ABSTRACT
Helcionelloid molluscs are described from the Gislöv Formation (Cambrian Series 2, Stage 4; Vergalian–Rausvian regional stages) in Scania, southern Sweden. Seven species are distinguished,including ‛Pelagiella’ sp., Helcionella antiqua, Davidonia puppis, Davidonia cf. rostrata, Latouchella cf. costata, Stenotheca norvegica, and an indeterminate helcionelloid. The c. 750 specimens were collected from a 30 cm interval with a diverse shelly fauna, encompassing the Ellipsostrenua spinosa Zone. The peak distribution of the molluscs is within the 2–3 mm size class, and there was limited sorting or transport prior to burial. Helcionella antiqua is characteristic of the helcionelloid assemblage, and together with Davidonia puppis, the only molluscs in the assemblage exceeding 10 mm in length. All species except ‘Pelagiella’ display full ontogenetic series providing verification of morphological transitions and direct ontogenetic ties between small helcionelloid juveniles or embryonic shells and their macro adults. Analysis of distributions indicates that the Gislöv assemblage is closely comparable to the Evjevik Member of the Mjøsa area, Norway, while sections in northern Sweden form a separate cluster. The distribution of the shelly assemblage in the E. spinosa Biozone is consistent with a temporal distribution of this fauna, rather than biofacies control.
Introduction
Lower Cambrian biozonation in Baltoscandia is largely based on trilobites, supported by additional records of organic-walled microfossils and trace fossils, particularly for the pre-trilobitic part of the succession (Ahlberg and Bergström Citation1993; Ahlberg et al. Citation1986, Citation2016; Cederström et al. Citation2012, Citation2022; Ebbestad et al. Citation2022; Jensen and Grant Citation1998; McLoughlin et al. Citation2021; Moczydłowska Citation1991, Citation1998; Moczydłowska et al. Citation2001; Nielsen and Schovsbo Citation2007, Citation2011; Palacios et al. Citation2022; Skovsted et al. Citation2021; see further references in these). Traditionally, four trilobite zones have been recognised for the lower Cambrian Series 2, encompassing the Ljubomlian to Kibartian Baltic stages sensu Nielsen and Schovsbo (Citation2011). Stratigraphical ranges of many trilobite taxa and the placement of trilobite zonal boundaries have generally been poorly constrained as a consequence of assemblages with a low taxonomic diversity, few available specimens and scattered outcrops (Martinsson Citation1974; Ahlberg Citation1985; Ahlberg et al. Citation1986; Axheimer et al. Citation2007; Cederström et al. Citation2012; Høyberget et al. Citation2015, Citation2019, Citation2022). Detailed sampling over many years of Cambrian Series 2 strata along the Swedish Caledonian front and Scania in southern Sweden has now allowed a revised biostratigraphy with a subdivision into four interval-zones, starting with the Schmidtiellus mickwitzi Zone, followed by the Holmia kjerulfi, Ellipsostrenua spinosa and Dellingia scanica–Kingaspidoides lunatus zones. The first appearance of the eponymous species marks the base of each zone (Cederström et al. Citation2012; Ahlberg et al. Citation2016, Citation2022).
Helcionelloid molluscs are rare in the traditional lower and middle Cambrian succession of Baltoscandia, and hitherto their biostratigraphical value has been marginal. Less than 10 species are described, with most being known from a single or a few specimens only, and generally represented by large specimens (Høyberget et al. Citation2015). Microscopic phosphatic moulds of molluscs, which commonly represent a taphonomical selection towards juvenile shells (Claybourn et al. Citation2019), are with one exception (Skovsted et al. Citation2021), essentially only known from the Miaolingian of Bornholm, Denmark (Berg‐Madsen and Peel Citation1978; Runnegar and Pojeta Citation1985, Citation1987). The Bornholm material is relatively rich, and only three other areas in Scandinavia show comparable diversity and abundance of macroscopical molluscan taxa and material; the Mjøsa area of southern Norway (Høyberget et al. Citation2015), Scania in southern Sweden (described herein), and the Lake Torneträsk area in northern Lapland, Sweden (Cederström et al. Citation2014).
The present study examines an abundant and diverse fauna of helcionelloid molluscs from the Gislöv Formation, encompassing the lower Cambrian Series 2, Stage 4 (Vergalian – Rausvian regional stages). The c. 750 specimens were collected mainly from a beach exposure near Gislöv on the south-eastern coast of Scania, southern Sweden (). The taxonomic composition of the assemblage is closely comparable to that of the Evjevik Member of the Mjøsa area in Norway (Høyberget et al. Citation2015), and the helcionelloid fauna from these sites is distinctive in its taxonomic composition and stratigraphical distribution. With seven taxa documented, the Gislöv record is the most diverse lower Cambrian assemblage in Baltoscandia. The co-occurring suite of trilobites is widespread in Scandinavia, dominated by ellipsocephalid taxa characterised by the presence of Ellipsostrenua linnarssoni (Kiær, Citation1917). In terms of relative abundance, Helcionella antiqua (Kiær, Citation1917) is characteristic of the helcionelloid assemblage. The extensive material gives a good basis for understanding the ontogeny and ecology of these molluscs. In the context of the associated trilobite assemblage, they contribute to our understanding of the biostratigraphy of this interval.
Geological setting
The stratotype for the Gislöv Formation is situated at Brantevik, just south-west of the town Simrishamn in Scania in south-eastern Sweden (); the unit is here 0.8 m thick (Bergström and Ahlberg Citation1981; Nielsen and Schovsbo Citation2007; Álvaro et al. Citation2010; Cederström et al. Citation2022). The Gislöv Formation is otherwise between 0.7 and 5.7 m thick in the outcrop area in Scania and subsurface in Kattegat and on Sjælland, Denmark (Nielsen and Schovsbo Citation2007). It is absent to the south on Bornholm but may have been reworked (Nielsen and Schovsbo Citation2007). The section studied herein is situated at Gislövshammar, which is about a kilometre south of the type section (Bergström and Ahlberg Citation1981, fig. 8; Cederström et al. Citation2022, fig. 7). Proximal facies of the Gislöv Formation are developed to the WNW at Hardeberga quarry and the distal facies to the ESE at the Brantevik–Gislövshammar coast with local modifications owing to sea floor topography (Álvaro et al. Citation2010; Cederström et al. Citation2022). Throughout its distribution area the Gislöv Formation rests unconformably, and with a sharp boundary, on the Rispebjerg Member of the Læså Formation. This is a regional erosive transgression surface, named discontinuity D1 by Álvaro et al. (Citation2010). A second discontinuity (D2) was recognised within the Gislöv Formation and a third (D3) truncates the formation, separating it from the shale of the Alum Shale Formation or locally the Forsemölla Limestone Bed developed at the base of the Alum Shale Formation. Discontinuity surface D3 has been associated with the Hawke Bay uplift, a global erosive regression expressed through differential uplift of western Baltica (Nielsen and Schovsbo Citation2011, Citation2015), or alternatively as the Baltic Basin hiatus with a more restricted regional and temporal character (Cederström et al. Citation2022).
The Gislöv Formation is developed as two deepening upward sequences (transgressive systems tracts TST1–STS2), bracketed between D1 and D3 and separated by D2 (Álvaro et al. Citation2010). The helcionelloid rich beds occur in the higher part of TST1, below discontinuity surface D2, in an interval corresponding to bed D of Bergström and Ahlberg (Citation1981, fig. 8; Cederström et al. Citation2022, fig. 7; herein). The equivalent is bed B of the second Brantevik section described in Bergström and Ahlberg (Citation1981, fig. 6; Álvaro et al. Citation2010, fig. 3, right column) and bed D of the Brantevik type section (Bergström and Ahlberg Citation1981, fig. 5; Cederström et al. Citation2022, fig. 6). Broadly defined, the lower part of TST1 comprises calcareous quartz-arenites and laminated siltstone, indicative of shallow water facies proximally (Hardeberga area) and deeper facies dominated by deposition of clay and distant shales (Brantevik area). In Scania, the Gislöv Formation encompasses Supersequence 2, sequences LC2:1–LC2:3 of Nielsen and Schovsbo (Citation2011). The sea level rise following D1 was named the Gislöv Drowning by these authors, and the TST1 sequence between D1 and D2 would correspond to LC2:1 and LC2:2.
Material and methods
Linnarsson (Citation1879) described Metoptoma barrandei – later placed within Scenella by Berg-Madsen and Peel (Citation1986) – from a middle Cambrian limestone near Simrishamn in Scania, southern Sweden. In the same paper he noted that the geologist Gustaf von Schmalensee had found fragmentary material of a closely related form at Gislöv further to the south along the coast. More than a century later Bergström and Ahlberg (Citation1981, fig. 14B) figured a specimen of Helcionella? sp. from the lower Cambrian Gislöv Formation at Kvasa north of Simrishamn (). They further reported occurrences from the same stratigraphical interval at the Hardeberga quarry near the city of Lund, from a locality just north of Gislövshammar promontory and from block material near Brantevik. Reinvestigations over several years of these sites by Peter Cederström, Carin H. Nilsson and John Ahlgren have now yielded c. 750 specimens of a diverse helcionelloid fauna from the Gislöv Formation, co-occurring with trilobites and other taxa (Cederström et al. Citation2009, Citation2022).
Figure 1. Geographical and geological maps. A, map of Scandinavia with location of allochthonous sections along the Caledonian front and distribution of surface and subsurface Lower Palaeozoic rocks (modified from Nielsen and Schovsbo Citation2011; Ebbestad et al. Citation2022). B, map of Scania, southern Sweden with Cambrian outcrop areas and localities discussed herein. C, map of the Brantevik–Gislövshammar area with location of the sites sampled for this study and the type section of the Gislöv Formation.
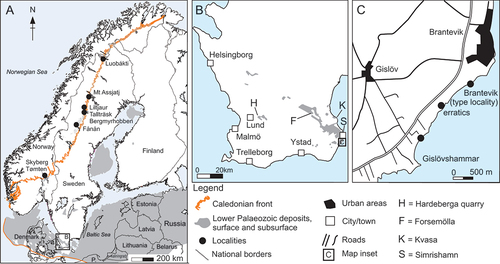
Most of the helcionelloid material, along with a diverse trilobite assemblage, comes from a c. 30 cm interval of laminated siltstone with intercalated calcareous nodules in a beach outcrop at Gislövshammar promontory (). This interval corresponds to bed D of Bergström and Ahlberg (Citation1981, fig. 8; see also Cederström et al. Citation2022, fig. 7) and the ‘Homogeneous to bedded shale facies’ of Álvaro et al. (Citation2010). Fossil remains are typically accumulated and densely packed in calcareous nodules, mostly representing internal moulds, sometimes with recrystallised shell material preserved as calcite. The diverse fauna at this level in Gislövshammar is numerically dominated by trilobites, with Calodiscus lobatus (Hall, Citation1847) being the most common trilobite species (Cederström et al. Citation2009, Citation2022). Bradoriids were described from the type locality at Brantevik, in a work also detailing the distribution of other shelly fossils, albeit no helcionelloid molluscs were recognised at this locality (Dies Álvarez et al. Citation2008, fig. 2). Recently, specimens of the camenellan tommotiid Lapworthella were described from Gislövshammar (Devaere and Skovsted Citation2021).
Figure 2. Stratigraphy and lithology of the Gislövshammar section, with previous trilobite zonation (left) and revised trilobite zonation (right) (modified from Bergström and Ahlberg Citation1981, fig. 8; Cederström et al. Citation2022, fig. 7).
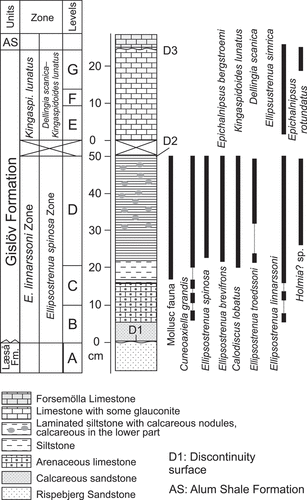
Figure 3. A–F, size frequency distribution of helcionelloid molluscs and Calodiscus sclerites from the Gislövshammar section. A, Helcionella antiqua. B, Stenotheca norvegica. C, Davidonia puppis. D, Davidonia cf. rostrata. E, Calodiscus lobatus (cephala). F, Calodiscus lobatus (pygidia). G, H, rarefaction diversity calculated from the Brantevik, Hardeberga, Gislövshammar and Mjøsa sections with trilobites only (G) and trilobites and helcionelloids combined (H). The x axis has been truncated to save space as the number of specimens exceeds 2200.
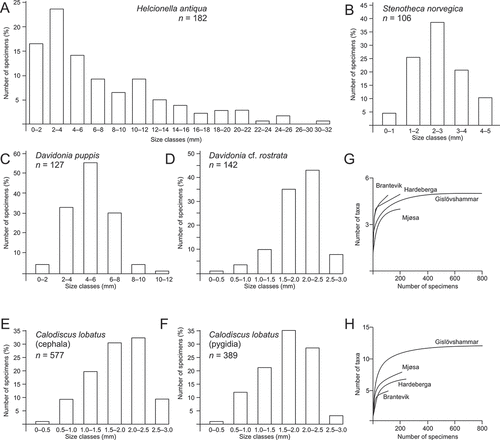
Figure 4. Quantitative visualisation of associations of taxa in Ellipsostrenua spinosa Zone in Norway and Sweden (paired group algorithm and the Dice similarity index). A, cluster analysis of trilobites only, with upper and lower beds of the Mjøsa and Mount Assjatj sections combined. B, cluster analysis of trilobites only, with upper and lower beds of the Mjøsa and Mount Assjatj sections separated. C, cluster analysis of trilobites and helcionelloids, with upper and lower beds of the Mjøsa and Mount Assjatj sections combined. D, cluster analysis of trilobites and helcionelloids, with upper and lower beds of the Mjøsa and Mount Assjatj sections separated.
The lack of helcionelloids in the type section at Brantevik is conspicuous because of the proximity to the similarly developed Gislövshammar section, where they are common. Collecting bias can be ruled out, and Cederström et al. (Citation2022) attributed differences in faunal composition in Scanian localities to different taphonomic conditions. The lack of helcionelloids in the Forsemölla section (see ) is explained by the helcionelloid-bearing level being absent, while the low diversity, low abundance distribution at Hardeberga quarry may be due to the more proximal facies development (Cederström et al. Citation2022). A second, much smaller collection studied herein, derives from a loose block of more compact siltstone found near Brantevik (), but with a similar assemblage to that at Gislövshammar promontory.
The distribution of helcionelloid molluscs and associated trilobites was analysed for six localities; two in Scania, the Mjøsa area in Norway, and three along the Swedish Caledonian front. These sections form the basis for the biozonation and associations discussed herein. A cluster analysis based on presence/absence data for both the samples (R-mode) and the variables (taxa) (Q-mode) was performed using the Dice and the Jaccard similarity indexes and paired group algorithm (Hammer and Harper Citation2006); these indexes yielded only different branch lengths. To assess the taphonomic influence and assemblage composition, size frequency distribution was calculated for the four most common helcionelloid taxa in the Gislövshammar section and compared to the distribution of the co-occurring trilobite Calodiscus lobatus. Total diversity relative to collection size was tested using individual rarefaction, and diversity indices were calculated to compare the diversity and abundance in the various sections. All calculations were made using Microsoft Excel© and PAST 3.19 (Hammer et al. Citation2001). The width of the protoconchs was measured at a shell length of 100 μm, following Nützel et al. (Citation2006). Most apices are laterally compressed, which would yield different widths if these were taken from anterior or lateral aspects. Measurements were therefore consistently made of the apex in lateral view, as this was most feasible. Only a few reliable measurements could be made, and only on macrophotos. The sizes are therefore estimates, and size variations cannot be calculated. Specimens were coated with ammonium chloride prior to photography.
Helcionelloid distribution and diversity
Regional distribution
The helcionelloids discussed herein are mainly known from Scania in southern Sweden and Mjøsa in southeastern Norway, within the Ellipsostrenua spinosa Zone. In Scania, all helcionelloid specimens occur in centimetre-sized limestone nodules in the 30 cm thick shale facies of the Gislöv Formation. Co-occurring trilobites are Ellipsostrenua linnarssoni (Kiær, Citation1917), E. spinosa (Ahlberg & Bergström, Citation1978), E. brevifrons Cederström et al., Citation2022, E. troedssoni Cederström et al., Citation2022, Cuneoaxiella grandis (Ahlberg & Bergström, Citation1978), Holmia? sp., and Calodiscus lobatus (Hall, Citation1847). In Norway, helcionelloids occur in the Evjevik Member of the Ringstrand Formation at the Skyberg Farm locality in the Mjøsa area. The member is 1.1 m thick with two bioclastic silty limestone beds bracketing a siliciclastic mudstone bed. The lower limestone bed is c. 30 cm thick with Helcionella antiqua, Stenotheca norvegica, Latouchella cf. costata, and Davidonia puppis. Associated trilobites are Ellipsostrenua gripi (Kautsky, Citation1945) and E. linnarssoni, both of which in the Mjøsa area are confined to the Evjevik Member, and Kjerulfia lata, Holmia cf. lapponica and H. kjerulfi. Note that Kjerulfia lata is known only from two well-preserved cephala, one from the classical Tømten Farm and the other from Skyberg Farm (; Nikolaisen Citation1986; Høyberget et al. Citation2015). The upper limestone bed is c. 20 cm thick, with Helcionella antiqua, Davidonia puppis and the unnamed conical taxon. Associated trilobites are E. gripi, E. linnarssoni, E. spinosa, E. sularpensis (Ahlberg & Bergström, Citation1978) and Calodiscus lobatus. Only Helcionella antiqua occurs in the mudstone between the lower and upper limestone beds, together with E. gripi, E. linnarssoni, E. spinosa, and H. cf. lapponica. In northern Sweden Helcionella rugosa was described from limestone lenses in the upper part of the siliciclastic mudstone beds of the Grammajukku Formation in the Mount Assjatj section (Kautsky Citation1945; Høyberget et al. Citation2015) occurring together with the trilobite E. gripi. The zonal trilobites E. linnarssoni and E. spinosa occur in limestone lenses at c. 8 m below this in the same section (Cederström et al. Citation2012, fig. 3). Additional helcionelloids were reported from the Grammajukku Formation at Mt Luobákte in northern Sweden (Cederström et al. Citation2014) but are so far not studied. A number of outcrops in Scania and along the Swedish Caledonian front contain the typical trilobite association of the Ellipsostrenua spinosa Zone, but despite a generally high collecting effort helcionelloid taxa have so far not been encountered. Part of the reason for this lies in the limited size of outcrops and sections, especially along the Caledonian front, but also because limestone may be missing. In Scania, the highly variable occurrences in nearly identical and close outcrops suggest even a taphonomic effect (Cederström et al. Citation2022).
Table 1. Distribution and number of specimens of helcionelloid molluscs and associated trilobites at six Scandinavian localities (see ), and associated diversity calculations for each locality. Data on trilobites from Cederström et al. (Citation2012, Citation2022).
Taphonomy
The size distribution of the four most common helcionelloids was analysed for the Gislövshammar section to evaluate the effect of post-mortem transportation and taphonomy on the composition of the assemblage (). The data were compared with the size frequency distribution of cranidia and pygidia of the trilobite Calodiscus lobatus (, re-measured here by Cederström, but see also Cederström et al. Citation2009).
Size counts for both Stenotheca norvegica (n = 106) and Davidonia puppis (n = 142) show a normal distribution (peak distribution in the 2–3 mm size classes). Size frequency distribution for Helcionella antiqua shells (n = 182) is right (positively) skewed with a possible second peak in the 10–12 mm size class (peak distribution in the 2–4 mm size classes). Size frequency distribution for Davidonia cf. rostrata (n = 142) is left (negative) skewed (peak distribution in the 2–2.5 mm size classes). A similar negative distribution is seen for both cranidia (n = 577) and pygidia (n = 389) of Calodiscus where the peak distribution is near the 2.0 mm size class.
Although the shape of the histograms varies from right-skewed, to normal and left-skewed, the results from the size frequency distribution support the conclusion by Cederström et al. Citation2009 that some degree of sorting and transport has effected the death assemblage. The depositional environment of the Gislöv Formation was calm (general deepening trend), and the helcionelloids occur in limestone nodules in a thin homogenous silty mudstone facies. However, most of the helcionelloid molluscs, as well as Calodiscus, are less than 3 mm in length or width, and were susceptible to winnowing even by weak hydrodynamic forces. A limited degree of sorting could explain the normal distribution for S. norvegica and D. puppis.
The positive distribution among the Helcionella specimens, on the other hand, seems at first to indicate a different taphonomic process. In marine populations small individuals will usually constitute the largest contribution to the death assemblage (high infant mortality), yielding a positively skewed distribution (Boucot Citation1953; Cadée Citation1982; Gosselin and Qian Citation1997). While this may suggest an in situ assemblage and limited sorting, this interpretation is not considered likely for the Gislöv helcionelloid distribution. An important aspect of the Helcionella distribution is that some specimens are 10 times larger than the other hellcionelid mollusc species and Calodiscus specimens in the same assemblage. In modern environments large shells are generally subjected to rapid fragmentation, with weak shells being preserved more locally, while larger, more robust shells have a broader spatial and temporal mixing in the sediment (Kowalewski Citation1997). Sorting may still have controlled the distribution of Helcionella specimens through accumulation of the smaller specimens, which would yield a positive size distribution when coupled with a selective loss of large specimens.
The negative distribution of Davidonia cf. rostrata and Calodiscus may reflect a larger input of older individuals for these taxa, although there are several factors that could produce such a distribution including sorting and diagenesis (Cadée Citation1982). An important observation is that both taxa are among the smallest in the assemblage, reaching no more than a maximum of c. 3 mm in length or width. Although numerous specimens have been collected, embryonic or possible larval specimens are lacking and, in general, the earliest sub-millimetre juvenile shells and embryonic shells are conspicuously missing in the Gislöv Formation. Such remains are frequently extracted as phosphatic moulds or phosphatic replacements, a type of preservation that is nearly absent in the Gislöv Formation, possibly due to the particular diagenetic processes. Most likely, the smaller size classes in these taxa are therefore under-represented, yielding an artificially negative distribution observed among these very small taxa. In fact, if the distributions of all measured taxa are considered, it is apparent that the peak distribution is within the 2–3 mm size class regardless of skewedness (some variation due to resolution of size class bins). Thus, the lack of separately preserved protoconchs or juvenile shells, preferential sorting of the 2–3 mm size classes and selective loss of larger specimens could explain the variation of the size frequency histograms within the thin facies interval of the Gislöv Formation.
Diversity and abundance
Geyer et al. (Citation2019) found that the abundance of helcionelloid molluscs from the Tannenknock Formation of the lower/middle Cambrian boundary succession in the Frankenwald area in southern Germany, was controlled by winnowing and size sorting. Deposition took place in a wave dominated environment with fragmentation of the shells, albeit with limited transportation (Geyer et al. Citation2019). The different facies at the Scanian and German sites suggest that the abundance and diversity in Scania was controlled by other factors, where weak sorting may mostly have affected the smaller sized individuals only. The Gislöv Formation is relatively thin and represents a short period of facies stability, with the assemblage possibly reflecting within-habitat time averaging (sensu Kidwell Citation1998). A death assemblage will nevertheless always have been effected by time-averaging, taphonomy and diagenesis (Kidwell and Bosence Citation1991) but may still capture the relative abundance well (Kidwell Citation2001, Citation2002). The abundance of taxa seen in the Gislövshammar section () may therefore closely reflect the relative abundance of the shelly fauna during the time of deposition. A simple test of diversity using rarefaction further suggests that the death assemblage collected in the Scanian sections also captures the diversity of shelly taxa fairly well ().
The Gislövshammar section is the best sampled of the sections discussed in this study, with c. 4000 specimens yielding 12 taxa (trilobites and helcionelloids) (); the abundance of Calodiscus from Fånån is an exception. The number of specimens collected in the Mjøsa section is comparable to that collected from the Hardeberga and Brantevik sections in Scania, and all three have a high number of taxa present. However, the Mjøsa samples are overwhelmingly dominated by E. linnarssoni, which despite the high total number of taxa gives a low diversity index compared to the other sections (). The high number of E. linnarssoni specimens in the Brantevik section also gives a lower diversity variable, but this is offset by a comparable high number of specimens among the other taxa. These patterns probably reflect a collection bias of the Mjøsa and Brantevik sections, reinforcing the assumption that the Gislövshammar section is the best sampled and that it captures relative diversity and abundances well.
Cederström et al. (Citation2022) suggested that taphonomic factors were behind what was considered a relatively low diversity (of trilobites) in the Scanian sections. With helcionelloids and other shelly fauna included, the overall diversity of shelly organisms and their individual abundance may instead be considered reasonably well represented in the Gislövshammar section. In the Mjøsa area part of the helcionelloid diversity is clearly missing, and typically the rare species are found only at Gislövshammar, whereas the diversity of trilobites at the two sections, as well as the Brantevik section, is similar.
Small juvenile shells, large adults and protoconchs
It is clear that many phosphatic steinkerns of tiny early Cambrian molluscs (preserved through preferential taphonomic selection) represent juveniles of larger adults (Dzik Citation1991; Peel Citation1991b; Martí Mus et al. Citation2008; Creveling et al. Citation2014; Dattilo et al. Citation2016; Jacquet and Brock Citation2016; Claybourn et al. Citation2019). Nevertheless, presumed adults of early and middle Cambrian molluscs generally appear diminutive in size (1–3 mm), while macro-molluscs (larger than 10 mm) are less common during this time (Runnegar and Jell Citation1976; Runnegar Citation1983; Runnegar and Pojeta Citation1985; Chaffee and Lindberg Citation1986; Hazprunar Citation1992; Jacquet and Brock Citation2016).
An obstacle to our understanding of the phylogenetic relationship between size-restricted juvenile phosphatic mollusc fossil and their potential adults has been the lack of preserved earliest growth stages in the large specimens. Studies by Martí Mus et al. (Citation2008) and Jacquet and Brock (Citation2016) found direct ontogenetic ties between small helcionelloid juveniles or embryonic shells and their macro adults, which is further demonstrated in the present material. Only Davidonia puppis and Helcionella antiqua are macro-molluscs among the Gislöv taxa, exceeding 10 mm in length, but both preserve specimens of all ontogenetic sizes, which allows verification of the ontogenetic transitions. Another Gislöv taxon that bridges the gap between small juvenile teleoconchs and small adult teleoconchs is D. cf. rostrata, which reaches a maximum length of c. 3 mm. The largest phosphatic steinkern of D. rostrata known is c. 1.3 mm in length (Claybourn et al. Citation2019), which is comparable in size with the smallest range of preserved specimens from the Gislöv Formation but less than half of the presumed adult maximum size of this taxon.
With the exception of Stenotheca norvegica and possibly Davidonia cf. rostrata, the steinkerns in the Gislöv material do not show distinct demarcations in the apical regions that could convincingly represent a transition from the protoconch to the teleoconch. In Davidonia puppis the c. 600 µm wide apex is set off by a comarginal constriction, although this may be a groove marking the development of the first rib. Generally, the apical regions of these taxa suggest the presence of a very large protoconch (230–330 µm in width), indicating non-planktotrophic (lecithotrophic) larval development. Larvae that feed (planktotrophic larval development) generally have a small two-stage protoconch (the embryonic shell or protoconch I, and the larval shell or protoconch II) in the size range of 60–120 µm. Non-planktotrophic larvae, on the other hand, do not feed but rely on an egg with a large supply of yolk and have larger protoconchs up to 300 µm or more in size. The large size of the protoconchs among the Gislöv helcionelloids is consistent with the suggestion that planktotrophic larval development first evolved in the latest Cambrian or Early Ordovician, with lecithotrophic larval development prevailing among the earliest molluscs (Nützel et al. Citation2006, Citation2007; Runnegar Citation2007; Nützel Citation2014 and references therein). Parkhaev (Citation2014) showed that protoconch size in some Cambrian molluscs ranges between 80 and 400 µm, which would not preclude the presence of small planktotrophic taxa, although Jacquet and Brock (Citation2016) pointed out that the presence of a larval shell (protoconch II) cannot be convincingly demonstrated in the material described by Parkhaev (Citation2014). Yet, variation in protoconch sizes in both planktotrophic and non-planktotrophic molluscs, as well as difficulties in distinguishing embryonic and larval shells in fossil material in general, hinders understanding of the distinction and timing of dispersal strategies among early molluscs. It has been suggested that lack of planktotrophy among early Cambrian molluscs could be reflected in a high degree of endemism as the non-planktotrophic larvae have lower dispersal potential (Chaffee and Lindberg Citation1986). However, many Cambrian molluscs are widespread, Davidonia rostrata being a case in point, which would support planktotrophic dispersal. Already Lochman (Citation1956) commented that Helcionella subrugosa showed a widespread distribution in the Dyerian (Cambrian Stage 4) of New York State, USA, comparable to that of planktonic forms; it was attributed to broad ecological tolerance. Gubanov (Citation1998, Citation2002) and Gubanov et al. (Citation2004) suggested instead that a wide dispersal of non-planktotrophic Cambrian molluscs could be explained by geographic proximity and distribution within similar climatic zones. The biogeographic distribution of Cambrian trilobites indicates that climatic belts were perhaps not essential to the dispersal pattern, but rather geographical distances and oceanic circulation patterns were important factors (Álvaro et al. Citation2013).
Correlation and biozonation
Baltoscandian biostratigraphy
Nielsen and Schovsbo (Citation2011) revised the lower Cambrian Vergalian–Kibartian biozonation (Cambrian Series 2, Stage 4) in Scandinavia and combined the traditional Holmia kjerulfi Zone of Bergström and Ahlberg (Citation1981) with the lower part of the Ellipsostrenua linnarssoni Zone into an informal Holmia kjerulfi–‘Ornamentaspis’ linnarsoni Assemblage Zone. This was followed by an informal Comluella?–Ellipsocephalus lunatus Assemblage Zone, incorporating the upper part of the traditional linnarssoni Zone and the subsequent undefined zone. Cederström et al. (Citation2012) retained a distinct H. kjerulfi Zone, followed by an Ornamentaspis? linnarssoni Zone, with the base defined by the first occurrence of the eponymous species. The latter zone was subdivided into informal lower and upper subzones, a division outlined already by Bergström and Ahlberg (Citation1981, p. 195). Høyberget et al. (Citation2015) adopted this zonation, but the upper range of the linnarssoni Zone was defined by the first occurrence of Ellipsostrenua lunatus. Ahlberg et al. (Citation2016) advocated a substantially simplified zonation for the Cambrian Series 2 in Scandinavia, consisting of four interval zones; the Schmiditelleus mickwitizi, Holmia kjerulfi, Ellipsostrenua spinosa and Chelediscus acifer zones, defined by the first occurrences of the eponymous species. Cederström et al. (Citation2022) revised the taxonomy of Scandinavian ellipsocephalids and adopted the Dellingia scanica–Kingaspidoides lunatus Zone instead of the C. acifer Zone. The D. scanica–K. lunatus Zone largely encompasses the Comluella?–Ellipsocephalus lunatus assemblage Zone proposed by Nielsen and Schovsbo (Citation2011).
The zonal taxa defining the new scheme were chosen because they were “distinctive, easily recognisable and, except for S. mickwitzi, known to be widespread” (Ahlberg et al. Citation2016, p. 16). It is worth noting that Ellipsostrenua spinosa only partly fulfils this criterion. Cederström et al. (Citation2011, Citation2012) found E. spinosa from the lower and middle part of the traditional E. linnarssoni Zone in several Scandinavian localities. It is a species with a distinct morphology and a relatively restricted stratigraphical range. The species is generally associated with the usually abundant E. linnarssoni and E. gripi in the Mjøsa area, Jämtland, and along the Caledonian front sections. Ellipsostrenua gripi is absent in Scania, and here, E. spinosa is instead associated with E. sularpensis, which may be a secondary index fossil for the E. spinosa Zone (Cederström et al. Citation2022); E. sularpensis is also found in the upper limestone bed of the Evjevik Formation in Mjøsa. However, in the Mjøsa area only two specimens of E. spinosa have ever been found, and in Scania this taxon is the second rarest species ().
The argument for replacing the abundant and widespread E. linnarssoni as the zonal fossil also partly stems from a supposed longer downwards range of E. linnarssoni than previously known and partial overlap with the underlying Holmia kjerulfi (Cederström et al. Citation2022). Contrary to this claim, there is in fact a very close correspondence of the ranges of E. linnarssoni and E. spinosa in sections, where they co-occur within centimetres of each other, and in addition, there is no firm evidence that the upper range of H. kjerulfi overlaps with the lower range of E. linnarssoni. The single known co-occurrence of these two in the Skyberg section in Lake Mjøsa is considered fortuitous, as the fragmented H. kjerulfi specimens are most likely reworked; other trilobite sclerites, brachiopods and helcionelloids in the same bed are well-preserved (Nikolaisen Citation1986; Høyberget et al. Citation2015). Note that Kjerulfia lata is only known from two well-preserved cephala, one from the locality Tømten farm and the other from Skyberg Farm (; Nikolaisen Citation1986; Høyberget et al. Citation2015).
Furthermore, with the lower occurrence of a zonal fossil defining a zone, the upper range of Holmia kjerulfi does not affect the definition. Thus, the closely corresponding lower ranges of E. spinosa and E. linnarssoni, the widespread and abundant occurrences of the latter, as well as the scarcity of E. spinosa in some sections, suggest that the lower range of E. linnarssoni may (still) be an equally good lower zonal marker for this interval.
Biozonation
Various trilobite taxa characterise the Ellipsostrenua spinosa Zone from Scania in the south and along the Scandinavian Caledonides to Lapland in the north (Cederström et al. Citation2022). Trilobites are known from several sections (Cederström et al. Citation2009, Citation2011, Citation2012), while the distribution of helcionelloid taxa is more restricted and so far only associated with the carbonate-rich facies. Although the number of taxa is low, a quantitative visualisation of association distributions using 18 species of trilobites and helcionelloids is attempted, and it shows a pattern somewhat similar to that outlined by Cederström et al. (Citation2022). When only trilobites are analysed, a northern Mjøsa–Lapland association and a southern Scania association are evident (). Typical taxa of the northern cluster are Kjerulfia lata, Epichalnipsus kullingi and Ellipsostrenua gripi. Although widely distributed, both E. linnarssoni and S. spinosa are associated with a northern group, reflecting their wide occurrence in the northern sections. The southern cluster includes Cuneoaxiella grandis, E. brevifrons, E. troedssoni, E. sularpensis, and Caldiscus lobatus. With combined data from both the upper and lower beds, the Mjøsa area is associated with the northern cluster. With a distinction of two beds for the Mjøsa and Mount Assjatj sections, the taxa of the upper bed in the Evjevik Member in Mjøsa instead cluster with the southern Scania association (). This is due to the presence of Ellipsostrenua sularpensis in both areas and reflects the pattern demonstrated by Cederström et al. (Citation2022).
The same variables plotted with helcionelloids included add some nuance to the distribution. Without a distinction of two beds for the Mjøsa and Mount Assjatj sections, the Mjøsa section is associated with a southern Scania association, while the remaining northern sections form a northern cluster (). Interestingly, the Mjøsa section still plots with a southern Scania association, more precisely the Gislövshammar section, even when the distribution in the two beds is differentiated (). In both these cluster variations, C. grandis and E. brevifrons are basal to the southern Scanian cluster, as they are hitherto only found in Scania. The upper records in Mount Assjatj have too few taxa and plot basal to both the northern and southern clusters (). The addition of the helcionelloids emphasises the close resemblance between the assemblages of the Evjevik Member and the Gislöv Formation, as represented by the Gislövshammar section, as noted already by Høyberget et al. (Citation2015). Furthermore, detailed range charts of trilobites in key sections in Sweden and Norway (Cederström et al. Citation2012; Høyberget et al. Citation2015, Citation2022) demonstrate this interval.
The spatial distribution of the Gislöv Formation deposits in Scania suggests that the Brantevik section represents deposition in proximal areas, while the Gislövshammar-Brantevik area has more distal deposits (Álvaro et al. Citation2010; Cederström et al. Citation2022). Local seafloor topography played an important role, and in the Forsemölla section the helcionelloid-bearing level has been cut out (non-deposition and/or erosion on a structural high) (Cederström et al. Citation2022). shows the correlation of six sections from Scania in the south to Lake Storuman in the north. The much thicker section at Mount Assjatj is not shown due to lack of space. At the type locality, and at Gislövshammar, the E. spinosa Zone is about 50 cm thick, although the basal contact is an unconformity with erosion and/or non-deposition of the lower zonal interval. Cederström et al. (Citation2022, fig. 4) suggested that nearly all of the LC2-1 sequence of Nielsen and Schovsbo (Citation2011) was cut out. A discontinuity surface (D2) is also found directly above the helcionelloid level. In Mjøsa the zone is slightly more than 100 cm thick. The bioclastic limestone of the Evjevik Member indicates reworked material (Høyberget et al. Citation2015) suggesting that even here some sediment may be missing although there is no obvious gap in the section. In the northern sections at Tallträsk and Jiltjaur, the zonal taxa occur in a 10–30 cm interval. In the composite Storuman area section, the zonal interval may be 130 cm thick. Gaps are not evident in any of these limited outcrops, which all have homogenous mudstone or siliciclastic mudstone preserved but without limestone in this interval (Cederström et al. Citation2012). In the northernmost section discussed here, at Mount Assjatj, E. linnarssoni and E. spinosa are only found in a thin limestone bed in an otherwise homogenous and thick sequence of siliciclastic mudstone. Both E. gripi and H. antiqua are found c. 8 m higher in the section, which suggests that here the E. spinosa Zone encompasses at least an 8 m interval.
The Gislöv Formation is generally a thin and condensed sequence encompassing two trilobite zones separated by an unconformity. Thus, both the lower and upper extension of the E. spinosa Zone may be truncated in Scania. The helcionelloid taxa occur in the upper part of the zone in Scania but throughout the range of the zone in Mjøsa. The only helcionelloids in the northern sections also occur high in the zone, in an otherwise seemingly continuous succession, but too few taxa are known to firmly establish this distribution. Except for K. lata, no taxa range across zonal boundaries between the H. kjerulfia and E. spinosa zones, which could otherwise be of help in determining the inevitable diachronism in the local range appearance and disappearance. The range of K. lata is consistent in sections where it is found (). Overall the diversity and abundance of the shelly assemblage in the E. spinosa Biozone are well documented and, with some variation, widely distributed, and confined temporally with the same taxa having comparable short ranges in several sections. This is consistent with a temporal distribution of this fauna, rather than biofacies. Within the narrow interval in question and the short temporal taxon range also in the condensed sections, diachronism is considered negligible. The two calcarenite beds of the Evjevik Member of the Ringstrand Formation were considered to represent sequences LC2-3 and LC2-4, respectively (Nielsen and Schovsbo Citation2011; Cederström et al. Citation2022), which is slightly higher than what is preferred herein.
Systematic palaeontology
The Swedish material is housed in the palaeontological collections at the Museum of Evolution, Uppsala University, Sweden (PMU) and the Geological Survey of Sweden, Uppsala (SGU). The nomenclature of measurements used in the descriptive part follows Jacquet and Brock (Citation2016).
Class Helcionelloida Peel, Citation1991a
Order Helcionelloida Geyer, Citation1994
Remarks
The preservation of the material presented here does not allow wider conclusions concerning the higher phylogenetic position of these molluscs. In addition to overall morphology, traits like functional morphology, muscle scars, and shell structures have been used to try to solve the Gordian knot of helcionelloid mollusc phylogeny (see for instance Peel Citation1991a, Citation1991b; Geyer Citation1994; Parkhaev Citation2002; Vendrasco et al. Citation2010; Vendrasco and Checa Citation2015; Thomas et al. Citation2020; Landing et al. Citation2021), but consensus is not imminent. Basic morphological delimitation is often exceedingly difficult with differences often defined by heterogeneous characters in specimens that may have one of the distinguishing traits mentioned (morphology, shell structure, muscle scars) but seldom a combination of these. Associations of micro- and macro-conchs are rare. A broader assessment of the higher phylogeny of these early molluscs is therefore beyond the scope of this paper. Some recent phylogenies favour affinity of most of the helcionelloid molluscs discussed herein with gastropods (e.g. Parkhaev Citation2002; Citation2017a, Citation2017b; Thomas et al. Citation2020). Alternatively, they have been considered to be untorted endogastric or exogastric molluscs, with discussions given by Vendrasco et al. (Citation2010), Ponder et al. (Citation2020) and Peel and Kouchinsky (Citation2022). Taxa described in the present material are treated as representing endogastrically coiled, untorted molluscs (unranked) of the Class Helcionelloida in the original sense of Peel (Citation1991a, Citation1991b).
Family Pelagiellidae Knight, Citation1956
Genus Pelagiella Matthew, Citation1895
Type species
By original designation of Matthew (Citation1895, p. 131), Cyrtolites atlantoides Matthew, Citation1893, p. 94, pl. 16, fig. 8, from the Hanford Brook Formation (probably the uppermost Cambrian provisional Series 2, Stage 4) at Hanford Brook, St. John, New Brunswick, Canada.
Remarks
Pelagiella is one of the more widespread small molluscs in the Cambrian. Parkhaev (Citation2001, Citation2004) listed and revised synonyms of 26 species from the lower and middle Cambrian worldwide and several others have been recognised since then. Although a wealth of morphological details are known, the supra-generic placement of Pelagiella has been disputed (see discussion in Landing et al. Citation2021). Recently a sharp distinction was made between the type species and other ‘pelagiellid’ taxa (sensu lato), where Pelagiella atlantoides was considered a gastropod and the only remaining taxon in the Pelagiellidae Knight Citation1956; (Landing et al. Citation2021). Other former pelagiellids with subtriangular to triangular apertures were referred to as polychaetes based on the superbly preserved chaetae fan-arrays in P. exigua Resser & Howell, Citation1938. This species was described as a gastropod by Thomas et al. (Citation2020) but placed in Pseudopelagiella by Landing et al. (Citation2021; see also Mghazli et al. Citation2023), a genus tentatively assigned to encompass other morphologically similar ‘pelagiellids’. Horný (Citation1964) introduced Cambretina and Costipelagiella for pelagiellids from the same horizon in the middle Cambrian of Bohemia, but subsequently these have been regarded as junior synonyms of Pelagiella (Kouchinsky et al. Citation2011, Citation2015; Thomas et al. Citation2020).
The two recently proposed supra-generic emendations are not embraced here. For the purpose of this study, they do not diminish the potential stratigraphical value of these shelly fossils which are referred here to ’Pelagiella’ sp. to indicate taxonomic uncertainty. For practical purposes, the taxon is included within the helcionelloid section and described as dextrally coiled.
‛Pelagiella’ sp.
()
Material
Mostly internal moulds, in total 35 specimens from Gislövshammar promontory, of which four are figured here (PMU 37517, 37518/1, 37520, 37524).
Description
Conch with 1.5 widely expanding whorls and apex sunken beneath the level of the last whorl. Height of the conch is about 80% of width, the largest specimen measures just over 2 mm in width. Upper surface, being slightly convex, slopes markedly inwards to deep sutures. Sutures are caused by the whorls barely touching on the internal mould due to the missing shell material. Outer surface of whorl well rounded and base of the shell broadly expanded. Protoconch is rounded and obtuse but not set off from the rest of the shell. Aperture radial, general shape unknown in detail but seemingly broadly oval. Inner margin straight, lip and base broad, reflected (as seen by groove on internal mould). Ornamentation on the upper surface consists of gently prosocyrt comarginal lines only.
Remarks
This species is comparable, but smaller than the Spanish species P. crassa Geyer, Citation1986 and this species also has a greater rate of expansion; it is similar in size and shape to P. atlasensis, Citation1986 from Morocco and P. subangulata (Tate, Citation1892) from Australia. The Swedish species differs from these three, and many other forms, by a lower rate of expansion and the low translation down the axis. The Australian P. subangulata seems to have a similar distinct sloping upper surface towards the axis as the Swedish form. Gently prosocyrt growth lines are preserved on the dorsal side in one external mould (). In species like P. subangulata from Australia and Greenland (see Skovsted Citation2004), the growth lines are more strongly prosocline.
Hitherto, P. kreklingensis Kobayashi, Citation1939 and P. broeggeri Grönwall, Citation1902 from the middle Cambrian of Norway and Denmark are the only known species of ‘Pelagiella’ in Scandinavia (Grönwall Citation1902; Kobayashi Citation1939; Runnegar and Pojeta Citation1985). The tentative identification of ‘Pelagiella’ in the Tremadocian of Norway and Sweden by Yochelson (Citation1962) is unresolved. The taxon was not identified by Høyberget et al. (Citation2015) in the material from the Evjevik Member in Norway.
Family Helcionelloidae Wenz, Citation1938
Genus Helcionella Grabau & Shimer, Citation1909
Type species
By original designation of Grabau and Shimer (Citation1909, p. 607), Metoptoma? rugosa Hall, Citation1847, p. 306, pl. 83, fig. 6, from the lower Cambrian near Troy, New York State, USA. See Knight (Citation1941) and Høyberget et al. (Citation2015) for discussion concerning the nomenclatural history of Hall’s species.
Remarks
The lower Cambrian Ilsanella Missarzhevsky, Citation1981 from Russia was considered a junior synonym of Helcionella by Geyer et al. (Citation2019), who argued that the emended diagnosis of Ilsanella provided by Høyberget et al. Citation2015 failed to adequately distinguish the type species of the two genera. The diagnosis given by Jacquet and Brock (Citation2016) was subsequently modified by Geyer et al. (Citation2019) but the modifications serve only to diffuse the distinction between Ilsanella and Helcionella suggested by the former authors. The diagnosis of Helcionella presented by Jacquet and Brock (Citation2016) is adopted here and consequently also their concept of Ilsanella.
Figure 5. Tentative correlation of the Ellipsostrenua spinosa Zone in Sweden and Norway. The range of helcionelloid molluscs indicates a strong correlation between the Gislövshammar and Mjøsa sections. The Mount Assjatj section is omitted for reasons of space as the interval is several metres thick compared to the centimetre scale for the other sections. See. for location of sections. Data added from Cederström et al. (Citation2012, Citation2022) and Høyberget et al. (Citation2015)
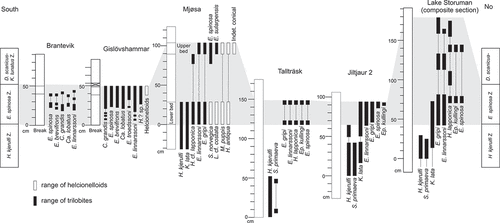
Figure 6. ‘Pelagiella’ sp. A, external mould showing ornamentation (PMU 37517). B, C, dorsal and dorsal oblique view of internal mould (PMU 37518/1). D, internal mould (PMU 37524). E, F, lateral oblique and lateral views of internal mould (PMU 37520). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.
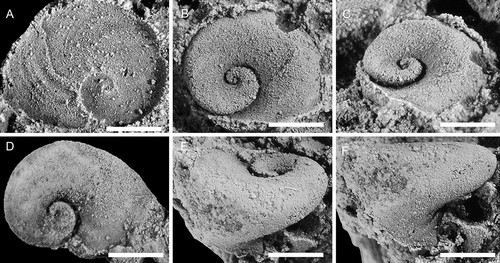
Figure 7. Helcionella antiqua (Kiær, Citation1917), with specimens arranged in an ontogenetic series. A, F, dorsal and left lateral view (PMU 37554). B, G, dorsal and posterior views (PMU 37555). C, H, dorsal and left lateral views (PMU 37556). D, E, I, dorsal,left lateral and posterior views (PMU 37557). J, K, L, dorsal, posterior oblique and detail of apex views (PMU 37558). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bar in D is 1 mm, and is common to all images.
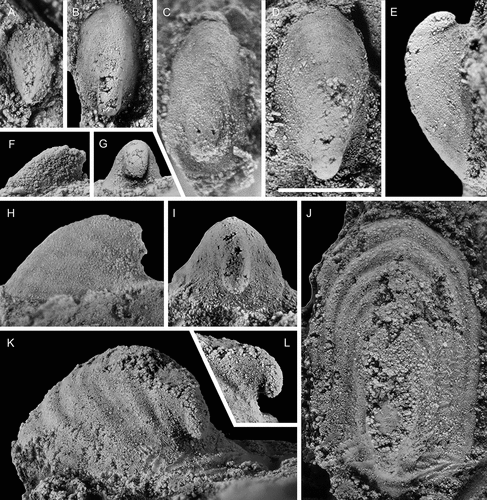
Figure 8. Helcionella antiqua (Kiær, Citation1917), with specimens arranged in an ontogenetic series. A–D, dorsal, left lateral, anterior and anterio-dorsal oblique views (PMU 37559). E, dorsal view (PMU 37561). F, dorsal view (PMU 37562/1). G–I, dorsal, posterior and detail views (PMU 37560). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bar in F = 1 mm and is common to all images.
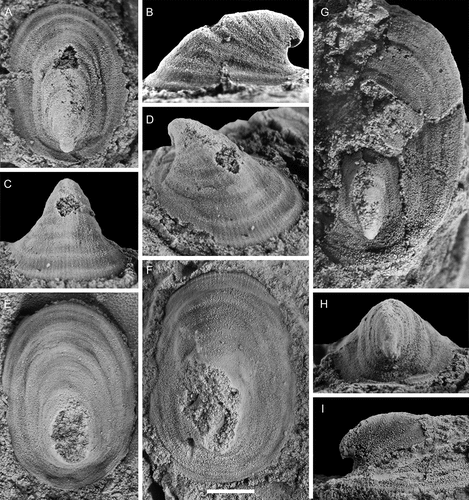
Figure 9. Large specimens of Helcionella antiqua (Kiær, Citation1917). A–C, dorsal, posterior and right lateral views (PMU 37563). D, G, dorsal and right lateral views of large specimen (PMU 37564/1) next to a medium sized specimen (PMU 37564/2). E, F anterior and dorsal views (PMU 37565). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bar in B = 10 mm and is common to all images.

Figure 10. Specimens from a loose block collected between Brantevik and Gislövshammar, Scania, southern Sweden. A–E, Helcionella antiqua (Kiær, Citation1917). A–C, dorsal, posterior oblique and right lateral views (PMU 38127). D, E, right lateral and dorsal views (PMU 38128). F, Davidonia puppis (Høyberget et al., Citation2015). Left lateral view (PMU 38133). From the Ellipsostrenua spinosa Zone. Scale bars = 5 mm.
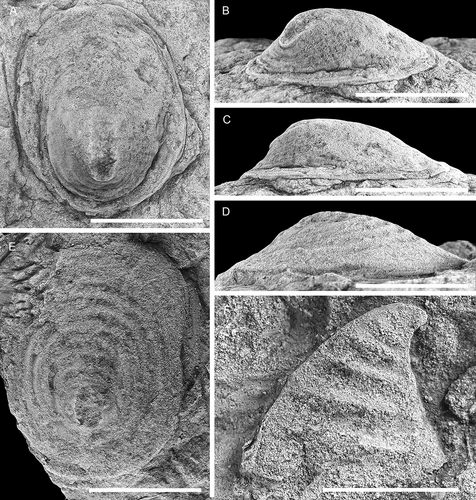
Figure 11. Comparison between Scenella barrandei (Linnarsson, Citation1879) and Helcionella antiqua (Kiær, Citation1917), showing dorsal, anterior, and lateral views and detail of shell ornamentation. A, B, E, F, Scenella barrandei, holotype (SGU 4532a), from the middle Cambrian Exsulans Limestone bed (Paradoxides paradoxisimus Zone) of the Alum Shale Formation at Kiviks-Esperöd in Scania, southern Sweden. C, D, G, H, Helcionella antiqua (PMU 37777) from the Ellipsostrenua spinosa Zone at Hardeberga, Scania, southern Sweden. Scale bars for A–E, H = 5 mm, scale bars for F, G = 1 mm.
Helcionella antiqua (Kiær, Citation1917)
(, 10A–E)
See Høyberget et al. (Citation2015) for a synonymy list.
Material
More than 250 specimens are available, mostly preserved as internal moulds, but some specimens retain some recrystallised shell or are composite, with ornamentation superimposed on the external mould. For this study 16 specimens were illustrated, PMU 37554–PMU 37562/1, 37563, 37564/1, 37564/2 and 37565 from Gislövshammar promontory, PMU 37777 from Hardeberga and PMU 38127 and 38128 from loose material at the beach near Brantevik.
Description
(Emended from Høyberget et al. Citation2015). Shell patelliform with low profile (H:L ration 0.4–0.5), aperture subrectangular in outline with width being ~50% of length in early ontogeny and ~80% of length in large specimens. Apex close to posterior margin in early ontogeny and at about posterior 1/5 of length in large specimens. Apertural plane is flat without apparent thickening of the apertural margin. Lateral areas of the shell steeply inclined. The supra-apical surface is slightly convex throughout ontogeny with lateral and posterior sides straight to slightly concave abapically. Supra-apical surface recurved towards the apex, with increased posterior shell expansion during ontogeny as a result of decreased rate of coiling. The presumed protoconch is cap-shaped, obtuse and about 280 µm in width. A slight sub-apical to lateral constriction is visible but does not extend to the dorsum. Sub-apical surface strongly concave. Surface of shell with gently rounded concentric rugae and fine concentric growth lines. Rugae may be suppressed in large specimens. Fine, densely spaced radial lirae appear early in ontogeny. The shell is thin and conforms to the shape of the internal mould.
Remarks
A description of the Norwegian-type material and synonyms was given by Høyberget et al. (Citation2015) but the Swedish material reveals additional details of the morphology and ontogeny. Apart from Scenella antiqua, two similar species were named by Kiær (Citation1917) from the Norwegian succession, and these were later partly revised by Resser (Citation1938). All three were placed within H. antiqua by Høyberget et al. (Citation2015). Kautsky (Citation1945) described Scenella sp. and H. rugosa var. lapponica from the Grammajukku Formation at Mount Assjatj, Swedish Lapland, and these were also placed within H. antiqua by Høyberget et al. (Citation2015); this is followed herein. Bergström and Ahlberg (Citation1981) illustrated an internal mould of Helcionella? sp. from the Holmia kjerulfi Assemblage Zone at Kvasa at the coast just south of Kivik, southern Sweden, and reported occurrences of this taxon from the same stratigraphical interval at Hardeberga quarry and at Gislövshammar (). The material of Bergström and Ahlberg (Citation1981) is treated as conspecific with Helcionella antiqua. The species Helcionella? antiqua Abaimova, Citation1976 from Russia was transferred to Purella Missarzhevsky, Citation1989 by Qian and Bengtson (Citation1989).
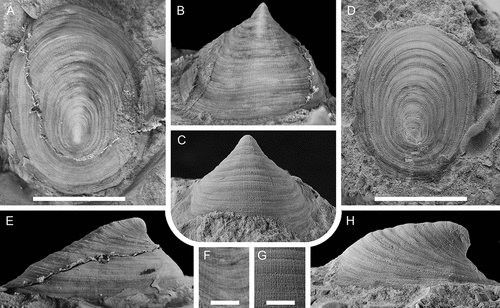
Helcionella antiqua is the most abundant helcionelloid from Gislövshammar, and also the largest, reaching up to 32 mm in length. It is the third most common species in the Evjevik Member in Mjøsa. During ontogeny the apertural shape changes from sub-rectangular to more sub-quadratic (compare with , ), the shell expansion increases so that the position of the apex moves from a marginal posterior position to an eccentric position about 1/5 back from the posterior margin ( vs. ). The fine lirae develop when the shell is about 2 mm long (). This nearly coincides with the development of the concentric rugae, which may appear slightly earlier (). In large specimens the rugae are less pronounced, but it is clear from the large material that the expression, regularity and strength of the first- and second-order ornamentation are quite variable, in part as a result of preservation.
The differences between Helcionella antiqua and the type species Helcionella subrugosa (d’Orbigny, Citation1850) were discussed by Høyberget et al. (Citation2015). The lowermost middle Cambrian species Helcionella oblonga Cobbold, Citation1921 from Britain and Morocco differs from H. antiqua in its much lower lateral profile and a strongly recurved apex (Jacquet and Brock Citation2016; Geyer et al. Citation2019). The lateral profile of H. rugosa var. comleyensis Cobbold, Citation1921 and development of the apex appears similar to that of H. antiqua but the aperture is circular in the British species. The largest known specimen is 17 mm long. Several species of Helcionella based on macro specimens, recently described from Germany and Australia (Jacquet and Brock Citation2016; Geyer et al. Citation2019; Geyer and Malinky Citation2020) are distinguished from H. antiqua by variations in profile, shape of aperture or expression of first- and second-order ornamentation.
Helcionella antiqua Kiær has been referred to Scenella Billings, Citation1872, Helcionella and Marocella Geyer, Citation1986. Scenella and Helcionella were considered to be closely related forms by Runnegar and Jell (Citation1976) who suggested that they could be interspecific variants. These are both comparatively large early molluscs (i.e. reaching 2–3 cm in length) with broadly similar patelliform shells with a sub-central apex, radial lirae and concentric growth lines shell. The presence of prominent rounded comarginal rugae in Helcionella may serve to distinguish it from Scenella (Conway Morris & Peel Citation2013). However, this could also reflect variation on a specific level of a broadly similar first order external ornamentation, which moreover shows variability in expression. This interpretation is supported by H. rugosa, where small to medium-sized specimens (~15 mm or less) are closely comparable to the type species of Helcionella, whereas the adumbilical concentric rugae can be effaced in larger specimens. For illustration, the c. 11 mm long Scenella barrandei (Linnarsson, Citation1879) from slightly younger beds in Scania than the Gislöv Formation, redescribed by Berg-Madsen and Peel (Citation1986), is compared with a similar sized specimen of H. antiqua ().
Marocella is a macro-mollusc with a low lateral profile, an eccentric apex and a sub-rectangular apertural outline. In overall shape it compares well with Helcionella, but Marocella is distinguished by a shell interior with a fine reticulate network. The shell also has rounded, comarginal rugae that may divide and are crossed by fine but widely spaced and irregularly developed radial lirae (Geyer Citation1986; Topper et al. Citation2009). Geyer (Citation1986) pointed out similarities in the ornamentation of Marocella with the monotypic Parmophorella Matthew, Citation1895 from Canada, in which the concentric rugae sometimes divide (Knight Citation1941). Knight and Yochelson (Citation1960) placed the genus in synonymy with Scenella but the name has been used infrequently since then (Runnegar and Jell Citation1976; Geyer Citation1986; Conway Morris and Peel Citation2013).
The lower Cambrian Minastirithella Jaquet and Brock, Citation2015 from Australia is another large patelliform shell comparable with Helcionella, but the sharp angular stepped to grooved rugae readily distinguish the Australian form. A similar morphology is mirrored in some compacted specimens of Helcionella from Sweden, as the early whorls are telescoped onto the later whorls. Totoralia Tortello & Sabattini, Citation2011 from the middle Cambrian of Argentina and Canada has stepped concentric rugae similar to those in Minastirithella, but is distinguished by an oval aperture and a sub-central and erect apex (Tortello and Sabattini Citation2011; Conway Morris and Peel Citation2013).
Genus Davidonia Parkhaev, Citation2017c
Type species
By original designation of Runnegar in Bengtson et al. (Citation1990): Mackinnonia davidi Runnegar in Bengtson et al. Citation1990, p. 233, from the lower Cambrian (Botoman) Parara Limestone at Horse Gully near Ardrossan, Yorke Peninsula, South Australia.
Remarks
Davidonia was introduced by Parkhaev (Citation2017c) as a replacement name for the pre-occupied Mackinnonia. The nomenclatural details are outlined in Peel (Citation2021), who correctly maintained Mackinnonia davidi Runnegar in Bengtson et al. Citation1990 as the type species of Davidonia. Parkhaev (Citation2001) considered the type species to be a junior synonym of Mackinnonia rostrata (Zhou & Xiao Citation1984, p. 132, pl. 3, figs 7–10), from the Atdabanian/Botoman Houjiashan Formation in Huoqui County, Anhui Province, of China, which has been accepted by subsequent authors (Claybourn et al. Citation2019; Li et al. Citation2021; Peel Citation2021). However, Mackinnonia davidi remains the type species in accordance with the International Commission of Zoological Nomenclature [ICZN] (Citation1999) Article 67.1.2. ‘The name of a type species remains unchanged even when it is a junior synonym or homonym, or a suppressed name’. (see also Høyberget et al. Citation2015).
Davidonia puppis (Høyberget, Ebbestad, Funke & Nakrem, Citation2015)
(figs 10F, )
2015 Mackinnonia puppis n. sp., Høyberget et al., pp. 50, 51, fig. 16A–J.
2015 Mackinnonia? sp., Høyberget et al., p. 52, fig. 17A, B, non fig. 17C, D.
2018 Mackinnonia puppis Høyberget et al., Citation2015; Jackson and Claybourn, p. 763.
2019 Mackinnonia puppis Høyberget, Ebbestad, Funke et Nakrem, Citation2015; Geyer et al., p. 225.
2019 Davidonia puppis Høyberget et al., Citation2015; Claybourn et al., p. 11.
Material
The Gislövshammar promontory material consists of 146 registered specimens, of which 20 are illustrated here (PMU 37862–37864, 37865/2, 37866–37868, 37869/1, 37870–37881), as well as one specimen (PMU 38133) from loose material at the beach near Brantevik.
Remarks
This species was described on the basis of about a dozen specimens from the Evjevik Member of Mjøsa, Norway (Høyberget et al. Citation2015). Norwegian and Swedish forms are considered conspecific, and the more abundant Swedish materials reveal new details about ontogeny and variability. This is the third most common species at Gislövshammar, while in the Evjevik Member it is the most common element. Two specimens were found in a loose block near Brantevik, and these are among the largest specimens preserved ().
The apex is preserved intact as internal moulds. It is narrow with a nearly symmetrical cap-shape and with a width of about 400 μm. At c. 600 μm in width, a weak groove separates it from the ensuing shell, and the first posterior knob or buttress develops immediately below the separating groove. The specimen in shows the thin shell covering the apex. The apex above the groove is large, most likely suggesting that it marks the development of an initial rib and not a transition between a protoconch and a teleoconch. Cederström et al. (Citation2014, pl. 4, fig. 5a) figured comparable cap-shaped apical regions in the mollusc Igorella emeiensis (Yu, Citation1979) from China where the embryonic shell is a small (250–350 μm) cap on top of an otherwise generally cap-shaped apical area. A similar morphology may be present in D. puppis.
Figure 12. Davidonia puppis (Høyberget et al., Citation2015), with specimens arranged in an ontogenetic series. A, D, right lateral and anterior views (PMU 37862). B, right lateral view (PMU 37863). The white arrows point to the thin outer shell. C, right lateral view (PMU 37864). E, dorsal view (PMU 37865/2). F, G, right lateral and dorsal views (PMU 37866). H, right lateral view (PMU 37867). I, right lateral view (PMU 37868). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.
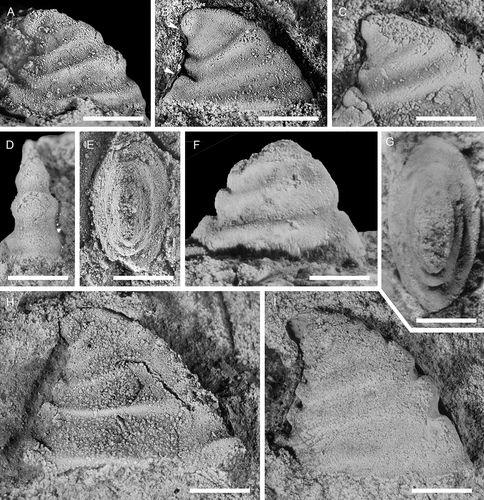
Figure 13. Davidonia puppis (Høyberget et al., Citation2015), with specimens arranged in an ontogenetic series. A, B, left lateral view and detail of posterior knobs (PMU 37869/1). Arrows in A point to thin outer shell layer; arrow in B points to shell material filling the space between knobs. C, left lateral view (PMU 37870). D, left lateral view (PMU 37871). E, right lateral view (PMU 37872). F, right lateral view (PMU 37873). G, left lateral view (PMU 37874). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.
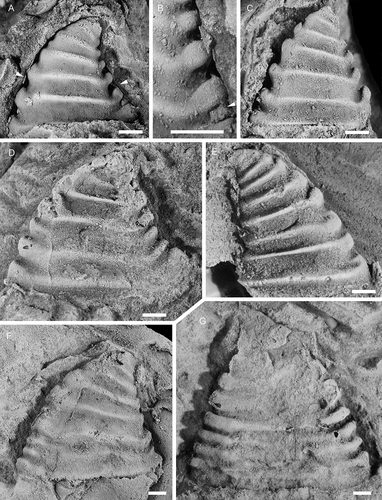
Figure 14. Davidonia puppis (Høyberget et al., Citation2015), showing details of shell morphology, shell mineralogy and ornamentation. A, B, right lateral and dorsal views (PMU 37875). C, dorsal view (PMU 37877). D, detail of ornamentation (external mould) (PMU 37876). E, F, anterior and posterior views (PMU 37878). G, H, silicon cast showing partly preserved shell ornamentation (PMU 37879). I, J, right lateral view of large internal mould with partially preserved shell layers (PMU 37880). K–M, dorsal, right lateral and posterior views of specimen with inner shell layer preserved (PMU 37881). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.
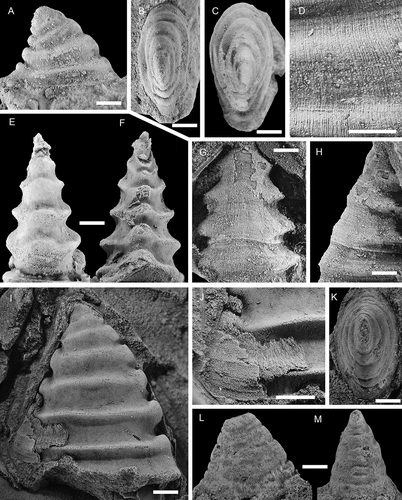
Figure 15. Davidonia cf. rostrata (Zhou & Xiao, Citation1984), with specimens arranged in an ontogenetic series. A, right lateral view (PMU 38253). B, right lateral view (PMU 37983). C, left lateral view (PMU 37984). D, right lateral view (PMU 38288). E, left lateral view (PMU 37987). F, G, right lateral and dorsal oblique views (PMU 37988/1). H, I, right lateral and anterior oblique views (PMU 37989). Arrow points to outer shell layer. J–L, left lateral, posterior and dorsal view of specimen with inner shell layer preserved (PMU 37992). M, left lateral view (PMU 37990). N, right lateral view (PMU 37991). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.

Figure 16. Davidonia cf. rostrata (Zhou & Xiao, Citation1984), showing details of morphology and shell preservation. A, B, left lateral view and detail of shell layers (PMU 37993/2). Arrows points to outer shell layer. C, left lateral view (PMU 37994/1). D, E, left lateral view and detail of shell layer (PMU 37993/1). F, right lateral view. Arrow points to outer shell layer (PMU 37995). G, left lateral view of specimen with partly recrystallised shell (PMU 38252). Arrow points to outer shell layer, obscuring the ribs on the internal mould. H, left lateral view of specimen showing two layers of recrystallised shell (PMU 38194/1). I, right lateral view of specimen showing inner shell structure of oblique bands (PMU 38289). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars for A, C, D, F–I = 1mm, scale bars for B, E = 0.5 mm
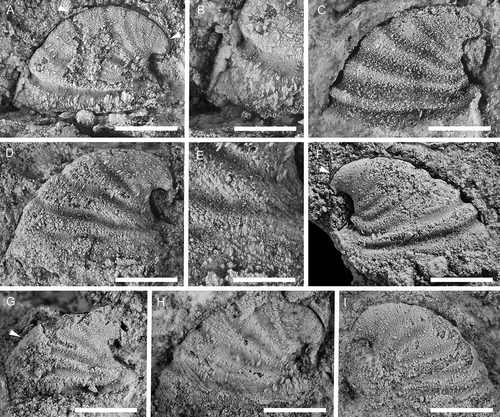
Figure 17. Latouchella cf. costata Cobbold, Citation1921, with specimens arranged in an ontogenetic series. A, left lateral view (PMU 38089). B, left lateral view (PMU 38090/1). C, right lateral view (PMU 38091). D–F, left lateral, posterior oblique and dorsal views (PMU 38092). G posterior view (PMU 38093). H, left lateral view (PMU 38094). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.
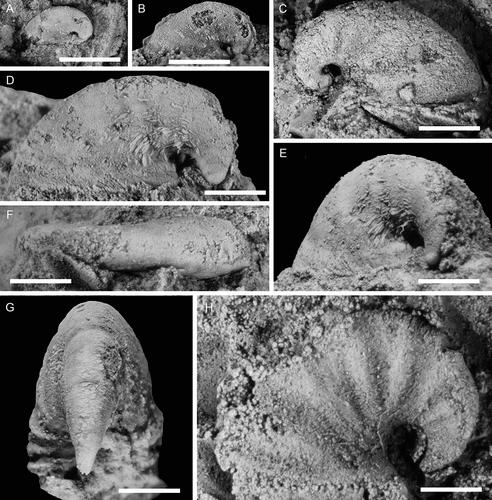
Figure 18. Stenotheca norvegica (Resser, Citation1938), with specimens arranged in an ontogenetic series. A, right lateral view (PMU 37778). B, right lateral view (PMU 37779). C, right lateral view (PMU 37780). D, right lateral view (PMU 37781). E, left lateral view (PMU 37782). F, right lateral view (PMU 37783/1). G–;J, anterior oblique, right lateral, dorsal, and dorsal oblique views (PMU 37784). K, silicon cast of specimen showing ornamentation, left lateral view (PMU 37785). L, left lateral view, internal mould (PMU 37786). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm.

Figure 19. Stenotheca norvegica (Resser, Citation1938), with specimens arranged in an ontogenetic series. A, external mould, left lateral view (PMU 37787). B, left lateral view (PMU 37778), C, D, right lateral and anterior views (PMU 37789/1). E, external mould, ventral view, showing key-hole morphology (PMU 37790). F, silicon cast of large specimen (PMU 37791). G, silicon cast showing detail of shell ornamentation (PMU 37792/1). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars = 1 mm
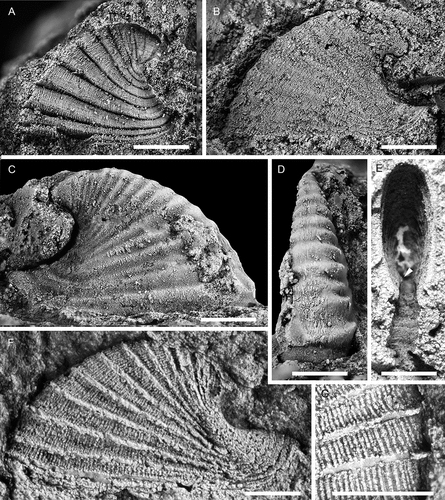
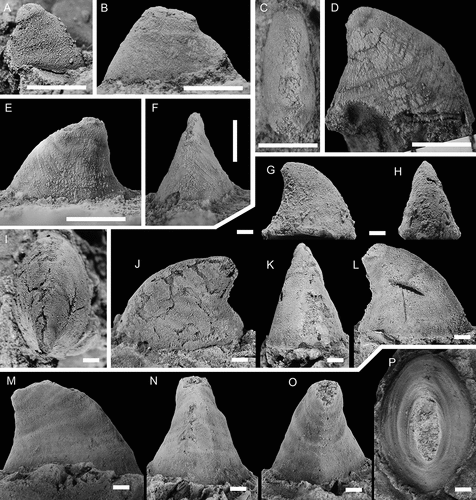
Figure 20. Conical Helcionelloid gen. and sp. indet., with specimens arranged in an ontogenetic series. A, right lateral view (PMU 37518/2). B, C, left lateral and dorsal views (PMU 38107). D, right lateral view (PMU 38108). E, F, left lateral and anterior views (PMU 38109). G, H, right lateral and anterior views (PMU 38110). I–L, dorsal, left, anterior and right lateral and views (PMU 38112). M–P, right lateral, anterior, posterior and dorsal views (PMU 38113). From the Ellipsostrenua spinosa Zone at Gislövshammar, Scania, southern Sweden. Scale bars in A–H = 1 mm, scale bars in I–P = 5 mm.
In several specimens from Gislövshammar the development of the co-marginal ribs deviates from their normal, robust, boat shape, in part reflecting taphonomic changes to underlying morphological variation. In PMU 37871 the third basal-most rib is effaced with only the anterior projection clearly developed (). In PMU 37873 three of the last four ribs are abnormally developed (). The uppermost of these ribs is sinuous towards the anterior. The one below this is also sinuous but nearly effaced with an under-developed anterior knob. Below this, the rib is vestigial and only discerned by an under-developed anterior knob but no posterior knob. Finally, the lowermost apertural rib seems to conform to a normal growth pattern. An even more unusual specimen, the large PMU 37874, has a higher number of ribs than any other specimen (). Furthermore, the last four ribs have generally slimmer anterior and posterior knobs and are more densely spaced than in other specimens. The abapical ribs are not well preserved but seem to conform to the normal robust type.
Deviation from normal growth development may have several reasons, such as genetically induced malformation or injury to the mantle cavity from infections or extrinsic factors such as predation. Skovsted (Citation2004, fig. 4) figured two specimens of Mackinnonia taconica (Landing & Bartowski, Citation1996) from North-East Greenland with rib irregularities which were tentatively attributed to repaired shell damage but the cause of the irregularities in the Swedish material is unclear.
Whereas the internal moulds of Davidonia puppis are strongly ribbed, these ribs are effaced on the outer shell surface. Vestiges of recrystallised shell are visible in several specimens and show how the grooves on the internal moulds are nearly filled with shell material, whereas the space between the posterior knobs are completely covered (). A few specimens preserve remnants of the outer shell ornamentation, which is developed as fine growth lines barely visible underneath densely spaced radial lirae (). Periodical growth increments may be visible as a shaper comarginal lines on the shell (). A single external mould from the upper bed of the Evjevik Member in Mjøsa shows similar ornamentation and growth increments. It was described as Mackinonnia? sp. by Høyberget et al. (Citation2015, fig. 17A, B) but is here treated as a specimen of D. puppis. At least two shell layers are visible (). The inner layer consists of fibrous calcite crystals oriented perpendicular to the aperture. This recrystallised layer is thick and fills the grooves on the internal mould and extends as a thin layer across the ribs (). The outer shell layer is thin, c. 70–80 microns thick, and is sometimes observed outlining the internal moulds ().
Davidonia cf. rostrata (Zhou & Xiao, Citation1984)
()
Material
A total of 162 specimens are catalogued, of which 17 are illustrated here (PMU 37983, 37984, 37987, 37988/1, 37989–37993/1, 37993/2, 37994, 37995, 38194/1, 38253, 38287–38289), all from Gislövshammar promontory.
Description
Shell recurved, of moderate height, with height being 2/3 of length. Ratio does not change much during ontogeny. Maximum length of the shell is 2.7 mm. Apical shell hook-like with the sub-apical area being vertical or slightly curved for a distance abapically, before curvature increases and leads into a short parietal train. In some cases this may project slightly posteriorly beyond the apex. Aperture outline ellipsoidal, width being 1/3 of length, with anterior margin more bluntly rounded than the slightly narrower and more pointed posterior margin. Apertural plane nearly flat but curving weakly adapically towards posterior end. Internal moulds of large specimens with five to six co-marginal ribs developed below the projecting apex, the last being at the apertural margin. Ribs are also visible in immature specimens but seemingly become effaced as the shell grows larger. All visible ribs are wide anteriorly and narrow posteriorly, albeit the rib closest to the aperture is only slightly wider anteriorly than posteriorly. The anterior portion of the ribs is weakly rounded, curving around the shell anteriorly, either straight across or with a slight adapical curve of the median part. The posterior end of the initial two–three ribs does not protrude beyond the margin of the mould, coinciding with the straight portion of the sub-apical area. Below this the ribs are upturned in lateral view and form a small knob at the posterior margin. Inter-rib areas are noticeably narrower than ribs, concave and of even width along the shell. Protoconch cap-shaped, laterally flattened, with a width of c. 270 μm. Weak groove may separate it from the ensuing shell. Ornamentation is unknown. Recrystallised shell indicates three shell layers. Shell material fills the lateral inter-rib areas and subdues or effaces the relief of the ribs.
Discussion
Davidonia rostrata (Zhou & Xiao, Citation1984) is a wide-spread mollusc, described from small, generally phosphatic juvenile shells and protoconchs in lower Cambrian (Stage 2–3) of China, Australia, Antarctica, Greenland, eastern North America and Spain (see Claybourn et al. Citation2019 for a synonymy list and distribution). It has been described as a species with variable morphology, showing variation in protoconch morphologies among dispersed geographical assemblages but convergence in the morphology of larger specimens (Jackson and Claybourn Citation2018). The largest specimens reach c. 1.0–1.3 mm in length (Skovsted Citation2004; Betts et al. Citation2016; Claybourn et al. Citation2019), which matches the size of the smallest specimens from Gislövshammar ().
The identification of this species in Scandinavia is tentative, as there is limited overlap in size between the small specimens from elsewhere and the Swedish specimens. Furthermore, the ribs differ from most other materials in being broader with more marked anterior expansions. In specimens from Antarctica the ribs may be effaced (Claybourn et al. Citation2019, fig. 4:6) while they are more clearly expressed in Greenland specimens but of more even width (Skovsted Citation2004, fig. 3B). Ribs in Australian specimens are more similar to those in the Swedish specimens (Betts et al. Citation2016, fig. 18 R, S).
As with Davidonia puppis, the ribbed morphology of the internal mould is largely obscured by the outer shell (). Three recrystallised shell layers are seen, starting with an inner layer composed of thin irregular bands forming an oblique curve across the shell, arching upwards from the posterior to the anterior (). This layer is covered by short fibrous crystals oriented perpendicular to the apertural margin, and represents the material that fills in the grooves in the internal mould (). A thin outer shell layer, 30–40 μm thick, is seen in some specimens ().
The cap-shaped and laterally flattened protoconch is similar to that in D. puppis, but the separation may be related to the transition to the teleoconch (), whereas the first groove beneath the apex in D. puppis is related to development of the first rib. However, this interpretation is conjectural as this feature is elusive and not consistently present. Claybourn et al. (Citation2019) determined the transition to the teleoconch in D. rostrata from Antarctica to lie at a change from a smooth shell to one with polygonal imprints.
Genus Latouchella Cobbold, Citation1921
Type species
By original designation, Latouchella costata Cobbold, Citation1921, p. 366, pl. 24, figs 41, 42, from the Comley Limestone (Cambrian provisional Series 2, Stage 4) at Comley, Shropshire, England.
Latouchella cf. costata Cobbold, Citation1921
()
Material
This is a rare species in the Gislövshammar fauna with only 20 specimens preserved, of which six (PMU 38089, 38090/1, 38091–38094) are illustrated herein.
Remarks
Høyberget et al. (Citation2015) described three poorly preserved specimens of Latouchella sp. from the Evjevik Member in Norway. In most respect, the Norwegian material was comparable to the type species but did not preserve the typical plications seen in the English species (see Gubanov and Peel Citation1998). The Norwegian material and the new specimens from Sweden are here considered to be conspecific, and inclusion of the Swedish material allows a closer comparison with the type species. As with the type material, the Scandinavian specimens are preserved as internal moulds with patches of recrystallised shell. Specimens of both are of the same size, with the largest specimen from Gislöv measuring 4 mm in length (). Furthermore, the general morphology, the asymmetrical coiling, and the plications of this specimen are similar to that of the larger paratype of L. costata figured by Gubanov (Citation1998, fig. 2). In the smaller specimens from Sweden, the plications are absent or very faintly marked (), just as in the Norwegian specimens.
Both the Swedish and British materials have similar hemispherical protoconchs that are clearly demarcated. The width of the protoconch (internal mould) in the Gislöv material is about 330 µm. In the type species the incurved initial shell juxtaposes the tip of the whorl against the sub-apical surface of the last part of the whorl, but this is not so prominent in the large Gislöv specimen. In other Swedish specimens the degree to which the tip is incurved varies, with specimens where it nearly touches the sub-apical surface () and others where it does not (). Gubanov and Peel (Citation1998) observed that the aperture of the larger paratype of L. costata was broader than that of the holotype. The Swedish material shows the same feature, with smaller specimen generally having narrow apertures.
Family Stenothecidae Runnegar and Jell, Citation1980
Remarks
Stenothecids are strongly laterally compressed univalved molluscs with a curved apertural margin. Vendrasco et al. (Citation2011) remarked that although a number of authors placed the Stenothecidae with the Helcionelloida, the stenothecid–helcionelloid relationship is uncertain, a statement echoed in the contrasting classifications of this taxon. Vendrasco et al. (Citation2011) selected to remove Watsonella Grabau, Citation1900 from the stenothecids, thus excluding Hammer et al. Citation2001, one of the two sub-families included in the stenothecids by Parkhaev (Citation2001, Citation2002, Citation2017a). See Jacquet and Brock (Citation2016) for further discussion on Watsonella. In comparison with Watsonella, the genus Anabarella Vostokova, Citation1962 was also omitted from the stenothecids by Vendrasco et al. (Citation2011), whereas Peel (Citation2021) argued that it should be retained in the family.
Genus Stenotheca Salter in Hicks, Citation1872
Type species
By original designation, Stenotheca cornucopia Salter in Hicks Citation1872, p. 180, pl. 7, figs 12, 13, from the middle Cambrian Porth-y-rhaw Group (Miaolingian) of Porth-y-rhaw, St. David’s, South Wales.
Stenotheca norvegica (Resser, Citation1938)
()
See Høyberget et al. (Citation2015) for a synonymy list.
Material
About 120 specimens are preserved as both internal and external moulds. Some specimens retain shell and ornamentation is often revealed on external moulds. For this study 15 specimens from Gislövshammar promontory are illustrated (PMU 37778–PMU 37783/1, 37784–37789/1, 37790–37792/1).
Discussion
The type material of this species was described by Kiær (Citation1917) based only on two poorly preserved specimens from Tømten farm in the Mjøsa area of Norway, but a dozen new specimens were described from the lower limestone bed of the Evjevik Member in Norway by Høyberget et al. (Citation2015). The Gislövshammar material agrees well with the Norwegian, and is considered conspecific. Stenotheca norvegica is the most common helcionelloid species in the Evjevik Member assemblage, while it is the fourth most common in the Gislövshammar assemblage. As in the Norwegian material, the height:length ratio varies between 0.62 and 0.50 (compare with Høyberget et al. Citation2015, fig. 15A, and with ibid., fig. 15 K). Another peculiarity is the distinct lateral constrictions on the posterior part of the parietal train, creating a key-hole shaped aperture (sensu Peel, Citation1991a, Citation1991b; ), which is reflected in the lateral constriction towards the posterior of the shell where the apertural margin of the parietal train turns adapically. The Gislövshammar specimens also show that the extent of the flared apertural increments is even greater than was revealed in the Norwegian material ().
The earliest juvenile shell preserved in the Swedish material is 1.6 mm in length. The internal cast of the apex is low, symmetrically cap-shaped and narrow. Its width is c. 230 µm, and the apex is separated from the rest of the shell by a concentric constriction. The apex is similar to that of Capitioconus from North-East Greenland (Skovsted Citation2004), but differs in being less bulbous and expanding faster.
The shell surface on the internal moulds appears to be smooth until the shell is just slightly longer than 1 mm, at which point the thin raised comarginal ribs start to appear. As many as 16 ribs can be seen in the large specimen in . The ribs are clearly separated by broader, slightly concave inter-rib areas. The ribs may correspond to the flared apertural increments on the shell; the specimen in shows at least 20 increments although far fewer are present in . The variability in the number of ribs, their density and spacing is also reflected in the Norwegian material, but does not seem to correspond to any ontogenetic differences.
Helcionelloid gen. and sp. indet.
?2015 Mackinnonia? sp., Høyberget et al., fig. 17C, D, not fig. 17 A, B.
Material
A total of 21 specimens are known, all preserved as internal moulds. Some specimens have remnants of shell layers but the external ornamentation is unknown. For this study seven specimens from Gislövshammar promontory are illustrated (PMU 37518/2, 38107–38010, 38012, 38013).
Description
Shell narrow, conical, height moderate to high (H:L between 0.60 and 0.78), evenly expanding. Aperture planar, its outline forming an ellipse in large specimens (W:L = 0.75) but more laterally compressed in small specimens (W:L = 0.45). Apex recurved, nearly reaching posterior margin. The sub-apical surface forms an even, gentle concave arch in some specimens. In other specimens, a marked change in curvature occurs slightly less than halfway down from apex, creating a kink in the lateral profile and a posterior expansion of the shell. Supra-apical surface evenly convex. Flanks of shell straight to weakly concave when viewed anteriorly. Internal moulds with irregular comarginal bands. Two recrystallised shell layers are seen; inner layer composed of thin irregular bands forming an oblique curve across the shell, arching upwards from the posterior to the anterior. Second layer outside of first, forms short fibrous crystals oriented perpendicular to the apertural margin.
Discussion
The shape of this broadly conical shell varies in the expression of the sub-apical curvature near the apertural edge, where it is either gently curved or develops into a distinct posterior expansion of the shell marked by a kink in the curvature. The specimens in are of the same size but show the differences clearly. It may be that the different development distinguishes between two species, but with the limited material and other shell features being similar, this is treated as interspecific variation. The poorly preserved external mould described as Mackinnonia? sp. by Høyberget et al. (Citation2015, fig. 17C, D) from the upper bed of the Evjevik Member in Mjøsa is tentatively placed with the Gislövshammar species.
During ontogeny, the outline of the aperture changes from a laterally compressed elliptical shape to a wider one (), but otherwise no other ontogenetic variation is discernible. Remnants of the shell () show at least two shell layers, remarkably similar in preservation and expression to the two inner layers seen in Davidonia cf. rostrata ().
Acknowledgments
PC thanks Erik and Signe Olsson (Simrishamn) for permission to excavate and sample the Gislövshammar locality. We thank Christian Skovsted (Stockholm) for stimulating discussions during the initial phase of this study. John ‘Jompa’ Ahlgren (Hällekis) and Carin H. Nilsson (Eslöv) kindly donated specimens to this study. We are grateful for comments by the editor and reviewers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abaimova GP. 1976. Samie drievnie gastropody sibiri [the oldest gastropods of Sibiria]. Trudy Instituta Geologii I Geofiziki SO AN SSSR. 296. 174–176. [In Russian].
- Ahlberg P. 1985. Lower cambrian trilobite faunas from the scandinavian caledonides–a review. In: Gee DG, Sturt BA, editors. the caledonide orogen – Scandinavia and related areas (Vol. 1). Chichester: Wiley; p. 339–346.
- Ahlberg P, Bergström J. 1978. Lower Cambrian ptychopariid trilobites from Scandinavia. Sveriges Geologiska Undersökning. 49:1–41.
- Ahlberg P, Bergström J. 1993. The trilobite Calodiscus lobatus from the lower Cambrian of Scania, Sweden. GFF. 115:331–334.
- Ahlberg P, Bergström J, Johansson J. 1986. Lower Cambrian olenellid trilobites from the Baltic Faunal Province. Geol fören Stockh förh. 108(1):39–56. doi: 10.1080/11035898609453745.
- Ahlberg P, Cederström P, Babcock LE. 2016. Cambrian Series 2 biostratigraphy and chronostratigraphy of Scandinavia: a reappraisal. Conference: Palaeo Down Under 2 11–15 July 2016 Adelaide. In: Laurie JR, Kruse PD, Garcia-Bellido DC, Holmes JD, editors. Hornsby: Geological Society of Australia abstracts. Vol. 117, p. 16.
- Álvaro JJ, Ahlberg P, Axheimer N. 2010. Skeletal carbonate productivity and phosphogenesis at the lower–middle Cambrian transition of Scania, southern Sweden. Geol Mag. 147(1):59–76. doi: 10.1017/S0016756809990021.
- Álvaro JJ, Ahlberg P, Babcock LE, Bordonaro OL, Choi DK, Cooper RA, Ergaliev G, Gapp K, Ghobadi Pour W, Hughes M, et al. 2013. Global Cambrian trilobite palaeobiogeography assessed using parsimony analysis of endemicity. In: Harper DAT, Servais T, editors. Early Palaeozoic biogeography and palaeogeography. 38 London: Geological Society of London Memoir, 273–296.
- Axheimer N, Ahlberg P, Cederström P. 2007. A new lower Cambrian eodiscoid trilobite fauna from Swedish Lapland and its implications for intercontinental correlation. Geol Mag. 144(6):953–961. doi: 10.1017/S0016756807003597.
- Bengtson S, Conway Morris S, Cooper BJ, Jell PA, Runnegar BN. 1990. Early Cambrian fossils from South Australia. Association of Australasian Palaeontologists Memoir. 9:1–364.
- Berg‐Madsen V, Peel JS. 1978. Middle Cambrian monoplacophorans from Bornholm and Australia, and the systematic position of the bellerophontiform molluscs. Lethaia. 11(2):113–125. doi: 10.1111/j.1502-3931.1978.tb01295.x.
- Berg-Madsen V, Peel JS. 1986. Scenella barrandei (mollusca) from the Middle Cambrian of Baltoscandia. Norsk geologisk tidsskrift. 66:81–86.
- Berg-Madsen V, Peel JS. 1987. Yochelcionella (mollusca) from the late Middle Cambrian of Bornholm, Denmark. Bull Geol Soc Denmark. 36:259–261.
- Bergström J, Ahlberg P. 1981. Uppermost lower Cambrian biostratigraphy in Scania, Sweden. Geol fören Stockh förh. 103(2):193–214. doi: 10.1080/11035898109454518.
- Betts MJ, Paterson JR, Jago JB, Jacquet SM, Skovsted CB, Topper TP, Brock GA. 2016. A new lower Cambrian shelly fossil biostratigraphy for South Australia. Gondwana Research. 36:176–208. doi: 10.1016/j.gr.2016.05.005.
- Billings FGS. 1872. On some fossils from the Primordial rocks of Newfoundland. Can Field-Nat. 6:465–479.
- Boucot AJ. 1953. Life and death assemblages among fossils. Am J Sci. 251(1):25–40. doi: 10.2475/ajs.251.1.25.
- Cadée GC. 1982. Low juvenile mortality in fossil brachiopods, some comments. Interne Verslagen Nederlands Instituut voor Onderzoek der Zee. 3:1–29.
- Cederström P, Ahlberg P, Ahlgren J, Høyberget M, Nilsson CH. 2011. Morphology, ontogeny and distribution of the Cambrian ellipsocephalid trilobite Strenuaeva spinosa from Scandinavia. GFF. 133:59–60.
- Cederström P, Ahlberg P, Babcock LE, Ahlgren J, Høyberget M, Nilsson CH. 2012. Morphology, ontogeny and distribution of the Cambrian Series 2 ellipsocephalid trilobite Strenuaeva spinosa from Scandinavia. GFF. 134(3):157–171. doi: 10.1080/11035897.2012.717963.
- Cederström P, Ahlberg P, Clarkson ENK, Nilsson CH, Axheimer N. 2009. The lower Cambrian eodiscoid trilobite Calodiscus lobatus from Sweden: morphology, ontogeny and distribution. Palaeontology. 52(3):491–539. doi: 10.1111/j.1475-4983.2009.00858.x.
- Cederström P, Ebbestad JOR, Ahlberg P. 2014. Helcionelloid molluscs from Cambrian Series 2 strata in Sweden: composition and stratigraphic implications. Abstracts, 31st Nordic Geological Winter Meeting, 8–10 January, Lund, Sweden, p. 66.
- Cederström P, Geyer G, Ahlberg P, Nilsson CH, Ahlgren J. 2022. Ellipsocephalid trilobites from Cambrian Series 2 and Stage 4. Fossils and Strata. 67:1–131.
- Chaffee C, Lindberg DR. 1986. Larval biology of Early Cambrian molluscs: the implication of small body size. Bull Mar Sci. 39:536–549.
- Claybourn TM, Jacquet SM, Skovsted TP, Holmer LE, Brock GA. 2019. Mollusks from the upper Shackleton Limestone (Cambrian Series 2), Central Transantarctic Mountains, East Antarctica. J Paleontol. 93:437–459.
- Cobbold ES. 1921. The Cambrian horizons of Comley (Shropshire), and their brachiopoda, pteropoda, gasteropoda, etc. Qua J Geol Soc. 76:325–386.
- Conway Morris S, Peel JS. 2013. A new helcionelloid mollusk from the middle Cambrian Burgess Shale, Canada. J Paleontol. 87:1067–1070.
- Creveling JR, Knoll AH, Johnston DT. 2014. Taphonomy of Cambrian phosphatic small shelly fossils. Palaios. 29:295–308.
- Dattilo BF, Freeman RL, Peters WS, Heimbrock WP, Deline B, Martin AJ, Kallmeyer JW, Reeder J, Argast A. 2016. Giants among micromorphs: were Cincinnatian (Ordovician, Katian) small shelly phosphatic faunas dwarfed? Palaios. 31:55–70.
- Devaere L, Skovsted CB. 2021. The youngest known tommotiid: Lapworthella bornholmiensis (Poulsen, 1942) from Cambrian Stage 4 to Guzhangian (Miaolingian) strata of Bornholm and southern Sweden. GFF. 143:151–167.
- Dies Álvarez ME, Gozalo R, Cederström P, Ahlberg P. 2008. Bradoriid arthropods from the lower–middle Cambrian of Scania, Sweden. Acta Palaeontologica Polonica. 53:647–656.
- d’Orbigny A. 1850. Prodrome de paléontologie stratigraphique universelle des animaux mollusques et rayonnés faisant suite au cours élémentaire de paléontologie. Vol. 1, Victor Masson, Paris. p. 394.
- Dzik J. 1991. Is fossil evidence consistent with traditional views of the early metazoan phylogeny? In: Simonetta AM, Morris SC, editors. The early evolution of Metazoa and the significance of problematic taxa. Cambridge: Cambridge University Press; p. 47–56.
- Ebbestad JOR, Hybertsen F, Högström AES, Jensen S, Palacios T, Taylor WL, Agić H, Høyberget M, Meinhold G. 2022. Distribution and correlation of Sabellidites cambriensis (Annelida?) in the basal Cambrian on Baltica. Geol Mag. 159:1262–1283.
- Geyer G. 1986. Mittelkambrishe Mollusken aus Marokko und Spanien. Senckenbergiana lethaea. 67:55–118.
- Geyer G. 1994. An enigmatic bilateral fossil from the lower Cambrian of Morocco. J Paleontol. 68:710–716.
- Geyer G, Malinky JM. 2020. Helcionelloid molluscs and hyoliths from the Miaolingian (middle Cambrian) of the subsurface of the Delitzsch–Torgau–Doberlug Syncline, northern Saxony, Germany. PalZ. 94:271–293.
- Geyer G, Valent M, Meier S. 2019. Helcionelloids, stenothecoids and hyoliths from the Tannenknock Formation (traditional lower middle Stage 4/Wuliuan boundary interval) of the Franconian Forest, Germany. PalZ. 93:207–253.
- Gosselin LA, Qian P-Y. 1997. Juvenile mortality in benthic marine invertebrates. Marine Ecological Progress Series. 146:265–282.
- Grabau AW. 1900. Palaeontology of the Cambrian terranes of the Boston Basin. Occas Pap Boston Soc Nat Hist. 4:601–694.
- Grabau AW, Shimer HW. 1909. North American Index Fossils; Invertebrates. 1. New York: A. G. Seiler and Company; p. 853.
- Grönwall KA. 1902. Bornholms Paradoxideslag og deres fauna. Danmarks geologiske undersøgelse, 2 række. 13:1–230.
- Gubanov AP. 1998. The Early Cambrian molluscan evolution and its palaeogeographic implications. Acta Universitatis Carolinae, Geologica. 42:419–422.
- Gubanov AP. 2002. Early Cambrian palaeogeography and the Iberia-Siberia connection. Tectonophysics. 352:153–168.
- Gubanov AP, Fernández-Remolar DC, Peel JS. 2004. Early Cambrian molluscs from Sierra de Córdoba (Spain). Geobios. 37:199–215.
- Gubanov AP, Peel JS. 1998. Redescription of the type species of Latouchella Cobbold, 1921 (Mollusca) from the Lower Cambrian of Comley (England). GFF. 120:17–20.
- Hall J. 1847. Containing descriptions of the organic remains of the lower division of the New York system (equivalent of the Lower Silurian rocks of Europe). Paleontol New York. 1:1–338.
- Hammer Ø, Harper DAT. 2006. Paleontological Data Analysis. Blackwell Publishing Malden: Blackwell Publishing. p. 351.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: palaeontological statistics software package for education and data analysis. Palaeontologia Electronica. 4:1–9.
- Hazprunar G. 1992. The first molluscs ‐ small animals. Bollettino di zoologia. 59:1–16.
- Hicks H. 1872. On some undescribed fossils from the Menevian Group of Wales. Q J Geol Soc London. 27:41–42.
- Horný RJ. 1964. The Middle Cambrian Pelagiellacea of Bohemia (Mollusca). Sborník Národniho Muzea v Praze. 20B:133–140.
- Høyberget M, Ebbestad JOR, Funke B. 2019. Re-evaluation of the stratigraphically important olenellid trilobite Holmia cf. mobergi from the Cambrian Series 2, Stage 3 and its implications for the lower Cambrian stratigraphy in the Mjøsa area, Norway. Nor J Geol. 99:1–29.
- Høyberget M, Ebbestad JOR, Funke B, Nakrem HA. 2015. The shelly fauna and biostratigraphy of the lower Cambrian (provisional Series 2, Stage 4) Evjevik Member, Ringstrand Formation in the Mjøsa area, Norway. Nor J Geol. 95:23–56.
- International Commission of Zoological Nomenclature [ICZN] 1999. International code of zoological nomenclature. Fourth edition. The International Trust for Zoological Nomenclature, c/o Natural History Museum, London, 306. [ online version 2012 at http://www.iczn.org/iczn/index. jsp].
- Jackson ISC, Claybourn TM. 2018. Morphometric analysis of inter- and intraspecific variation in the Cambrian helcionelloid mollusc Mackinnonia. Palaeontology. 61:761–773.
- Jacquet SM, Brock GA. 2016. Lower Cambrian helcionelloid macromolluscs from South Australia. Gondwana Research. 36:333–358.
- Jensen S, Grant SWF. 1998. Trace fossils from the Dividalen Group, northern Sweden: implications for Early Cambrian biostratigraphy of Baltica. Norsk geologisk tidsskrift. 78:305–317.
- Kautsky F. 1945. Die Unterkambrische Fauna vom Aistjakk in Lappland. Geol fören Stockh förh. 67:129–211.
- Kiær J. 1917. The Lower Cambrian Holmia fauna at Tømten in Norway. Norske Videnskapsselskapets Skrifter, I Matematisk-Naturvidenskabelig Klasse. 1916(10):1–140.
- Kidwell SM. 1998. Time-averaging in the marine fossil record: overview of strategies and uncertainties. Geobios. 30:977–995.
- Kidwell SM. 2001. Preservation of species abundance in marine death assemblages. Science. 294:1091–1094.
- Kidwell SM. 2002. Time-averaged molluscan death assemblages: palimpsests of richness, snapshots of abundance. Geology. 30:803–806.
- Kidwell SM, Bosence DWJ. 1991. Taphonomy and time-averaging of marine shelly faunas. In: Allison PA, Briggs DEG, editors. Taphonomy, releasing the data locked in the fossil record. New York: Plenum Press; p. 115–209.
- Knight JB. 1941. Paleozoic Gastropod genotypes. Geol Soc Am Spec Pap. 32:1–510.
- Knight JB. 1956. New families of gastropods. J Wash Acad Sci. 46:41–42.
- Knight JB, Yochelson EL. 1960. Monoplacophora. In: Moore RC, editor. Treatise on Invertebrate Paleontology, Volume I, Mollusca 1. Lawrence: Geological Society of America and University of Kansas Press; p. 177–184.
- Kobayashi T. 1939. Restudy on Lorenz’s Raphistoma bröggeri from Shantung with a note on Pelagiella. J Geol Soc London. 3(1):283–288.
- Kouchinsky A, Bengtson S, Clausen S, Gubanov A, Malinky JM, Peel JS. 2011. A middle Cambrian fauna of skeletal fossils from the Kuonamka Formation, northern Siberia. Alcheringa. 35:123–189.
- Kouchinsky A, Bengtson S, Clausen S, Vendrasco MJ. 2015. An early Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia. Acta Palaeontologica Polonica. 60:421–512.
- Kowalewski M. 1997. The reciprocal taphonomic model. Lethaia. 30:86–88.
- Landing E, Bartowski KE. 1996. Oldest shelly fossils from the Taconic allochthon and late Early Cambrian sea-levels in eastern. J Paleontol. 70:741–761.
- Landing E, Geyer G, Jirkov IA, Schiaparelli S. 2021. Lophotrochozoa in the Cambrian evolutionary radiation and the Pelagiella problem. Pap Palaeontol. 7:2227–2244.
- Linnarsson G. 1879. Om faunan i kalken med Conocoryphe exsulans (“Coronatuskalken”). Sveriges Geologiska Undersökning. 35:1–31.
- Li L, Zhang X, Skovsted CB, Yun H, Pan B, Li G. 2021. Revisiting the molluscan fauna from the Cambrian (Series 2, stages 3–4) Xinji Formation of North China. Papers in Palaeontology. https://onlinelibrary.wiley.com/doi/full/10.1002/spp2.1289.
- Lochman C. 1956. Stratigraphy, paleontology, and paleogeography of the Elliptocephala asaphoides strata in Cambridge and Hoosick quadrangles, New York. Geol Soc Am Bull. 67:1331–1396.
- Martí Mus M, Palacios T, Jensen S. 2008. Size of the earliest mollusks: did small helcionellids grow to become large adults? Geology. 36:175–178.
- Martinsson A. 1974. The Cambrian of Norden. In: Holland CH, editor. Lower Palaeozoic Rocks of the World (Vol. 2). London: Cambrian of the British Isles, Norden, and Spitsbergen. Wiley–Interscience; p. 185–283.
- Matthew GF. 1893. Illustrations of the fauna of the St. John Group, No. VIII. Proc Trans R Soc Can. 11:85–129.
- Matthew GF. 1895. The Protolenus fauna. Trans N Y Acad Sci. 14:101–153.
- McLoughlin S, Vajda V, Topper TP, Crowley JL, Liu F, Johansson O, Skovsted CB. 2021. Trace fossils, algae, invertebrate remains and new U-Pb detrital zircon geochronology from the lower Cambrian Torneträsk Formation, northern Sweden. GFF. 143:103–133.
- Mghazli K, Lazreq N, Geyer G, Landing E, Boumehdi MA, Youbi N. 2023. Cambrian microfossils from the High Atlas, Morocco: taxonomic, biostratigraphic, palaeobiogeographic, and depositional significance of the Brèche à Micmacca limestone beds. J Afr Earth Sci. 197:1–27. 104751.
- Missarzhevsky VV. 1981. A new name for a gastropod genus. Paleontologicheskiy Zhurnal. 15:118. [In Russian].
- Missarzhevsky VV. 1989. The earliest skeletal fossils and stratigraphy of the Precambrian–Cambrian boundary beds. Trudy Geologicheskogo Instituta, Akademiya Nauk SSSR. 443: 1–237. [In Russian].
- Moczydłowska M. 1991. Acritarch biostratigraphy of the lower Cambrian and the Precambrian-Cambrian boundary in southeastern Poland. Fossils and Strata. 29:1–127.
- Moczydłowska M. 1998. Lower Cambrian acritarch biochronology in Baltoscandia. In: Ahlberg P, editor. Guide to excursions in Scania and Västergötland, southern Sweden (Vol. 141). Lund: Lund Publications in Geology; p. 9–16.
- Moczydłowska M, Jensen S, Ebbestad JOR, Budd G, Martí Mus M. 2001. Biochronology of the autochthonous lower Cambrian in the laisvall-storuman area, Swedish Caledonides. Geol Mag. 138:435–453.
- Nielsen AT, Schovsbo NH. 2007. Cambrian to basal Ordovician lithostratigraphy in southern Scandinavia. Bull Geol Soc Denmark. 53:47–92.
- Nielsen AT, Schovsbo NH. 2011. The lower Cambrian of Scandinavia: depositional environment, sequence stratigraphy and palaeogeography. Earth Sci Rev. 107:207–310.
- Nielsen AT, Schovsbo NH. 2015. The regressive early-mid Cambrian “Hawke Bay Event” in Baltoscandia: epeirogenic uplift in concert with eustacy. Earth Sci Rev. 151:288–350.
- Nikolaisen F. 1986. Olenellid trilobites from the uppermost Lower Cambrian Evjevik Limestone at Tømten in Ringsaker, Norway. Norsk geologisk tidsskrift. 66:305–309.
- Nützel A. 2014. Larval ecology and morphology in fossil gastropods. Palaeontology. 57:479–503.
- Nützel A, Lehnert O, Frýda J. 2006. Origin of planktotrophy – Evidence from early molluscs. Evol Dev. 8:325–330.
- Nützel A, Lehnert O, Frýda J. 2007. Origin of planktotrophy—Evidence from early molluscs: a response to Freeman and Lundelius. Evol Dev. 9:313–318.
- Palacios T, Högström AES, Jensen S, Ebbestad JOR, Agić H, Høyberget M, Meinhold G, Taylor WL. 2022. Organic-walled microfossils from the Kistedalen Formation, Norway: acritarch chronostratigraphy of the Baltic Miaolingian and evolutionary trends of placoid acritarchs. Pap Palaeontol. 1457:1–41.
- Parkhaev PY. 2001. Molluscs and siphonoconchs. In: Gravestock DI, Alexander EM, Demidenko YE, Esakova NV, Holmer LE, Jago JB, Lin TR, Melnikova LM, Parkhaev PY, Rozanov AY, et al., editors. The Cambrian biostratigraphy of the Stansbury Basin, South Australia (Vol. 282). Moscow: Transactions of the Palaeontological Institute, Academy of Science; p. 133–210.
- Parkhaev PY. 2002. Phylogenesis and the system of the Cambrian univalved mollusks. Paleontol J. 36. 25–36. Translated from Paleontologicheskiy Zhurnal, 2002(1), 27–39.
- Parkhaev PY. 2004. Malacofauna of the Lower Cambrian Bystraya Formation of Eastern Transbaikalia. Paleontol J. 38. 590–608. Translated from Paleontologicheskii Zhurnal’, 2004(6), 9–25.
- Parkhaev PY. 2014. Protoconch morphology and peculiarities of the early ontogeny of the Cambrian Helcionelloid mollusks. Paleontol J. 48. 369–379. Translated from Paleontologicheskii Zhurnal’, 2014(4), 32–40.
- Parkhaev PY. 2017a. On the position of Cambrian archaeobranchians in the system of the class Gastropoda. Paleontol J. 51. 453–463. Translated from Paleontologicheskii Zhurnal’, 2017(5), 3–12.
- Parkhaev PY. 2017b. Origin and the Early Evolution of the Phylum Mollusca. Paleontol J. 51. 91–112. Translated from Paleontologicheskii Zhurnal’, 2017(6), 91–112.
- Parkhaev PY. 2017c. Davidonia nom. nov., a new substitute name for a Cambrian gastropod genus. Paleontol J. 51. 574. Translated from Paleontologicheskii Zhurnal’, 2017(5), 115.
- Peel JS. 1991a. Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca. In: Simonetta AM, Morris SC, editors. The early evolution of metazoa and the significance of problematic taxa. Cambridge: Cambridge University Press; p. 157–177.
- Peel JS. 1991b. The Classes Tergomya and Helcionelloida, and early molluscan evolution. Bulletin Grønlands Geologiske Undersøgelse. 161:11–65.
- Peel JS. 2021. An outer shelf shelly fauna from Cambrian Series 2 (Stage 4) of North Greenland (Laurentia). J Paleontol. 95(83):1–41.
- Peel JS, Kouchinsky A. 2022. Middle Cambrian (Miaolingian Series, Wuliuan Stage) molluscs and mollusc-like microfossils from North Greenland (Laurentia). Bull Geol Soc Denmark. 70:69–104.
- Ponder WF, Lindberg DR, Ponder JM. 2020. Biology and evolution of the Mollusca, Volume 2. CRC Press Boca Raton: Florida; p. 870.
- Qian Y, Bengtson S. 1989. Palaeontology and biostratigraphy of the early Cambrian Meishucunian Stage in Yunnan Province, South China. Fossils and Strata. 24:1–156.
- Resser CE. 1938. Fourth contribution to nomenclature of Cambrian fossils. Smithsonian Misc Collect. 97:1–43.
- Resser CE, Howell BJ. 1938. Lower Cambrian Olenellus zone of the Appalachians. Geol Soc Am Bull. 49:195–248.
- Runnegar B. 1983. Molluscan phylogeny revisited. Mem Assoc Australas Palaeontol. 1:121–144.
- Runnegar B. 2007. No evidence for planktotrophy in Cambrian molluscs. Evol Dev. 9:311–312.
- Runnegar B, Jell PA. 1976. Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa. 1:109–138.
- Runnegar B, Jell PA. 1980. Australian Middle Cambrian molluscs: corrections and additions. Alcheringa. 4:111–113.
- Runnegar B, Pojeta J. 1985. Origin and diversification of the Mollusca. In: Trueman ER, Clarke MR, editors. The Mollusca (Vol. 10). Orlando (Florida): Evolution. Academic Press; p. 1–57.
- Skovsted CB. 2004. Mollusc fauna of the early Cambrian Bastion Formation of North East Greenland. Bull Geol Soc Denmark. 51:11–37.
- Skovsted CB, Topper TPM, Johansson S, Liu F, Vajda V. 2021. First discovery of small shelly fossils and new occurrences of brachiopods and trilobites from the early Cambrian (Stage 4) of the Swedish Caledonides, Lappland. GFF. 143:134–150.
- Tate R. 1892. The Cambrian fossils of South Australia. Trans R Soc S Aust. 15:183–189.
- Thomas RDK, Runnegar B, Matt K. 2020. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: lophotrochozoan heritage and conchiferan novelty. Palaeontology. 63:601–627.
- Topper TP, Brock GA, Skovsted CB, Paterson JR. 2009. Shelly fossils from the lower Cambrian Pararaia bunyerooensis Zone, Flinders Ranges, South Australia. Mem Assoc Australas Palaeontol. Cambro-Ordovician Studies III. 37:199–246.
- Tortello M, Sabattini N. 2011. Totoralia, a new conical-shaped mollusk from the middle Cambrian of western Argentina. Geologica Acta. 9:175–185.
- Vendrasco MJ, Checa AC. 2015. Shell microstructure and its inheritance in the calcitic helcionellid Mackinnonia. Est J Earth Sci. 64:99–104.
- Vendrasco MJ, Kouchinsky AV, Porter SM, Fernandez CZ. 2011. Phylogeny and escalation in Mellopegma and other Cambrian molluscs. Palaeontologia Electronica. 14:1–44.
- Vendrasco MJ, Porter SM, Kouchinsky A, Guoxiang LI, Fernandez CZ. 2010. New data on molluscs and their shell microstructures from the middle Cambrian Gowers Formation, Australia. Palaeontology. 53:97–135.
- Vostokova VA. 1962. Cambrian gastropods from Siberian platform and Taimyr. Stateiy po paleontologii i biostratigrafii. 28:51–74. [In Russian].
- Wenz W. 1938. Handbuch der Paläozoologie; Gastropoda. Handbuch der Paläozoologie, Allgemeiner Teil und Prosobranchia. Schindewolf OH, editor. Handbuch der Paläozoologie. Vol. 6 1: Berlin, Verlag Gebrüder Borntæger; P.1–240.
- Yochelson EL. 1962. Early Ordovician gastropods from the Oslo Region, Norway. Norsk geologisk tidsskrift. 42:239–250.
- Yu W. 1979. Earliest Cambrian monoplacophorans and gastropods from western Hubei with their biostratigraphical significance. Acta Palaeontologica Sinica. 3:233–274.
- Zhou B, Xiao L. 1984. Early Cambrian monoplacophorans and gastropods from Huainan and Huoqiu counties, Anhui Province. Pap Stratigr paleontol. 13. 125–140. [In Chinese with English abstract]
