ABSTRACT
The Yixian Formation (Barremian to Aptian, Lower Cretaceous) of China has yielded many fossils of early angiosperms, but not all of these angiosperms are completely understood. The seeds of angiosperms are attached at different positions in the fruits than those previously reported from the Early Cretaceous, which suggests various provenances for these carpels and indicates the necessity of further investigation of the angiosperms present in the formation to elucidate the diversity and evolution of early angiosperm flowers. Here, we report a novel fossil angiosperm, Varifructus lingyuanensis gen. et sp. nov, which has seeds attached on the dorsal sides of its fruits. The fossils are rendered more intriguing by the fact that there appear to be perianths in the flowers, the fruits are frequently arranged in asymmetrical pairs, and two branching patterns are seen in this single specimen. This unique combination of characteristics provides a rare raw material for evaluating flower evolution in the Early Cretaceous.
Introduction
The evolution of flowers can be reliably elucidated based only on their real history, which is determined mainly by fossil evidence. How and from where did the carpels and perianths originate? Did early flowers have perianths? What kind of perianths did these early flowers have? What kinds of leaves did these early flowering plants have? What types of branching patterns did these early flowering plants have? Are these early flowers similar to or different from gymnosperms (such as Gnetales) and, if so, in what terms? These questions were rarely answered in previous studies, which is mostly due to the fragmentary preservation of specimens, although the Yixian Formation has yielded various early fossil angiosperms that shed unique light on their early evolution (Duan Citation1998; Sun et al. Citation1998, Citation2001, Citation2002; Leng and Friis Citation2003, Citation2006; Ji et al. Citation2004; Wang and Zheng Citation2009, Citation2012; Wang and Han Citation2011; Han et al. Citation2013, Citation2017; Liu and Wang Citation2017). To shed additional light on these questions, a new angiosperm, Varifructus gen. nov, from the Yixian Formation is described here. Apparently, the diversified gynoecia of early angiosperms in Yixian Formation are suggestive of independent provenances for these gynoecia and an earlier origin of angiosperms; this is a conclusion that is compatible with the recent discovery of an Early Jurassic angiosperm, Nanjinganthus (Fu et al. Citation2018) and the molecular clock (Li et al. Citation2019).
Materials and methods
Geological setting
The Yixian Formation of Liaoning, China is famous worldwide for its early angiosperm fossils (Sun et al. Citation1998, Citation2001, Citation2002; Leng and Friis Citation2003, Citation2006; Ji et al. Citation2004; Wang and Zheng Citation2009, Citation2012; Han et al. Citation2013, Citation2017; Liu and Wang Citation2017; Wang Citation2017, Citation2018a, Citation2018b; Han et al. Citation2017) and animal fossils (including insects) (Wang et al. Citation1989; Li and Luo Citation2006; Liu et al. Citation2007, Citation2008). These plants and animals together constitute the world famous Jehol Biota, which is also the biological background for angiosperm diversification in the Early Cretaceous. There was once some controversy over the age of the Yixian Formation (Sun et al. Citation1998; Swisher et al. Citation1998), but now there is a general consensus on the age of the Yixian Formation, namely, approximately 125 Ma (Barremian-Aptian, Early Cretaceous) (Dilcher et al. Citation2007; Wang Citation2017).
Sample collection
The specimens of Varifructus are compressions/impressions preserved on two facing siltstone slabs with certain coalified residues (Duan Citation1998). The specimens are approximately 32 cm long and 22 cm wide. The slabs were collected from Dawangzhangzi, Lingyuan, Liaoning, China (), which is a location that has yielded specimens of Archaefructus sinensis, Sinocarpus decussatus, Callianthus dilae, Nothodichocarpum lingyuanensis, and Neofructus lingyuanensis (Duan Citation1998; Sun et al. Citation2002; Leng and Friis Citation2003, Citation2006; Ji et al. Citation2004; Wang and Zheng, Citation2009; Wang and Wang Citation2010; Han et al. Citation2017; Liu and Wang Citation2017; Wang Citation2018a). The specimens were photographed using a Nikon D810 digital camera and a Leica M205FA stereomicroscope that was equipped with a DFC550 digital camera. Some fragments of the fruits were removed, coated with gold, and observed using a Leo 1530 VP SEM (scanning electron microscope) at the Nanjing Institute of Geology and Palaeontology, CAS. All the figures were organised using Photoshop 7.0.
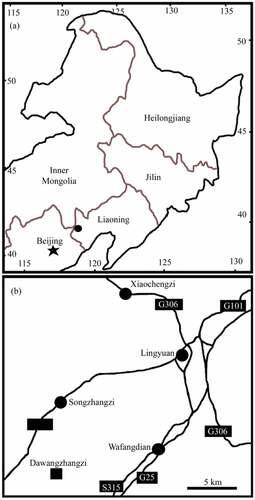
Figure 1. Geographical position of the locality of the Varifructus gen. nov in Liaoning, China (Drawn by X.W.). Reproduced from Han et al. (Citation2017) with permission and courtesy of Acta Geologica Sinica (English edition). (a) Fossil locality (black dot) in northeastern China. (b) Detailed position of fossil locality Dawangzhangzi (black square) in a suburb of Lingyuan, Liaoning
Results
Varifructus gen. nov.
Generic diagnosis: Distal portion of plant bearing leaves and terminating in a cluster of fructifications. The branches rigid, straight, axillary, opposite or not. Fructifications usually in pairs, including one to several follicles. The follicle one or more in a whorl, on the terminals of peduncles, with short tip. The seeds oval, probably orthotropous, attached on the dorsal side of the fruit.
Type species: Varifructus lingyuanensis gen. et sp. nov.
Etymology: Vari-, for variable morphology; -fructus, for fruit in Latin.
Stratigraphic horizon
Yixian Formation (equivalent to the Barremian-Aptian) Lower Cretaceous (125 Ma).
Type locality
Dawangzhangzi, Lingyuan, Liaoning, China (41º15ꞌN, 119º15ꞌE).
Varifructus lingyuanensis gen et sp. nov.
()
Figure 2. Distal portion of the plant of Varifructus gen. nov, Ma007. Note the nodes (1, 2), a flower bud (3), and fruits of varying morphologies (4 − 9). Bar = 2 cm
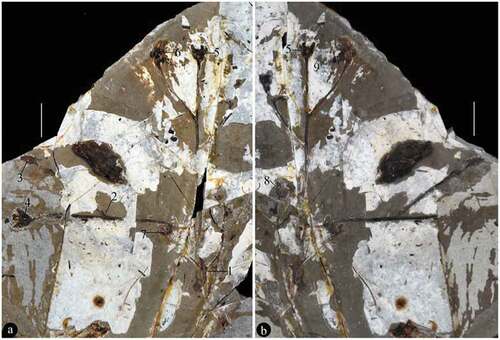
Figure 3. Branching of Varifructus gen. nov. (a) Detailed view of Node 1 in ), which exhibits opposite axillary branching. Note the two subtending bisected leaves (black arrows) and axillary branches (white arrows). Bar = 5 mm. (b) Detailed view of Node 2 in ), which shows axillary branching. Note the subtending leaf (black arrow) and axillary branch (white arrow), on the counterpart. Bar = 2.5 mm
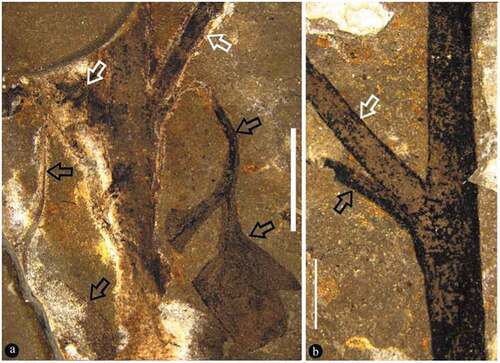
Figure 4. Fruit pair and flower bud of Varifructus gen. nov
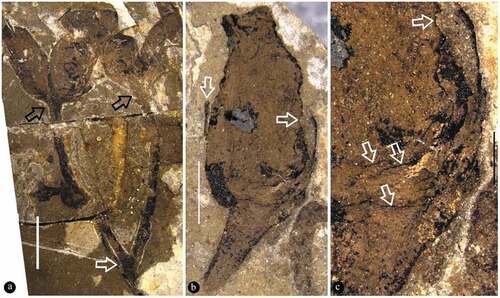
Figure 5. Fruit pair and its details

Figure 6. Fruit pair and its details
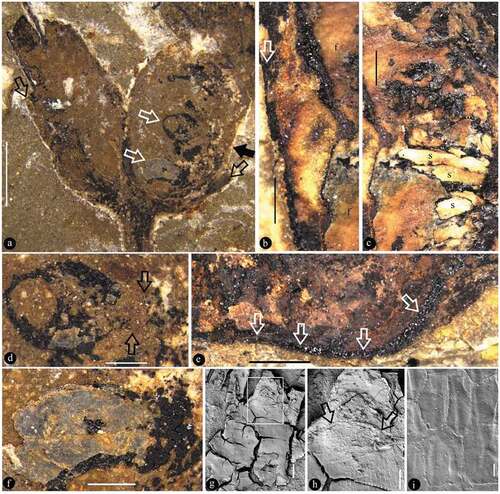
Figure 7. Fruit marked as 5 in Figure 2b (on the counterpart) and its details

Specific diagnosis: same as the genus.
Description
The specimen is 16.9 cm long and 12.2 cm wide and includes main branch bearing leaves, lateral branches, flower buds, and fruits (). The main branch preserved in the specimen is rigid and slightly curved, 3.6 mm wide at the base, and tapering distally to 1.7 mm wide (). The lateral branches are axillary and are opposite or not () – (b)). The subtending leaves are petiolate megaphylls, lobate with a slender pedicel up to 7 mm long and only 0.6 mm wide, and the venation is vague ()). Some lobate leaves are attached at the base of fruit pairs ()). The flower buds and fructifications are present on the termini of the branches ()), 5(a), 6(a), 7(a)). The flower bud is approximately 9 mm long and 4.5 mm wide with a truncated−appearing tip and peripheral foliar parts (perianth?) ()). The fructifications are of two types and are usually arranged in pairs, and each includes either a single follicle (or one more) on an elongate slender stalk or at least three follicles whorled on a short 1.6 mm wide stalk (), 6(a), 7(a)). The single fruit is relatively large, e.g., 8.9–10.3 mm long and 3.3–3.9 mm wide, with a short tip, and its stalk is approximately 5 mm long and 0.3 mm wide (–c)). More than three fruits are attached on a stout stalk in a whorl with peripheral foliar parts (perhaps perianth), and each has an arcate ventral margin and a relatively straight dorsal margin (), 6(a,b), 7(a,b)). The ovules/seeds are probably orthotropous and are 1.7–1.8 mm long and 1.1 mm wide; they are attached along the dorsal margin of the fruit, which usually has a strong vascular bundle ()). An in situ megaspore membrane with rectangular cells is seen in some ovules/seeds (, d, f–i)).
Etymology
Lingyuan− for the city of Lingyuan, where the fossil was collected.
Holotype
Ma007A (), Ma007B.
Depository
Key Laboratory of National Forestry and Grassland Administration for Orchid Conservation and Utilization, College of Landscape, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China.
Remark
Our interpretation of ‘megaspore membrane’ is based on its position in the ovule/seed within the carpel/fruit, its membrane-like morphology, and anatomical details (–i)). This information does not resemble any known seed coat/integument of a seed/ovule.
Discussions
Angiosperms are the most diverse plant group in the current ecosystem, and they are defined, and thus distinguished from other seed plants (gymnosperms), by ovule(s)/seeds that are enclosed before pollination (Wang Citation2018a, Citation2018b). The seeds of Varifructus gen. nov are apparently enclosed by its fruit wall ()), which suggests that Varifructus gen. nov is an angiosperm. The morphology of Varifructus gen. nov distinguishes it from previously reported angiosperms from the Yixian Formation (Sun et al. Citation1998, Citation2001, Citation2002; Leng and Friis Citation2003, Citation2006; Ji et al. Citation2004; Wang and Zheng Citation2009, Citation2012; Han et al. Citation2017) (). Therefore, Varifructus gen. nov is a new member of early angiosperms in the Yixian Formation. The physically connected parts of Varifructus gen. nov () provide the raw data and a rare opportunity to evaluate the evolution of flowering plants in the Early Cretaceous.
Table 1. Comparison between Varifructus gen. nov and other angiosperms from the Yixian Formation
Other than Liaoningfructus (Han et al. Citation2017), angiosperms previously reported from the Yixian Formation have no typical physically connected angiospermous megaphylls. Although Leng and Friis reported a leaf associated with Sinocarpus (Leng and Friis Citation2006), this requires further confirmation, as association is not a strong evidence for reliable reconstruction. The leaves of Varifructus gen. nov are physically connected with other parts of the plant and provide solid evidence of the relationship between these plant parts. Although, unfortunately, the venation of the leaf is not well-preserved, the general morphology, such as the deeply lobed leaf shape of Varifructus gen. nov, is apparently unlike that of any of the leaves of the assumed primitive angiosperms, including Magnolia and Amborella, which prompts reconsideration of the systematic conclusions based solely on living angiosperms.
Only the distal portion of Varifructus gen. nov is preserved. The occurrence of both flower buds ()) and fructifications () in this limited portion of the plant implies that the anthesis of Varifructus gen. nov is quite long. Such a feature is apparently conducive to the successful reproduction of Varifructus gen. nov as it provides the plant with more time to pollinate and produce more seeds.
Branching patterns are important features in plant taxonomy. It is interesting to note that, in the limited preserved part of Varifructus gen. nov, two different branching patterns are present ()). While the branching shown in ) is opposite, but it is not opposite in ). These variable branching patterns within a single plant generally reflect the morphological plasticity of Varifructus gen. nov, as seen in the fructification morphology (below), during the early evolving period of angiosperms.
The fructifications of Varifructus gen. nov are usually in pairs ()). The pairs are sometimes symmetrical ((i), (e), two fructifications are similar to each other in morphology and organisation) ()) or sometimes asymmetrical ((i), (e), two fructifications are different in morphology and organisation) ()). In the latter case, a fructification may include only one single fruit. This observation seems compatible with the plasticity in branching pattern discussed above. Both branching pattern and fructification morphology indicate the great morphological plasticity of Varifructus gen. nov.
Some foliar parts are present in the flower buds ()) and fructifications ()) of Varifructus gen. nov. These foliar parts conjure to perianth frequently seen in typical flowers and thus suggest that there is a perianth in the flower of Varifructus gen. nov. This perianth-like structure is noteworthy in that to date, there has been no well-defined perianth or perianth-like structure in previously known fossils of angiosperms from the Yixian Formation. Therefore, the perianth-like structure of Varifructus sheds unique light on the evolution of perianths in the Early Cretaceous. However, if the Early Jurassic Nanjinganthus with a typical ‘derived’ flower morphology is considered (Fu et al. Citation2018), it seems that perianths originated much earlier than has been assumed or have originated independently multiple times. This makes the evolutionary roadmap of early angiosperms more complicated and requires more effort for clarification.
We have not seen any traces of anthers or stamens in Varifructus gen. nov. Thus, it is very likely that the flowers of Varifructus gen. nov are dioecious. Therefore, how and when the male and female organs were integrated into a perfect flower is still a question awaiting answers.
Megaspore membranes are frequently seen in gymnosperms but are very rarely seen in angiosperms. In the latter group, probably due to their enclosed status, the ovules live in a niche controlled by ovaries, and a thick and conspicuous megaspore membrane became surplus and reduced during evolution. Thus, the lack of a megaspore membrane is very likely a result of long-term evolution in angiosperms, and the presence of a megaspore membrane in Varifructus gen. nov (, d, f–i)) reflects its plesiomorphic status in the evolutionary lineage. This is coherent to the Early Cretaceous age of Varifructus gen. nov.
For the derivation of carpels in angiosperms, there are two competing hypotheses. Arber and Parkin proposed that carpels in angiosperms are equivalent to so-called ‘megasporophyll’, which bears ovules along its margins (Arber and Parkin Citation1907). This proposal remains speculative as Bennettitales, the proposed relatives of angiosperms, lack any trace of ‘megasporophylls’. The dorsally attached ovules/seeds in Archaefructus, Nothodichocarpum, Neofructus and here in Varifructus gen. nov ()) (all from the Yixian Formation) (Ji et al. Citation2004; Wang and Zheng Citation2012; Liu and Wang Citation2017; Han et al. Citation2017) and also studies on Magnoliaceae (Liu et al. Citation2014; Zhang et al. Citation2017) further reject this hypothesis. Another hypothesis states that carpels comprise placentas and ovarian walls (Taylor Citation1991; Taylor and Hickey Citation1996; Wang Citation2018a, Citation2018b). This hypothesis is favoured by increasing amounts of fossil evidence (Wang and Wang Citation2010; Liu and Wang Citation2017; Han et al. Citation2017), studies of living Magnoliaceae (Liu et al. Citation2014; Zhang et al. Citation2017), the developmental genetics of Arabidopsis (Rounsley et al. Citation1995; Roe et al. Citation1997; Skinner et al. Citation2004; Mathews and Kramer Citation2012). The different (ventral or dorsal) ovule attachment in Archaefructus and Sinocarpus, respectively, suggest that the placenta is independent of the enclosing foliar part (e.g., ovarian wall or carpel wall) and may be fused either to the margins or to the midvein of the enclosing foliar part; this at least may have been the case in the Early Cretaceous. Here, the existence of Varifructus gen. nov with dorsally attached ovules/seeds further strengthens this generalisation.
Conclusion
Varifructus gen. nov is characterised by its lobed leaves, variable branching patterns and fructification morphology with ovules attached along the dorsal fruit margin and represents a new element of flowering plants in the Yixian Formation (the Lower Cretaceous). With its morphological fluidity, Varifructus gen. nov may well represent a typical state of plants during the early evolution of angiosperms. Along with previous fossil angiosperms, the existence of Varifructus gen. nov favours an earlier origin of angiosperms and refutes an early Cretaceous origin for angiosperms.
Acknowledgments
We appreciate Mr Yan Fang for help with SEM. And we appreciate two anonymous reviewers for their detailed and constructive suggestions.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- Arber EAN, Parkin J. 1907. On the origin of angiosperms. J Linnean Soc London Bot. 38(203):29–80. doi:10.1111/j.1095-8339.1907.tb01074.x.
- Dilcher DL, Sun G, Ji Q, Li H. 2007. An early infructescence Hyrcantha decussata (comb. nov.) from the Yixian Formation in northeastern China. Proc Nat Acad Sci USA. 104(22):9370–9374. doi:10.1073/pnas.0703497104.
- Duan S. 1998. The oldest angiosperm—a tricarpous female reproductive fossil from western Liaoning Province, NE china. Sci China Ser D. 41(1):14–20. doi:10.1007/BF02932415.
- Fu Q, Diez JB, Pole M, García Ávila M, Liu Z-J, Chu H, Hou Y, Yin P, Zhang G-Q, Du K. 2018. An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife. 7:e38827. doi:10.7554/eLife.38827.
- Han G, Fu X, Liu Z-J, Wang X. 2013. A new angiosperm genus from the lower cretaceous Yixian Formation, Western Liaoning, China. Acta Geol Sin. 87(4):916–925. doi:10.1111/1755-6724.12100.
- Han G, Liu ZJ, Wang X. 2017. A Dichocarpum-like angiosperm from the early cretaceous of China. Acta Geol Sin. 90:1–8. doi:10.1111/1755-6724.13059.
- Ji Q, Li HQ, Bowe M, Liu YS, Taylor DW. 2004. Early Cretaceous Archaefructus eoflora sp. nov. with bisexual flowers from Beipiao, Western Liaoning, China. Acta Geol Sin. 78:883–896. doi:10.1111/j.1755-6724.2004.tb00210.x.
- Leng Q, Friis EM. 2003. Sinocarpus decussatus gen. et sp. nov., a new angiosperm with basally syncarpous fruits from the Yixian Formation of Northeast China. Plant Syst Evol. 241(1−2):77–88. doi:10.1007/s00606-003-0028-8.
- Leng Q, Friis EM. 2006. Angiosperm leaves associated with Sinocarpus infructescences from the Yixian Formation (Mid−Early Cretaceous) of NE China. Plant Syst Evol. 262(3−4):173–187. doi:10.1007/s00606-006-0461-6.
- Li G, Luo ZX. 2006. ACretaceous symmetrodont therian with some monotreme-like postcranial features. Nature. 439(7073):195–200. doi:10.1038/nature04168.
- Li HT, Yi T-S, Gao L-M, Ma P-F, Zhang T, Yang J-B, Gitzendanner MA, Fritsch PW, Cai J, Luo Y, et al. 2019. Origin of angiosperms and the puzzle of the Jurassic gap. Nat Plants. 5(5):461–470.
- Liu WZ, Hilu K, Wang YL. 2014. From leaf and branch into a flower: Magnolia tells the story. Bot Stud. 55(1):28. doi:10.1186/1999-3110-55-28.
- Liu Y, Sinitshenkova ND, Ren D. 2007. A new genus and species of stonefly (Insecta: Plecoptera) from the Yixian formation, Liaoning Province, China. Cretaceous Res. 28(2):322–326. doi:10.1016/j.cretres.2006.08.003.
- Liu YS, Ren D, Sinitshenkova ND, Shih CK. 2008. Three new stoneflies (Insecta: Plecoptera) from the Yixian formation of Liaoning, China. Acta Geol Sin. 82(2):249–256.
- Liu ZJ, Wang X. 2017. Yuhania: A unique angiosperm from the Middle Jurassic of Inner Mongolia, China. Hist Biol. 29(4):431–441. doi:10.1080/08912963.2016.1178740.
- Mathews S, Kramer EM. 2012. The evolution of reproductive structures in seed plants: A re−examination based on insights from developmental genetics. New Phytol. 194(4):910–923. doi:10.1111/j.1469-8137.2012.04091.x.
- Roe JL, Nemhauser JL, Zambryski PC. 1997. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell. 9:335–353. doi:10.2307/3870486.
- Rounsley SD, Ditta GS, Yanofsky MF. 1995. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell. 7:1259–1269. doi:10.2307/3870100.
- Skinner DJ, Hill TA, Gasser CS. 2004. Regulation of ovule development. Plant Cell. 16(suppl_1):S32−S4. doi:10.1105/tpc.015933.
- Sun G, Zheng S, Dilcher D, Wang Y, Mei S. 2001. Early angiosperms and their associated plants from Western Liaoning, China. Shanghai Technology & Education Press: Shanghai.
- Sun G, Dilcher DL, Zheng S, Zhou Z. 2002. Archaefructaceae, a new basal angiosperm family. Science. 296(5569):899–904. doi:10.1126/science.1069439.
- Sun G, Dilcher DL, Zheng S, Zhou Z. 1998. In search of the first flower: a Jurassic angiosperm, Archaefructus, from Northeast China. Science. 282:1692–1695. doi:10.1126/science.282.5394.1692.
- Swisher CC, Wang Y, Wang X, Xu X, Wang Y. 1998. 40Ar/39Ar dating of the lower Yixian Fm, Liaoning Province, northeastern China. Chin Sci Bull. 43(S1):125−125. doi:10.1007/BF02891590.
- Taylor DW. 1991. Angiosperm ovule and carpels: their characters and polarities, distribution in basal clades, and structural evolution. Postilla. 208:1–40.
- Taylor DW, Hickey LJ. 1996. Flowering plant origin, evolution & phylogeny. New York: Chapman & Hall; p. 116–140. doi:10.1007/b102239.
- Wang WL, Zheng S, Zhang L, Po R, Zhang W, Wu H, Ju R, Dong G, Yuan H. 1989. Mesozoic stratigraphy and paleontology in Western Liaoning. Beijing: Geological Publisher.
- Wang X. 2017. A biased, misleading review on early angiosperms. Nat Sci. 9:399–405. doi:10.4236/ns.2017.912037.
- Wang X. 2018a. The dawn angiosperms. Heidelberg: Springer. doi:10.1007/978-3-319-58325-9.
- Wang X. 2018b. An era of errors: unveiling the truth of Archaeanthus and its implications for angiosperm systematics. ChinaXiv. 201804:201934.
- Wang X, Han G. 2011. The earliest ascidiate carpel and its implications for angiosperm evolution. Acta Geol Sin. 85:998–1002.
- Wang X, Wang SJ. 2010. Xingxueanthus: an enigmatic Jurassic seed plant and its implications for the origin of angiospermy. Acta Geol Sin. 84(1):47–55. doi:10.1111/j.1755-6724.2010.00169.x.
- Wang X, Zheng SL. 2009. The earliest normal flower from Liaoning Province, China. J Integr Plant Biol. 51:800–811. doi:10.1111/j.1744-7909.2009.00838.x.
- Wang X, Zheng XT. 2012. Reconsiderations on two characters of early angiosperm Archaefructus. Palaeoworld. 21:193–201. doi:10.1016/j.palwor.2012.10.002.
- Zhang X, Liu W, Wang X. 2017. How the ovules get enclosed in magnoliaceous carpels. Plos One. 12:e0174955. doi:10.1371/journal.pone.0174955.

