents from the Old World during the Tertiary: new insights into the emergence of modern ‘phiomorph’ families. In: Cox FG, Hautier L, editors. Evolution of the rodents: advances in phylogenetics, functional morphology and development. Cambridge University Press; p. 87–138.
- Baskin J. 1996. Systematic revision of Ctenodactylidae (Mammalia, Rodentia) from the Miocene of Pakistan. Paleovertebrata. 25:1–49.
- Baudelot S, de Bonis L. 1968. Contribution à l’étude des rongeurs de Γ Aquitanien moyen et supérieur de l’Agenais. Société d’Histoire Naturelle de Toulouse, 104(1/2):160–164.
- Bendukidze OG. 1993. Small mammals from the Miocene of southwestern Kazakhstan and Turgai. Metsniereba Tiblissi. in Russian, 1–139.
- Bendukidze OG, de Bruijn H, van den Hoek Ostende LW. 2009. A revision of Late Oligocene associations of small mammals from the Aral Formation (Kazakhstan) in the National Museum of Georgia. Tbilissi Palaeodiversity. 2:343–377.
- Berthet D, Escuillié F, Hugueney M. 2005. Les faunes de petits mammifères de Billy-Créchy (Allier) à la transition Oligocène-Miocène. Cahiers scientifiques du Muséum d’histoire naturelle de Lyon - Centre de conservation et d’étude des collections. Tome. 8:7–31.
- Bohlin B. 1946. The fossil mammals from the Tertiary deposit of Taben-Buluk, Western Kansu Part 1I: simplicidentata, Carnivora, Artiodactyla, Perissodactyla, and Primates. Palaeontologica Sinica New Series C. 8b:1–259.
- Bowdich TE. 1821. An analysis of the natural classifications of mammalia for the “use of students and travelers. J Smith Paris. 1–115.
- Comte B. 1985. Eléments nouveaux sur l’évolution des genres Eucricetodon et Pseudocricetodon (Eucricetodontinae, Rodentia, Mammalia) de l’Oligocène d’Europe occidentale. Palaeovertebrata. 15(1):1–69.
- D’Elía G, Fabre P-H, Lessa EP. 2019. Rodent systematics in an age of discovery: recent advances and prospects. J Mammal. 100(3):852–871. doi:10.1093/jmammal/gyy179.
- Daams R. 1976. Miocene rodents from Cetina de Aragon (Prov. Zaragoza) and Buñol (Prov, Valencia), Spain. Proceedings Koninklijke Nederlandse Akademie Van Wetenschappen Series B. 79(3):152–182.
- Daams R, de Bruijn H. 1995. A classification of the Gliridae (Rodentia) on the basis of dental morphology. Hystrix. 6(12):3–50.
- Daams R, Freudenthal M, Lacomba JL, Alvarez MA. 1989. Upper Oligocene micromammals from Pareja, Loranca Basin, prov. of Guadalajara, Spain. Scripta Geologica. 89:27–56.
- De Bruijn H, Daams R, Daxner-Höck G, Fahlbusch V, Ginsburg L, Mein P, Morales J. 1992. Report of the RCMNS working group on fossil mammals, Reisensburg 1990. Newsl Stratigr. 26(2/3):65–118. doi:10.1127/nos/26/1992/65.
- De Bruijn H, Hussain NT, Leinders JJM, 1981. Fossil rodents from the Murree formation near Banda Daud Shah, Kohat, Pakistan. Proceedings Koninklijke Nederlandse Akademie van Wetenschappen B, 84: 71–99; Amsterdam.
- De Bruijn H, Marković Z, Wessels W. 2013. Late Oligocene rodents from Banovići (Bosnia and Herzegovina). Palaeodiversity. 6:63–105.
- De Bruijn H, Marković Z, Wessels W, Milivojević M, van de Weerd AA. 2018a. Rodent faunas from the Paleogene of south-east Serbia. Palaeobiodiversity Palaeoenvironments. 98(3):441–458. doi:10.1007/s12549-017-0305-0.
- De Bruijn H, Marković Z, Wessels W, van de Weerd AA. 2018b. Pappocricetodontinae (Rodentia, Muroidea) from the Paleogene of south-east Serbia. Paleobiodiversity Paleoenvironments. 99(3):511–526. doi:10.1007/s12549-018-0343-2.
- De Bruijn H, Mayda S, van den Hoek Ostende L, Kaya T, Saraç G. 2006. Small mammals from the Early Miocene of Sabuncubeli (Manisa, S.W. Anatolia, Turkey). Beiträge zur Paläontologie Österreichs. 30:57–87.
- De Bruijn H, Rümke CG. 1974. On a peculiar mammalian association from the Miocene of Oschiri (Sardinia) 1 and 2. Proc K Ned Akad Wet B. 77(1):44–79.
- De Bruijn H, Ünay E, Saraç G, Klein Hofmeier G. 1987. An unusual new eucricetodontine from the lower Miocene of the Eastern Mediterranean. Proc K Ned Akad Wet B. 90(21):119–132.
- De Bruijn H, Ünay E, Saraç G, Yilmaz A. 2003. A rodent assemblage from the Eo/Oligocene boundary interval near Süngülü, Lesser Caucasus, Turkey. In: López-Martínez N, Peláez-Campomanes P, Hernández Fernández M, editors. En torno a Fósiles de Mamíferos: datación, Evolución y Paleoambiente. Coloquios de Paleontología, (Universidad Complutense de Madrid) Volumen Extraordinario no 1. En honor al dr. Remmert Daams; p. 47–76. ISSN: 1132-1660.
- De Bruijn H, van den Hoek Ostende LW, Donovan SK. 2007. Mirrabella, a new name for the genus Mirabella de Bruijn et al., 1987 (Mammalia): preoccupied by Mirabella Emeljanov, 1982 (Insecta). Contrib Zool. 76(4):279–280. doi:10.1163/18759866-07604006.
- De Gregorio A. 1887. Intorno a un deposito di roditori e di carnivori sulla vetta di Monte Pellegrino. Atti Della Società Toscana Scienze Naturali Pisa. 8:3–39.
- De Leeuw A, Mandic O, de Bruijn H, Marković Z, Reumer J, Wessels W, Šišić E, Krijgsman W. 2011. Magnetostratigraphy and small mammals of the Late Oligocene Banovići basin in NE Bosnia and Herzegovina. Palaeogeography, Palaeoclimatol, Palaeoecol. 310(34):400–412. doi:10.1016/j.palaeo.2011.08.001.
- Dienemann A. 1987. Die Gattungen Eucricetodon und Pseudocricetodon (Rodentia, Mammalia) aus dem Oligocän, Süddeutschlands. Verlag Bayerische Akademie.der Wissenschaften. Abhandelungen Neue Folge. 165:1–158.
- Doukas CS, Theocharopoulos CD. 1999. Smaller mammals from the Oligocene of Kyprinos (Thrace, N. Greece). In: Reumer JWF, de Vos J, editors. Elephants have a snorkel! Papers in honour of Paul Y. Sondaar. Deinsea. Vol. 7; p. 133–145. [ISSN 0923-9308].
- Engesser B. 1985. Die Gattung Eucricetodon (Mammalia, Rodentia) im Grenzbereich Oligozän/Miozän. Eclogae Geol Helv. 78(3):669–692.
- Engesser B. 1987. New Eomyidae, Dipodidae and Cricetidae (Rodentia, Mammalia) of the Lower Freshwater Molasse of Switzerland and Savoy. Eclogae Geologicae Helvetiae. 80(3):945–993.
- Flynn LJ, Cheema IU. 1994. Baluchimyine rodents from the Zinda Pir Dome, western Pakistan: systematic and biochronologic implications. In: Tomida Y, Li C, Setoguchi T, editors. Rodent and Lagomorph Families of Asian Origins and Diversification. Kyoto: National Science Museum Monographs; p. 115–129.
- Flynn LJ, Jacobs LL, Cheema IU. 1986. Baluchimyinae, a new ctenodactyloid rodent subfamily from the Miocene of Baluchistan. Am Mus Novit. 2841:1–58.
- Flynn LJ, Jacobs LL, Kimura Y, Lindsay EH. 2019. Rodent Suborders. Fossil Imprint. 75(3–4):292–298. Praha. 2533–4050 (print), 2533–4069 (on-line). doi:10.2478/if-2019-0018.
- Freudenthal M. 1994. Cricetidae (Rodentia, Mammalia) from the Upper Oligocene of Mirambueno and Vivel del Río (prov. Teruel, Spain). Scripta Geologica. 104:1–55.
- Freudenthal M. 1996. The Early Oligocene rodent fauna from Olalla 4A (Teruel, Spain). Scripta Geologica. 112:1–67.
- Freudenthal M, Lacomba JI, Sacristán MA. 1992. Classification of European Oligocene cricetids. Revista Espagnola Paleontología Extra Volume. 49–57.
- Freudenthal M, Martín-Suárez E. 2016. A review of Oligocene and early Miocene European Cricetidae (Mammalia). Spanish J Palaeontol. 31(2):341–352. doi:10.7203/sjp.31.2.17160.
- Gabunia L, Bendukidze O. 1990. On small mammals from the Oligocene of Benara (Akhalzikhe region, south Georgia). Acad Sci Georgia, Inst Paleobiol. 1–11.
- Gervais MP. 1848-1852. Zoologie et Paléontologie Françaises. Nouvelles Recherches sur les Animaux Vivants et Fossiles de la France. Arthus Bertrand Paris. 13:1–271.
- Gervais P. 1853. Description ostéologique de l’Anomalurus, et remarques sur la classification naturelle des rongeurs. Annales Sciences Naturalis. 20:238–246. Paris (3e série).
- Gingerich PD. 2003. Stratigraphic and micropaleontological constraints on the middle Eocene age of the mammal-bearing Kuldana Formation of Pakistan. J Vertebr Paleontol. 23(3):643–651. doi:10.1671/2409.
- Gomes Rodrigues H, Marivaux L, Vianey-Liaud M. 2012. The Cricetidae (Rodentia, Mammalia) from Ulantatal area (Inner Mongolia, China): new data concerning the evolution of Asian cricetids during the Oligocene. J Asian Earth Sci. 56:160–179. doi:10.1016/j.jseaes.2012.05.007.
- Gomes Rodrigues H, Marivaux L, Vianey-Liaud M. 2013. On the status of early Eucricetodontinae (Muroidea, Rodentia) with a special focus on the Atavocricetodon vs Eucricetodon issue: morphometrical aspects. Spanish J Paleontol, Sociedad Española De Paleontología. 28(1):17–27. doi:10.7203/sjp.28.1.17813.
- Gomes Rodrigues H, Marivaux L, Vianey-Liaud M. 2014. Rodent paleo communities from the Oligocene of Ulantatal (Inner Mongolia, China). Palaeovertebrata. 38(1):1–11. doi:10.18563/pv.38.1.e3.
- Hartman J, van de Weerd AA, de Bruijn H, Wessels W. 2019. An exceptional large sample of the early Miocene ctenodactyline rodent Sayimys giganteus, specific variation and taxonomic implications. Fossil Imprint. 75(3–4):359–382. Praha. 2533–4050 (print), 2533–4069 (on-line). doi:10.2478/if-2019-0023.
- Hinton MAC. 1933. Diagnoses of new genera and species of rodents from Indian Tertiary Deposits. Ann Mag Nat Hist Ser. 10(12):620–622. doi:10.1080/00222933308673728.
- Huchon D, Catzeflis FM, Douzery JP. 2000. Variance of molecular datings, evolution of rodents, and the phylogenetic affinities between Ctenodactylidae and Hystricognathi. Proc Royal Soc B. 267(1441):393–402. doi:10.1098/rspb.2000.1014.
- Hugueney M. 1969. Les rongeurs (Mammalia) de l’Oligocène supérieur de Coderet-Bransat (Allier). Documents Laboratoire Géologique Faculté Sciences Lyon. 34:1–227.
- Hugueney M. 1980. La faune de mammifères de l’Oligocène moyen de Saint-Menoux (Allier). Première partie: rongeurs (Mammalia, Rodentia). Revue Scientifique Du Bourbonnais. 1980. 57–71.
- Hugueney M. 1999. Genera Eucricetodon and Pseudocricetodon. In: Rössner GE, Heissig K, editors. The Miocene land mammals of Europe. Munich: Friedrich Pfeil; p. 347–358.
- Hugueney M, Bulot C. 2011. Les petits Mammifères du Burdigalien (MN3; Miocène) d’Estrepouy (Gers, France): liste faunique actualisée. Estudios Geologicos. 67(2):427–442. doi:10.3989/egeol.40584.200.
- Hussain ST, de Bruijn H, Leinders JM. 1978. Middle Eocene rodents from the Kala Chitta Range (Punjab, Pakistan). Proceedings Koninklijke Nederlandse Akademie Van Wetenschappen Series B. 81:74–112.
- Illiger C 1811: Prodromus systematis mammalium et avium additis terminis zoographicis utriusque classis, eorumque versione germanica p. 1–301. Berolini: Sumptibus C. Salfeld.
- Jones RW. 1999. Marine invertebrate (chiefly foraminiferal) evidence for the palaeogeography of the Oligocene-Miocene of western Eurasia, and consequences for terrestrial vertebrate migration. In: Agusti J, Rook L, Andrews P, editors. Hominid evolution and climatic changes in Europe Volume 1. The Evolution of Neogene Terrestrial Ecosystems in Europe. Cambridge University Press; 274–308.
- Kalthoff DC. 2000. Die Schmelzmikrostructur in den Incisiven der hamsterartigen Nagetiere und anderer Myomorpha (Rodentia, Mammalia). Palaeontographica (A). 259:1–193.
- Karadenizli L. 2011. Oligocene to Pliocene palaeogeographic evolution of the Çankırı-Çorum Basin, central Anatolia, Turkey. Sediment Geol. 237(12):1–29. doi:10.1016/j.sedgeo.2011.01.008.
- Kayseri-Özer MS, Karadenizli L, Akgün F, Oyal N, Saraç G, Şen S, Tunoğlu C, Tuncer A. 2017. Palaeoclimatic and palaeoenvironmental interpretations of the Late Oligocene, Late Miocene–Early Pliocene in the Çankırı-Çorum Basin. Palaeogeogr Palaeoclimatol Palaeoecol. 467:16–36. doi:10.1016/j.palaeo.2016.05.022.
- Kordikova EG, de Bruijn H. 2001. Early Miocene rodents from the Aktau Mountains (south- eastern Kazakhstan). Senckenbergiana Lethaea. 81(2):391–405. doi:10.1007/BF03042791.
- Krijgsman W, Duermeijer CE, Langereis CG, de Bruijn H, Saraç G, Andriessen PAM. 1996. Magnetic polarity stratigraphy of Late Oligocene to Middle Miocene mammal-bearing continental deposits in Central Anatolia (Turkey). Newsl Stratigr. 34(1):13–29. DOI:007S-00421/96/0034–0013. doi:10.1127/nos/34/1996/13.
- Lavocat R. 1961. Le gisement de vertébrés miocènes de Beni Mellal (Maroc): étude systématique de la faune de mammifères. Notes et Mémoires du Service Géologique du Maroc. 155:29–77.
- Li C, Qiu Z. 1980. Early Miocene mammalian fossils of the Xining basin. Qinghai province, Vertebrata Palasiatica. 18(3):198–209.
- Li Q, Meng J, Wang Y. 2016. New Cricetid Rodents from Strata near the Eocene-Oligocene Boundary in Erden Obo Section (Nei Mongol, China). PLoS ONE. 11(5):e0156233. doi:10.1371/journal.pone.0156233.
- Lindsay EH, Flynn LJ. 2016. Late Oligocene and early Miocene Muroidea of the Zinda Pir Dome. Hist Biol. 28(12):215–236. doi:10.1080/08912963.2015.1027888.
- Lopatin AV. 1996. Stratigraphy and small mammals from the Aral Formation of the Altyn Schokysu locality (North Aral region). Stratigrafiya, Geologicheskaya Korrelyatsiya. 4:65–79.
- López-Antoñanzas R, Knoll F, Maksoud S, Azar D. 2015. First Miocene rodent from Lebanon provides the ‘missing link’ between Asian and African gundis (Rodentia: ctenodactylidae). Sci Rep. 5(1):12871. doi:10.1038/srep12871.
- López-Antoñanzas R, Sen S, Koufos GD. 2005. A ctenodactylid rodent (Mammalia: rodentia) from the Middle Miocene of Chios Island (Greece). Geobios. 38(1):113–126. doi:10.1016/j.geobios.2003.08.006.
- López-Antoñanzas R, Sen S, Saraç G. 2004. New large ctenodactylid species from the lower Miocene of Turkey. J Vertebr Paleontol. 24(3):676–688. doi:10.1671/0272-4634(2004)024[0676:ANLCSF]2.0.CO;2.
- López-Guerrero P, Maridet O, Daxner-Höck G. 2017. Evolution of the genus Eucricetodon (Rodentia, Mammalia) from the Valley of Lakes (Mongolia): a taxonomical description and update on the stratigraphical distribution. In: : Daxner-Höck Göhlich U, editor. The Valley of Lakes in Mongolia, a key area of Cenozoic mammal evolution and stratigraphy. Palaeobiodiversity and Palaeoenvironments. Vol. 97. (1);67-87. doi:10.1007/s12549-016-0251-2.
- Lucas SG, Kordikova EG, Emry RJ. 1998. Oligocene stratigraphy, sequence stratigraphy, and mammalian biochronology north of the Aral sea, western Kazakhstan. In: Beard C, Dawson MR, editors. Dawn of the age of mammals in Asia. Bulletin of Carnegie Museum of Natural History. Vol. 34; p. 313–348.
- Maridet O, Wu W, Ye J, Bi S, Ni X, Meng J. 2009. Eucricetodon (Rodentia, Mammalia) from the Late Oligocene of the Junggar basin, northern Xinjiang. China:American Museum Novitates. Vol. 3665; 1–21.
- Marivaux L, Benammi M, Ducrocq S, Jaeger J-J CY. 2000. A new baluchimyine rodent from the Late Eocene of the Krabi Basin (Thailand): paleobiogeographic and biochronologic implications. Comptes rendus de l’Academie des Sciences. 331:427–433.
- Marivaux L, Boivin M. 2019. Emergence of hystricognathous rodents: palaeogene fossil record, phylogeny, dental evolution and historical biogeography. Zool J Linn Soc. 20:1–36.
- Marivaux L, Vianey-Liaud M, Jaeger -J-J. 2004. High-level phylogeny of early Tertiary rodents: dental evidence. Zool J Linn Soc. 142:105–134. doi:10.1111/j.1096-3642.2004.00131.x.
- Marivaux L, Vianey-Liaud M, Welcomme JL. 1999. Premiere découverte de Cricetidae (Rodentia, Mammalia) Oligocènes dans le synclinal sud de Gandoe (Bugti Hills, Balouchistan, Pakistan). Comptes Rendus de l’Académie des Sci. 329:839–844.
- Marivaux L, Welcomme J-L. 2003. New diatomyid and baluchimyine rodents from the Oligocene of Pakistan (Bugti Hills, Balochistan): systematic and paleobiogeographic implications. J Vertebr Paleontol. 23(2):420–434. doi:10.1671/0272-4634(2003)023[0420:NDABRF]2.0.CO;2.
- Marivaux L, Welcomme J-L, Vianey-Liaud M, Jaeger -J-J. 2002. The role of Asia in the origin and diversification of hystricognathous rodents. Zool Scr. 31:225–239. doi:10.1046/j.1463-6409.2002.00074.x.
- Marković A, de Bruijn H, van de Weerd AA, Wessels W. 2019. Deperetomys (Rodentia, Muridae) from the Oligocene of Serbia and Bosnia and Herzegovina. Palaeobiodiversity Palaeoenvironments. doi:10.1007/s12549-019-00389-0.
- Martin T. 1992. Schmelzmikrostruktur in den Inzisiven alt- und neuweltlicher hystricognather Nagetiere. Palaeovertebrata, Mémoire extraordinaire. 1–168.
- Matthew WD, Granger W. 1923. Nine new rodents from the Oligocene of Mongolia. Am Mus Novit. 102:1–10.
- Mayo NA. 1982. Bemerkungen zur Systematik und Evolution einiger Theridomyidae und Cricetidae (Rodentia, Mammalia) des Oligozäns: antwort an M. Vianey Liaud. Eclogae Geologicae Helvetiae. 75:697–719.
- Mein P, Freudenthal M. 1971a. Une nouvelle classification des Cricetidae (Mammalia, Rodentia) du Tertiaire de l’Europe. Scripta Geologica. 2:1–35.
- Mein P, Freudenthal M. 1971b. Les Cricetidae de Vieux-Collonges, partie 1. Scripta Geologica. 5:1–51.
- Métais G, Albayrak E, Antoine P-O, Erdal O, Karadenizli L, Oyal N, Saraç G, Büyükmeriç Y, Sen S. 2016. Oligocene ruminants from the Kızılırmak Formation, Çankırı-Çorum Basin, central Anatolia, Turkey. Palaeontologia Electronica. 19(3.37A):1–23.
- Métais G, Antoine P-O, Hassan Baqri SR, Crochet J-Y, De Franceschi D, Marivaux L, Welcomme J-L. 2009. Lithofacies, depositional environments, regional biostratigraphy and age of the Chitarwata Formation in the Bugti Hills, Balochistan, Pakistan. J Asian Earth Sci. 34(2):154–167. doi:10.1016/j.jseaes.2008.04.006.
- Métais G, Sen S, Sözeri K, Peigné S, Varol B. 2015. Late Paleogene terrestrial fauna and paleoenvironments in Eastern Anatolia: new insights from the Kağızman-Tuzluca Basin. J Asian Earth Sci. 107:96–109. doi:10.1016/j.jseaes.2015.03.048.
- Meulenkamp JE, Sissingh W. 2003. Tertiary palaeogeography and tectonostratigraphic evolution of the Northern and Southern Peri-Tethys platforms and the intermediate domains of the African–Eurasian convergent plate boundary zone. Palaeogeography, Palaeoclimatolol, Palaeoecolol. 196(12):209–228. doi:10.1016/S0031-0182(03)00319-5.
- Misonne X. 1957. Mammifères oligocènes de Hoogbutsel et Hoeleden; 1 – rongeurs et Ongulés. Bulletin De l’Institut Royal Des Sciences Naturelles De Belgique. 33(51):1–15.
- Muirhead L. 1819. Mazology. In: Brewster D, editor. The Edinburgh Encyclopedia W (Vol. 13). Edinburgh: Blackwood; p. 393–486.
- Okay AI, Özcan E, Hakyemez A, Siyako M, Sunal G, Kylander-Clark ARC. 2019. The Thrace Basin and the Black Sea: the Eocene–Oligocene marine connection. Geol Mag. 156(1):39–61. doi:10.1017/S0016756817000772.
- Oliver A, Sanisidro O, Bayarmaa B, Ichinnorov N, Daxner-Höck G. 2017. Turnover and diversification rates in Ctenodactylidae (Rodentia, Mammalia) from Mongolia. Palaeobiodiversity Palaeoenvironments. 97(1):51–65. doi:10.1007/s12549-016-0265-9.
- Peláez-Campomanes P. 1995. Valdecollares: a rodent fauna from the lower Oligocene of the Loranca Basin (Cuenca, Spain). Proc K Ned Akad Wet. 98(3):265–289.
- Sallam HM, Seiffert ER. 2016. New phiomorph rodents from the latest Eocene of Egypt, and the impact of Bayesian ‘‘clock’’-based phylogenetic methods on estimates of basal hystricognath relationships and biochronology. PeerJ. 4:e1717. doi:10.7717/peerj.1717.
- Sallam HM, Seiffert ER, Simons EL. 2011. Craniodental morphology and systematics of a new family of hystricognathous rodents (Gaudeamuridae) from the Late Eocene and Early Oligocene of Egypt. PLoS ONE. 6:e16525. doi:10.1371/journal.pone.0016525.
- Sallam HM, Seiffert ER, Simons EL. 2012. A basal phiomorph (Rodentia, Hystricognathi) from the Late Eocene of the Fayum Depression, Egypt. Swiss J Palaeontol. 131(2):283–301. doi:10.1007/s13358-012-0039-6.
- Sallam HM, Seiffert ER, Steiper ME, Simons EL. 2009. Fossil and molecular evidence constrain scenarios for the early evolutionary and biogeographic history of hystricognathous rodents. Proc Natl Acad Sci USA, USA. 106(39):16722–16727. doi:10.1073/pnas.0908702106.
- Schaub S. 1925. Die hamsterartigen Nagetiere des Tertiärs und ihre lebenden Verwandten. Abhandlungen Schweizerischen Paläontologische Gesellschaft. 45:1–114.
- Schlosser M. 1884. Die Nager des Europäischen Tertiärs nebst Betrachtungen über die Organisation und die geschichtliche Entwicklung der Nager überhaupt. Palaeontographica. 31:1–143.
- Schmidt-Kittler N, ed. 1987. European reference levels and correlation tables. Münchner Geowissenschaftliche Abhandlungen, (A), Vol. 10: 13–20. (München)
- Shevyreva NS. 1967. Cricetodon from middle Oligocene of central Kazakhstan. Paleontologicheskia Zhurnal. 2:90–98. [in Russian].
- Thaler L. 1966. Les rongeurs fossiles du Bas-Languedoc dans leur rapports avec l’histoire des faunes et la stratigraphie d’Europe. Mémoires Musée Histoire Naturelles nouvelle série C. 17:1–295.
- Tullberg T. 1899. Über das System der Nagetiere: eine phylogenetische Studie. Nova Acta Regiae Societatis Scientiarum Upsaliensis. 18:1–514.
- Ünay E, de Bruijn H, Saraç G. 2003a. The Oligocene rodent record of Anatolia: a review. Reumer JWF, Wessels W, editors. Distribution and migration of Tertiary mammals in Eurasia. Special Issue. Deinsea (Rotterdam), ISSN 2468-8983: 531–538.
- Ünay E, de Bruijn H, Saraç G. 2003b. A preliminary zonation of the continental Neogene of Anatolia based on rodents. In: Reumer JWF, Wessels W, editors. Distribution and migration of Tertiary mammals in Eurasia. Rotterdam: Deinsea, ISSN 2468-8983: 539–547. Special Issue.
- Ünay-Bayraktar E. 1989. Rodents from the Middle Oligocene of Turkish Thrace. Utrecht Micropaleontological Bulletins, Special Publication. 5:1–119.
- van de Weerd AA, de Bruijn H, Wessels W, Marković Z (in prep) New late Oligocene rodent faunas from the Pannonian basin.
- Vianey-Liaud M. 1972. Contribution à l’ étude des Cricétidae oligocènes d’ Europe occidentale. Palaeovertebrata. 5(1):1–44. doi:10.18563/pv.5.1.1-44.
- Vianey-Liaud M, Gomes Rodrigues H, Marivaux L. 2010. A new Oligocene Ctenodactylinae (Rodentia: mammalia) from Ulantatal (nei Mongol): new insight on the phylogenetic origins of the modern Ctenodactylidae. Zool J Linn Soc. 160(3):531–550. doi:10.1111/j.1096-3642.2010.00615.x.
- Vianey-Liaud M, Schmidt-Kittler N, Marivaux L. 2006. The Ctenodactylidae(Rodentia) from the Oligocene of Ulantatal (Inner Mongolia, China). Palaeovertebrata. 34:111–205.
- Viret J, Roman F, Viret J. 1930. Le Miocène continental de l’Armagnac et le gisement burdigalien de La Romieu (Gers). Société Géologique de France. Livre Jubilaire. 2:577–604.
- Wang B. 2001. Late Eocene Ctenodactyloids (Rodentia, Mammalia) from Qujing, Yunnan, China. Vertebrata PalAsiatica. 39(1):24–42.
- Wang B-Y. 1984. Dianomys gen. nov. (Rodentia, Mammalia) from the lower Oligocene of Qujing, Yunnan, China. Mainzer geowissenschaftlicher Mitteilungen. 13:37–48.
- Wang B-Y. 1988. Distylomyidae fam. nov. (?Ctenodactyloidea, Rodentia) from Nei Mongol, China. Vertebrata Palasiatica. 26(1):35–49.
- Wang B-Y. 1997. The Mid-Tertiary Ctenodactylidae (Rodentia, Mammalia) of Eastern and Central Asia. Bull Am Mus Nat His. 234:1–88.
- Wessels W, Fejfar O, Peláez-Campomanes P, van der Meulen A, de Bruijn H. 2003. Miocene small mammals from Jebel Zelten, Libya. In: López-Martínez N, Peláez-Campomanes P, Hernández Fernández M, editors. En torno a Fósiles de Mamíferos: datación, Evolución y Paleoambiente. Coloquios de Paleontología, Volumen Extraordinario no 1. En honor al dr: Remmert Daams; p. 699–715.
- Wood AE. 1937. Fossil rodents from the Siwalik beds of India. Am J Sci. 34:64–76. doi:10.2475/ajs.s5-34.199.64.
- Ziegler R. 1994. Rodentia (Mammalia) aus den oberoligozänen Spaltenfüllungen Herrlingen 8 und Herrlingen 9 bei Ulm (Baden-Württemberg). Stuttgarter Beiträge Naturkunde, B. 196:1–81.
