ABSTRACT
The fossil record of macroscelidean mammals is notoriously patchy, with a significant spatial and temporal gap separating faunas from the early Oligocene localities of northern Africa and the early Miocene localities of eastern and southern Africa. Here we describe fossil macroscelideans representing Myohyracinae and Rhynchocyoninae recovered from a rift-fill sequence of richly fossiliferous sandstones in the late Oligocene Nsungwe Formation in the Rukwa Rift Basin of southwestern Tanzania. Radiometrically dated to 25.2 Ma, a new Palaeogene myohyracine taxon (Rukwasengi butleri) is represented by a partial maxilla (RRBP 05409) preserving a lightly worn M2-M3. The M2 exhibits a less hypsodont and mesiodistally elongate morphology than the early Miocene Myohyrax oswaldi, and the three-rooted M3 exhibits a tiny mesially positioned fossette. A new rhynchocyonine (Oligorhynchocyon songwensis) is represented by specimens more brachyodont than the early Miocene Miorhynchocyon. Taken together these finds document a rare window into macroscelidean evolutionary history with diversification of the group near the Palaeogene-Neogene Transition (PNT). Continued exploration offers a refined perspective on mid-Cenozoic faunal and ecosystem dynamics on continental Africa, expanding opportunities for recognising trends in palaeobiological diversity across habitat types and through time.
Introduction
Unravelling the origin and affinities of macroscelideans (also termed sengis, or elephant shrews) has long presented a palaeontological puzzle. Modern sengis are small insectivorous mammals characterised by rapid hind limb-dominant locomotion and an elongate flexible snout. Extant sengis consist of 20 species within six described genera, all restricted to continental Africa (Heritage et al. Citation2020). Often described as ‘living fossils’, their evolutionary history has been linked with mixodectid insectivores (Schlosser Citation1910), Glires (Novacek Citation1984), Menotyphla (Haeckel Citation1866; Gregory Citation1910), condylarthrans (Hartenberger Citation1986; Simons et al. Citation1991; Zack et al. Citation2005; Tabuce et al. Citation2007; Penkrot et al. Citation2008), and prior to a more expanded African Cenozoic fossil record, some of their members were originally described as hyracoids or even marsupials. More recently, macroscelideans have been grouped among afrotherians, an array of endemic African mammals that includes modern tubulidentates, tenrecoids, hyracoids, sirenians, and proboscideans united first on the basis of molecular sequence data (e.g., Stanhope et al. Citation1998; Murphy et al. Citation2001; Asher et al. Citation2003; Asher Citation2007; Hedges et al. Citation2015) and later supported by morphological features (e.g., Sánchez‐Villagra et al. Citation2007; Seiffert Citation2007; Tabuce et al. Citation2007, Citation2008; Wible et al. Citation2007; Asher and Lehman Citation2008; O’Leary et al. Citation2013).
Importantly, temporal and spatial sampling gaps in early Cenozoic fossil-bearing sites on continental Africa have obscured macroscelidean evolutionary history across the Palaeogene-Neogene Transition (PNT). Palaeogene sengi discoveries were long limited to two now-extinct subfamilies from localities in northern Africa. Taxonomy follows Holroyd (Citation2010). Metoldobotinae includes a single described genus (Metoldobotes) from the early Oligocene Fayum Depression of Egypt (Schlosser Citation1910; Simons et al. Citation1991). Herodotiinae includes Chambius from the late Early or early Middle Eocene Chambi Massif in Tunisia (e.g., Hartenberger Citation1986; Tabuce Citation2018), Nemenchatherium from the mid-late Eocene Bir el Ater, Algeria, and the late Middle or Late Eocene locality of Dur At-Talah, Libya (Tabuce et al. Citation2001), Eotmantsoius represented by a single tooth also from Dur At-Talah, Libya (Tabuce et al. Citation2012), and Herodotius from Oligocene localities in the Fayum Depression of Egypt (Simons et al. Citation1991). Localities in Namibia have revealed interesting materials including an enigmatic tooth from the Silica North locality (Pickford et al. Citation2008) and other materials interpreted as Eocene in age (but see Seiffert Citation2010; Coster et al. Citation2012; Marivaux et al. Citation2012; Sallam and Seiffert Citation2016 for a range of alternative age interpretations extending into the Oligocene or even the Miocene).
Metoldobotes has remained an uncontested sengi by most. Seiffert (Citation2007) and Asher and Seiffert (Citation2010) reviewed arguments for and against including herodotiines (Nementchatherium, Chambius and Herodotius) in the Macroscelidea. In recent years, additional significant and well-preserved herodotiine specimens have been described from sites in northern Africa (Tabuce et al. Citation2012; Tabuce et al. Citation2008). But until recently, the subsequent record for the group has been interrupted by a ~ 9 Ma hiatus in the fossil record until the recovery of Miocene and more recent faunas of eastern (Kenya) and southern (South Africa, Namibia) Africa, with localities that preserve an array of forms more similar to modern rhynchocyonine sengis (see review in Holroyd Citation2010). Also common in Miocene faunas are members of the Myohyracinae (Patterson Citation1965), the now-extinct subfamily of macroscelideans originally interpreted as hyracoids with tooth specialisations reflecting herbivorous diets (e.g., Butler Citation1984). The sengi fossil record expands considerably throughout the Neogene, with macrosceledines documented by at least 18 Ma (e.g., Butler Citation1984) and mylomygalines documented in the early Pleistocene of South Africa (reviewed in Holroyd Citation2010). Today only rhynchocyonines and macroscelidines remain.
Fossils from the Rukwa Rift Basin of southwestern Tanzania offer a rare window into the mid-Cenozoic of the African continental vertebrate record in general and that of sengis specifically. Specimens recovered from the ~25 Ma Nsungwe Formation reveal a rich fauna of late Oligocene invertebrates (Feldmann et al. Citation2007; Roberts et al. Citation2016; Epa et al. Citation2018), fishes (Stevens et al. Citation2016; Claeson et al. Citation2021), anurans (Blackburn et al. Citation2015 & Citation2019), lepidosaurs (McCartney et al. Citation2014; McCartney et al. Citation2021; Müller et al. Citation2018), and a diversity of mammals (Stevens et al. Citation2005, Citation2006, Citation2008, Citation2009a, Citation2009b, Citation2013) including sengis.
Here we describe Palaeogene evidence of both Myohyracinae and Rhynchocyoninae from Africa south of the equator. These discoveries offer insight into the geographic and temporal expansion and evolutionary significance of Palaeogene macroscelideans from the interior of the African continent.
Location
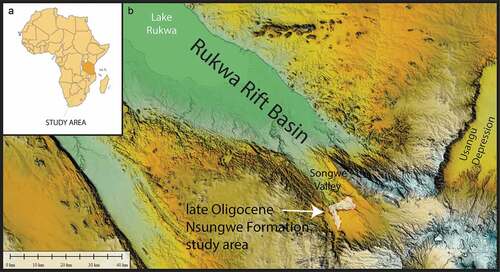
Specimens described herein derive from continental strata in the Rukwa Rift Basin of southwestern Tanzania, from a rock unit that represents the earliest known Cenozoic sedimentary record of rifting (Roberts et al. Citation2012) in the Western Branch of the East African Rift System (). Fossil-bearing localities are situated in the Songwe River valley at approximately 8º 56ʹ S, 33º 12ʹ E and are part of a laterally continuous series of interbedded fluvial sandstones, floodplain and channel fill palaeosols, lacustrine siltstones, and devitrified airfall tuffs (bentonites) comprising the Songwe Member of the Nsungwe Formation (Roberts et al. Citation2004 & Citation2010; Lawrence et al. Citation2021). Specimens were recovered from variable discharge, flashy fluvial deposits (some of which have been overprinted by paedogenesis) at localities TZ-01, TZ-01S, and TZP-2. The fauna is dominated by small (<4 cm) specimens, including numerous teeth, jaws, and postcranial elements from microsites that preferentially preserve isolated elements (Stevens et al. Citation2005, 2006, Citation2008, Citation2009a, Citation2009b, Citation2013; Stevens et al. Citation2016; McCartney et al. Citation2014, 2021; Blackburn et al. Citation2015, Citation2019; Müller et al. Citation2018; Claeson et al. Citation2021). Fossil localities are bracketed by a series of well-dated volcanic ash beds that demonstrate a roughly similar age of ~25 Ma for all three localities (Roberts et al. Citation2012; Stevens et al. Citation2013) from which the sengi specimens were recovered.
Figure 1. Geological context. Specimens were recovered from the Songwe Member of the late Oligocene Nsungwe Formation in the Rukwa Rift Basin of southwestern Tanzania. A, Geographic position of the Rukwa Rift Basin in eastern Africa (Tanzania shaded) and B, Digital elevation model showing the outcrop distribution of the Nsungwe Formation study area at the southern end of the Rukwa Rift Basin in the Songwe Valley.
Materials and methods
Specimens were collected by standard palaeontological hand-quarrying methods. Fieldwork was conducted under permits issued by the Tanzanian Commission for Science and Technology (COSTECH) and the Tanzania Antiquities Unit. Specimens are designated with RRBP, Rukwa Rift Basin Project (identifier used by the Tanzania Antiquities Unit), Dar es Salaam, Tanzania.
After mechanical preparation at the Ohio University Palaeobiology Preparation and Imaging Facility, specimens were µCT scanned at the Shared Materials Instrumentation Facility (SMiF) at Duke University, Durham, NC on a Nikon XTH 225 ST scanner. Specimens were scanned using an isotropic voxel size of 0.010 mm, at a voltage of 113 kV and amperage of 124 µA. Digital models were constructed through segmentation and visualised using the volume rendering and isosurface modules in Avizo Lite 9.2.0. Digital data including DICOM stacks and mesh models are available on MorphoSource. The following standard sengi tooth measurements were obtained on dental specimens (following Butler Citation1984) using a Nikon SMZ-1500 stereomicroscope bundled with a Motic 10.0 MP digital camera (Motic ImagePlus Version 3.0): on molars, maximum length (mesiodistal), maximum breadth (buccolingual); on premolars: maximum length (mesiodistal), maximum buccolingual breadth at distal cusps and at mesial cusps. Comparative material consisted primarily of reference specimens and casts of representative fossil and Recent macroscelideans examined in person by NJS at the following institutions: National Museums of Kenya (NMK/KNM), Duke University Division of Fossil Primates (DPC), United States National Museum of Natural History-Smithsonian Institution (USNM). Specimen casts were kindly provided by R. Asher, P. Holroyd, and R. Tabuce. For less accessible specimens, observations were supplemented with photographic comparisons.
Results
Systematic palaeontology
Order Macroscelidea Butler Citation1956
Family Macroscelididae Bonaparte Citation1838
Subfamily Myohyracinae Andrews 1914
Rukwasengi butleri new genus and species
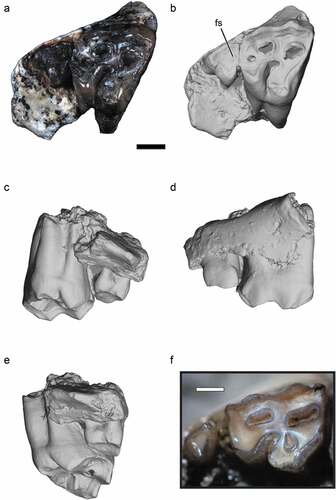
Figure 2. Nsungwe formation Myohyracine. Photograph (A) and digital renderings (B–E) of the late Oligocene Rukwasengi butleri (RRBP 05409, holotype) upper right molars (M2-3) in occlusal (A, B), lingual (C), buccal (D), and posterior (E) views. Photograph of Myohyrax oswaldi (KNM-RU 3763) in occlusal view (F) for reference. The lead line to “fs” calls out the small fossette on M3. Scale bars equal 1 mm.
Type Specimen
RRBP 05409, partial right maxilla preserving M2-M3 ().
Type Locality
TZP-2, late Oligocene Songwe Member of the Nsungwe Formation, Mbeya Region, southwestern Tanzania.
Etymology
Generic epithet incorporates the name of the rift segment from which the specimen derives (Rukwa), and the common term for macroscelidean (sengi). Specific epithet in honour of sengi expert Percy Butler.
Diagnosis
Differs from rhynchocyonine macroscelideans in possessing pronounced fossettes on upper molar teeth. Cheek teeth are less hypsodont and shorter mesiodistally than early Miocene Myohyrax and Protypotheroides. Further differs from Myohyrax in M2 buccolingual breadth exceeding mesiodistal length, M2 with a single more buccally positioned anterior fossette and two posterior fossettes (one buccal and one more centrodistally positioned), and a relatively larger and three-rooted M3 exhibiting a small mesially positioned fossette. Differs from Namasengi in exhibiting less individuated upper molar cusps, and from both Namasengi and Promyohyrax in lacking a buccal sinus on upper molars. Further differs from Promyohyrax in being larger, relatively more buccolingually broad, and having less buccally inflated M2. Further differs from herodotiines in lacking a buccal cingulum on upper molars, and in exhibiting a relatively smaller M3.
Description
RRBP 05409 is a partial right maxilla preserving moderately worn and closely approximated M2-M3 (). The M2 measures 2.45 mm in mesiodistal length, and 3.3 mm in buccolingual breadth at the broadest point near the base of the mesial cusps. Although worn, it is apparent that the paracone is slightly taller and more buccally positioned than is the metacone. A strong, mesially curved lingual sulcus is present. The lingual margins of the protocone and hypocone are sharp in outline and directed anteriorly. Three rounded fossettes are present in the M2 occlusal surface, two positioned in the buccal half of the tooth, and a third smaller and more central fossette distally. The third molar is three-rooted and triangular in outline, measuring 1.2 mm by 1.5 mm in maximum mesiodistal and buccolingual dimensions, respectively. The lightly worn M3 closely approximates M2 within the maxilla and exhibits a round, very small, mesially positioned fossette.
Remarks
Rukwasengi resembles Palaeogene herodotiines and metoldobotines (and not early Miocene myohyracines; ) in exhibiting an M2 that is broader buccolingually than mesiodistally. Notably, the Rukwasengi M3 is also three rooted and exhibits a distinctive, small mesially positioned fossette. It is relatively smaller than the M3 in herodotiines, yet relatively larger than the M3 in Myohyrax. Rukwasengi expands the eastern African record for myohyracines earlier by at least 4 Ma, offering a glimpse into the mid-Cenozoic gap in the sengi fossil record.
Order Macroscelidea Butler Citation1956
Family Macroscelididae Bonaparte Citation1838
Subfamily Rhynchocyoninae Gill, 1872
Oligorhynchocyon songwensis new genus and species.
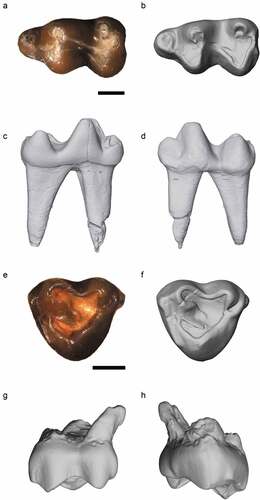
Figure 3. Nsungwe formation Rhynchocyonine. Photograph (A) and digital rendering (B–D) of the late Oligocene Oligorhynchocyon songwensis (RRBP 08086, holotype) left lower fourth premolar in occlusal (A, B), buccal (C), and lingual (D) views. Photograph (E) and digital rendering (F-H) of Oligorhynchocyon songwensis (RRBP 07433, referred specimen) upper left molar (M2 or M3) in occlusal (E, F), buccal (G), and lingual (H) views. Scale bars equal 1 mm.
Type Specimen
RRBP 08086, left p4 ().
Type Locality
TZ-01S, late Oligocene Songwe Member of Nsungwe Formation, Mbeya Region, southwestern Tanzania.
Etymology
Generic epithet incorporates the age of the rock unit from which the specimen derives (Oligo), and a modern sengi taxon (rhynchocyon). Specific epithet references the Songwe River along which the fossil locality is located.
Diagnosis
Cheek teeth larger and more mesiodistally elongate than Palaeogene metoldobotines and herodotiines; lacking characteristic fossettes/fossettids observed in myohyracines; more brachyodont than mylomygalines, Pronasilio, and modern Rhynchocyon. Distinct from Miocene Miorhynchocyon species in lacking p4 anterobuccal cingulid, and in possessing weaker p4 cristid obliqua that meets the posterior wall of the trigonid lower and closer to the metaconid (rather than in a position midway between protoconid and metaconid). Further differs from M. meswae in relatively lower paraconid height in relation to metaconid, and from both M. meswae and M. rusingae in smaller size. Differs from M. clarki in being more brachyodont, with p4 relatively longer mesiodistally in relation to buccolingual breadth, sharper margins of the buccal cusps, and a pronounced p4 posterior cingulid. More brachyodont than M. gariepensis, with a much lower paraconid and less pronounced and basally inflated trigonid cusps. More brachyodont than materials referred to Eorhynchocyon and Namasengi. Further differs from Namasengi in exhibiting a wider p4 talonid.
Description
RRBP 08086 is a lightly worn two-rooted and molariform left p4 () measuring 4.1 mm in mesiodistal length, 1.95 mm buccolingual breadth at the broadest point near the distal cusps, and 1.8 buccolingual breadth across the protoconid and metaconid. RRBP 08086 lacks a strong anterobuccal cingulid, exhibiting a very faint rugosity in that position. The low, wide paraconid is lingually positioned and reaches only half the height of the other trigonid cusps. It is well-individuated and linked to the protoconid by a small paracristid that ascends along the posterobuccal aspect of the cusp to a notch between the paraconid and protoconid. A prominent preprotocristid descends along the anterolingual aspect of the protoconid to terminate in the same notch, meeting the paracristid just posterobuccal to the paraconid. The transversely aligned protoconid and metaconid are tall and subequal in size, and connected by a narrow protocristid, forming a steep and unbroken posterior trigonid wall, at the base of which a weak cristid obliqua terminates just buccal to the rise of the metaconid. The hypoconid and entoconid are transversely aligned and subequal in size, although the entoconid is sharper in outline and there is no evidence of an entostylid near its anterior slope. Trigonid and talonid basins are steeply sloped lingually and cusp heights are uneven across the tooth, with the protoconid and metaconid approximately 50% taller than the paraconid, entoconid and hypoconid. Distal to the entoconid, a posterior cingulid rises to terminate at a hypoconulid expansion along the posthypocristid.
Referred specimen
Collected nearby in locality TZ-01, RRBP 07433 is a lightly worn three-rooted left upper molar (M2 or M3) (). The molar measures 2.8 mm in mesiodistal length, and 2.6 mm in buccolingual breadth. The tooth is brachyodont yet crestiform, with cusps largely subsumed in crests. The large paracone connects through a preparacrista to the well-developed and anteriorly projecting parastyle. A pronounced anterior cingulum is visible. The preprotocrista is wide and extends approximately halfway to a point between the paracone and the parastyle. A strong postprotocrista extends from the protocone, branching to form a basin distolingual to the metacone. RRBP 07433 preserves the bases of three roots that appear subequal in size. More brachyodont than early Miocene rhynchocyonines (including M. clarki), RRBP 07433 is consistent in size with RRBP 08086 and so provisionally referred to the same taxon here.
Remarks
Two specimens, RRBP 08086 (locality TZ-01S) and RRBP 07433 (locality TZ-01) document the presence of rhynchocyonines in the Oligocene Nsungwe Formation. RRBP 08086 is a well-preserved lower p4 and RRBP 07433 is a lightly worn isolated upper molar. Smaller than Miorhynchocyon meswae and M. rusingae, these specimens are clearly rhynchocyonine although more brachyodont and distinctive from early Miocene forms. Rukwa specimens expand the record for Rhynchocyoninae into the Palaeogene of eastern Africa, further addressing the mid-Cenozoic gap in the sengi fossil record.
Discussion and Conclusions
The Rukwa macroscelideans are significant in representing the earliest evidence of both myohyracines and rhynchocyonines from eastern Africa. Sengi fossils have been reported from localities as early as Eocene in age (e.g., Hartenberger Citation1986) yet for decades only a handful of pre-Miocene genera were known (Simons et al. Citation1991; Hartenberger Citation1986; Tabuce et al. Citation2001, Citation2012, 2017; Seiffert Citation2007; Pickford et al. Citation2008). Palaeogene taxa have generally been assigned to either Metoldobotinae or Herodotiinae (e.g., Simons et al. Citation1991; Seiffert Citation2007 but see Senut and Pickford Citation2021), with morphological evidence documenting well-differentiated rhynchocyonines and myohyracines by the early Miocene (Butler Citation1995). Molecular studies retrieve divergence estimates for crown macroscelidids (rhynchocyonines, macroscelidines) by the early Oligocene (Heritage et al. Citation2020).
Until recently, the oldest definitive fossil evidence of rhynchocyonines (Miorhyncocyon meswae, Butler Citation1984) in eastern Africa derived from the early Miocene Meswa Bridge locality in Kenya. Two different species (Miorhynchocyon clarki, Butler Citation1969; M. rusingae, Butler and Hopwood Citation1957) are recognised from the nearby Songhor locality with an age estimate of ~20 Ma. Miorhynchocyon is found in a host of localities with the last occurrence recorded at the ~14 Ma Fort Ternan locality (M. rusingae, Butler Citation1969). Two high-crowned rhynchocyonines (Brachyrhynchocyon and Hypsorhynchocyon; Senut Citation2008) have been recognised from the early Miocene locality of Northern Sperrgebiet in Namibia, documenting a wide geographic distribution for the group. Interesting materials have recently been described from Eocliff in the Sperrgebiet, Namibia, interpreted as Eocene in age (Senut and Pickford Citation2021). Additional species have also been postulated but not formally described from the early Miocene of Uganda (Butler Citation1984).
Myohyracines (represented by Myohyrax oswaldi) are common in the ~20 Ma Chamtwara and Songhor localities in Kenya and in sites from the Miocene of Namibia (e.g., Senut 2003), persisting until at least ~13 Ma based on fossils from Fort Ternan, Kenya and Bosluts Pan, South Africa (Holroyd Citation2010). A second species of Myohyrax (M. pickfordi, Senut Citation2008) and a larger myohyracine genus (Protypotheroides) have been described from the ~20 Ma Langental deposits in Namibia (Stromer Citation1922; Patterson Citation1965; Pickford et al. Citation2008), along with materials from the Eocliff in the Sperrgebiet, Namibia (Senut and Pickford Citation2021), suggesting a more diverse radiation awaits discovery for that group.
Presence of rhynchocyonine and myohyracine fossils from the Nsungwe Formation in the Rukwa Rift Basin documents that both groups were present in eastern Africa by 25 Ma, prior to the PNT. Continental rift-fill deposits of the Nsungwe Formation offer an important window into the evolutionary history of late Oligocene terrestrial and freshwater biotas in eastern Africa, providing data on mammalian diversification against the backdrop of rift development in the Western Branch of the East African Rift System.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Data statement and data availability
This published work, including the novel genus Rukwasengi (urn:lsid:zoobank.org:act:E60CFB69-1F55-41F2-BC9A-79079C6EBE62), novel species butleri (urn:lsid:zoobank.org:act:E360C064-23CA-45DC-B9A8-C2012241584A), novel genus Oligorhynchocyon (urn:lsid:zoobank.org:act:6ACC3E77-2D4A-4806-ACE1-440ADDAC3B2E), and novel species songwensis (urn:lsid:zoobank.org:act:9B8C3D70-EFCB-424F-BC65-A4FDCD879130), along with the associated nomenclatural acts, have been registered in ZooBank under urn:lsid:zoobank.org:pub:7525A261-F757-4917-96EA-ADC6128D1535. µCT data and associated mesh (PLY) files are hosted at Morphosource (www.morphosource.org).
Acknowledgments
We appreciate excellent suggestions from Reviewer 1 (anonymous) and Reviewer 2 (Rodolph Tabuce) that improved the manuscript. We thank F. Bassange, J. Temu, and E. Bwasiri (Tanzania Antiquities Unit), E. Mshui and E. Mbede (University of Dar es Salaam), and the Tanzania Commission for Science and Technology; K. Whitman for mechanical specimen preparation; H. Sallam and M. Borths for µCT scanning; L. Myerholtz and J. Groenke for digital model development; H. and M. Fässler, T. Plattner for field support; E. Seiffert for helpful discussions; G. Gunnell and J.G. Fleagle for guidance and encouragement; F. Manthi, E. Mbua, M. Muungu, and R. Nyaboke (National Museums of Kenya) for specimen access; all RRBP field team members from 2002-present.
Additional information
Funding
References
- Asher RJ. 2007. A web-database of mammalian morphology and a reanalysis of placental phylogeny. BMC Evol Biol. 7(1):108. doi:10.1186/1471-2148-7-108.
- Asher RJ, Lehmann T. 2008. Dental eruption in afrotherian mammals. BMC Biol. 6(1):14. doi:10.1186/1741-7007-6-14.
- Asher RJ, Novacek MJ, Geisler JH. 2003. Relationships of endemic African mammals and their fossil relatives based on morphological and molecular evidence. J Mamm Evol. 10(1):131–194. doi:10.1023/A:1025504124129.
- Asher RJ, Seiffert ER. 2010. Systematics of endemic African mammals. Cenozoic mammals of Africa. Berkeley: University of California Press; p. 911–928.
- Blackburn DC, Paluh DJ, Krone I, Roberts EM, Stanley EL, Stevens NJ. 2019. The earliest fossil of the African clawed frog (Genus Xenopus) from Sub-Saharan Africa. J Herpetol. 53(2):125–130, 126. doi:10.1670/18-139.
- Blackburn DC, Roberts EM, Stevens NJ. 2015. The earliest record of the endemic African frog family ptychadenidae from the Oligocene Nsungwe formation of Tanzania. J Vertebr Paleontol. 35(2):e907174. doi:10.1080/02724634.2014.907174.
- Bonaparte CL. 1838. Synopsis vertebratorum systematis. Nuovi Annali delle Scienze Naturali, Bologna. 1:105–133.
- Butler PM. 1956. Erinaceidae from the Miocene of East Africa. Fossil Mammal Afr. 11:1–75.
- Butler PM. 1969. Insectivores and bats from the Miocene of East Africa: new material. In: Fossil vertebrates of Africa. (ed. LSB Leakey) Vol. 1. London and New York: Academic Press; p. 1–37.
- Butler PM. 1984. Macroscelidea, insectivora and Chiroptera from the Miocene of East Africa. Palaeovertebrata. 14:117–198.
- Butler PM. 1987. Fossil insectivores from Laetoli. Laetoli, A Pliocene site in Northern Tanzania. Oxford: Oxford University Press; p. 85–87.
- Butler PM. 1995. Fossil Macroscelidea. Mammal Rev. 25(1‐2):3–14. doi:10.1111/j.1365-2907.1995.tb00432.x.
- Butler PM, Hopwood AT. 1957. Insectivora and Chiroptera from the Miocene rocks of Kenya. Fossil Mammal Afr. 13:1–35.
- Claeson KM, Ngasala S, Gottfried MD, Roberts EM, O’Connor PM, Stevens NJ. 2021. A new assemblage of Cenozoic lungfishes (Dipnoi: lepidosirenidae) from the late Oligocene Nsungwe formation, Rukwa Rift Basin, southwestern Tanzania. Geobios. doi:10.1016/j.geobios.2020.09.004
- Coster P, Benammi M, Mahboubi M, Tabuce R, Adaci M, Marivaux L, Bensalah M, Mahboubi S, Mahboubi A, Maameri C, et al. 2012. Chronology of the Eocene continental deposits of Africa: magnetostratigraphy and biostratigraphy of the El Kohol and Glib Zegdou formations, Algeria. Geol Soc Am Bull. 124(9–10):1590–1606. doi:10.1130/B30565.1
- Epa YR, Stigall AL, Roberts EM, O’Brien HD, Stevens NJ. 2018. Morphological diversification of ampullariid gastropods (Nsungwe Formation, Late Oligocene, Rukwa Rift Basin, Tanzania) is coincident with onset of East African rifting. Pap Palaeontol. 4(3):327–348. doi:10.1002/spp2.1108.
- Feldmann R, O’Connor P, Stevens N, Gottfried M, Roberts E, Ngasala S, Rasmusson EL, Kapilima S. 2007. A new freshwater crab (Decapoda: brachyura: potamonautidae) from the Paleogene of Tanzania, Africa. Neues Jahrbuch Fur Geologie Und Palaontologie-abhandlungen. 244(1):71–78. doi:10.1127/0077-7749/2007/0244-0071.
- Gregory WK. 1910. The orders of mammals. Bull Amer Mus Nat Hist. 27:1–524.
- Haeckel E. 1866. Generelle Morphologie der Organismen. 2, Aligemeine Entwicklungsgeschichte der Organismen. Berlin: Reimer.
- Hartenberger J-L. 1986. Hypothèse paléontologique sur l’origine des Macroscelidea (Mammalia). Comptes Rendus de l’Académie des Sciences, Paris. 302:247–249.
- Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. 2015. Tree of life reveals clock-like speciation and diversification. Mol Biol Evol. 32(4):835–845. doi:10.1093/molbev/msv037.
- Heritage S, Rayaleh H, Awaleh DG, Rathbun GB. 2020. New records of a lost species and a geographic range expansion for sengis in the Horn of Africa. PeerJ. 8:e9652. doi:10.7717/peerj.9652.
- Holroyd PA. 2010. Macroscelidea. Cenozoic mammals of Africa. Berkeley: University of California Press; p. 89–98.
- Lawrence L, Spandler C, Roberts EM, Hilbert-Wolf HL. 2021. Mineralogy and origin of alkaline Nsungwe tuffs of the Rukwa Rift Basin, southwestern Tanzania. Lithos. 380-381:105885. doi:10.1016/j.lithos.2020.105885.
- Marivaux L, Lihoreau F, Manthi FK, Ducrocq S. 2012. A new basal phiomorph (Rodentia, Hystricognathi) from the late Oligocene of Lokone (Turkana Basin, Kenya). J Vertebr Paleontol. 32(3):646–657. doi:10.1080/02724634.2012.657318.
- McCartney JA, Bouchard SN, Reinhardt JA, Roberts EM, O’Connor PM, Mtelela C, Stevens NJ. 2021. The oldest lamprophiid (Serpentes, Caenophidia) fossil from the late Oligocene Rukwa Rift Basin, Tanzania and the origins of African snake diversity. Geobios. doi:10.1016/j.geobios.2020.07.005
- McCartney JA, Stevens NJ, O’Connor PM. 2014. The earliest colubroid-dominated snake fauna from Africa: perspectives from the late Oligocene Nsungwe formation of southwestern Tanzania. PLOS ONE. 9(3):e90415. doi:10.1371/journal.pone.0090415.
- Müller J, Roberts E, Naylor E, Stevens N. 2018. A Fossil Gekkotan (Squamata) from the late Oligocene Nsungwe formation, Rukwa Rift Basin, Tanzania. J Herpetol. 52(2):223–227, 225. doi:10.1670/17-123.
- Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, O’Brien SJ. 2001. Molecular phylogenetics and the origins of placental mammals. Nature. 409(6820):614–618. doi:10.1038/35054550.
- Novacek M. 1984. Evolutionary stasis in the elephant-shrew, rhynchocyon. In: Eldredge N, Stanley SM, editors. Living Fossils. New York: Springer New York; p. 4–22.
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-X, Meng J, et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 339(6120):662–667. doi:10.1126/science.1229237.
- Patterson B. 1965. The fossil elephant shrews (family Macroscelididae). Bulletin of the Museum of Comparative Zoology, HarvardUniversity. Vol. 133: 297–335.
- Penkrot TA, Zack SP, Rose KD, Bloch JI. 2008. Postcranial morphology of apheliscus and haplomylus (condylarthra, apheliscidae): evidence for a paleocene holarctic origin of macroscelidea. In: Sargis EJ, Dagosto M, editors. Mammalian evolutionary morphology: a tribute to Frederick S szalay. Dordrecht: Springer Netherlands; p. 73–106.
- Pickford M, Senut B, Morales J, Mein P, Sanchez IM. 2008. Mammalia from the Lutetian of Namibia. Mem Geol Surv Namibia. 20:465–514.
- Roberts EM, O’Connor PM, Gottfried MD, Stevens NJ, Kapilima S, Ngasala S. 2004. Revised stratigraphy and age of the red sandstone group in the Rukwa Rift Basin, Tanzania. Cretac Res. 25(5):749–759. doi:10.1016/j.cretres.2004.06.007.
- Roberts EM, O’Connor PM, Stevens N, Gottfried MD, Jinnah ZA, Ngasala S, Choh AM, Armstrong RA. 2010. Sedimentology and depositional environments of the Red Sandstone Group, Rukwa Rift Basin, southwestern Tanzania: new insight into Cretaceous and Paleogene terrestrial ecosystems and tectonics in sub-equatorial Africa. J Afr Earth Sci. 57(3):179–212. doi:10.1016/j.jafrearsci.2009.09.002.
- Roberts EM, Stevens NJ, O’Connor PM, Dirks PHGM, Gottfried MD, Clyde WC, Armstrong RA, Kemp AIS, Hemming S. 2012. Initiation of the western branch of the East African Rift coeval with the eastern branch. Nat Geosci. 5(4):289–294. doi:10.1038/ngeo1432.
- Roberts EM, Todd CN, Aanen DK, Nobre T, Hilbert-Wolf HL, O’Connor PM, Tapanila L, Mtelela C, Stevens NJ. 2016. Oldest evidence of fungus-farming termites reveals Paleogene African origin of agriculture. PLoS ONE. 11(6):e0156847. doi:10.1371/journal.pone.0156847.
- Sallam HM, Seiffert C. 2016. New phiomorph rodents from the latest Eocene of Egypt, and the impact of Bayesian “clock”-based phylogenetic methods on estimates of basal hystricognath relationships and biochronology. PeerJ. 4:e1717. doi:10.7717/peerj.1717.
- Sánchez‐Villagra MR, Narita Y, Kuratani S. 2007. Thoracolumbar vertebral number: the first skeletal synapomorphy for afrotherian mammals. Syst Biodivers. 5(1):1–7. doi:10.1017/S1477200006002258.
- Schlosser M. 1910. Uber einige fossiler Saugetiere aus dem Oligocan von Aegypten. Zoolischer Anzeiger. 35:500–508.
- Seiffert ER. 2007. A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence. BMC Evol Biol. 7(1):224. doi:10.1186/1471-2148-7-224.
- Seiffert ER. 2010. Chronology of paleogene mammal localities. In: Werdelin L, Sanders WJ, editors. Cenozoic mammals of Africa. Berkeley: University of California Press; p. 19–26.
- Senut B. 2008. Macroscelididae from the lower Miocene of the Northern Sperrgebiet, Namibia. Mem Geol Surv Namibia. 20:185–225.
- Senut B, Pickford M. 2021. Micro-cursorial mammals from the late Eocene tufas at Eocliff, Namibia. Mem Geol Surv Namibia. 23:90–160.
- Simons EL, Holroyd PA, Bown TM. 1991. Early tertiary elephant-shrews from Egypt and the origin of the Macroscelidea. Proc Natl Acad Sci. 88(21):9734–9737. doi:10.1073/pnas.88.21.9734.
- Stanhope MJ, Waddell VG, Madsen O, De Jong W, Hedges SB, Cleven GC, Kao D, Springer MS. 1998. Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. Proc Natl Acad Sci. 95(17):9967–9972. doi:10.1073/pnas.95.17.9967.
- Stevens NJ, Gottfried MD, Roberts EM, Kapilima S, Ngawala S, O’Connor PM. 2008. Paleontological Exploration in Africa: a view from the Rukwa Rift Basin of Tanzania. In: Fleagle JG, Gilbert CC, editors. Elwyn Simons: a search for origins. New York (NY): Springer; p. 159–180.
- Stevens NJ, Holroyd PA, Roberts EM, O’Connor PM, Gottfried MD. 2009a. Kahawamys mbeyaensis (n. gen., n. sp.) (Rodentia: thryonomyoidea) from the late Oligocene Rukwa Rift Basin, Tanzania. J Vertebr Paleontol. 29(2):631–634. doi:10.1671/039.029.0219.
- Stevens NJ, O’Connor PM, Gottfried MD, Roberts EM, Ngasala S. 2005. An anthropoid primate humerus from the Rukwa Rift Basin, Paleogene of Southwestern Tanzania. J Vertebr Paleontol. 25(4):986–989. doi:10.1671/0272-4634(2005)025[0986:AAPHFT]2.0.CO;2.
- Stevens NJ, O’Connor PM, Gottfried MD, Roberts EM, Ngasala S, Dawson MR. 2006. Metaphiomys (Rodentia: phiomyidae) from the Paleogene of southwestern Tanzania. J Paleontol. 80(2):407–409. doi:10.1666/0022-3360(2006)080[0407:MRPFTP]2.0.CO;2
- Stevens NJ, O’Connor PM, Roberts EM, Gottfried MD. 2009b. A hyracoid from the late Oligocene red sandstone group of Tanzania, Rukwalorax jinokitana (gen. and sp. nov.). J Vertebr Paleontol. 29(3):972–975. doi:10.1671/039.029.0302.
- Stevens NJ, Seiffert ER, O’Connor PM, Roberts EM, Schmitz MD, Krause C, Gorscak E, Ngasala S, Hieronymus TL, Temu J. 2013. Palaeontological evidence for an Oligocene divergence between old world monkeys and apes. Nature. 497(7451):611–614. doi:10.1038/nature12161.
- Stevens WN, Claeson KM, Stevens NJ. 2016. Alestid (Characiformes: alestidae) fishes from the late Oligocene Nsungwe Formation, Rukwa Rift Basin, of Tanzania. J Vertebr Paleontol. 36(5):e1180299. doi:10.1080/02724634.2016.1180299.
- Stromer E. 1922. Erste Mitteilung über tertiare Wirbeltier-Reste aus Deutsch-Südwest Afrika. Sitz Ber bayerischen Akad Wiss München. ii:331–340.
- Tabuce R. 2018. New remains of Chambius kasserinensis from the Eocene of Tunisia and evaluation of proposed affinities for Macroscelidea (Mammalia, Afrotheria). Hist Biol. 30(1–2):251–266. doi:10.1080/08912963.2017.1297433.
- Tabuce R, Asher RJ, Lehmann T. 2008. Afrotherian mammals: a review of current data. Annals of Human Genetics. 72(1):2–14. doi:10.1111/j.1469-1809.2007.00402.x.
- Tabuce R, Coiffait B, Coiffait P-E, Mahboubi M, Jaeger -J-J. 2001. A new genus of Macroscelidea (Mammalia) from the Eocene of Algeria: a possible origin for elephant-shrews. J Vertebr Paleontol. 21(3):535–546. doi:10.1671/0272-4634(2001)021[0535:ANGOMM]2.0.CO;2.
- Tabuce R, Jaeger -J-J, Marivaux L, Salem M, Bilal AA, Benammi M, Chaimanee Y, Coster P, Marandat B, Valentin X, et al. 2012. New stem elephant-shrews (Mammalia, Macroscelidea) from the Eocene of Dur At-Talah, Libya. Palaeontology. 55(5):945–955. doi:10.1111/j.1475-4983.2012.01163.x.
- Tabuce R, Marivaux L, Adaci M, Bensalah M, Hartenberger J-L, Mahboubi M, Mebrouk F, Tafforeau P, Jaeger -J-J. 2007. Early tertiary mammals from North Africa reinforce the molecular Afrotheria clade. Proc Biol Sci. 274(1614):1159–1166.
- Wible JR, Rougier GW, Novacek MJ, Asher RJ. 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 447(7147):1003–1006. doi:10.1038/nature05854.
- Zack SP, Penkrot TA, Bloch JI, Rose KD. 2005. Affinities of ‘hyopsodontids’ to elephant shrews and a holarctic origin of Afrotheria. Nature. 434(7032):497–501. doi:10.1038/nature03351.
