ABSTRACT
Polyneopteran clade of insects is very diverse and has a remarkable fossil history starting in the Late Palaeozoic. Representatives of Dictyoptera comprise roaches, termites and mantids and their stem group relatives like the common Palaeozoic and Mesozoic roachoids, a group well known for its problematic taxonomy. In this study, two new taxa are described based on forewing venation well displaying convexity or concavity of the veins due to preservation in spherosideritic concretions. A new paoliid, Stephanopsis testai sp. nov. (Blattinopsidae), from Moscovian deposits at Mazon Creek (Illinois, U.S.A.), supplements Stephanopsis mirandus found at the same locality, which mainly differs in the branching patterns of MP and anal veins. A new stem dictyopteran, Sosnowiecia dareki gen. nov. et sp. nov. (?Mesorthopteridae), from Langsettian deposits at Sosnowiec-Klimontów (Lower Silesia, Poland) is problematic in terms of taxonomy as it has a mosaic of characters characteristic of Paoliida, Eoblattida and Grylloblattida, but retains affinities with roachoid insects, such as Miroblatta sp. Hence, these two new species increase the diversity of Carboniferous polyneopteran insects, but reveals the problem of depending only on alar characters in taxonomy.
LSID urn:lsid:zoobank.org:pub:49299826-0F1E-4892-8D88-82FBDCC192BF
Introduction
Dictyoptera is a well-supported clade of polynepteran insects comprising crown groups of Mantodea (mantids) and Blattodea (cockroaches and termites) with long evolutionary histories and stem group relatives in the Late Palaeozoic and Mesozoic strata (Legendre et al. Citation2015; Wipfler et al. Citation2019). These include particularly well-known roachoids, the females of which had very long ovipositors (Grimaldi and Engel Citation2005; Hörnig et al. Citation2018). Although these insects were very abundant and diverse in Late Palaeozoic ecosystems, their systematics is unsatisfactory mainly due to large intraspecific variability in wing venation, which is frequently used as characters for their classification. On the other hand, the alar characters of the stem group of Dictyoptera, such as Paoliida, are not so variable at the intraspecific level and therefore more suitable for taxonomy (Prokop et al. Citation2014). Paoliida currently consists of three families, Paoliidae, Blattinopsidae and Anthracoptilidae, but the position of some others remains uncertain (Guan et la. Citation2016).
In this paper two new Pennsylvanian taxa of stem-Dictyoptera are designated: Stephanopsis testai sp. nov. (Blattinopsidae) from Mazon Creek in Illinois, U.S.A and Sosnowiecia dareki gen. et sp. nov. (?Mesorthopteridae) from Sosnowiec-Klimontów in Lower Silesia, Poland. In addition, problems with the classification of Palaeozoic polyneopteran insects are discussed.
Materials and methods
Materials examined in this study came from spherosideritic concretions/nodules from two Pennsylvanian deposits; Mazon Creek Lagerstätte in Illinois, U.S.A. (Moscovian, Carbondale Formation) and Sosnowiec-Klimontów in Lower Silesia, Poland (Langsettian, Mudstone series – Załęże beds, Coal-bearing Mudstone). While Mazon Creek is a world-famous site with a diverse entomofauna (Carpenter Citation1997; Kukalová-Peck Citation1997), insects from Sosnowiec are only reported in a few publications (Krawczyncski et al. Citation1997; Prokop et al. Citation2012, Citation2014, Citation2017, Citation2022; Dvořák et al. Citation2019).
The specimens were observed in a dry state using a Nikon SMZ 745T stereomicroscope. Line drawings of venation were made using a Zeiss SteREO Discovery.V20 with camera lucida attachment. Habitus drawing of S. dareki gen. nov. et sp. nov. was done by a scientific illustrator, Zuzana Čadová (Liberec, Czech Republic). Photographs of fossil specimens in a dry state were taken with a Canon EOS 550D digital camera equipped with either a MP-E 65 mm or EF-S 60 mm macro-lens. Original photographs were processed using the image-editing software Adobe Photoshop CS and some were processed using the stacking software Helicon Focus.
Both type specimens are housed in the institutional collections of The Field Museum of Natural History (Chicago, U.S.A.) and the Natural History Museum of the Institute of Systematics and Evolution of Animals PAS (Cracow, Poland).
The nomenclature of the wing venation generally follows the scheme of Brodsky (Citation1994) and Schubnel et al. (Citation1961). Abbreviation of veins: A1/A2 – anal veins; CuA/CuP – cubitus anterior/posterior; M – stem of media; MP – media posterior; PCu – postcubital vein; RA/RP – radius anterior/posterior; ScA/ScP – subcosta anterior/posterior.
Results
Order Paoliida Handlirsch Citation1906
Family Blattinopsidae Bolton, 1925
Genus Stephanopsis Kukalová, Citation1958
Stephanopsis testai sp. nov. ()
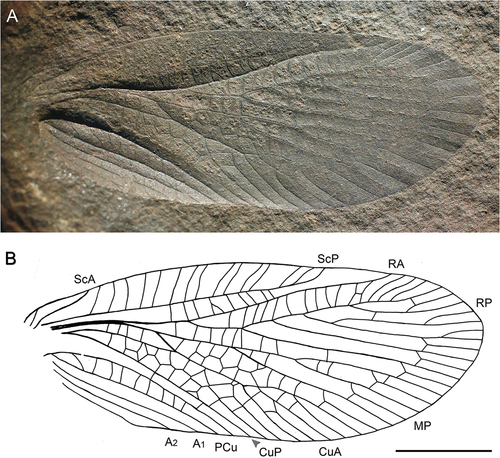
Figure 1. Stephanopsis testai sp. nov., holotype TVT 4023 (originally in Thomas V. Testa coll., currently deposited in FMNH), Moscovian: Middle Pennsylvanian, Mazon Creek, Illinois U.S.A. (A) photograph of forewing venation; (B) line drawing of venation of forewing. Scale bars = 5 mm.
ZooBank LSID: urn:lsid:zoobank.org:act:ECF9934E-1F55-46E1-9FA7-A59DADFD6796
Etymology
Patronymic; named after Thomas V. Testa in honour of his fieldwork at Mazon Creek.
Diagnosis
Area between C and ScP twice as wide as the area between ScP and RA. First branches of RP and MP at the same level of about half of the length of the wing. RP posteriorly pectinated with five primary branches. Two rows of cells between CuA and CuP below the arculus. PCu vein well developed. Anal veins simple.
Description
Based on forewing venation. Wing very well preserved with numerous cross veins and noticeable reticular pattern with two rows of large irregular cells in cubital and partially in medial area. Wing length about 23.9 mm, and maximum width 9 mm. Noticeable short weakly sigmoidal ScA ending at the costal margin 3.7 mm from the wing base. ScP with numerous anterior and slightly oblique veinlets, ending at the costal margin two-thirds along the length of the wing. Area between C and ScP wide (1.8 mm), about twice the width at the widest part of the area between ScP and RA. RA apparently convex, slightly sigmoidal and simple. RP emerging from RA 8.7 mm from the base of the wing, regularly posteriorly pectinated with five main branches and several sigmoidal anterior terminal shoots that reach the apical margin of the wing. MP with three branches, with the first at the same level as the first branch of RP, around half of the length of the wing. CuA with five primary posterior branches. Branches of RP, MP and CuA run parallel and form a regular pattern distally with veinlets between adjoining branches about 3 mm in length. Strong oblique cross vein mp-cua (arculus) present between MP and CuA. CuP simple and distally nearly straight. Convex PCu running parallel to CuP (sensu Schubnel et al. Citation2020). Three simple anal veins discernible.
Type material
Holotype TVT 4023 (originally in Thomas V. Testa coll., currently deposited in FMNH), positive (B) and negative imprint (A) of nearly complete forewing preserved in siderite nodule.
Type locality
Mazon Creek, Peabody Coal Pit 11, Grundy County, Illinois, U.S.A.
Type strata
Francis Creek Shale Member, Carbondale Formation, Will-Kankakee-Grundy Co., Illinois, U.S.A.; Moscovian (315.2–307.0 Ma), Middle Pennsylvanian, Carboniferous (Shabica and Hay Citation1997).
Discussion
This fossil wing clearly belongs to Blattinopsidae (Paoliida) because ScP ends on the costal margin with exception in Avionblattinopsis Quispe et al., Citation2021, stem of R sigmoidal, RP pectinated with high number of posterior branches and slightly, but not extensively extends the area between MP and CuA, which differentiates Blattinopsidae from Paoliidae (Prokop et al. Citation2014; Quispe et al. Citation2021). There is also a superficial resemblance in the venation with some Archaeorthoptera, but this group differs in a common stem for M + CuA and the fusion of CuA and CuP(a), which are missing in this fossil (Béthoux and Nel Citation2002).
The general pattern of venation S. testai sp. nov. resembles some other blattinopsid like species of the genus Glaphyrophlebia Handlirsch, Citation1906, but Glaphyrophlebia has a regular parallel pattern of R, M and CuA branches with many supplementary veins. For example, in Glaphyrophlebia wettinensis Fritsch, Citation1899 the first branch of RP and MP is at the same level and RP is posteriorly pectinated and has a similar number of branches (Hörnschmeyer and Stapf Citation2001). But there is a marked difference between S. testai sp. nov. and Glaphyrophlebia in that it lacks the typical vein-bow and has a distinctly narrower space between veins ScP and RA (Hörnschemeyer and Stapf Citation2001; Aristov et al. Citation2021; Aristov and Rasnitsyn Citation2022a).
The present forewing can be clearly assigned to the genus Stephanopsis Kukalová, Citation1958 as the width of the costal area (between C and ScP) is much wider than the area between ScP and RA (Hörnschemeyer and Stapf Citation2001). Stephanopsis testai sp. nov. clearly differs from Stephanopsis incerta Laurentiaux, Citation1950 and Stephanopsis elegans Schlechtendal in Handlirsch Citation1906 by the position of the first branch of RP being about half way along the wing, whereas in the latter species, the first branch of RP is about a third of the way along the length of the wing and more anterior to the first branch of MP. Moreover, S. elegans has a much longer ScP reaching far beyond two-thirds of the way along the length of the wing and in S. incerta there is a much finer reticular pattern between veins CuA and CuP. But as the preserved fossil of S. incerta is probably a hind-wing (Kukalová Citation1958), an exact comparison is difficult. The venations of S. testai sp. nov. and S. mirandus (Richardson, Citation1956) are similar and they are described from the same locality. The vein RA has a similar sigmoidal shape, first branch of RP and MP occur at the same level of about half way along the length of the wing and the general branching pattern of RP is similar (Richardson Citation1956). However, MP has the same number of branches in both species, but their branching pattern is different in respect of topology. The most remarkable difference is the posterior branching of the anal veins in S. mirandus, whereas in S. testai sp. nov. they are clearly all simple. Hörnschmeyer and Stapf (Citation2001) also consider the venation of Klebsiella extincta (Meunier, Citation1908) to be similar to that of the genus Stephanopsis (Meunier Citation1908). As the holotype of K. extincta was recently reviewed by Quispe et al. (Citation2021), we compared both these species. The general pattern of veins is comparable, especially the primary branching of the main veins. However, a better developed medial area with six terminal branches instead of three as in S. testai sp. nov. and a less developed cubital area clearly distinguish these two species. Also, the regular pattern of terminal veinlets is absent in K. extincta.
Superorder Dictyoptera
Family ?Mesorthopteridae
Genus Sosnowiecia gen. nov.
ZooBank LSID: urn:lsid:zoobank.org:act:1EEACE1C-BDE9-4332-83A7-9D255F5C1858
Type species. Sosnowiecia dareki sp. nov.
Diagnosis
Based on forewing characters: RA and RP branch near the middle of the wing behind the first and second branches of M. Long stem of Cu. CuA posteriorly pectinated with four convex branches. Area between MP and CuA very narrow with one row of cells. Arculus (cross vein mp-cua) weakly developed. CuP terminally bifurcated. Area between CuA and CuP slightly enlarged with numerous irregular cells.
Etymology
Named after the locality, Sosnowiec (Lower Silesia, Poland), where the specimen was found; gender feminine.
Sosnowiecia dareki sp. nov. ()
Figure 2. Sosnowiecia dareki gen. et sp. nov., holotype MP ISEA I−F/MP/1488/22-08 (Natural History Museum of the Institute of Systematics and Evolution of Animals PAS, Cracow coll.), Langsettian (Westphalian A): Pensylvannian, Sosnowiec-Klimontów, originally Porąbka-Klimontów Mine, Poland. (A) line drawing of habitus, (B) line drawing of forewing venation. Both line drawings were done by Zuzana Čadová. Scale bars = 5 mm.
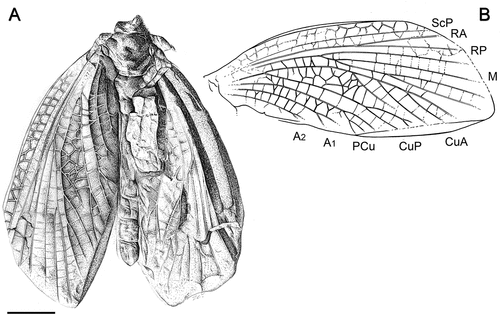
Figure 3. Sosnowiecia dareki gen. et sp. nov., holotype MP ISEA I−F/MP/1488/22-08 (Natural History Museum of the Institute of Systematics and Evolution of Animals PAS, Cracow coll.), Langsettian (Westphalian A): Pensylvannian, Sosnowiec-Klimontów, originally Porąbka-Klimontów Mine, Poland. (A) photograph of habitus – imprint, (B) photograph of habitus – counter-imprint. Scale bars = 5 mm.

ZooBank LSID: urn:lsid:zoobank.org:act:8151F945-C84D-41FA-BB68-E259C6178014
Etymology
Named after Darek (diminutive of Polish first name Dariusz) the collector of this fossil; gender feminine.
Diagnosis
By monotypy, that of the genus.
Description
Body preserved with arched mesonotum and both mesothoracic wings flexed and slightly open, partially preserved metathorax hidden under the anal areas of wings and four slender segments of abdomen (proximal part of abdomen hidden as well). Forewing incompletely preserved with about one-third of the distal part missing. Regular net of transverse veinlets or irregular cells, the so-called archedictyon, present. ScP slightly oblique, running parallel to stem of R and ending in distal third of the length of the wing. Costal area between anterior wing margin and ScP with a coarse network and two rows of cells. Costal area 1.4 mm wide without apparent widening near wing base; costal area about one and half times wider than the widest part of the area between ScP and RA. RA/RP bifurcation relatively distant, 13.9 mm from base of wing, which is approximately between one-third and half of the length of the wing. RA and RP simple in preserved basal parts of the wing. Stem of M/MP clearly concave and anteriorly pectinated with four visible branches. First division of MP is 3.3 mm from the bifurcation of RA and RP. Straight or oblique simple cross veins in area between branches of MP. Stem of Cu relatively long with the division into CuA and CuP about 4.6 mm from wing base. Space between CuA and CuP widely open with irregular network of cells. Strongly convex CuA posteriorly pectinated and ending with four simple branches on posterior wing margin. First division of CuA nearly level with division of RA and RP. CuP concave with distal branches and anterior branch ending with short twig. Convex PCu distally bifurcated and posterior branch ending with a short twig on posterior margin of wing. Two anal veins ending with terminal twigs. Numerous simple or Y shaped cross veins between branches of CuP, PCu and anal veins.
Type material
Holotype MP ISEA I−F/MP/1488/22-08 (print and counter imprint of thorax with mesothoracic wings and partially preserved abdomen in sideritic concretion).
Type locality
Sosnowiec-Klimontów, originally Porąbka-Klimontów Mine, Upper Silesian Coal Basin, Poland (Krawczyński et al. Citation1997; Prokop et al. Citation2012).
Type strata
Langsettian (Westphalian A), Pensylvannian, Mudstone series (Załęże beds), Coal-bearing Mudstone Series (Pacyna and Zdebska Citation2002).
Discussion
The pattern of forewing venation of this fossil corresponds to that of polyneopteran insects and clearly differs from that of Archaeorthoptera in the absence of a basal division of CuP, absence of distal fusion of anterior branch of CuP with CuA and also lack of basal fusion of veins M with CuA (Béthoux and Nel Citation2002). There is a clear resemblance to ‘blattoid’ lines Dictyoptera (Blattodea + Mantodea) and especially Paollida, but not an exact match. The most remarkable characters shared with Paoliida are a long common stem of Cu in front of the bifurcation into CuA and CuP, M running closely parallel to R near base of wing and relatively broad area with irregular network of cross veins between CuA and CuP. On the other hand, a short weakly developed convex arculus between M/MP and CuA is not very distinct and there are no convex anterior branches of CuA, which are typical for roachoid-like or paoliid insects (e.g. Zhang et al. Citation2013; Prokop et al. Citation2014; Correia et al. Citation2019). Other typical features of Paoliidae are oblique anterior veinlets of ScP or a broad area between MP and CuA, which are not present in S. dareki gen. et sp. nov. (Prokop et al. Citation2014), but are present in some Blattinopsidae and also some Palaeozoic roachoids, like Miroblatta costalis Laurentiaux-Vieira & Laurentiaux, Citation1987 (see Béthoux et al. Citation2011). However, the position of these insects remains uncertain, but they most probably belong to the stem group of Dictyoptera (Correia et al. Citation2019).
Possible affinities to Eoblattida, including Gryloblattida sensu Storozhenko (Citation1997), are also apparent, but the concept of this rich group is still questionable as the type species, Eoblatta robusta (Brongniart, Citation1893), seems to belong to Archaeorthoptera (Schubnel et al. Citation2020). General arrangement of the main veins in the mid wing area, for example, resemble ‘eoblattid’ Permula lebachensis Schlechtendal, Citation1913, with the first branch of M close to that of the first branch of R and CuA posteriorly pectinated (Aristov Citation2015). However, in S. dareki the width of the costal area between ScP and C clearly narrows distally and there is a different pattern of cross veins. In contrast, sigmoidal CuA and the costal field narrows towards wing base and the wing tip differs in P. lebachensis Schlechtendal, Citation1913. Moreover, a large part of the wing is missing and thus an exact comparison is impossible. There is a most definite resemblance to Paridelia pusilla Sharov, Citation1961 (see Aristov Citation2015). The R division in both taxa is located beyond the first third of the length of the wing and behind the first branch of M. Both veins RA and RP run simple for a long distance. They also share posteriorly pectinated CuA with four branches and anterior branches of ScP forming a cellular meshwork instead of simple oblique veinlets. But P. pussila differs from S. dareki in the more basal placement of the first CuA branch in relation to the first branch of M, CuP lacks a distal bifurcation and the branching pattern of M is different. Taxonomic placement of P. pussila has been changed several times. Initially it was placed in the family Ideliidae (Sharov Citation1961) and subsequently in Kortshakoliidae, which are both part of Grylloblattida (Storozhenko Citation1997, Citation1998). Later, Aristov (Citation2015) transferred Paridelia to the family Mesorthopteridae within Eoblattida. However, due to uncertain position of Eoblattida, the placement of the family Mesorthopteridae must be re-examined. Another problem complicating a correct taxonomic placement arises from the uncertain relationships between extinct Grylloblattida and presumably crown group Grylloblattodea with Grylloblattidae (Storozhenko Citation2002; Cui et al. Citation2015). A mosaic of characters (particularly resembling Paoliida, Dictyoptera and Grylloblattida) and uncertain, probably very basal position (within Polyneoptera) of the most similar species P. pussila, lead us to consider S. dareki as a representative of a stem group of Dictyoptera with affinity to blattoids, but without an exact placement in currently described orders. This statement is congruent with the recent analysis supporting a relatively close phylogenetic relationship of extant Grylloblattodea and Dictyoptera (Wipfler et al. Citation2019).
Conclusion
In this study one new genus and two new species of polyneopteran insects are described based on the venation of their forewings. Stephanopsis testai sp. nov. is based on well-preserved venation, which clearly placed it in Blattinopsidae (Paollida). Sosnowiecia dareki gen. nov. et sp. nov. is based on an incomplete forewing which has a combination of characters that prevents an accurate placement, but affinities with blattoids are clearly apparent. The situation is further complicated by the uncertain status of Grylloblattida, the stem group of Dictyoptera (like roachoids) and especially Eoblattida. In order to determine the Palaeozoic evolutionary history of Polyneoptera, a comprehensive phylogenetic study and clarification of the status of these high ranked taxa is needed.
Acknowledgments
We are indebted to the scientific illustrator Zuzana Čadová for the magnificent line drawing of Sosnowiecia dareki. We cordially thank Darek Wojciechowski for his fieldwork at Sosnowiec. The authors are grateful to Paul Mayer (The Field Museum, Chicago, U.S.A.), who kindly provided access to the collection under his care. The authors are grateful to Anthony F. G. Dixon (University of East Anglia, Norwich, United Kingdom) for improving the English and an anonymous referee for suggestions for improving an earlier version of the manuscript. TD & JP were supported by the Grant Agency of the Czech Republic (No. 18-03118S).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aristov DS. 2015. Classification of the order Eoblattida (Insecta: Blattidea) with description of new taxa. Far Eastern Entomologist. 301:1–56.
- Aristov DS, Rasnitsyn AP. 2022a. New Blattinopsidae (Insecta: Blattinopsida) from the Middle Permian of European Russia. Paleontological Journal. 56(2):187–193. doi:10.1134/S0031030122020034.
- Aristov DS, Rasnitsyn AP. 2022b. New species of the genus Glaphyrophlebia (Insecta, Blattinopsida: Blattinopsidae) from the Upper Carboniferous of Ukraine. Far Eastern Entomologist. 450:9–11. doi:10.25221/fee.450.2.
- Aristov DS, Rasnitsyn AP, Naugolnykh SV. 2021. New Blattinopsidae (Insecta: Blattinopsida) in the Permian of the Pechora Basin (Komi Republic, Russia) in the context of landscape and vegetation evolution. Paleontological Journal. 55(6):641–649. doi:10.1134/S0031030121060022.
- Béthoux O, Nel A. 2002. Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera Sensu Nov. Zootaxa. 96:1–88. doi:10.11646/zootaxa.96.1.1
- Béthoux O, Schneider JW, Klass KD. 2011. Redescription of the holotype of Phyloblatta gaudryi (Agnus, 1903) (Pennsylvanian; Commentry, France), an exceptionally well-preserved stem-dictyopteran. Geodiversitas. 33(4):625–635. doi:10.5252/g2011n4a4.
- Brodsky AK. 1994. The Evolution of Insect Flight. Oxford (New York, Tokyo): Oxford University Press.
- Brongniart C. 1893. Recherches pour servir à l’histoire des insectes fossiles des temps primaires précédées d’une étude sur la nervation des ailes des insectes. Bulletin de la Société d’Industrie Minérale de Saint-Etienne. 7(4):1–491.
- Carpenter FM. 1997. Insecta. Chapter 14A. In: Shabica CW, Hay AA, editors. Richardson’s guide to the fossil fauna of Mazon creek. Chicago, llinois: Northeastern Illinois University; p. 184–193.
- Correia P, Schubnel T, Nel A. 2019. What is the roachoid genus Eneriblatta (Dictyoptera: Phyloblattidae) from the Carboniferous of Portugal. Historical Biology. 33(6):777–782. doi:10.1080/08912963.2019.1661407.
- Cui YY, Béthoux O, Klass KD, Ren D. 2015. The Jurassic Bajanzhargalanidae (Insecta: Grylloblattida?): new genera and species, and data on postabdominal morphology. Arthropod Structure & Development. 44(6 Pt B):688–716. doi:10.1016/j.asd.2015.04.008.
- Cui YY, Béthoux O, Ren D. 2011. Intraindividual variability in Sinonamuropteridae forewing venation (Grylloblattida; Late Carboniferous): taxonomic and nomenclatural implications. Systematic Entomology. 36(1):44–56. doi:10.1111/j.1365-3113.2010.00545.x.
- Dvořák T, Pecharová M, Krzemiński W, Prokop J. 2019. New archaeorthopteran insects from the Carboniferous of Poland: insights into tangled taxonomy. Acta Palaeontologica Polonica. 64:787–796. doi:10.4202/app.00614.2019
- Fritsch A. 1899. Beschreibung einiger neuen fossilen Insekten in Schleferton des Steinkohlengebirge v. In: Beyschlag F, Fritsch KW, editors. Das Jüngere Steinkohlengebirge und das Rothliegende in der Provinz Sachsen und den Angrenzenden Gebieten. Abhandlungen der Königlich Preußischen-Geologischen Landesanstalt Berlin: Im Vertrieb der S. Schropp'schen Hof-Landkartenhandlung (J.H. Neumann). p. 1–263. Wettin.
- Grimaldi DA, Engel MS. 2005. Evolution of the insects. Cambridge: Cambridge University Press.
- Guan Z, Prokop J, Roques P, Lapeyrie J, Nel A. 2016. Revision of the enigmatic insect family Anthracoptilidae enlightens the evolution of Palaeozoic stem-dictyopterans. Acta Palaeontologica Polonica. 61:71–87.
- Handlirsch A. 1906. Die fossilen Insekten und die Phylogenie der rezenten Formen. In: Ein Handbuch für Paläontologen und Zoologen. Leipzig: Wilhelm Engelman; p. 1430.
- Hörnig MK, Haug C, Schneider JW, Haug JT. 2018. Evolution of reproductive strategies in dictyopteran insects—clues from ovipositor morphology of extinct roachoids. Acta Palaeontologica Polonica. 63:1–24. doi:10.4202/app.00324.2016.
- Hörnschemeyer T, Stapf H. 2001. Review of Blattinopsidae (Prothortoptera) with description of new species from the Lower Permian of Niedermoschel (Germany). Neue Jahrbuch für Geologie und Paläontologie, Abhandlungen. 221(1):81–109. doi:10.1127/njgpa/221/2001/81.
- Krawczyński W, Filipiak P, Gwoździewicz M. 1997. Zespoł skamie−niałości z karbońskich sferosyderitów (westfal A) NE części Górno−śląskiego Zagłębia Węglowego. Przeglad Geologiczny. 45:1271–1274.
- Kukalová J. 1958. Remarks to the family Blattinopsidae Bolton, 1925 (Insecta, Protorthoptera). Vestnik Ustredniho Ustavu Geologickeho. 33:129–131.
- Kukalová-Peck J. 1997. Mazon Creek insect fossils: the origin of insect wings and clues about the origin of insect metamorphosis. Chapter 14B. In: Shabica CW, Hay AA, editors. Richardson’s Guide to the Fossil Fauna of Mazon Creek. Chicago, Illinois: Northeastern Illinois University; p. 194–207.
- Laurentiaux D. 1950. Les insectes des bassins houillers du Gard et de la Loire. Annales de Paléontologie. 36:63–84.
- Laurentiaux-Vieira F, Laurentiaux D. 1987. Un remarquable Archimylacridae du Westphalien inférieur belge. Ancienneté du dimorphisme sexuel des blattes. Annales de la Société Géologique du Nord. 106:37–47.
- Legendre F, Nel A, Svenson GJ, Robillard T, Pellens R, Grandcolas P. 2015. Phylogeny of Dictyoptera: dating the origin of cockroaches, praying mantises and termites with molecular data and controlled fossil evidence. PLos ONE. 10(7):1–27. doi:10.1371/journal.pone.0130127.
- Meunier F. 1908. Quatrième note sur de nouveaux insectes du Stéphanien de Commentry. Bulletin du Muséum National d’Histoire Naturelle. 14:244–249.
- Pacyna G, Zdebska D 2002. Upper Carboniferous plant macrofossils from sideritic concretions in Sosnowiec (Upper Silesian Coal Basin) and Mazon Creek (Illinois, USA). In: Lipiarski I (ed.), Proceedings XXV Symposium Geology of Coal−Bearing Strata of Poland, 123–127. University of Mining and Metallurgy, Cracow.
- Prokop J, Krzemińska E, Wojtechowski D, Wojciechowski D. 2012. Paoliida, a putative stem-group of winged insects: morphology of new taxa from the Upper Carboniferous of Poland. Acta Palaeontologica Polonica. 57(1):161–173. doi:10.4202/app.2010.0064.
- Prokop J, Krzeminski W, Krzeminska E, Hörnschemeyer T, Ilger J-M, Brauckmann C, Grandcolas P, Nel A. 2014. Late Palaeozoic Paoliida is the sister group of Dictyoptera (Insecta: Neoptera). Journal of Systematic Palaeontology. 12(5):601–622. doi:10.1080/14772019.2013.823468.
- Prokop J, Pecharová M, Nel A, Hörnschemeyer T, Krzemińska E, Krzemiński W, Engel MS. 2017. Paleozoic nymphal wing pads support dual model of insect wing origins. Current Biology. 27(2):263–269. doi:10.1016/j.cub.2016.11.021.
- Prokop J, Rosová K, Krzemińska E, Krzemiński W, Nel A, Engel MS. 2022. Abdominal serial homologues of wings in Paleozoic insects. Current Biology. 32(15):3414–3422. doi:10.1016/j.cub.2022.06.024.
- Quispe L, Roques P, Garrouste R, Nel A. 2021. Carboniferous Blattinopsidae: revision of Klebsiella and new genus and species from Avion (Insecta, Paoliida). Historical Biology. 34(3):383–389. doi:10.1080/08912963.2021.1916817.
- Richardson ES Jr. 1956. Pennsylvanian invertebrates of the Mazon Creek area, Illinois. Insects. Fieldiana Geology. 12:15–56.
- Schlechtendal DHR. 1913. Untersuchung ueber die karbonischen Insekten und Spinnen von Wettin unter Berucksichtigung berwandter Faunen (Revision der Originale von Germar, Giebel und Goldenberg). Nova Acta Akademia Leopoldina-Carolinae. 98(1):1–186.
- Schubnel T, Desutter-Grandcolas L, Legendre F, Prokop J, Mazurier A, Garrouste R, Grandcolas P, Nel A. 2019. To be or not to be: postcubital vein in insects revealed by microtomography. Systematic Entomology. 45(2):327–336. doi:10.1111/syen.12399.
- Schubnel T, Roberts D, Roques P, Garrouste R, Desutter-Grandcolas L, Nel A. 2020. Moscovian fossils shed light on the enigmatic polyneopteran families Cacurgidae and Eoblattidae (Insecta: ‘Eoblattida. Archaeorthoptera). Journal of Systematic Palaeontology. 18(6):499–511. doi:10.1080/14772019.2019.1627595.
- Shabica CW, Hay AW. 1997. Richardson’s guide to the fossil fauna of Mazon Creek. Northeastern Illinois Studies. 1–307.
- Sharov AG. 1961. Order Paraplecoptera. In: Rohdendorf BB, Becker-Migdisova EE, Martynova OM, Sharov AG. Paleozoiskie nasekomye Kuznetskogo basseina [Paleozoic insects of the Kuznetsk basin.] Trudy Paleontologicheskogo Instituta Akademii nauk SSSR. (Vol: 85, pp. 164–224).
- Storozhenko SY. 1997. Classification of order Grylloblattida, with description of new taxa. Far Eastern Entomologist. 42:1–20.
- Storozhenko SY. 1998. Sistematika, filogeniya i evolyutsiya grilloblattidovykh nasekomykh (Insecta: Grylloblattida) [Systematics, phylogeny and evolution of the grylloblattids (Insecta: grylloblattida).] Dal’nauka. Dordrecht (Boston, London): Kluwer Academic Publishers; p. 517.
- Storozhenko SYu. 2002. Order Grylloblattida Walker, 1914 (= Notoptera Crampton, 1915, = Grylloblattodea Brues et Melander, 1932, + Protorthoptera Handlirsch, 1906, = Paraplecoptera Martynov, 1925, + Protoperlaria Tillyard, 1928). In: Rasnitsyn AP, and Quicke DLI, editors. History of insects. Dordrecht, Boston, London: Kluwer Academic Publishers; pp. 278–284.
- Wipfler B, Letsch H, Frandsen PB, Kapli P, Mayer C, Bartel D, Buckley TR, Donath A, Edgerly-Rooks JS, Fujita M, et al. 2019. Evolutionary history of Polyneoptera and its implications for our understanding of early winged insects. Proceedings of the National Academy of Sciences. 116:3024–3029. doi:10.1073/pnas.18177941.
- Zhang Z, Schneider JW, Hong Y. 2013. The most ancient roach (Blattodea): a new genus and species from the earliest Late Carboniferous (Namurian) of China, with a discussion of the phylomorphogeny of early blattids. Journal of Systematic Palaeontology. 11(1):27–40. doi:10.1080/14772019.2011.634443.
