ABSTRACT
The presence of Dromornithidae in the Australian Cenozoic fossil record was first reported in 1872, yet although eight species and hundreds of specimens are known, key information on their morphology remains elusive. This is especially so for their skulls, which contributes to a lack of resolution regarding their relationships within Galloanserae. The skull of the Pleistocene dromornithid, Genyornis newtoni, was initially described in 1913. Additional fossils of this species have since been discovered and understanding of avian skull osteology, arthrology, and myological correlates has greatly advanced. Here we present a complete redescription of the skull of Genyornis newtoni, updating knowledge on its morphology, soft-tissue correlates, and palaeobiology. We explore the diversity within Dromornithidae and make comprehensive comparisons to fossil and extant galloanserans. Furthermore, we expand on the homologies of skull muscles, especially regarding the jaw adductors and address the conflicting and unstable placement of dromornithids within Galloanserae. Findings support generic distinction of Genyornis newtoni, and do not support the close association of Dromornithidae and Gastornithidae. We thus recommend removal of the dromornithids from the Gastornithiformes. Considering character polarities, the results of our phylogenetic analyses, and palaeogeography, our findings instead support the alternative hypotheses, of dromornithids within, or close to, the Suborder Anhimae with Anseriformes.
Introduction
The fossil evidence for an endemic Australian avian radiation of evolutionarily distinct giant birds, the Dromornithidae, shows this family had a long temporal range, extending from at least the Eocene (Vickers-Rich and Molnar Citation1996) to the late Pleistocene (Rich Citation1979; Wells and Tedford Citation1995; Murray and Vickers-Rich Citation2004; Miller et al. Citation2005; Worthy et al. Citation2016a; McInerney et al. Citation2022). Named taxa span a shorter interval, however, and include late Oligocene-early Miocene Barawertornis tedfordi Rich Citation1979; late Oligocene Dromornis murrayi Worthy, Handley, Archer and Hand Citation2016a; middle Miocene D. planei (Rich Citation1979); middle to late Miocene species Ilbandornis woodburnei Rich Citation1979 and I. lawsoni Rich Citation1979; late Miocene D. stirtoni Rich Citation1979; as well as possibly Pliocene D. australis Owen Citation1872 (see Murray and Vickers-Rich Citation2004). The youngest representative is Genyornis newtoni Stirling and Zietz Citation1896, the only dromornithid species currently known to have survived into the late Pleistocene; a range of dated and undated fossil localities support a Pleistocene occurrence of G. newtoni (e.g. as reviewed in Saltré et al. Citation2016). The most recent and robustly dated site, Lake Callabonna shows G. newtoni survived minimally 48 – ~45 thousand years ago (Ka) in the north-eastern region of South Australia (McInerney et al. Citation2022). Lake Callabonna is a unique fossil locality in Australia and spans many square kilometres wherein thousands of animals (mostly large mammals) were mired. The unusual deposition has allowed for near-complete, articulated fossils to be uncovered (see Stirling Citation1894 for original descriptions of Lake Callabonna). It is the type locality for G. newtoni, and the source of the specimens described in this paper.
Dromornithids are represented primarily by an abundance of postcranial material (Rich Citation1979). Historically, cranial fossils were rare, extremely fragmented, and/or inadequate for a comprehensive morphological analysis (Murray and Megirian Citation1998; Worthy et al. Citation2016a). This is evidenced by the first described dromornithid skull material: that of G. newtoni was reported by Stirling (Citation1913; see Appendix One: ), redescribed by Murray and Megirian (Citation1998), and again by Murray and Vickers-Rich (Citation2004), despite recognition of the specimen being ‘a friable mass of broken fragments … [that] had suffered such great distortion from pressure affecting it laterally that anything approaching a satisfactory reconstruction was quite impossible’ (Stirling Citation1913, p. 111). Following numerous attempts at stabilising and reconstructing the skull, Stirling concluded that the specimen failed to yield important information on shape and structure, although other described elements, i.e. quadrates and a mandible, were better preserved and remained relatively informative. Regardless, the 1913 description of this skull has formed the basis of all reconstructions of G. newtoni, in association with interpretations that were misled by an early consensus that the Dromornithidae had ratite affinities (see Owen Citation1874; Stirling and Zietz Citation1896, Citation1900, Citation1905; Stirling Citation1913; Lambrecht Citation1933; Rich Citation1979: fig. 1; Rich Citation1980; Murray and Vickers-Rich Citation2004; Nguyen et al. Citation2010).
Rich (Citation1979) described five additional dromornithid species in three genera, yet did not attribute cranial material to any taxa, nor discuss Stirling’s (Citation1913) skull material of G. newtoni, as noted by Olson (Citation1985). In 1998, the first skull materials for species of Dromornis and Ilbandornis was described by Murray and Megirian (Citation1998), and those of G. newtoni redescribed despite the authors not directly examining the fossils themselves (p. 74). They concluded this latter material contributed important proportional information for dromornithid morphology, identifying elements which aligned the dromornithids with the Galloanserae. Crown group galloanserans form two diverse orders of birds, that from most to least basal, include the Megapodiidae, Cracidae, Numididae, Odontophoridae, and Phasianidae – galliforms – and the Anhimidae, Anseranatidae, and Anatidae – anseriforms. They further suggested that G. newtoni closely resembled a goose (Anser, Anatidae) or screamer (Chauna, Anhimidae) and concluded anseriform affinities; subsequent publications, therefore, treated the dromornithids as anseriforms (see Nguyen et al. Citation2010; Angst and Buffetaut Citation2017). Analysis of phylogenetic relationships among extinct Galloanserae by Worthy et al. (Citation2016b; Citation2017b) instead resolved dromornithids as sister to the ordinal-level lineages, Anseriformes and Galliformes.
Murray and Vickers-Rich (Citation2004) discussed the skull morphology of the dromornithids, and in 2016 new skull material for species of Ilbandornis, Dromornis, and Barawertornis were subsequently described (Worthy et al. Citation2016a). Additional key dromornithid cranial characters were identified, and Worthy et al. (Citation2016a) reiterated the lack of informative skull material available for G. newtoni. This is supported by the exclusion of Genyornis newtoni from a study on dromornithid endocranial anatomy by Handley and Worthy (Citation2021) and recent observations of the original G. newtoni skull material (now SAMA P10838). Although the ratite affinities of the Dromornithidae have since been dismissed (Olson Citation1985; Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004; Mayr Citation2011, Citation2022a; Worthy et al. Citation2017b), the lack of new skull material for G. newtoni over the past 109 years has led to misconceptions on morphology, derived from photos and descriptions of the original skull (e.g. the shape of the upper bill), that have only been partially resolved through inferences from other dromornithids. As a result, despite the species being known for more than one hundred years and one of the longest known and best represented dromornithids – much of the leg, sternum, pelvis, and vertebral column have been described (Stirling and Zietz Citation1896, Citation1900, Citation1905; Stirling Citation1913; Murray and Vickers-Rich Citation2004) – the morphology of the skull of Genyornis newtoni requires a review. This is now possible due to discoveries made during a series of expeditions to Lake Callabonna from 2013–2019 wherein multiple skull elements were recovered.
It is evident that an analysis of the skull of G. newtoni based on modern knowledge of dromornithids and updated literature on the skull of Aves is necessary, and so we present detailed descriptions of the skull of Genyornis newtoni based on the first new material discovered since 1913. These specimens provide novel information on the morphology of the braincase, upper bill, palate, quadrate, and lower bill. We additionally use these skeletal elements as a framework of osteological correlates for soft tissue structures which enable justified inferences on kinetic capabilities, and skull myology and syndesmology. This most especially relates to the adductor chamber – homological understanding of which has grown substantially for Aves since the turn of the century, and has been facilitated by digital dissections (e.g. Zusi and Livezey Citation2000; Holliday and Witmer Citation2007; Lautenschlager et al. Citation2014; Jones et al. Citation2019; Smith-Paredes and Bhullar Citation2019). The results of this study are used to refine the systematic placement of dromornithids, make inferences on dromornithid evolution, and infer the ecology of G. newtoni.
Materials and methods
Institutional Abbreviations. AMNH, American Museum of Natural History, New York, U.S.A.; ANSTO, Australian Nuclear Science and Technology Organisation, Sydney, New South Wales, Australia; FU, Flinders University, Adelaide, Bedford Park, South Australia; FUR, Flinders University Vertebrate Collection, Palaeontology Laboratory, Flinders University, Adelaide, South Australia; MHNT, Muséum d’Histoire naturelle de Toulouse, France; NMV, Museums Victoria, Melbourne, Victoria; NTM, Museum and Art Gallery of the Northern Territory, Alice Springs, Northern Territory; QM, Queensland Museum, Brisbane, Queensland; QVM, Queen Victoria Museum and Art Gallery, Launceston, Tasmania; SAHMRI, South Australian Medical and Health Research Institute, Adelaide, South Australia; SAMA, South Australian Museum, Adelaide, South Australia; USNM, United States National Museum, Washington D.C., U.S.A..
Specimen collection, preparation, and morphological analysis
We studied the morphology of the skull including the braincase, upper bill, palate, parts of the jugal arches, hyoid skeleton, lower bill, and quadrates of Genyornis newtoni from six specimens (see ), all from Lake Callabonna Fossil Reserve, Pirlatapa and Adnyamathanha Country, South Australia, Australia. For additional specimen photos, see Appendix One. Specimens were collected during expeditions in 2013, 2014, 2018, and 2019, authorised by permits from the Department of Environment, Water, and National Resources, Government of South Australia (U26313–1, U26313–2, and in 2018 and 2019 File ref. DEWNRF-26892) and the South Australian Museum, and were prepared at FU; fossils were mechanically cleaned of matrix using both dental and PalaeoTools pneumatic Micro Jack® tools and stabilised by infusion with ParaloidTM B-72 dissolved in acetone. Additional material of G. newtoni, stored in FU and SAMA collections, were also examined, including those available from the 1913 descriptions (see Stirling Citation1913). Descriptions are based off morphological observations and comparisons with other dromornithids and galloanserans. Measurements, made using Erskine Oral Care Dentagauge 2 callipers with results rounded to the nearest 0.1 mm, are given in Appendix Two ().
Table 1. Preserved skull elements, damage and taphonomic distortion in recently recovered Genyornis newtoni skull material from Lake Callabonna. Note that all of these specimens are also associated with post-cranial material not described here.
Specimens SAMA P59516 and SAMA P59521 were scanned (neutron beam scanning at ANSTO, Sydney by Joseph Bevitt and µCT scanning at SAHMRI, Adelaide, respectively) to assess internal structures although in neither case could the bone be reliably distinguished from the infilling matrix (a mix of kaolin and smectite clays, calcite concretions, and paraloid, as ascertained by X-ray diffraction analysis, authors’ unpublished data for SAMA P53833, field ID: Geny10) in subsequent reconstructions in Materialise Mimics (Materialise’s Interactive Medical Image Control System) Innovation Suite (versions 18.0–22.0).
Assignment of skull material to taxa follows Murray and Megirian (Citation1998) for Dromornis planei, D. stirtoni, Ilbandornis species, and Worthy et al. (Citation2016a) for D. murrayi, Barawertornis and Ilbandornis ?woodburnei. All Genyornis newtoni skull material is identified by its association with partial skeletons including the diagnostic leg bones, comparison with material described by Stirling (Citation1913) and furthermore, G. newtoni is the only dromornithid species known from the Pleistocene.
A three-dimensionally modelled Ilbandornis woodburnei cranium was produced via segmentation of CT data in Materialise Mimics (Materialise’s Interactive Medical Image Control System) Innovation Suite 22.0 (for scan data, see Handley & Worthy, Citation2021), digitally altered and reconstructed to be more complete using Blender v. 2.93.2, and images of perspectives subsequently adapted using Adobe Photoshop v. 24.
Comparative material
Dromornithid and galloanseran specimens were loaned to THW at FU from SAMA, NTM, QM, and QVM. A range of comparative specimens were also accessed using the Flinders University Vertebrate Collection. Where specimens were unavailable for direct observation, e.g. gastornithids and other fossil galloanserans, comparisons were made using the available literature, photographs supplied by THW (e.g. Gastornis giganteus, Presbyornis pervetus), and a scan of Presbyornis pervetus (supplied by L. M. Witmer). All comparative material used can be found listed in SI 1. All comparative specimens including some with dried musculature intact, and targeted dissections of single Gallus gallus, Anas superciliosa and Chenonetta jubata specimens (unregistered), were used to support interpretations on the myology which was derived from the published literature.
Nomenclature
Higher taxonomic nomenclature follows Worthy et al (Citation2017b; Citation2017a; Sun et al. Citation2017) except we use Galloanserae due to taxonomic priority and thus, galloanseran, as the adjectival form and to refer to members of Galloanserae. In addition, the Superfamily Anatoidea, and its distinction with respect to the Suborder Anseres, is used in the sense of Livezey (Citation1997), as advocated by Field et al. (Citation2020). For anatomical nomenclature, we follow Baumel and Raikow (Citation1993) unless indicated otherwise. Selected updated and synonymised terminology, i.e. for the bony palate proposed by Zusi and Livezey (Citation2006), the maxillary bone by Mayr (Citation2018a), the quadrate by Elzanowski et al. (Citation2001) and Elzanowski and Stidham (Citation2010), and the myology of the adductor chamber by Holliday and Witmer (Citation2007), has been used when necessary. The prefix os is omitted from the names of skull bones and the term cranium is used to refer specifically to the neurocranium or braincase as per Baumel and Witmer (Citation1993: annot. 8a, p. 68), not to be mistaken for the term skull, which is used here to refer to the entire head skeleton including the cranium, jaws, quadrates, hyoid apparatus, and other associated bones of the combined neurocranium and splanchnocranium (see Zusi Citation1993). While maxilla refers to the total structure comprising the upper jaw (Baumel and Witmer Citation1993: annot. 93; Zusi Citation1993, p. 394–395), we opt to use the term ‘rostrum’ herein when referring to this complex (as per Mourer-Chauviré and Balouet Citation2005), to avoid confusion with the maxillary bones (ossa maxillaria) that participate in its composition (however, see Clarke Citation1993: annot 12, regarding the proper anatomical use of the term rostrum). As per Mayr (Citation2018a), we follow the correct Latin spelling for the bilaterally paired upper jaw bones, the praemaxilla, rather than ‘premaxilla’. For myological homologies, see Appendix Three (), additional skeletal homologies are present in SI 2.
A notable subject of contention is the use of osteological terms associated with the ‘fossa temporalis’ or ‘temporal fossa’, resulting from imprecisions in referring to it through interpretation of the associated myological homology in different clades (see Zusi and Livezey Citation2000, pp. 165–166). In general, the temporal fossa accommodates adductor muscle origins in various configurations across different groups of Neornithes, including musculus pseudotemporalis superficialis, bellies associated with musculus adductor mandibulae externus (AME) profundus, and musculus AME superficialis (using terminology of Holliday and Witmer Citation2007), the latter of which being the most superficial of the external adductor musculature and attaching to the caudolateral region of the cranium (e.g. see Goodman and Fisher Citation1962; Elzanowski Citation1987; Baumel and Witmer Citation1993; Vanden Berge and Zweers Citation1993; Weber Citation1996; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007). Use of this term has also included association with musculus depressor mandibulae (van Gennip Citation1986, p. 5; Baumel and Witmer Citation1993: fig. 4.1; see below). To address this issue, Zusi and Livezey (Citation2000) advocated the use of ‘fossa muscularis temporalium’ as well as terms that specifically relate to the musculature occupying each region (e.g. impressio musculi adductor mandibulae externus (AME) profundus, pars coronoideus, using applied nomenclature of Holliday and Witmer Citation2007), which we support. However, when referring to the complete region of attachment for the musculus AME on the lateral squamosum, we opt to use the generalised topographic term ‘temporal fossa’ (Holliday and Witmer Citation2007). Additionally, following Holliday and Witmer (Citation2007), directional terms are used to refer to specific structural regions within this (i.e. ‘dorsotemporal fossa’ and ‘caudotemporal fossa’). Hereon, in names for certain muscles and nerves, ‘musculus’ (plural ‘musculi’) is abbreviated to ‘m.’ (‘mm.’) and ‘nervus’ to ‘n.’ respectively.
The term ‘fossa subtemporalis’ (impressio temporalis, sensu van Gennip Citation1986) is similarly ambiguous. Following Zusi and Livezey (Citation2000, p. 166), we refer to this depression with regards to the associated musculature, although note that here (in the context of the Dromornithidae) it is instead solely related to the origin of m. depressor mandibulae (i.e. impressio musculi depressor mandibulae).
Phylogenetic analyses
To qualitatively assess some of the hypotheses present herein, and to precede future, more extensive analyses assessing the phylogeny of Dromornithidae and their relationships to other galloanseran families, we only present preliminary analyses using a limited character and taxon sample. Our ingroup includes 22 modern species from across the Galloanserae radiation and 12 relevant fossil taxa that are hypothesised to have galloanseran or near-galloanseran affinities, while the outgroup consists of three palaeognaths (Eudromia elegans, Dromaius novaehollandiae and Casuarius casuarius) and four neoavians (Antigone rubicunda, Fulmarus glacialoides, Accipiter fasciatus and Colius striatus). We have reassessed and added to the characters of Worthy et al. (Citation2017b; see Appendix Four) and produced a character matrix comprising characters of the skull only. These 100 morphological/standard discrete characters address variation across the complete skull (3), cranium (28), lacrimal (3), quadrate (22), mandible (29), and rostrum (15). Of these characters, 51 are ordered. Missing data was coded as ‘?’, and no gaps (‘-’) were coded. See SI 3 and 4 for the corresponding NEXUS files that were used in phylogenetic analyses. These analyses are summarised below. In consideration of the likely extensive convergence in postcranial material and the modifications derived from the giant, flightless nature of the dromornithids, a reassessment of dromornithid postcranial morphology is required to inform on the characters and methods used in a more complete analysis. This is beyond the scope of this study which specifically focuses on testing the phylogenetic information pertaining to the skull.
Currently, a lack of directly comparable skull elements for some dromornithid species (resulting in missing data) and our exclusion of postcranial material from these analyses, restrict quantitative assessment of intrafamilial dromornithid relationships. These phylogenetic analyses are thus focused on testing the interfamilial relationships in the context of Galloanserae. All analyses in the present study were constrained to molecular-based topological relationships for modern taxa following a combination of Wang et al. (Citation2013), Burleigh et al. (Citation2015), Prum et al. (Citation2015), and Kimball et al. (Citation2019), and appropriated to the taxon-selection herein at specific or generic levels. Molecular-based constraints for Anatidae were relaxed following Worthy et al. (Citation2022).
Parsimony analyses were conducted using PAUP* v. 4.0a169 (Swofford Citation2003). The search for optimal trees involved a heuristic approach, with 10,000 replicates of random stepwise taxon addition using the tree-bisection-reconnection (TBR) branch-swapping algorithm, holding 10 trees at each step, and saving no more than 100 trees of a length greater than or equal to 1 in each replicate. Subsequent bootstrapping involved 10,000 bootstrap replicates with the following parameters: 100 random-addition sequence replicates per bootstrap replicate; no more than 1,000 trees at a score equal to or greater than 1 saved per bootstrap replicate; holding 10 trees each step; and TBR branch swapping implemented. Multistate characters were treated as uncertainty.
An undated Bayesian analysis of the morphological data was also performed using MrBayes v. 3.2.7 (Ronquist and Huelsenbeck Citation2003), appropriating the same character and taxon selection, ordering settings, and molecular-based topological constraints as the parsimony analysis. The Mk model (Lewis Citation2001) was used to apply maximum likelihood phylogeny inference to the variable, discrete morphological dataset (coding = variable). Evolutionary rate variability was distributed according to gamma parameter (rates = gamma). Four independent analyses were simultaneously run for a total of 50,000,000 generations, and sampled every 5,000 generations, to confirm convergence. The heating parameter was set as 0.1, and four chains per analysis, one cold and three incrementally heated, were used to better explore the tree topology space. The first 20% of sampled trees from all runs were discarded as relative burn-in, and the remaining samples combined to produce a consensus tree.
Systematic palaeontology
AVES Linnaeus, Citation1758
NEORNITHES Gadow, Citation1892
NEOGNATHAE Pycraft, Citation1900
GALLOANSERAE Sibley, Ahlquist and Monroe, Citation1988
DROMORNITHIDAE Fürbringer, Citation1888
GENYORNIS Stirling and Zietz, Citation1896
GENYORNIS NEWTONI Stirling and Zietz, Citation1896
Referred material
Lake Callabonna Fossil Assemblage. SAMA P59516, articulated cranium, rostrum, quadrates, pterygoids, elements of the hyoid apparatus, and mandible associated with a complete postcranial skeleton of individual 1 of CB2018.75 field collection number. SAMA P59517, flattened rostrum and part mandible associated with several postcranial elements from individual 2 of CB2018.75. SAMA P59520, fragmented, articulated partial left cranium, quadrate, and caudal mandible, disarticulated rostral mandibular fragment, and other associated cranial fragments, associated with postcranial elements and gizzard stones of individual 3 of CB2019.14. SAMA P59521, complete rostrum associated with postcranial elements from either individual 1 or 2 of CB2019.14. NMV P256893, partial articulated left side of cranium, jugal arch, ceratobranchial, quadrate and caudal mandible, disarticulated right partial quadrate, jugal arch, and cranial and mandibular fragments, all associated with the near complete, articulated postcranial skeleton of individual CB2018.23. SAMA P53830, right partial quadrate with disarticulated caudal part of the jugal arch and condylus occipitalis, associated with postcranial elements of individual CB2014.Geny5. Skull fossil previously attributed to Genyornis newtoni include SAMA P10838, a fragmented skull, SAMA P10788, a partial mandible and unregistered quadrates (Stirling Citation1913).
Descriptions and comparisons
Fossils
Complete fusion of the constituent bones of each of the cranium, rostrum and mandible is evident in all specimens signifying that they belonged to mature individuals (Zusi Citation1993). This is supported for specimen SAMA P59516 by histological interpretations by Chinsamy and Worthy (Citation2021: Table 2) of the leg bones. All fossils described herein are variably affected by crushing, deformation, and fragmentation (see ), which limits morphological interpretation, although together they allow for a reliable and detailed description of nearly all features of the skull of G. newtoni. Descriptions are facilitated by comparisons with the morphology of other dromornithid fossils throughout.
Proportions
The articulated skull of specimen SAMA P59516 (), provides a basis for proportional estimates of the skull in this species. Specimen SAMA P10838 (see Appendix One: ) was, according to the original descriptions, 290 mm long from the condylus occipitalis to the symphysial apex of the mandible, and 150 mm high (Stirling Citation1913, p. 112). Comparatively, the most complete skull described here, specimen SAMA P59516, is slightly larger, approximately 296 mm in length from the rostral apex of the rostrum to the condylus occipitalis. This is perhaps slightly foreshortened by the disarticulation of the craniorostral hinge (sensu Mourer-Chauviré and Balouet Citation2005) and the caudal movement of the rostrum to overlap the cranium by maximally 33 mm. The maximum height of the cranium from the dorsal surface to the ventral tip of the processus paroccipitalis is 135.5 mm. Our observations support Stirling’s contention that only a total length and maximum depth of the skull could be ascertained from specimen SAMA P10838 due to poor preservation limiting identification of the margins of important structural features. However, Murray and Megirian (Citation1998) inferred goose-like proportions of the skull with the rostrum accounting for 50% of complete rostrocaudal skull length. Contrary to this, specimen SAMA P59516, instead shows that the rostrum is closer to 1.8 times the length of the cranium.
Figure 1. The near-complete, articulated skull of Genyornis newtoni (SAMA P59516): A. Left lateral view; B. Left lateral view outlined with major parts distinguished; C. Right lateral view; D. Right lateral view outlined with major parts distinguished; E. Dorsal view; F. Caudal view; G. Ventral view; H. Rostral view. Annotations: cb., ceratobranchial; cr., cranium; ma., mandible; or., orbit; pt., pterygoid; ro., rostrum; qu., quadrate; ve., articulated vertebrae. Scale bars: 50 mm, E. to G. all to same scale.
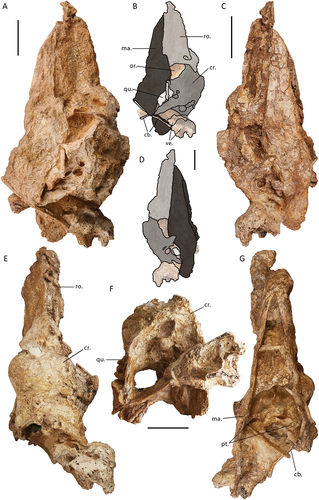
The proportions of the cranium and rostrum within the skull of G. newtoni differ from other dromornithids; the rostra of Dromornis stirtoni and D. planei are near three times as long as the cranium. This is most extreme in D. stirtoni, wherein the entire skull would have been approximately just over 500 mm long with 460 mm taken up in beak length (Murray and Megirian Citation1998: fig. 28). There are currently no rostra known for species of Ilbandornis or Barawertornis, precluding comparisons. Marked rostrocaudal compression of the cranium is characteristic for dromornithids, with the dorsoventral depth of the cranium considerably greater than the rostrocaudal length (Worthy et al. Citation2016a; Handley and Worthy Citation2021). This feature is increasingly extreme in Dromornis species; least in D. murrayi, greater in D. planei, and especially so regarding D. stirtoni, wherein the cranium length of the latter is only half the dorsoventral depth, with the impact of this also evident in the subtle signs of brain compression (Handley and Worthy Citation2021). Although, as in Ilbandornis, some rostrocaudal compression is evident, the cranium of G. newtoni lacks such exaggerated compression and, as no cranium is known for the early Miocene Barawertornis tedfordi, a trend, if any, in non-Dromornis dromornithids cannot be ascertained.
Craniorostral hinge
The skull of G. newtoni is prokinetic (the rostrum is inflexible but bending occurs at a craniofacial hinge: see Hofer Citation1949; Frazatta Citation1962; Bock Citation1964; Bühler Citation1980; Bühler et al. Citation1988; Gussekloo et al. Citation2001; Pecsics et al. Citation2017). The zona flexoria craniofacialis is one of the primary flexion zones associated with cranial kinesis in birds (Fisher Citation1955; Bock Citation1964; Bühler Citation1980; Zusi Citation1984, Citation1993; Bühler et al. Citation1988; Baumel and Raikow Citation1993; Bailleul et al. Citation2017), generally composed of overlapping, thinned premaxillary and frontal processes of the nasalia (nasals) and praemaxillaria (premaxillaries), respectively, from the rostrum, which are fused with the rostral ends of the frontalia (frontals) and the dorsal lamina of the mesethmoidale of the cranium, and sometimes stabilised by laterally bounding lacrimalia (lacrimals; Fisher Citation1955; Bock Citation1964; Bühler Citation1980; Zusi Citation1984; Baumel and Raikow Citation1993). This region is instead represented by a completely mobile synovial joint (Bühler Citation1980, p. 449, 451–452; as defined by Baumel and Raikow Citation1993: Arthr. Intro., annot. 1, 4, 46) in Genyornis newtoni, other dromornithids, gastornithids, and sylviornithids among galloanserans (Witmer and Rose Citation1991; Andors Citation1992; Murray and Megirian Citation1998; Worthy Citation2000; Mourer-Chauviré and Balouet Citation2005; Worthy et al. Citation2016a). As such, specific referral to this articulation as a craniorostral hinge (Murray and Vickers-Rich Citation2004; craniorostral joint, sensu Mourer-Chauviré and Balouet Citation2005) throughout this document, is considered more appropriate for these taxa. This morphology is rare among bird families and is comparable to that seen in some Psittaciformes (and others e.g. Podargidae and Microcarbo melanoleucos; Andors Citation1992; Zusi Citation1993).
The only specimen which preserves the craniorostral hinge in near articulation is SAMA P59516, although the joint is overlapped by the dorsocaudally displaced rostrum, and thus not visible in its entirety. The articular surface on the dorsocaudal rostrum is preserved in specimens SAMA P59521 and SAMA P59517 (Appendix One: ), although significantly damaged in the latter. The caudal regions of the praemaxillaries and the nasals (and the lacrimals, see below) are synostosed to form a surface for articulation with the cranium. This is less robust and appears proportionally more dorsoventrally narrow and caudally flattened than that of Dromornis planei (NTM P9973–2), in which, robust, bulbous and caudally prominent lateral articular ‘condyles’ (internal processes of nasolacrimals, sensu Murray and Vickers-Rich Citation2004: fig. 181, 184) lie either side of a distinct medial fossa. These condyles were considered to be reciprocal structures to the deep lateral fossae seen on several dromornithid crania (which also were identified for Sylviornis neocaledoniae CitationPoplin Citation1980 and Megavitiornis altirostris Worthy Citation2000 by Mourer-Chauviré and Balouet Citation2005; D. planei and D. stirtoni, by Murray and Vickers-Rich Citation2004: figs. 52, 76, 77), and may be formed in part from the frontal processes of the nasal bones. Unlike the completely mobile, true diarthrosis in S. neocaledoniae, the dromornithid form would have provided relative lateral stability for the craniorostral hinge, while allowing significant free movement of the rostrum in the occlusal plane (Murray and Vickers-Rich Citation2004, p. 235; as was similarly observed for M. altirostris by Mourer-Chauviré and Balouet Citation2005). Genyornis newtoni appears to have homologous condyles on the dorsocaudal rostrum (see 4.6), although they are much smaller evidencing less stability was required for the relatively smaller rostrum.
The medial section of the dorsocaudal-most rostrum is occluded in specimens SAMA P59516 and SAMA P10838, and post-mortem damage is present in this region in specimen SAMA P59521. Thus, the presence or absence of a sulcus to receive a median prominence as observed in D. planei (see median process/tuberosity of Murray and Vickers-Rich Citation2004: figs. 52, 76, 77, 181–184), cannot be confidently assessed. However, the median part of the dorsocaudal rostrum of SAMA P59521 has an abraded surface approximately rostrocaudally-level with the previously described articulatory condyles and suggests that in a better-preserved state it would have caudally exceeded these condyles and dorsally overlapped any median prominence of the dorsal cranium. In SAMA P10838 and P59516, there is no evidence of an obvious dorsal depression on the rostral cranium that participates in the craniorostral articulation, opposing the median dorsocaudal rostrum (see ‘frontal groove’ of Murray and Vickers-Rich Citation2004, p. 235), however, this area is not fully exposed in either specimen. The craniorostral hinge divides the rostrocaudal skull length into two dorsally convex sections (as viewed laterally), the rostrum and the cranium; when the rostrum is occluded with the mandible, the hinge would form a shallow dorsal notch in lateral aspect, becoming deeper as the upper jaw is opened (also see Murray and Vickers-Rich Citation2004). In contrast, reconstructions of Gastornis giganteus (Cope Citation1876) suggest the transition in lateral aspect across the hinge is flat (see Witmer and Rose Citation1991: fig. 1).
The caudal foreshortening of the anteorbital region of the cranium and the concomitant caudal repositioning of the craniorostral hinge in dromornithids (Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004, p. 126; Worthy et al. Citation2016a) is evident in G. newtoni. The result is a lack of preorbital zone in the cranium, contrasting with all other galloanserans. The hinge transects the orbit just rostral to the processus postorbitales in G. newtoni but is located further caudal in species of Dromornis, in rostrocaudal alignment with the process.
There have been no lacrimals previously unambiguously identified for G. newtoni; the lacrimal and its processes identified and figured for the original skull (SAMA P10838) by Murray and Vickers-Rich (Citation2004: fig. 107, p. 60, 127) cannot be verified by direct examination of the relevant material. Furthermore, we do not recognise any separate and unfused lacrimals in the new skull material presented herein. Murray and Vickers-Rich (Citation2004, p. 127–128) did not find evidence of orbital processes of the lacrimals in other dromornithids. However, they inferred that the supraorbital processes may have fused to the dorsorostral cranium as the ‘anterolateral processes’ and that the lacrimals contributed to the lateral dorsocaudal rostrum as ‘nasolacrimal processes’ (Murray and Vickers-Rich Citation2004, p. 235, fig. 180, 184). Instead, considering the material at hand, we interpret the area caudolateral of apertura nasi ossea as representative of the synostosis between the head of the lacrimal and the caudal rostrum, whereby the caudoventral projection on the rostrum dorsal of angulus tomialis and laterally adjacent to processus jugalis of the maxillare, may be homologous with the processus orbitalis of the lacrimal. This interpretation is supported by the distinct, dorsoventrally elongate process on the caudal margin of the Dromornis planei rostrum NTM P932–2. The latter is linked with the more ventral arcus jugalis by a narrow channel and appears ventrally separate from the more rostral nasal bar, forming an opening (i.e. fenestra antorbitalis), and is consistent with a ventrocaudal process of the lacrimal.
Thus, we do not support the hypothesis regarding fusion of the lacrimal to the cranium in dromornithids. This lack of synostosis is typical of basal galliforms, such as megapodiids, and some basal anseriforms, i.e. Anhimidae, Anachronornithidae, Anseranatidae, Presbyornithidae, and even basal anatids, e.g. species of Biziura, Dendrocygna, and Coscoroba (see Olson and Feduccia Citation1980; Zelenkov and Stidham Citation2018; Tambussi et al. Citation2019; De Mendoza et al. Citation2020; Field et al. Citation2020: SI p. 26–27; Houde et al. Citation2023). Consequently, this state is considered plesiomorphic for members of Galloanserae (Tambussi et al. Citation2019). However, the lacrimals of S. neocaledoniae, gastornithids, Danielsavis nazensis Houde et al. Citation2023, and some galliforms are fused to the frontals and span the craniorostral hinge to articulate with the nasals (pers. obvs. photos of specimen AMNH 6169 of Gastornis giganteus; Matthew and Granger Citation1917; Andors Citation1992; Murray and Megirian Citation1998; Mourer-Chauviré and Balouet Citation2005; Houde et al. Citation2023; Mayr et al. Citation2023). The fusion of the lacrimal to the frontals which occurs in anatids is considered a feeding adaptation (see Fisher Citation1955; Dzerzhinsky Citation1982; Zelenkov and Stidham Citation2018).
In the megapodiid species with the largest rostrum, Macrocephalon maleo, no synostosis of the lacrimal is present and instead, there is a very small frontal articulation and a much larger one with the rostrum. In G. newtoni specimens which retain the craniorostral hinge, there are no apparent laterally facing articulatory facets, processes or osseous lobes on the cranium that could articulate with a more lateral lacrimal head or supraorbital process; nor have any been identified in the crania of other dromornithids (Murray and Megirian Citation1998; Worthy et al. Citation2016a). The only articulatory surfaces on the cranium are those rostrally facing, as part of the hinge joint (i.e. anterolateral processes of Murray and Vickers-Rich Citation2004, p. 235). Considering the form of M. maleo, an alternative hypothesis to that above is that the lateral edge of the caudal rostrum in G. newtoni may have been a mediolaterally thin, dorsoventrally tall facet that adjoined an unfused lacrimal. However, an implication of this separate or discrete lacrimal scenario would have been a severely restricted orbital space in G. newtoni, and since no separate lacrimals have been reliably identified for any dromornithid, this hypothesis is considered unlikely.
Cranium
Orbit and rostrodorsal region of cranium
The cranium of G. newtoni, as for other dromornithids, is marked by large (relative to the cranium), widely separated orbits extending caudolaterally from the craniorostral hinge, that occupy the rostral 45% of total cranial rostrocaudal length and 35% of cranial dorsoventral depth, in lateral view. Specimen SAMA P59516 preserves both orbits with an estimated interorbital width across the dorsal surface of the frontals of minimally 74.4 mm, although poor preservation of the cranium likely underestimates that of the undistorted skull, and precludes standard interorbital width measurements (measurements are restricted to the region just caudal of the hinge). As for all dromornithid crania, there is no depressio frontalis, the roof of the cranium is smoothly curved in sagittal section, contrary to most galloanserans.
The crista supraorbitalis (; sensu Livezey and Zusi Citation2006) of G. newtoni is a sharp crest along its entire length that encloses about a third of the circumference of the orbit from the hinge to the processus postorbitalis. In rostral view, the crest flares laterally as a convex arc far more than in species of Dromornis. When viewed laterally, the caudal part of the crest appears straight as in Ilbandornis woodburnei, and less rounded than in D. planei. This lack of curvature of the orbit in lateral aspect is enhanced by the medially convergent rostral half of the supraorbital crests that are also relatively dorsoventrally flattened. Its dorsal and ventral surfaces are penetrated by numerous neurovascular foramina, in addition to the unique vesicular surface texture (most apparent on specimen NMV P256893, see , see also Stirling Citation1913, p. 113). The crista supraorbitalis of Gastornis giganteus is a robust, laterally-prominent crest of bone (Matthew and Granger Citation1917; also pers. observ. from photographs), more so than that of the dromornithids. The wide mediolateral width between supraorbital crests contrasts with the narrower form typical of Anseres, as well as the late Paleocene anseriform Anachronornis anhimops Houde et al. Citation2023 (see Houde et al. Citation2023). Contrary to Murray and Vickers-Rich (Citation2004, p. 263), we find no evidence of an impression or fossa for ‘nasal salt glands’ (fossa glandulae nasalis, also see King Citation1993) on the dorsal frontal area in any G. newtoni specimens. However, we recognise the presence of a vascularised depression in species of Dromornis, where it is largest and most obviously depressed in D. planei (see Murray and Vickers-Rich Citation2004, p. 263), which may or may not be associated with a nasal gland. The anhimid Chauna torquata has a morphology comparable with the latter, especially.
Figure 2. Genyornis newtoni caudal orbit, NMV P256893, rostral view showing the caudal orbit and quadrate articulation with the skull and mandible: A. Image; B. Annotated outline. Annotations: ap.zyg.oss., aponeurosis zygomatica ossificans; cr.or., crista supraorbitalis; f.pseu., fossa pseudotemporalis; mand., mandible; m.lev., origin for m. levator palpebrae dorsalis; n.for., foramen neurovasculare; quad., quadrate. Scale bar: 10 mm. Dark grey shading indicates regions where damage precludes morphological assessment, and light grey indicates foramina and fossae. Dotted lines provide approximate region corresponding to labelled area, and do not indicate exact boundaries.
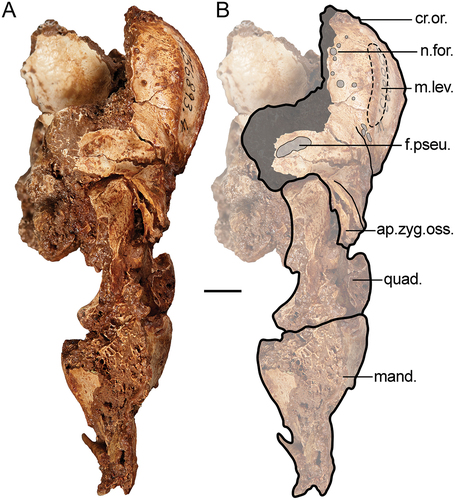
Figure 3. Lateral view of the left side of the cranium of Genyornis newtoni: A. SAMA P59516 with shaded indication of focus region; B. SAMA P59516 image and annotated outline, digitally removed from the image of the entire skull; C. NMV P256893 image (includes part of the mandible and quadrate ventrally) and annotated outline; D. Left rostrolateral view of Anhima cornuta specimen NMV B12574, box denotes region of focus in E., Quadrate disarticulated; E. Rostrolateral view of the left lateral cranium of Anhima cornuta specimen NMV B12574. Annotations: an.tymp., annulus tympanicus; ap.dep.m., an aponeurotic site of origin of m. depressor mandibulae; ap.zyg.oss., aponeurosis zygomatica ossificans; cr.zyg., crista zygomatica; cr.nu.trans., crista nuchalis transversa; cr.or., crista supraorbitalis; cr.art., crista aponeurosis articularis; f.pseu., fossa pseudotemporalis; imp.dm., impressio m. depressor mandibulae; imp.sup., impressio m. AME superficialis; jug., jugal arch; mand., mandible; m.a.e., osseous meatus acusticus externus; pr.par., processus paroccipitalis; pr.post., processus postorbitalis; pr.sup., processus suprameaticus; quad., quadrate; t.pseu., tubercle for m. pseudotemporalis (specifically aponeurosis pseudotemporalis superficialis). Scale bars: A. 50 mm, B., C. 20 mm, D., E. 10 mm. Dark grey shading indicates regions where damage precludes confident morphological assessment, and light grey indicates foramina and fossae. Dotted lines provide approximate regions corresponding to labelled areas, and do not represent accurate boundaries.
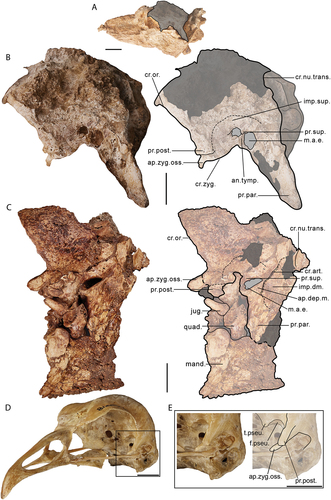
Caudal orbit
Just ventromedial of the caudal part of the crista supraorbitalis, on the dorsocaudal surface of the orbit, is a dorsoventrally tall, rugose surface, pitted by numerous foramina, that likely corresponds to the origin of m. levator palpebrae dorsalis (m.lev., see also Shufeldt Citation1890, p. 55–56; Fisher and Goodman Citation1955: fig. 2; Elzanowski Citation1987). This is consistently present among dromornithid crania that preserve this region.
The caudoventral part of the orbit, which includes the area muscularis aspera, is marked laterally by a distinct fold of bone formed by the processus postorbitalis and aponeurosis zygomatica ossificans (see ‘Temporal region’ for discussion of the osteology and myological correlates of this region). The area muscularis aspera is not completely visible in any specimen of Genyornis newtoni, but is well-preserved in specimens of Dromornis murrayi, D. planei and Ilbandornis woodburnei. In these taxa, a rostrally projected tubercle sits, dorsal to the foramen n. maxillomandibularis (identified in Worthy et al. Citation2016a), and medial of a distinct fossa; this fossa is identifiable in G. newtoni specimen NTM P256893 but not the parts more mesad. The tuberculum likely hosts the aponeurosis pseudotemporalis superficialis (see also Davids Citation1952a, p. 89–90, fig. 10 a, b, ‘aponévrose 10, tuber 10’; sensu Dzerzhinsky and Potapova Citation1974; Weber Citation1996), in association with the m. pseudotemporalis superficialis (Vanden Berge and Zweers Citation1993: annot. 19), which is a significant component of the adductor mandibulae internus group (e.g. Dzerzhinsky Citation1982; Vanden Berge and Zweers Citation1993; Murray and Vickers-Rich Citation2004; Holliday and Witmer Citation2007), in recognition of a similarly located crest or attachment region in other galloanserans (e.g. Anhima cornuta, Anseranas semipalmata, Alectura lathami). Prominent cristae associated with the aponeuroses of m. pseudotemporalis, have been identified in other birds that also have proportionally heavy mandibles. These include heavy-billed finches (e.g. Geospiza fortis [Thraupidae], see Genbrugge et al. Citation2011: fig. 4, Cr7, Cr8), species of Phoenicopterus [Phoenicopteridae], and Porphyrio [Rallidae] (pers. observ.; Baumel and Witmer Citation1993, annot. 89). Discussion on the variation in the morphology of this region between species of Dromornis and Ilbandornis is covered by Worthy et al. (Citation2016a), although we suggest a different musculature arrangement following a reassessment of the relevant osseous correlates.
We also associate the aforementioned fossa with m. pseudotemporalis superficialis (e.g. Dzerzhinsky Citation1982, also see above references), and the feature termed the ‘fossa pseudotemporalis’ by Murray and Vickers-Rich (Citation2004, p. 240, figs. 52, 76, 77, 92, 188). Murray and Megirian (Citation1998) attributed this fossa to ‘mm. protractor quadratus and pterygoideus’ and Worthy et al. (Citation2016a), to ‘mm. AME medialis et superficialis’. This particularly well-developed cavity in dromornithids has been recognised as characteristic of this family (Murray and Vickers-Rich Citation2004, p. 76), but is also typical of anseriforms, in support of systematic affinities with that group (Murray and Megirian Citation1998, p. 80, 96; Murray and Vickers-Rich Citation2004, p. 240). An especially similarly deep fossa is present in anhimids (pers. observ., also see Dzerzhinsky Citation1982). Other anseriforms, such as Anseranas semipalmata and Anser caerulescens, have a distinct depression for the muscle origin in the same position. That in the latter species is notably more like that of Dromornis murrayi, in which the fossa is shallower than in other dromornithids.
In association with the fossa pseudotemporalis and the medially adjacent tubercle, the impression for the origin of m. pseudotemporalis superficialis appears to maximally extend to the laterally adjacent aponeurosis zygomatica ossificans and processus postorbitalis on the caudal orbit in all dromornithids. The area of origin extends to a rostroventral portion of the lateral cranium (including the temporal fossa, see ‘Nomenclature’) in several avian clades, e.g. palaeognaths (Elzanowski Citation1987, p. 83; Holliday and Witmer Citation2007: and references therein), although, is typically restricted or confined behind the eye to the area muscularis aspera in anseriforms and galliforms (e.g. Lakjer Citation1926; Hofer Citation1950; Davids Citation1952a; Starck and Barnikol Citation1954; Goodman and Fisher Citation1962; Fujioka Citation1963; Dzerzhinsky and Belokurova Citation1972; Zweers Citation1974; Dzerzhinsky Citation1982; Weber Citation1996; Matsuoka et al. Citation2008). The latter, in effect, forms the fossa pseudotemporalis, similar to the apparent dromornithid condition. The incidence and variable development of cristae on the area muscularis aspera, and expansion of the pseudotemporal fossa presumably acts to increase the attachment surface area for this muscle within its restricted region of origin. The relative expression of the muscle origin area appears to correlate with size of the mandible, being deeper in species with larger mandibles.
Temporal region
As in all galliforms and anhimids, the dromornithids have relatively small processus postorbitales that project ventrally and slightly rostrally; in contrast, in non-anhimid anseriforms they are more elongate and rostrally directed and contribute to the ventral margin of the orbit (Baumel and Witmer Citation1993, annot. 14; Zusi and Livezey Citation2000; Field et al. Citation2020). The basal anseriform Anachronornis anhimops also has postorbital processes that project only slightly rostrally and are not as rostrocaudally extensive as those in anatoids (however, see below, Houde et al. Citation2023).
The aponeurosis zygomatica (sensu Elzanowski Citation1987; Weber Citation1996; Zusi and Livezey Citation2000) is one of the major aponeuroses of the external adductor musculature complex (specifically m. AME profundus, pars zygomaticus) in Neornithes, although its ossification to form aponeurosis zygomatica ossificans (AZO; sensu Zusi and Livezey Citation2000, p. 166) only occurs in some neornithine species (Zusi and Livezey Citation2000). Additionally, the interaction and development of the ossified and unossified aponeurosis, processus zygomaticus and processus postorbitalis, and the consequent impacts on the arrangement of the associated musculature, varies across Galloanserae, and has systematic importance (see Zusi and Livezey Citation2000; Murray and Vickers-Rich Citation2004: fig. 136). To adequately illustrate the morphology presented in G. newtoni and other dromornithids, contextual description of the state in other galloanserans is required. The myology of the adductor chamber in galliforms has been extensively researched (e.g. Burggraaf Citation1954a; Fujioka Citation1963; Dzerzhinsky and Belokurova Citation1972; Dzerzhinsky Citation1974, Citation1980; Weber Citation1996; Zusi and Livezey Citation2000). In most galliforms, ossification of aponeurosis zygomatica occurs and the resultant AZO extends rostrally from the processus zygomaticus; in many, the aponeurosis connects rostrally with the ventral tip of the processus postorbitalis (see Kirikov Citation1944; Olson and Feduccia Citation1980; Dzerzhinsky Citation1982, Citation1995; Zusi and Livezey Citation2000). Complete connection forms the orbitozygomatic junction (sensu Elzanowski and Mayr Citation2017) and encloses a secondary temporal fenestra (see Elzanowski and Mayr Citation2017). This morphology is notably extreme in gastornithids, in which the AZO forms a large, robust bridge, traversing more than half the side of the cranium (Matthew and Granger Citation1917; Troxell Citation1931; Andors Citation1992; Murray and Vickers-Rich Citation2004; Elzanowski and Mayr Citation2017).
In the basal anseriform family Anhimidae, the aponeurosis zygomatica also ossifies to form the AZO, however, unlike galliforms, there is no zygomatic process (Zusi and Livezey Citation2000). The aponeurosis zygomatica has a linear attachment along the rostrocaudal length of the lamina lateralis cranii (sensu Zusi and Livezey Citation2000), and passes medial to, and rostrally beyond, the processus postorbitalis (see Dzerzhinsky Citation1982; Zusi and Livezey Citation2000: fig. 6, 7; Murray and Vickers-Rich Citation2004, p. 166–167, fig. 136). The aponeurosis is ossified along much of this length in adult anhimids, except for in its rostral-most part (Zusi and Livezey Citation2000, p. 173–175). Through myological, ontogenetic and homological studies relating to the osseous structures of the adductor chamber in these birds (Dzerzhinsky Citation1982, Citation1995; Zusi and Livezey Citation2000), it is currently understood that the wedge-shaped temporal region corresponding to the AZO and postorbital process is related to the evolutionary medial migration of the origin of m. AME profundus, pars coronoideus (sensu Zusi and Livezey Citation2000; terminology of Holliday and Witmer Citation2007), relative to a typical galliform state. This medial retreat of fibres that would normally arise from the dorsotemporal fossa effectively truncates the lateral squamosum rostrally from the caudoventral orbit, while impressio m. AME superficialis (caudotemporal fossa) remains on the caudolateral squamosum. Consequently, the AZO is closely associated with the processus postorbitalis in anhimids and contributes to the laterally adjacent crista m. AME superficialis (sensu Zusi and Livezey Citation2000; using terminology of; Holliday and Witmer Citation2007), and laterally delimits the impressio m. AME profundus, pars coronoideus (Dzerzhinsky Citation1982; crista zygomatica sensu Zusi and Livezey Citation2000).
Dzerzhinsky (Citation1982, p. 1031) originally interpreted this complex as a sesamoidal ossification and superimposition upon the processus zygomaticus (i.e. AZO), fused to a caudoventrally expanded base of the processus postorbitalis, effectively closing the dorsotemporal fossa, and termed the resultant composite osseous structure the processus sphenotemporalis (see also Dzerzhinsky Citation1995). Using ontogenetic evidence, Zusi and Livezey (Citation2000, p. 175–177, 180–181) further honed interpretations on the homology of this structure, regarding the contribution of the AZO. They noted that the processus zygomaticus does not contribute to this morphology in anseriforms and that the morphology was better explained as an evolutionary product of the rostral extension of the origin for aponeurosis zygomatica to the processus postorbitalis and the aforementioned ventromedial migration of the origin of m. AME profundus, pars coronoideus (see above). Recently, Houde et al. (Citation2023) dismissed Zusi and Livezey’s interpretations and advocated for a sphenotemporal process, and the presence of a processus zygomaticus that abuts the laterosphenoidale ventrally at sutura laterospheno-squamosa in anseriforms. We instead find the hypotheses stemming from the comprehensive homological and ontogenetic study of this region by Zusi and Livezey (Citation2000) to be more compelling, especially in consideration of the following important observations: (1) identification of a potential incipient processus zygomaticus at the caudal-most area corresponding to the origin of the aponeurosis zygomatica in some immature anseriforms; (2) the recognition that in anatids, the rostroventral extension of squamosum and its shape in its interaction with the laterosphenoidale (including processus postorbitalis) at sutura laterospheno-squamosa can falsely resemble a processus zygomaticus; and (3) the region of the squamosum which interacts with the laterosphenoidale is non-homologous with the area from which the processus zygomaticus arises in many other Neornithes (see Zusi and Livezey Citation2000, p. 170, fig. 4, 5; Mayr and Manegold Citation2021). We thus opt to follow the homological interpretations of Zusi and Livezey (Citation2000), herein, owing to lack of evidence that rejects the findings of their focused study, nor robustly supports alternative hypotheses. Zusi and Livezey (Citation2000) further noted that while the impressio m. AME profundus, pars coronoideus, was also similarly medially located in other anseriforms, its relationship to osseous structures was different in non-anhimid anseriforms. There is little to no ossification present for the zygomatic aponeurosis (crista zygomatica sensu Zusi and Livezey Citation2000, see below) in addition to a distinct processus zygomaticus being absent (the homologous locus may be represented by a tubercle, see Zusi and Livezey Citation2000), and the processus postorbitalis (of the laterosphenoidale bone) is rostroventrally developed and orientated. The impressio m. AME profundus, pars coronoideus, effectively has greater association with the processus postorbitalis in Anseres (that is anseriforms exclusive of anhimids, see Livezey Citation1997; Worthy et al. Citation2017b), compared to arising from the ventromedial AZO as in species of Chauna and Anhima.
In dromornithids, it is clear that considerable ossification of the aponeurosis zygomatica occurs and produces a morphology, including a distinct, conspicuously rostrally projected AZO, which we interpret to be a near identical osteological arrangement to that of anhimids (also previously mentioned by Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a). This morphology is observed in all dromornithid crania with adequate preservation. As a result, in G. newtoni, the m. AME profundus, pars coronoideus, is interpreted to originate ventromedially on the AZO (also inferred for species of Dromornis and Ilbandornis by Murray and Vickers-Rich Citation2004: fig. 188) and the impressio m. AME superficialis is representative of the only part of the m. AME complex to remain on the lateral squamosal (and the ‘temporal fossa’). The various features of this region are best preserved in specimen NTM P256893 () of G. newtoni, as well as Ilbandornis (OMV2000:GFV:20 pers. observ.) and D. planei (NTM P9464–106, see Murray and Vickers-Rich Citation2004).
In G. newtoni, the impressio m. AME profundus, pars coronoideus, is a wide and deep, mediodorsally expanded depression, which is more similar in form to Chauna torquata than Anhima cornuta, and although slightly differs in osteological homologies (see below), is similar in shape to the same impression in Anseranas semipalmata and Anachronornis anhimops. It is more dorsoventrally expansive than in Ilbandornis woodburnei, and more comparable to Dromornis planei. The impression is bordered dorsomedially by a rostrocaudally elongate crest, and laterally by the crista zygomatica which may be nearly confluent and continuous with the crista m. AME superficialis in the rostral-most area, but not caudally (see below, also Zusi and Livezey Citation2000: fig. 6, 7, p. 175). The crista zygomatica is formed from the crest-like ossification of the aponeurosis zygomatica where it meets the cranium, ventral or ventromedial of the dorsolateral bounds of the origin of m. AME superficialis, and is not mutually exclusive with respect to the more extensive aponeurotic ossification that characterises AZO in many galloanserans (see Zusi and Livezey Citation2000: fig. 6, p. 175, ). Scarring, present both laterally and medially on the rostroventral AZO in specimen NMV P256893 of G. newtoni, suggests that it predominantly or exclusively supported the unossified aponeurosis zygomatica. Comparably, in D. planei, the unossified aponeurosis zygomatica appears to have additionally emanated from the postorbital process (see Murray and Vickers-Rich Citation2004: fig. 188, ‘crista AME superficialis’). In anatids (e.g. Anser caerulescens, and Tadorna tadornoides), the minor ossification of the aponeurosis zygomatica (if present) typically produces only a low, ridge-like crista zygomatica which is ventrally separated from, and medial to, the crista m. AME superficialis (Zusi and Livezey Citation2000, p. 175, fig. 6).
It is likely that aponeurosis zygomatica emanated from the rostrocaudal length of lamina lateralis cranii in the late Paleocene anseriform Anachronornis anhimops, as it does in anhimids, and a small caudal impression, rostrally adjacent to processus suprameaticus would have accommodated the origin for m. AME superficialis. The latter state is also comparable to that of anhimids, while the relatively expansive impressio m. AME profundus, pars coronoideus, best resembles the anseranatid and dromornithid condition. Like anseranatids, but unlike anhimids and dromornithids, there is no conspicuous rostral projection with apical ossified fibres or scarring, and related mediolateral thickening of the ventral lamina lateralis cranii, that would be typical of an extensively ossified aponeurosis zygomatica. This is further supported by the observable sutura laterospheno-squamosa ventrally on Anachronornis anhimops, which comparatively appears obscured by the development of AZO in anhimids and dromornithids, but importantly denotes that the more rostral area corresponds to the laterosphenoid and processus postorbitalis (see Houde et al. Citation2023: fig. 1 A). Ontogenetic evidence suggests that rostral development of the laterosphenoid bone, not associated with the AZO, is characteristic of Anseres, where rostral projection of this region is additionally accomplished through the development of the AZO in anhimids and dromornithids (Zusi and Livezey Citation2000). Additionally, as evidenced by some incomplete fusion of cranial elements in the cranium of A. anhimops, its cranium shape and pattern of suture arrangement closely resembles the skull of juvenile Chauna torquata, before the AZO has entirely developed (compare Zusi and Livezey Citation2000: fig. 5 A; Houde et al. Citation2023: figs. 1D, S3). While the rostral projection of processus postorbitalis in A. anhimops is superficially similar to the state of anhimids and dromornithids, it is thus likely analogous, in support of this taxon possessing an intermediate condition between extant anhimids and Anseres (Houde et al. Citation2023, p. 16).
A foramen temporale venosum (sensu Mayr et al. Citation2021) has been recognised within the region of origin for m. AME profundus, pars coronoideus, in Galliformes, leading this feature to be considered a cranial autapomorphy of the group (see Mayr et al. Citation2021: fig. 4). Despite variation in the location of the non-ossified aponeurosis zygomatica, a seemingly homologous foramen is also present in the region of attachment for this muscle in all anatids compared in this study, and possibly Presbyornis pervetus Wetmore Citation1926 (USNM 299846). This foramen was not identified in dromornithids or Anhima cornuta. Considering this foramen is aligned with the sutura laterospheno-squamosa, most visible in younger individuals (e.g. Cygnus olor, see Houde et al. Citation2023: fig. S2), it may be associated with the fusion of the two bones in adulthood. Presence or absence in dromornithids and anhimids cannot be confirmed potentially due to the AZO deforming this region.
The partes superficialis et zygomatica of m. AME profundus (Zusi and Livezey Citation2000; Holliday and Witmer Citation2007; Appendix Three) originate on the lateral and medial sides of the unossified aponeurosis zygomatica, respectively (Zusi and Livezey Citation2000, p. 169), and are located well rostrad in galloanserans compared to the condition in other avian orders due to the relatively large size of m. AME superficialis (see Zusi and Livezey Citation2000, p. 177, fig. 8). In anseriforms, these two distinct bellies, partes superficialis et zygomaticus, uniquely originate from the unossified aponeurosis zygomatica rostral of its attachment to the processus postorbitalis (Zusi and Livezey Citation2000, p. 177, figs. 6 and 8). The morphology in dromornithids appears similar to anhimids (see Murray and Vickers-Rich Citation2004: fig. 136), so the bellies are deemed likely to arise from the unossified aponeurosis zygomatica in G. newtoni, in a similarly rostral position. Any attachment surface(s) corresponding with m. AME medialis cannot be identified and may be interrelated with those of m. AME profundus, considering that this muscle is not easily distinguishable from the latter muscle part in birds (Holliday and Witmer Citation2007, p. 481; Appendix Three).
As aforementioned, the impressio m. AME superficialis (caudotemporal fossa) is the only part of the temporal fossa retained on the lateral squamosal in dromornithids (see ‘Nomenclature’, and also Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a). It is characterised by a depression that extends caudally from processus postorbitalis and the rostral end of the AZO to a variable position dorsal of the dorsocaudal margin of the osseous meatus acusticus externus (external auditory canal) and is relatively medially positioned like in Anhimidae (see Zusi and Livezey Citation2000). The dorsal bounds of this muscle origin are rounded and poorly defined on the lateral squamosal in all dromornithids, and clearly dorsolateral of crista zygomatica (see ). In contrast, crista m. AME superficialis is pronounced ventrally, crest-like in its association with aponeurosis mediosuperficialis (sensu Dzerzhinsky Citation1982; Dzerzhinsky and Grintsevichene Citation2002), and nearly confluent with the crista zygomatica across most of its rostrocaudal length in both anhimids and anseranatids, and also apparently in anachronornithids (see above and Zusi and Livezey Citation2000, p. 175; Houde et al. Citation2023). Presumably, the lack of a distinct crista m. AME superficialis caudal of the AZO in dromornithids may have myological implications with regards to superficial aponeurotic attachment and proportional forces acting in this region (Bernhard Citation1924; Bryant and Seymour Citation1990 and references therein). While the degree of ossification of aponeurosis zygomatica in anatids clearly differs from that of dromornithids and anhimids (and anseranatids and anachronornithids to a lesser extent, see above), the dorsoventral separation between the dorsal bounds of impressio m. AME superficialis and crista zygomatica in anatids is more comparable to the dromornithid condition. The caudal extent of the impressio m. AME superficialis in dromornithid taxa is variously defined by a pronounced tubercle or dorsoventrally aligned ridge. This tubercle (in part) relates to an aponeurotic attachment of the caudal m. AME superficialis (crista aponeurosis articularis, sensu Murray and Vickers-Rich Citation2004: fig. 188; fig. 4). Compared to the large and rugose form of D. planei, the crest is less pronounced in Ilbandornis woodburnei and even less distinct, and more of a low ridge in G. newtoni. Several galliform taxa have an angular, rostrally protruding ossified aponeurotic fibre mass associated with this region caudal of the processus suprameaticus (e.g. Lagopus lagopus), whereas in anseriforms, including anhimids, the caudal-most attachment of the m. AME superficialis is represented by an incurvate and variably pronounced ridge, dorsal of the suprameatic process.
Figure 4. Soft-tissue attachment sites on the cranium of a dromornithid, illustrated using a digitally modified representation of Ilbandornis woodburnei (QMV 2000:gfv:20) due to the relative lack of deformation in this skull and the conservative nature of dromornithid skull morphology: A. Rostral aspect; B. Caudal aspect; C. Ventral aspect; D. Right lateral aspect; E. Oblique (rostrolateral) aspect. Abbreviations: ap.art., origin site of aponeurosis articularis; ap.dep.m., an aponeurotic site of origin of m. depressor mandibulae; ap.med.sup., origin site of aponeurosis mediosuperficialis; ap.sup., origin site of aponeurosis superficialis; ap.zyg., origin site of aponeurosis zygomatica (muscle fibres of m. AME profundus, pars zygomaticus, and m. AME profundus, pars superficialis, originate from the medial and lateral surfaces of this aponeurosis, respectively); lig.oc.mand., attachment area corresponding to the origin of ligamentum occipitomandibulare, continuous with that of membrana postmeatica; lig.post.orb., attachment area of origin of ligamentum postorbitale; m.AME.prof.cor., origin area of m. AME profundus, pars coronoideus; m.AME.sup., origin area of m. AME superficialis; m.biv.cerv., insertion area of m. biventer cervicis; m.c.c., origin area of m. cucullaris capitis; m.comp., insertion area of m. complexus; m.dep.mand., origin area of m. depressor mandibulae; m.pro.pter.quad., origin area of m. protractor pterygoidei et quadrati; m.ps.tem.s., origin area of m. pseudotemporalis superficialis; m.rec.cap.dors., insertion areas for slips of m. rectus capitis dorsalis; m.rec.cap.lat., insertion area of m. rectus capitis lateralis; m.rec.cap.vent.lat., area for aponeurosis of insertion of m. rectus capitis ventralis, pars lateralis; m.rec.cap.vent.med., insertion area of m. rectus capitis ventralis, pars medialis; m.spl.cap., insertion area of m. splenius capitis; mem.at.oc.dors., attachment area of membrana atlantooccipitalis dorsalis; mem.at.oc.vent., attachment area of membrana atlantooccipitalis ventralis; mem.pm., attachment site of membrana postmeatica, which incorporates and is not separatable with respect to the more ventromedial ligamentum occipitomandibulare. Scale bar: 50 mm. Illustrated attachment areas on the cranium are non-extensive estimates; only the sites corresponding to selected muscles, aponeuroses, membranes and ligaments are indicated.
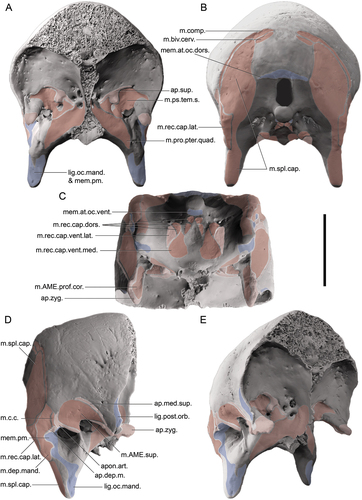
The inferred myological topology associated with the dromornithid adductor chamber (), as evidenced by patterns of fossae and cristae upon the skull (discussed above), are similar to the anhimid condition, as explored and illustrated by several authors (e.g. Dzerzhinsky Citation1982; Zusi and Livezey Citation2000; Dzerzhinsky and Grintsevichene Citation2002). The primary variations between these two lineages, aside from greater rostrocaudal foreshortening of the dromornithid cranium, is present in the proportional sizes of the processus postorbitalis and AZO, which are enlarged and more robust, the dorsoventral separation between crista zygomatica and the dorsal bounds of m. AME superficialis, and the development of crista aponeurosis articularis. The greater size and depth of the impression m. AME superficialis (caudotemporal fossa), the proportionally enlarged rostral end of the AZO, and evidence for further attachment for the non-ossified portion of the aponeurosis zygomatica on the postorbital process in species of Dromornis, may be also linked to the larger, heavier mandible relative to skull size, compared to G. newtoni and species of Ilbandornis. In D. planei, we associate the relative reduction of the processus postorbitalis and the process tip not ventrally overhanging the ossified zygomatic aponeurosis with the requirement for greater adductor musculature throughout this region; this may imply that the ligamentum postorbitale was absent, as observed for the large-billed finch Geospiza fortis (see Genbrugge et al. Citation2011, p. 692). This ligament is also absent in the erismaturine anatid Oxyura jamaicensis (see Goodman and Fisher Citation1962). Specimen SAMA P59516 of G newtoni, shows the ventral extent of the processus postorbitalis was variable in this lineage; we recognise three potential drivers: (a) intraspecific variation associated with an absence of selection pressures on the shape of the processus postorbitalis and presence of the ligamentum postorbitale, (b) sexual dimorphism in the mass of the muscles in this region, as SAMA P59516 is considered a likely female (Chinsamy and Worthy Citation2021) and NMV P256893, sex unknown, and (c) ontogenetic variation. Although the ligamentum postorbitale serves to coordinate motion of both upper and lower jaws as the mandible is depressed, it is not required as it is only one of several means of doing this (Zusi Citation1967; Bout and Zweers Citation2001).
Lateral aspect of the caudal cranium
In all three partially preserved crania of G. newtoni, specimens SAMA P59516, NMV P256893, and SAMA P59520, the otic head of the quadrate remains articulated in the quadratic cotyla of the cranium, ultimately obscuring it. For other dromornithids where it is visible, e.g. I. woodburnei specimen QMV:2000:GFV:20, I. ?lawsoni NTM P907–27, and D. planei specimen QM F57947, we use the term, cotyla quadratica squamoso-otica (named for the close approximation of the two quadratic cotylae which, together form a single articular facet, see also Baumel and Raikow Citation1993, annot. 25; also termed recessus quadratica, sensu Worthy et al. Citation2016a). In species of Ilbandornis and Barawertornis, the residual homolog of recessus tympanicus dorsalis extends dorsolaterally as a shallow fossa, partly separating the constituent cotylae, at their ventromedial margins, although it is thinnest and deepest in B. tedfordi (specimen QM F58013). An exceedingly small foramen is present centrally on the cotyla quadratica squamoso-otica in D. murrayi, D. planei, and I. woodburnei, likely a remnant of the associated foramen pneumaticum dorsale (Baumel and Witmer Citation1993: annot. 25; Mayr Citation2020) along with a second small foramen at the medioventral margin of the aforementioned dorsal tympanic depression. A large foramen just medial of this and between the articulatory surfaces is associated with the foramen for ramus occipitalis of arteria ophthalmica externa. Due to similarities in quadrate morphology (see ‘Quadrate’), and relatively consistent morphology for this region across all dromornithid specimens where it can be observed, we assume that G. newtoni likely had a similar cotyla quadratica squamoso-otica and associated features. This morphology is uncommon within Galloanserae and differs dramatically from the condition in non-anhimid anseriforms, and megapodes which typically possess two distinct quadratic cotylae, cotyla quadratica squamosi and cotyla quadratica otici, separated by a deep recessus tympanicus dorsalis, and an associated, generally large foramen pneumaticum dorsale (sensu Mayr Citation2020; e.g. see Anseranas semipalmata, Tadorna tadornoides, and Alectura lathami). In many non-megapodiid galliforms and S. neocaledoniae (see Mourer-Chauviré and Balouet Citation2005), the cotylae are only separated by a thin depression continuous with a dorsally located recessus tympanicus dorsalis consistent with the more closely applied condyles on the quadrate head (e.g. Acryllium vulturinum and Lagopus lagopus).
In Genyornis newtoni, SAMA P59516, the quadratic capsule is bounded laterally by a thin osseous wall, perforated by a small, ovular (9.4 mm high, 7.1 mm wide) fenestra, through which the pars otica of the quadrate, still in articulation, can be viewed. This lamina extends caudoventrally from the AZO, to join the thin, bar-like annulus tympanicus (processus postglenoidalis, sensu Stellbogen Citation1930; see also Baumel and Witmer Citation1993: annot. 77; Mayr Citation2020). There is little evidence for this lamina being an artefact of preservation or damage in specimen SAMA P59516, and damage to both SAMA P59520 and NMV P256893 precludes a non-ambiguous assessment of this feature in these G. newtoni specimens. This feature may then reflect unusual intraspecific variation, arising from an extension of the squamosum, or ossification of aponeurosis zygomatica, m. AME superficialis, a membrane, ligament, or cartilage. Alternatively, the absence of this character in other dromornithid taxa, and all other assessed galloanserans, may imply that this character is autapomorphic to G. newtoni.
The processus suprameaticus is mediolaterally flattened in G. newtoni, as in D. planei, and less prominent than the more rounded, ventrally protruding process in I. woodburnei, which is more distinct from the annulus tympanicus, a bony ridge connecting the process ventrally with the ala parasphenoidalis (Stellbogen Citation1930; Mayr Citation2020). In all dromornithids, the lateral overhang of the small processus suprameaticus relative to the caudolateral margin of the cotyla quadratica squamoso-otica is minimal (D. murrayi) to lacking (D. planei, I. woodburnei, G. newtoni). This is uncommon within Galloanserae, even those with an annulus tympanicus (see below), due to variation in the rostrocaudal location of the process relative to the cotyla. The processus suprameaticus of Gastornis giganteus and S. neocaledoniae, considerably laterally overhangs the caudolateral margin of the cotyla quadratica squamosi.
In species of Ilbandornis and Dromornis, the annulus tympanicus is especially robust compared to G. newtoni. Ossification of this bar also occurs in some anatids (Anser caerulescens, Cereopsis novaehollandiae, and Cnemiornis calcitrans; see also Worthy et al. Citation1997, Citation2017b: app. 1, char. 16), odontophorids (Callipepla californica), and phasianids (Perdix perdix and Syrmaticus soemmerringii, for the latter see Mayr Citation2020: fig. 8), suggesting variable development among galloanserans. In dromornithids, the ventromedial extension of the annulus tympanicus from processus suprameaticus, to fuse to the enlarged and laterally projecting ala parasphenoidalis, encloses the osseous meatus acusticus externus ventrorostrally. The enclosed nature of the osseous meatus acusticus externus, differs considerably from most galloanserans, including the gastornithids and sylviornithids, which appear to lack an ossified annulus tympanicus associated with the processus suprameaticus. In G. newtoni, the lateral opening of the ear (the osseous meatus acusticus externus) is relatively small and circular, approximately 10.8 mm high and 10.05 mm wide in SAMA P59516, although it is more oval shaped in NMV P256893. Species of Dromornis and Ilbandornis have a proportionally larger and less enclosed (especially ventrally) osseous meatus acusticus externus relative to the rostrocaudal length of the cranium, and the laterally flaring and broadly curved margins give a more funnel-shaped appearance in these taxa (Murray and Megirian Citation1998; Worthy et al. Citation2016a). Much of the otic region, and the more medial cavum tympanicum proper (see Witmer Citation1990; Baumel and Witmer Citation1993: annot. 21), is obscured by sediment and compression in all specimens of G. newtoni and, therefore, cannot be described in further detail (see Mayr Citation2020 for an assessment of the otic region in Neornithes), although as is typical for Galloanserae, the pila otica is observed to be non-trabeculated (see Mayr Citation2020).
The processus paroccipitalis projects far ventrally and slightly laterally, terminating 58.5 mm past the ventral margin of the osseous meatus acusticus externus in specimen SAMA P59516 of G. newtoni. The process is mediolaterally compressed and flattened with subparallel rounded rostral and caudal edges. In lateral view, the process is proportionally slenderer and more dorsoventrally elongate than in other dromornithids. In these species, e.g. I. woodburnei, more extensive fusion of the process with the ala parasphenoidalis creates a rostrocaudally wide and robust triangular structure, when viewed laterally, like that of non-anhimid anseriforms; anhimids and galliforms have far smaller processus paroccipitalis, which extend maximally to a point level with the ventral floor of the otic region. The elongated length of the processus paroccipitalis in G. newtoni, resembles that of the processes in Ga. giganteus and S. neocaledoniae. In G. newtoni, a marked attachment region for the ligamentum occipitomandibulare on the rostrolateral surface of the ventral part of the processus paroccipitalis is largely obscured or damaged in all specimens that preserve this area, but it is visible on the left processus paroccipitalis of SAMA P59516. This attachment is an ovoid, well defined mark in G. newtoni but is comparatively better developed in both D. planei and D. murrayi as a circular, flattened surface. In I. woodburnei, the shallow depression indicating the ligamentous attachment area is relatively smaller and less clearly delimited than in all these species.
On the dorsolateral surface of the processus paroccipitalis, the fossa subtemporalis, or more specifically, the impressio m. depressor mandibulae (van Gennip Citation1986; Weber Citation1996; terminology of Zusi and Livezey Citation2000; see ‘Nomenclature’), is a dorsoventrally elongate, impressed surface, variably obscured by taphonomic damage in all G. newtoni specimens. In most dromornithids, including G. newtoni, the impression is well-defined rostrally in partial association with crista aponeurosis articularis (of m. AME superficialis, see above); however, in D. planei the impression is especially obvious and well delimited on all sides, such as in specimen NTM P9464–106, where the origin of m. depressor mandibulae expands dorsoventrally and is extensively scarred. It is positioned rostrally adjacent to the crista nuchalis transversa as in most birds (Baumel and Witmer Citation1993, annot. 104). In galliforms, the attachment surface is small, more dorsal relative to the bony external auditory canal, and lacks defined margins, making it seem confluent with the temporal fossa in some taxa. Comparatively, anseriforms have a much larger impressio m. depressor mandibulae. In the most extreme forms (e.g. Anhima cornuta, Anseranas semipalmata, Melanitta perspicillata, Aythya australis), the impression extends dorsally along the crista nuchalis transversa to near its dorsal terminus with raised ridges delimiting all sides. Although predominantly hosting the m. depressor mandibulae (van Gennip Citation1986; Vanden Berge and Zweers Citation1993), the impression m. depressio mandibulae may also have hosted fibres relating to the caudal-most origin of the m. AME superficialis, as it does in most neognaths, including Galloanserae (see Holliday and Witmer Citation2007). Concomitantly, in G. newtoni as in other dromornithids, the rostral-most extent of the origin of m. depressor mandibulae is indicated in part by crista aponeurosis articularis. Comparatively, in Anhima cornuta, the crista associated with the rostral-most origin of m. depressor mandibulae is distinct, while the crest indicating the caudal extent of the m. AME superficialis located further rostrally. The rostrocaudal foreshortening of the caudal part of the cranium in dromornithids has seemingly narrowed the distance between the two muscles. This suggests that the greater size and number of associated cristae is likely to accommodate both the caudal-most origin of the m. AME superficialis and the dorsorostral-most origin of the m. depressor mandibulae (see 4.4.4 for further descriptions of impressio m. depressor mandibulae). Unlike in anhimids, the caudal m. AME superficialis and rostral m. depressor mandibulae are closely spaced in anseranatids, and in anatids they abut each other at a single common crest similar to the dromornithid state (see above).
In G. newtoni, the rostral bounds of impressio m. depressor mandibulae are also continued ventrorostrally by a pronounced crest that ventrocaudally borders the osseous meatus acusticus externus. The neck muscle m. cucullaris capitis attaches to the cranium just rostrad and superficial of the rostral-most origin of m. depressor mandibulae in anseriforms, in close association with the rim of the bony external auditory canal (m. dermotemporalis sensu Goodman and Fisher Citation1962; Dzerzhinsky Citation1982; Vanden Berge and Zweers Citation1993: annot. 7; Dzerzhinsky and Grintsevichene Citation2002) and is perhaps related to this crest in G. newtoni. The m. cucullaris capitis originates more dorsorostrally in many galliforms (e.g. Dzerzhinsky Citation1980; Weber Citation1996). Presumably, the relatively pronounced crest that separates impressio m. depressor mandibulae from impressio m. AME superficialis (see above) may have hosted this attachment in other dromornithids.
Occipital region, caudal view
Delimiting the caudal extent of the impressio m. depressor mandibulae, in dorsoventral alignment with the condylus occipitalis, at the lateroventral junction of the crista nuchalis transversa and crista ventralis (crista occipitalis, sensu Dullemeijer, 1951; sensu Ghetie et al. Citation1976; crista occipitalis, sensu Landolt and Zweers Citation1985; linea nuchalis transversa, sensu Weber Citation1996), are bilaterally paired prominentiae exoccipitales (Owen Citation1873, p. 512; Mivart Citation1896: fig. 9; Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a: fig. 1; SI T.4). The two crests form a V-shaped notch ventrally upon each prominentia, likely associated with the insertion of the m. rectus capitis lateralis, which attaches here in many bird groups including anseriforms and galliforms, superficial to the insertion of the lateral most part of m. splenius capitis on the caudal cranium (see Boas Citation1929; Davids Citation1952b, Citation1952c; Goodman and Fisher Citation1962, p. 118; Zusi and Storer Citation1969; Ghetie et al. Citation1976; Landolt and Zweers Citation1985; Vanden Berge and Zweers Citation1993: annot. 43, 44; Lautenschlager et al. Citation2014; Jones et al. Citation2019; Böhmer et al. Citation2020). The prominences are well developed in Anhima cornuta and many other anseriforms (e.g. Anseranas semipalmata, Aythya australis), but are to a greater extent in the dromornithids, especially species of Dromornis, where they contribute to the laterocaudal flare of the cranium margins.
As in all dromornithids, anhimids, and most galliforms, the crista nuchalis transversa is identifiable, although it is not particularly distinct in G. newtoni. Comparatively, the crest is more prominent in anseriforms including Anachronornis anhimops (see Houde et al. Citation2023: fig. 1E), enhanced by deeper fossae dorsal and ventral to it (for an extreme, see Oxyura australis), and the developmental effects of the forces associated with muscles which insert upon or near it, i.e., the m. complexus (m. cucullaris caput part, sensu Goodman and Fisher Citation1962, p. 116) along its edge, the deeper m. splenius capitis, and the dorsally situated m. biventer cervicis (Ostrom Citation1961, p. 88, and references therein; Goodman and Fisher Citation1962, p. 116–117; Landolt and Zweers Citation1985; Vanden Berge and Zweers Citation1993: annot. 41–43; Lautenschlager et al. Citation2014; Böhmer et al. Citation2020). The shape of the crest, although distorted, suggests that the caudal dorsal profile of the cranium of G. newtoni is rounded, like all other dromornithids. With the exception of some specimens such as NTM P9464–106, the mid-dorsal parietal region of the caudal cranium in dromornithids is not well-known as most specimens either do not preserve this region (e.g. NTM P907–27) or have damage precluding visibility (e.g. QMV:2000:GFV:20, QM F57974, SAMA P59516). Based on all available evidence, this region in all dromornithids appears to be distinctly flattened, and lacks (1) a prominentia cerebellaris, (2) a crista nuchalis sagittalis (develops to differing degrees primarily in goose-type non-anhimid anseriforms, and generally absent in other galloanserans, Landolt and Zweers Citation1985; Tambussi et al. Citation2019), and (3) fonticuli occipitales (considered a synapomorphic feature for most anseriforms; lateral occipital fontanelles sensu Beddard Citation1898; Landolt and Zweers Citation1985; Baumel and Witmer Citation1993: annot. 87; Ellrott and Schmitz Citation2010; Tambussi et al. Citation2019).
The crista ventralis is well developed in dromornithids, as in most anseriforms, although to a lesser extent than in the gastornithid Ga. giganteus. The crista ventralis distinctly separates relative dorsal and ventral regions of the caudal face, as it extends laterally, from a short distance above the dorsal margin of the foramen magnum, to converge with the crista nuchalis transversa at the prominentia exoccipitalis (described above). The crista ventralis overhangs the ventral region of the caudal cranium which is steeply angled rostroventrally and encloses a deep depression on either of its ventrolateral sides by the more lateral prominentia exoccipitalis and processus paroccipitalis. This depression is more common in anseriforms than in galliforms in which the crista ventralis is less distinct and more rounded, although some, e.g. Sylviornis neocaledoniae, and to a slight extent, Alectura lathami, also have this region depressed and the crista, rostroventrally angled. Differing from dromornithids however, this region in anseriforms appears almost dorsoventrally compressed, and in many (e.g. Melanitta perspicillata), the condylus occipitalis is ventrally displaced nearer to dorsoventral alignment with the lamina parasphenoidalis.
The condylus occipitalis is rounded laterally and ventrally and has a shallow incisura mediana condyli flattening the dorsal surface and creating a smooth transition into the ventral floor of the foramen magnum (see also in Stirling Citation1913: plate XXXVI, ). The incisura is intermediate in depth in G. newtoni compared to other dromornithids; in I. woodburnei, this dorsal concavity is extremely shallow, yet in D. planei and D. murrayi, it is distinct, more like the deep, curved incisura in many extant and extinct galloanserans, including S. neocaledoniae and Ga. giganteus (Andors Citation1988, p. 138). The condylus occipitalis is pedestalled on a slightly constricted column in G. newtoni, to approximately the same extent as in I. woodburnei, although slightly less so than in species of Dromornis. Compared to the large, rounded foramen magnum found in most galloanserans, that of all dromornithids is a dorsally tall, rectangular opening with sub-parallel sides and a size that only slightly exceeds that of the condylus occipitalis. Ga. giganteus also has a foramen magnum proportionally small compared to the condylus occipitalis (Matthew and Granger Citation1917, p. 312; Andors Citation1988, p. 183; pers. obvs). As in I. woodburnei (see Worthy et al. Citation2016a) and D. planei, the condylus occipitalis and foramen magnum are orientated directly rostrocaudally on the caudal cranium of G. newtoni. The placement of the condylus occipitalis and foramen magnum in D. murrayi is unique among dromornithids in that it is more ventrorostrally positioned and directed, similar to the plane observed in anhimids. In all dromornithids, a fossa subcondylaris is lacking or very shallow; there is no identifiable depression in the region ventral of the condylus occipitalis. The depth of the fossa in other galloanserans is variable with some species developing a distinct cavity (e.g. Tadorna tadornoides).
The paired fossae parabasalis () are shallow in G. newtoni and most other dromornithids; in D. planei they are deeper. The fossae are bordered medially by a distinct crest, likely the crista fossae parabasalis, which is considered present in many anseriforms (Baumel and Witmer Citation1993: annot. 86), as it separates the foramina within the fossa parabasalis from those more medial. It is less distinct in D. planei and species of Ilbandornis and indistinct in D. murrayi. The two foramina for the n. hypoglossi (XII) are represented by a larger foramen just medial to the fossa parabasalis, near the condylus occipitalis, and a second smaller foramen, more ventrally, on the medial margin of the crista fossae parabasalis. Within the fossa, are the foramen n. vagi (X), the foramen n. glossopharyngealis (IX), the ostium canalis carotici (for the carotid and the branch of cranial nerve VII), and the ostium canalis ophthalmici externi (for venae and arteria ophthalmica externa; Baumel and Witmer Citation1993; Weber Citation1996: fig. 6; Worthy et al. Citation2016a; Handley and Worthy Citation2021). The foramen n. vagi and ostium canalis ophthalmici externi sit dorsally within the fossa parabasalis, with the former being the largest of the two, and both medial-most and dorsal-most of three foramina that are nearly aligned dorsoventrally, including the foramen n. glossopharyngealis, and ostium canalis carotici most ventrally. Damage and sediment infilling limit further interpretation on the morphology of this region in G. newtoni. The placement and arrangement of the fossa parabasalis and the associated foramina does not appear to vary much from that of other dromornithids. Variation is present between dromornithids and Ga. giganteus, as well as extant galliforms and anseriforms, where the fossa is located further lateroventrally from the condylus occipitalis; in some anseriforms, the location transitions to be nearly on the ventral surface of the cranium (e.g. Oxyura australis, Cygnus atratus, Tadorna tadornoides, and Melanitta perspicillata).
Figure 5. Caudal and ventral views of the cranium of Genyornis newtoni, digitally cropped from the images of the entire skull: A. SAMA P59516 image and annotated outline in caudal view; B. SAMA P59516 fossa parabasalis and associated foramina for nerves, focus region outlined in A.; C. Complete ventral view of SAMA P59516 with box indicating region featured in D.; D. Ventral cranium, SAMA P59516 image and annotated outline, rotated anti-clockwise from C. Annotations: can., canal tentatively identified as canalis orbitalis (see Baumel and Witmer Citation1993: annot. 95); cfp., crista fossae parabasalis; coe., ostium canalis ophthalmici externi; cond.oc., condylus occipitalis; cr.bas.lat., crista basilaris lateralis; cr.nu.trans., crista nuchalis transversus; cr.ven., crista ventralis; f.para., fossa parabasalis; fng., foramen n. glossopharyngealis (IX); fnh., foramen n. hypoglossi (XII); fnv., foramen n. vagi (X); inc.mc., incisura mediana condyli; for.mag., foramen magnum; i.para., lamina parasphenoidalis; m.rec., insertion for musculus rectus capitis dorsalis; occ., ostium canalis carotici and branch of nerve VII; pr.basi., processus basipterygoideus; pr.par., processus paroccipitalis; prom.ex., prominentia exoccipitalis; r.para., rostrum parasphenoidalis (basis rostri parasphenoidalis); s.int., septum interorbitale; tu.au., tuba auditiva communis; tubc.bas., tuberculum basilare. Scale bars: A., D. 20 mm, B. 10 mm, C. 40 mm. Dark grey shading indicates regions where damage precludes morphological assessment, and light grey indicates foramen and fossae. Dotted lines in D. indicate the alignment and distortion of the sagittal plane of each region.
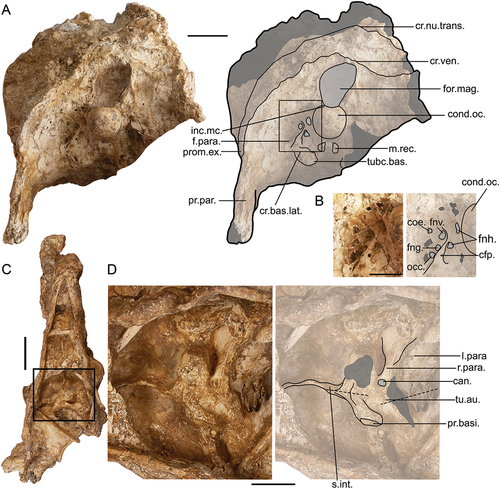
Notably like the condition observed in Anhima cornuta, ventral of the fossa parabasalis in all dromornithids, the crista basilaris transversa and crista basilaris lateralis (see Landolt and Zweers Citation1985, p. 625) are relatively indistinct, although where they intersect is a prominent (10.4 mm wide in G. newtoni) and rounded tuberculum basilare. The bilaterally paired structures here identified as tubercula basilaria (see Baumel and Witmer Citation1993: annot. 83) are synonymous with the mamillar tuberosities as described by Worthy et al. (Citation2016a). Each represents the area for attachment of the heavy aponeuroses for insertion of m. rectus capitis ventralis, pars lateralis, and for slips of the m. rectus capitis dorsalis on the caudal side (see Landolt and Zweers Citation1985, p. 646–649; see ). The tuberculum basilare forms a ventral protuberance of the basioccipitale at its rostrolateral junction with the exoccipital, prooticum, and basisphenoidale (Worthy and Scofield Citation2012), which are overlaid during ontogenetic development by the lamina parasphenoidalis (Jollie Citation1957; Baumel and Witmer Citation1993). Differing from our use, Landolt and Zweers (Citation1985, p. 625, Citation1987) instead used the term tuberculum basilare to refer to the bulla basitemporalis (sensu Davids Citation1952b). This emanates from the lamina parasphenoidalis in many anseriforms, e.g. Anas platyrhynchos, and galliforms, e.g. Alectura lathami, and is here considered absent in dromornithids. We choose to follow Baumel and Witmer (Citation1993) for nomenclatural consistency, and as disparity exists over whether the term ‘mammillary processes’ (sensu Pycraft Citation1902) refers to the tubercles, or to the processus medialis parasphenoidalis (Bock Citation1960b; sensu Baumel and Witmer Citation1993: annot. 97; Livezey and Zusi Citation2006: char. 0123, p. 44, fig. 8); confusion is likely due to the two coinciding in some birds (see Baumel and Witmer Citation1993: annot. 83). Bock (Citation1960b) considered the processus medialis parasphenoidalis to be absent in all Galloanserae, and thus we also assume its absence in dromornithids.
The attachment surfaces of the cervical muscles on the basis cranii externa (basicranium) are not well-pronounced in G. newtoni although they are in I. woodburnei, specimen QMV:2000:GFV:20 (see ), and D. planei, specimen NTM P9464–106, so inferences of muscle attachments are facilitated by these two specimens (also see Boas Citation1929; Davids Citation1952b; Goodman and Fisher Citation1962: fig. 6–7, p. 118–122; Fujioka Citation1963: pl. VI; Zweers et al. Citation1987; Böhmer et al. Citation2020; Worthy et al. Citation2016a). The ventral surface of the tuberculum basilare acts as the insertion point of m. rectus capitis ventralis, pars lateralis. The m. rectus capitis ventralis, pars medialis, attaches further rostrally on a large, round area of the flattened surface of the lamina parasphenoidalis. A distinct impression for the insertion of one of three paired branches of m. rectus capitis dorsalis is, in D. planei, on the caudodorsal surface of the tuberculum basilare, and appears as a slight depression in G. newtoni. The remaining two paired heads of the m. rectus capitis dorsalis each have distinct, small, and ovular correspondingly paired attachment depressions (also see Lautenschlager et al. Citation2014; Worthy et al. Citation2016a). The most rostroventral of the two sits medially to the tuberculum basilare, on the caudal side of crista basilaris transversa, whereas the more caudomedial insertions are just ventral of the condylus occipitalis, in an area that would relate to just rostral of fossa subcondylaris if it was marked, near the mediolateral midline of the skull. In non-anhimid anseriforms, aponeurotic attachment marks associated with the insertions of the m. rectus capitis dorsalis branches are formed on the caudal side of, and medial to the tubercula basilaria, and in some taxa, e.g. Cygnus atratus, these structures become continuous along the crista basilaris transversus. Additionally, each tuberculum basilare is ventrolaterally extended along each respective crista basilaris lateralis, relating to a more developed region of insertion for the m. rectus capitis ventralis, pars lateralis. Comparatively, in galliforms, the two cristae, the pair of tubercula basilaria, and the aponeurotic attachment surfaces associated with insertion of the rectus capitis ventralis muscles, are relatively flattened or less defined/pronounced. The crista basilaris transversa extends mediolaterally, forming a low or indistinctive, near-straight band across the caudal basicranium, as defined by the rostral boundary of fossa subcondylaris (see Landolt and Zweers Citation1985, p. 625).
Ventral aspect
The lamina parasphenoidalis ( C-D) presents a smooth uninterrupted surface, curving laterally onto each parasphenoid process of the ventral cranium. In several other dromornithids, such as D. planei and I. woodburnei, the lamina parasphenoidalis merges rostrolaterally with ala parasphenoidalis, however, this transition is relatively abrupt in G. newtoni, specimen SAMA P59516, and is most similar to D. murrayi in this regard. Damage and obfuscation in all other available specimens of G. newtoni prevents more thorough assessment of this condition. In I. woodburnei, the lamina parasphenoidalis tapers rostrally into a point ventrorostral of the tuba auditiva communis (see Worthy et al. Citation2016a). This is inferred for G. newtoni and species of Dromornis, as although the region is damaged, the remaining bone suggests a similar morphology. Aside from specimen SAMA P59516 of G. newtoni, the rostrum parasphenoidalis is only preserved in D. planei (NTM P9464–106), in which it is mediolaterally wider, dorsoventrally flatter, and proportionally more robust. The rostrum parasphenoidalis is truncated just rostral of both processus basipterygoidei in G. newtoni, whereas it ends caudal to the rostral terminus of the processes in D. planei. Additionally, unlike in D. planei, in ventral view, the rostrum parasphenoidalis in G. newtoni does not extend rostrally past the craniorostral hinge, which is likely a result of the lesser rostrocaudal compression in the cranium relative to D. planei. The septum interorbitale extends rostrally and dorsally from the rostral-most rostrum parasphenoidalis as a thin osseous wall between the orbits and is well developed in SAMA P59516. The processus basipterygoidei in G. newtoni are smaller relative to cranium size than in D. planei although, in both, they are widely separated by the rostrum parasphenoidalis, with flat, elliptical articular surfaces, which protrude laterally from the rostrum parasphenoidalis on very short, stout pedestals. The location, shape, and sessile nature of the processus basipterygoidei in dromornithids is a conclusive feature supporting their placement within Galloanserae (Elzanowski Citation1977; Baumel and Witmer Citation1993: annot. 93; Weber Citation1993; Dzerzhinsky Citation1995; Livezey Citation1997; Murray and Vickers-Rich Citation2004, p. 163; Mayr Citation2017, p. 107) and has been used several times to assist in the placement of other fossil birds within this radiation (e.g. S. neocaledoniae, see Mourer-Chauviré and Balouet Citation2005).
Quadrate
The quadrates () of G. newtoni that were described by Stirling (Citation1913: pl. XXXVI, fig. 4–8), and again by Murray and Vickers-Rich (Citation2004, p. 60–62, fig. 38), were near complete. However, they could not be located for this study so, consequently, we redescribe the quadrates using new material (specimens SAMA P59516, SAMA P59520, NMV P256893: fig. 6, and SAMA P53830) in conjunction with the description and plates published by Stirling (Citation1913). The specimens appear to be of comparable size, although differential preservation restricts quantitative comparisons. Previously, Murray and Megirian (Citation1998) suggested that the quadrates of G. newtoni correspond most closely with those of D. planei although we recognise clear proportional differences between the two species; the quadrate of G. newtoni is less robust, more tapered dorsally, and has a more elongate pars otica and processus orbitalis. The proportions and morphology of the quadrates of G. newtoni are, instead, more like those attributed to species of Ilbandornis. Regardless, all dromornithid quadrates are medially concave, forming a deep c-shape from the head of the pars otica down the medial side of the quadrate. This curvature is extreme in D. stirtoni (NTM P3202). Only the quadrates of Anhima cornuta appear to show a similar curvature although this is coupled with mediolateral flattening that is absent in dromornithids.
As per the quadrate morphology of Ilbandornis sp. (NTM P3237), D. stirtoni (NTM P5401, NTM P3202) and D. planei (NTM P9464–100, NTM P9464–118; for all see Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a), the head of the pars otica in G. newtoni also has a single, sub-oval and dorsally convex compound structure comprising two adjoined capitula, capitulum squamoso-oticum (new term, referring to the fused capitulum squamosum and capitulum oticum). Paired with the receiving singular joint cavity of the cranium (see cotyla quadratica squamoso-otica and recessus quadratica, above) this is characteristic of articulatio quadrato-squamoso-otica, as in species of Gallus, for example (see Baumel and Raikow Citation1993: annot. 25). Due to the lack of a distinct vallecula intercapitularis, only a dorsally raised angularity, which represents the rostrolaterally orientated lateral rim of the capitulum oticum articular surface, indicates the location of the junction between the two capitula in G. newtoni. Similarly, only the variable pronunciation of the edges of the articular surfaces of the capitula in rostral view reveal the extent of each capitulum in other dromornithids. In extant galloanserans, the capitula are distinct with a variably deep and wide vallecula intercapitularis. In Sylviornis neocaledoniae and species of Gastornis, the vallecula intercapitularis is shallow and the capitula are closely abutting (Matthew and Granger Citation1917; Andors Citation1988; Mourer-Chauviré and Balouet Citation2005; Bourdon et al. Citation2016). In many anatids, the pars otica is functionally single-headed (Zelenkov and Stidham Citation2018).
The shape of the conjoined capitulum in G. newtoni is similar to that of the smaller quadrates attributed to D. planei (NTM P9464–118) and D. stirtoni (NTM P5401); more rounded mediolaterally and dorsally than the quadrates of Ilbandornis sp. (NTM P3237, NTM P3235). In G. newtoni, Ilbandornis sp., and D. stirtoni (NTM P5401), a ridge runs ventrally from the capitulum squamosum to become confluent with the tuberculum subcapitulare (sensu Elzanowski and Stidham Citation2010: ; see also SI 2). However, the quadrates of D. planei and D. stirtoni contrast with those in species of Ilbandornis and G. newtoni, in that the capitulum squamosum variably overhangs the shaft of pars otica; this is to the greatest extent in D. stirtoni, where it markedly overhangs laterally the tuberculum subcapitulare.
The tuberculum subcapitulare (eminentia articularis, sensu Lowe Citation1926; Weber Citation1996; adductor crest, sensu Murray and Vickers-Rich Citation2004) is considered a typical galloanseran character, despite its variability in shape and size, and absence in some species of Crax (as discussed by Elzanowski and Stidham Citation2010; Elzanowski and Boles Citation2012; Field et al. Citation2020: SI, p. 28). In G. newtoni, the tuberculum forms a long crest, starting from near to the lateral capitulum squamoso-oticum and spanning up to one third of the dorsoventral height of the quadrate. This may be associated with a well-developed aponeurosis articularis (sensu Weber Citation1996), as described for several extant galloanserans (e.g. Burggraaf Citation1954a; Dzerzhinsky and Belokurova Citation1972; Dzerzhinsky and Potapova Citation1974; Dzerzhinsky Citation1982; Weber Citation1996; Zusi and Livezey Citation2000; see SI 2) and aponeurosis quadrata (sensu Dzerzhinsky and Potapova Citation1974; Dzerzhinsky Citation1982; aponeurosis Q2, sensu Weber Citation1996), related to a large point of origin for a slip of the m. AME superficialis (sensu Holliday and Witmer Citation2007), that also originates on the dorsally adjacent cranium (see ‘Cranium’; e.g. Hofer Citation1950; Zusi and Storer Citation1969; Dzerzhinsky Citation1982; Weber Citation1996; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007 and references therein; Elzanowski and Boles Citation2012). This muscle origin area is particularly large in galliforms and anhimids (Weber Citation1996). Comparatively, in Anseres, the m. AME superficialis does not originate upon the quadrate. Instead, a similarly located, but non-homologous, tubercle predominantly corresponds to the origin of m. adductor mandibulae posterior lateralis (Goodman and Fisher Citation1962; Vanden Berge and Zweers Citation1993; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007; Appendix Three). In galliforms and anhimids, the m. adductor mandibulae posterior muscle bellies instead arise ventrally and rostrally near the base of the orbital process (e.g. compare Davids Citation1952a; Fuchs Citation1954a; Fujioka Citation1963: figs. 3–8, 10; Dzerzhinsky Citation1982; Weber Citation1996; Zusi and Livezey Citation2000: fig. 8; Dzerzhinsky and Grintsevichene Citation2002: fig. 7). Considering the many similarities observed between anhimids and dromornithids, particularly pertaining to the external adductor musculature (see ‘Cranium’), we expect homological association of the tuberculum subcapitulare with m. AME superficialis to be more likely. In support of this assessment, the G. newtoni quadrates with complete orbital processes figured by Stirling (Citation1913: pl. XXXVI) possess a well-developed crest near the basal orbital process which is consistent with the more typical origin of m. adductor mandibulae posterior (see below; also e.g. Davids Citation1952a; Weber Citation1996; Holliday and Witmer Citation2007).
An especially extensive tuberculum subcapitulare is typical for dromornithids and is proportionally shorter but still elongate in species of Presbyornis and some megapodiids, compared to the smaller form of other galloanserans (Elzanowski and Stidham Citation2010). The dromornithid form is also generally more robust (Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a), and although proportionally variable in size, it is uniquely unpronounced on the quadrate of D. murrayi (see Worthy et al. Citation2016a: fig. 4 A, B). The bipartite tubercle of anhimids is potentially associated with a fragmented ancestral crest (Elzanowski and Stidham Citation2010; Elzanowski and Boles Citation2012), which may be derived from a structure not dissimilar to that of G. newtoni. In species of Gastornis, the tuberculum (‘process for m. AME profundus and associated aponeuroses’, sensu Andors Citation1992; tuberculum musculi adductor mandibulae, sensu Bourdon et al. Citation2016) is dorsoventrally shorter than that of dromornithids, developing more as a prominent, conical tubercle which is hooked ventrally in G. parisiensis (see Bourdon et al. Citation2016; Mourer-Chauviré and Bourdon Citation2020). This tuberculum is also likely related to the origin of m. AME superficialis in gastornithids and many galloanserans, compared to the relatively atypical association with m. adductor mandibulae posterior that is characteristic of Anseres (see above).
In specimen SAMA P53830 (see Appendix One: Figure A3 C), on the medial side of pars otica, a small foramen is present, which is absent in other specimens. The medial face in this specimen appears flat relative to both SAMA P59520 (see Appendix One: Figure A3 F) and NMV P256893 (see Appendix One: ) which both have a distinct depressio rostromedialis (sensu Elzanowski et al. Citation2001), covering most of the medial side of the pars otica. In specimen NMV P256893, yet absent in SAMA P59520, a large (7.3 mm high, 3.6 mm wide), rostrally situated and oval foramen is present within this depression. A similarly located and obvious foramen is also found in D. planei, D. murrayi and Ilbandornis sp. and absent from the imperforate quadrates of D. stirtoni (see Worthy et al., Citation2016a: fig. 4F). In several of the dromornithid quadrates, there appears to be a ridge which traverses dorsally from the region of articulation with the pterygoid and curves rostrally around the caudal edge of fossa basiorbitalis prior to continuing along the foramen’s ventrocaudal margin towards the caudal-most part of the capitulum squamoso-oticum on the head of the pars otica. This strut-like ridge is distinct in some specimens (e.g. D. planei NTM P5401; Ilbandornis sp. NTM P3237), including in specimens of G. newtoni (SAMA P59520; NMV P256893, SAMA P53830, as well as the complete quadrates described by Stirling Citation1913: pl. XXXVI), but is rounded and inconspicuous in others (e.g. D. planei NTM P9464–118, only visible by manipulating specimens under a light source). Should this ridge be homologous with the crista medialis, then the foramen can be matched with that of the galliform foramen type (following descriptions by Elzanowski et al. Citation2001; Elzanowski and Stidham Citation2010) and thus, following Worthy et al. (Citation2016a), we tentatively assign the term foramen pneumaticum rostromediale.
Comparably, most extant anseriforms have a foramen pneumaticum caudomediale; in anhimids, the foramen is considered vestigial and is often absent (Elzanowski and Stidham Citation2010, p. 315). While typically in the caudomedial position, in some anseriforms it appears rostrally displaced (e.g. species within Anserinae and Mergini, Elzanowski and Stidham Citation2010). In fossil taxa, Anachronornis anhimops and Presbyornis species have a variably developed foramen pneumaticum caudomediale or deep associated depression (Elzanowski and Stidham Citation2010: fig. 5; Houde et al. Citation2023, p. 17, fig. 1 L – M); Conflicto antarcticus Tambussi et al. Citation2019 has both a rostromedial as well as a caudomedial pneumatic foramen (Tambussi et al. Citation2019: fig. 6); early Eocene Danielsavis nazensis has neither foramen but forms a deep depressio caudomedialis (Houde et al. Citation2023: fig. 5 H – I, 7 D, p. 29; Mayr et al. Citation2023: fig. 3); the Upper Cretaceous Asteriornis maastrichtensis Field et al. Citation2020, of near-galloanseran affinities (Field et al. Citation2020) has only the rostromedial pneumatic foramen.
The proposed homology of the foramen is further supported by the confident identification of the crista tympanica on the caudal pars otica in some dromornithid specimens, which is especially pronounced in G. newtoni SAMA P53830 (see also Elzanowski and Stidham Citation2010). On specimen NTM P256893, a short (6.4 mm), component of the crista tympanica is present approximately 4.8 mm caudolateral of the crista medialis and laterally delimits depressio caudomedialis (sensu Elzanowski et al. Citation2001), ventral to the compound capitular heads. The small, shallow, and triangular depressio caudomedialis is comparatively more dorsoventrally extensive in specimen SAMA P53830. In other dromornithids, the caudomedial depression is ventrally confluent with a broad depression on the caudal pars mandibularis, that is especially bowl-like in species of Dromornis. In quadrates of G. newtoni, this ventral depression is variably deeper.
The foramen pneumaticum basiorbitale is common among crownward birds, including those in the genera Ichthyornis, Conflicto, Pelagornis, Presbyornis (see Field et al. Citation2020: SI p. 28), Danielsavis and Anachronornis (see Houde et al. Citation2023; Mayr et al. Citation2023), as well as galliforms (Elzanowski and Stidham Citation2010). The foramen is occasionally present in D. planei (e.g. NTM P9464–100) and absent in D. stirtoni yet remains unknown for G. newtoni and Ilbandornis sp. due to taphonomic damage. Variably located and often vestigial foramina have been identified in a similar region of the derived quadrates of anhimids, although the foramen pneumaticum basiorbitale is absent in anatoids (Elzanowski and Stidham Citation2010). The foramen is also absent in gastornithids (Mourer-Chauviré and Bourdon Citation2020) and likely absent in S. neocaledoniae (see Mourer-Chauviré and Balouet Citation2005: fig. 6).
Small tubercles in G. newtoni just ventral to the foramen pneumaticum rostromediale are equivalent to what was interpreted as the attachment for the ‘posterior ligament of the quadrate’ by Murray and Megirian (Citation1998) for dromornithids. This medial area, and the more rostral fossa basiorbitalis, corresponds to the insertion of m. protractor pterygoidei et quadrati and interrelated aponeuroses in many studied avian taxa (e.g. Fuchs Citation1954b; Starck and Barnikol Citation1954), including the megapodiid Aepypodius arfakianus by Weber (Citation1996: Q3). Regardless of the exact homology, this rugose attachment surface is observed in all dromornithid specimens, although the shape of the attachment region slightly varies. For example, in D. planei, the attachment region is small and triangular.
Despite consistent damage to the caudal side of all G. newtoni quadrates, the presence of a well-developed prominence projecting dorsocaudally from the pars quadratojugalis of the processus lateralis (sensu Elzanowski et al. Citation2001) is evident, most especially in specimen SAMA P59520. This correlates with the massive quadratojugal eminence described by Murray and Vickers-Rich (Citation2004, p. 62), is visible in the quadrate images figured by Stirling (Citation1913: pl. XXXVI), and is also indicated by the preserved structure of the quadrates of species of Ilbandornis and Dromornis despite also having consistent damage to the region. Articulated quadrates of G. newtoni (SAMA P59516, NMV P256893, SAMA P59520), show this projection to be directed towards the dorsal processus paroccipitalis and aligned with the ventrocaudal bounds of the osseous meatus acusticus externus. The position and orientation of this structure suggests it is homologous with the prominentia submeatica (sensu Elzanowski Citation1987) of anatoids (also noted by Elzanowski and Boles Citation2012, p. 908), however, it is a comparatively inflated structure in these taxa, instead of the strong projection that appears characteristic of dromornithids (pers. observ.; see also Elzanowski and Stidham Citation2010). The prominence likely provided attachment for the membrana postmeatica (Elzanowski Citation1987; sensu Baumel and Raikow Citation1993: annot. 37) and was closely associated with the m. depressor mandibulae and incorporated aponeuroses (Elzanowski Citation1987). Together, these soft-tissue structures would have effectively participated in the caudal wall of the external auditory canal, as it does in anatoids and other taxa which possess a homologous prominentia, e.g. tinamous (see Davids Citation1952a; Elzanowski Citation1987 and references therein; Elzanowski and Stidham Citation2010). A more medial processus submeaticus (sensu Elzanowski and Stidham Citation2010), a feature common among anseriforms including anhimids, which hosts tissues that connect with the processus suprameaticus of the cranium (and more closely associated with the rostral bounds of meatus acusticus externus), is absent in G. newtoni and all dromornithids (see ligamentum postquadratum, sensu Dzerzhinsky Citation1982; Elzanowski Citation1987). In galliforms, the prominentia submeatica occurs only in cracids, and a processus submeaticus is absent (Elzanowski and Stidham Citation2010). Additionally, quadrates of Presbyornis species (see Elzanowski and Stidham Citation2010), Ga. parisiensis (see Bourdon et al. Citation2016: fig. 2), Ga. giganteus (see Matthew and Granger Citation1917: Pl. XXII, fig. 2a) and S. neocaledoniae (see Mourer-Chauviré and Balouet Citation2005: fig. 6 E-F) all lack both the prominentia submeatica and processus submeaticus.
The only complete orbital processes of the quadrate known for G. newtoni are those presented by Stirling (Citation1913), from which this updated description is based. The processus orbitalis of D. stirtoni (specimen NTM P3202) and the quadrates described by Stirling (Citation1913) for G. newtoni are similar in length to that of the pars otica from which it extends rostrally at a wide angle. The rostral tip of the orbital process in G. newtoni is slightly curved ventrally and medially in association with the origin of m. pseudotemporalis profundus along its edge (e.g. see Davids Citation1952a; Fuchs Citation1954a). The straight dorsal margin intercepts the pars otica just ventral of the capitulum squamoso-oticum, the latter is only comparable to that of Anseranas semipalmata; in anatids (but not Dendrocygna), this dorsal margin is markedly concave. Comparably, the right angle between the processus orbitalis and pars otica in gastornithids and S. neocaledoniae, is like many other anseriforms, and smaller than most galliforms (see also Elzanowski and Stidham Citation2010).
As in many galloanserans, including species of Anhima, Anseranas, Oxyura and Cygnus, and G. parisiensis (see Bourdon et al. Citation2016), the fossa basiorbitalis on the medial side of the processus orbitalis is large and deeply excavated for insertion of m. protractor pterygoidei et quadrati (e.g. Davids Citation1952c; Fuchs Citation1954b; see Stirling Citation1913). Rostrally, a second depression, likely the depressio protractoris (sensu Elzanowski et al. Citation2001) is evident and separated from the fossa basiorbitalis by a prominent, rostrocaudally narrow ridge. On the lateral surface of the orbital process, a sharply-ridged crista orbitalis (sensu Elzanowski et al. Citation2001) traverses longitudinally along the long axis from the rostral apex before turning sharply obliquely to extend close to the dorsal margin of the process. While probably associated with m. pseudotemporalis profundus rostrally, the latter structure and flattened surface in between evidences an especially well-developed attachment site predominately corresponding to the origin of m. adductor mandibulae posterior (see above, also e.g. Davids Citation1952a; Fuchs Citation1954a; Goodman and Fisher Citation1962; Weber Citation1996).
There is no evidence for a distinct facies articularis pterygoidea ventrally on the processus orbitalis. The morphology of this region is instead more like the anseriform and cracid conditions: the raised condylus pterygoideus is dorsally confluent with, or adjacent to, the facies articularis pterygoidea (see Dzerzhinsky Citation1982; Elzanowski and Stidham Citation2010; Zelenkov and Stidham Citation2018). In G. newtoni, the condylus pterygoideus is a small oval tuberosity (Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004), less distinct than in other dromornithid quadrates (see Worthy et al. Citation2016a). In all, the ventral margin of the condylus pterygoideus meets the rostral edge of the condylus mandibularis medialis (see also Stirling Citation1913, p. 119; Elzanowski and Stidham Citation2010; Worthy et al. Citation2016a).
All dromornithids have the galloanseran bicondylar form of the mandibular process (Murray and Vickers-Rich Citation2004; Mayr Citation2017; Worthy et al. Citation2017b), although the pars mandibularis is considerably larger proportionally in species of Dromornis than it is in G. newtoni and Ilbandornis species. The complete articular surface is narrow, the condyles are near equal in size, and both are rostrocaudally elongate ovate shapes, tapering towards the midline of the articular surface with overlap of their long axes just greater than half the length of the condyles. Unlike other galloanserans, the articular surface is relatively flat, the two condyles are not well defined individually, and the vallecular intercotylaris is shallow and dorsoventrally wide. The condylus mandibularis lateralis is markedly less ventrally convex and the condylus mandibularis medialis, slightly more so (see also Worthy et al. Citation2016a). Comparatively, in gastornithids and S. neocaledoniae, both condyles are ventrally convex, separated by a deep vallecula intercondylaris (Bourdon et al. Citation2016; Worthy et al. Citation2016a; Mourer-Chauviré and Bourdon Citation2020).
For articulation with the jugal arch, galloanseran quadrates, including in Ga. parisiensis and S. neocaledoniae (see Bourdon et al. Citation2016; Mourer-Chauviré and Bourdon Citation2020) typically have a deep bowl-like fovea quadratojugalis linking the distinct facies quadratojugalis ventralis and facies quadratojugalis dorsalis. Comparatively, in G. newtoni and other dromornithids, the fovea quadratojugalis is a large, shallow, plate-like structure at the base of the prominentia submeatica with no distinct facies quadratojugalis dorsalis. Only in Ilbandornis sp. specimen NTM P3235 is there a small, raised shelf which indicates the potential presence of the facies articularis ventralis. In G. newtoni, an angular ridge defines the ventral margin of the fovea quadratojugalis and overhangs the lateral mandibular condyle. Whether the rim extends dorsally cannot be determined due to taphonomic damage in all quadrates we observed for G. newtoni. An embayment, separated from the depressio subcondylaris by a ridge, is present between the fovea quadratojugalis and the lateral mandibular condyle. This is relatively shallow in G. newtoni, as in species of Ilbandornis, whereas in D. stirtoni, it is especially distinct. A depressio supracondylaris (sensu Elzanowski and Stidham Citation2010) appears to be present just rostromedial to the fovea quadratojugalis in the Stirling (Citation1913: pl. XXXVI, fig. 4–8) quadrates of G. newtoni; however, equivalents cannot be verified in any available specimens.
Pterygoid
There are two poorly preserved, disarticulated pterygoids in the caudal area of specimen SAMA P 59516, which are visible from ventral perspective, on left and right sides of the skull (). The right pterygoid appears to present a cup-like facies articularis quadratica, similar to the pterygoids of other dromornithids. The fragmentary nature of these elements prevents more comprehensive description.
Rostrum
The rostrum () of Genyornis newtoni is composed of completely synostosed elements (praemaxillaria, maxillaria, nasalia, palatina, and vomer), indicating that all studied specimens represent adult individuals. The lacrimalia also appear to be completely fused to this structure. There is no complete arcus jugalis preserved for any specimen although fragments of the jugal arches and caudal maxillare (i.e. mainly contributed to by the processus jugalis of the maxillare) are used to interpret morphology (see ). In dorsal view, the rostrum is nearly three times longer than its maximum width (SAMA P59521, see Appendix Two) and has a broad and rounded tip. The lateral margins narrowly diverge and are notably convex, the culmen is sharply angled over the mid-length zone, the sides are flat or slightly shallowly concave, and in lateral aspect, the tomial surface is flat. The rostrum of specimen SAMA P59521 is larger than SAMA P59517, and greater in dorsoventral height although the height of both specimens is proportionate to length. SAMA P59517 is likely from a male (Chinsamy and Worthy Citation2021), with the SAMA P59521, thus presumed to be of the smaller female morph.
Figure 6. Genyornis newtoni right quadrate, NMV P256893: A. Lateral view, image and annotated outline; B. Medial view, image and annotated outline; C. Dorsal view; D. Ventral view; E. Rostral view; F. Caudal view. Annotations: cap.s-o., capitulum squamoso-oticum; cond.l., condylus mandibularis lateralis; cond.m., condylus mandibularis medialis; con.pt., condylus pterygoideus including facies articularis pterygoidei; cr.med., crista medialis; cr.tymp., crista tympanica; d.caud., depressio caudomedialis; d.rost., depressio rostromedialis; d.sup., depressio supracondylaris; for.rost., foramen pneumaticum rostromediale; f.basi., fossa basiorbitalis; fov.q., fovea quadratojugalis; musc., tubercles for insertion of m. protractor pterygoidei et quadrati; o.cap., otic part of capitulum squamoso-oticum; p.oti., pars otica; p.sub., prominentia submeatica; s.cap., squamosal part of capitulum squamoso-oticum; t.sub., tuberculum subcapitulare; v.inco., vallecular intercotylaris. Scale bars: 10 mm. Dark grey shading indicates regions where damage precludes morphological assessment, and light grey indicates the foramen pneumaticum rostromediale.
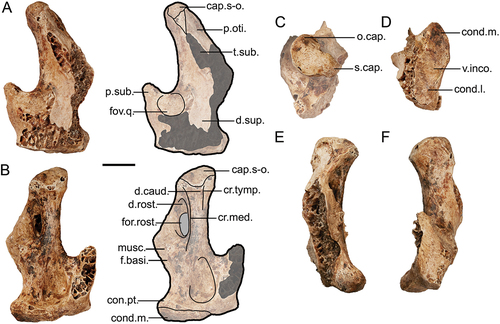
Figure 7. Genyornis newtoni rostrum, lateral views: A. SAMA P59521 left lateral view image and annotated outline; B. SAMA P59521 right lateral view; C. SAMA P59517 left and right lateral views. Annotations: ang., angulus tomialis; ca., casque; cr.tom., crista tomialis; fov., foveae corpusculorum nervosorum; lac., lacrimal ; margo.lat., margo lateralis palatini; nas., apertura nasi ossea; pala., palatinum; p.o.l., processus orbitalis of lacrimale; pr.jug., processus jugalis of the maxillare; pr.max., processus maxillopalatinus of the maxillare; pr.n.m., processus nasalis of the maxillare; sul.para., sulcus paratomialis. Scale bars: 20 mm. Dark grey shading indicates regions where damage precludes morphological assessment, and light grey indicates fenestrae, foveae, and aperturae.
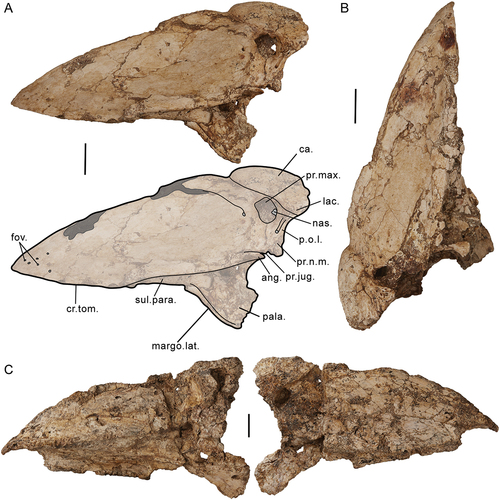
Dorsal and lateral view: os praemaxillare et os nasale
On the dorsocaudal surface of the rostrum, a well-defined, lowly-raised, triangular structure, interpreted as a ‘casque’, abuts the entire width of the craniorostral hinge and rostrally terminates in a rounded point at the dorsal culmen. Complete synostosis of the processus praemaxillaris nasalis (sensu Livezey and Zusi Citation2006) and the processus frontalis praemaxillaris (sensu Livezey and Zusi Citation2006) contribute to the casque. The casque may be associated with the conchal and or maxillary diverticula of the antorbital sinus (Witmer Citation1990; Mayr Citation2018b) and the presence of paired depressions covered with dimples on the dorsal surface, bilateral to the midline, may suggest this area, at least, was highly vascularised. Although the casque is absent in other dromornithid species with identified rostra (Dromornis planei and D. stirtoni), two specimens of D. planei (NTM P9973–2 and NTM P9464–107) have distinct grooves which follow the same triangular shape of the casque from the dorsal borders of the aperturae nasi osseae (external nares; sensu Zusi Citation1993) to meet rostrally along the culmen. The triangular space within is also heavily vascularised in D. planei specimens, most conspicuously in NTM P.9973–2 and NTM P932–2. The casque of S. neocaledoniae is similarly located on the rostrum (rather than the cranium), although it is a much larger, dorsally prominent, ornament (Mourer-Chauviré and Balouet Citation2005). Few other galloanserans have such bony ornamentation on the skull and rostrum with exceptions being species of Melanitta, and some species of Numididae and Cracidae (e.g. Mitu mitu; see Mayr Citation2018b for a review).
The rostrum, as per other dromornithids, is dominated by large, flattened plates of the paired praemaxillaria and maxillaria, which fuse into a single robust structure, a feature shared with other giant galloanserans such as the gastornithids and sylviornithids (see Andors Citation1988, Citation1992; Mourer-Chauviré and Balouet Citation2005), as well as anseriforms, and some cracids. Comparatively, most galliforms have weakly fused upper jaw elements (see Field et al. Citation2020). Aside from taphonomic fragmentation, especially prominent in SAMA P59517, the surface texture of the plates is smooth and likely were shallowly depressed (accommodating for slight taphonomic deformation). There is no evidence of the extensive cavitation and folding that is prominent on specimen NTM P932–2 of Dromornis planei () and NTM P9245 of D. stirtoni (Murray and Megirian Citation1998: fig. 15; Murray and Vickers-Rich Citation2004: fig. 195). Due to differential preservation on both sides of these rostra, the cause of this surface texture remains unknown, although it could be associated with a soft-tissue structure on the dorsal rostrum, or alternatively be pathological in nature. On the Genyornis rostrum, there is no indication of the extent of the rhamphotheca – unlike the horizontal groove present in Dromornis specimen NTM P.9464–107. This may suggest that the rhamphothecal sheath covered the complete rostrum aside from the casque.
Figure 8. Dromornis planei rostrum, specimen NTM P932–2, shows distortion and cavitation on the surface of the bone: A. Right side; B. Left side; C. Close-up image of the distortion on the rostrodorsal-most part of the left side of the rostrum. Annotations: ar.jug., arcus jugalis; cr.tom., crista tomialis; fen., fenestra; lac., lacrimal; p.o.l., processus orbitalis of lacrimal. Scale bars: A., B. 50 mm and C. 20 mm.
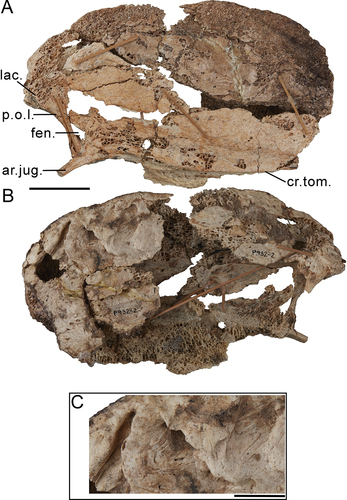
The rostrum is widely triangular in transverse section, contra Murray and Megirian (Citation1998, p. 59, 67) who described the rostrum of G. newtoni as laterally compressed, reminiscent of the extremely narrow rostrum attributed to species of Dromornis. However, reassessment of the latter, suggests taphonomic mediolateral compression may have been underestimated. In specimen NTM P.9973–2 (D. planei), the only seemingly uncompressed rostrum of Dromornis, the angle at which the lateral sides diverge from the culmen increases ventrally, suggesting the maximum mediolateral width of the ventral rostrum (not preserved), would be far greater than that of the preserved part, and so at least equal in width to the cranium. Should this be the case, the relative width of the rostrum may not vary so drastically from that of G. newtoni.
The culmen in lateral aspect, gently curves ventrally towards its tip, lacking the hemispherical curvature of the dorsal profile characteristic of species of Dromornis (see Murray and Megirian Citation1998: fig. 6, 7, 15, 28, 30) and the relatively planar deep gastornithid rostra (Andors Citation1988: pl. 1; Andors Citation1992: fig. 1, 2; Bourdon et al. Citation2016: fig. 1). Conversely, the dorsoventral shallowing of the culmen towards the rostral apex is absent in Dromornis. The rostral apex is mediolaterally wide and rounded with numerous foveae corpusculorum nervosorum perforating it, as common among anseriforms (Baumel and Witmer Citation1993: annot. 41), which Murray and Megirian (Citation1998) used to argue the presence of a thinly cornified and well-developed nail on the bill tip. Although absent or very subtle in G. newtoni, there is a slight rostral hook on the end of the rostrum of D. stirtoni (see Murray and Megirian Citation1998). Both Sylviornis neocaledoniae (see Mourer-Chauviré and Balouet Citation2005: fig. 5), the juvenile gastornithid Omorhampus storchii (previously synonymised with Ga. giganteus by Andors Citation1988; Louchart et al. Citation2021), and specimens of Ga. parisiensis show a ventral curve of crista tomialis suggesting a small hook-like structure. A rostrally hooked bill is common among the galliforms and some anseriforms, including Anhima cornuta, Anseranas semipalmata, Presbyornis species (see Zelenkov and Stidham Citation2018), Dendrocygna species and Oxyura australis, although in non-anhimid anseriforms the hook is not a sharp point but instead generally forms a rounded lip.
The external nares (aperturae nasi osseae) are holorhinal (the caudal border of the nostrils end rostral to the craniofacial hinge and are generally oval in shape, e.g. Bock Citation1964), as is common among galloanserans (Sibley and Ahlquist Citation1990; Dzerzhinsky Citation1995). Relative to the size of the rostrum, they are exceedingly small, circular, and located dorsocaudally, differing in location from the further ventral but similar shaped nares of gastornithids (Matthew and Granger Citation1917; Andors Citation1988, Citation1992). The external nares are slightly more rostrocaudally elongate in SAMA P59516, compared to that of SAMA P59517 and P59521. Three shallow grooves radiate rostrally from a neurovascular foramen, located on the lateral surface, just rostrad of the nasal aperture. These correspond well with passages for nerves and blood vessels (see Murray and Vickers-Rich Citation2004, p. 237, fig. 185), potentially terminal ramifications of the ophthalmic (V1, rami rostri maxillaris from praemaxillary rami of ramus medialis and rami nasales interni of ramus lateralis) and maxillary nerves (V2, specifically rami nasales externi of nervi nasopalatinus), which can be extensive and well developed in this area of the rostrum in anseriforms (see Bubień-Waluszewska Citation1980). A second foramen and deep, dorsoventrally aligned vascular groove are located caudolaterally from the nares, on each post-narial bar composed of the fused processus nasalis of the maxillare, processus maxillaris of the nasal, and the lacrimal. The region surrounding and rostral of the nares is more heavily vascularised in species of Dromornis, with a ‘narial groove’ Murray and Vickers-Rich (Citation2004) attribute to functional ‘nasal salt glands’ (i.e. fossa glandulae nasalis). These grooves may be better compared with the sulcus nasi that is typical of gastornithids (see Bourdon et al. Citation2016; Mourer-Chauviré and Bourdon Citation2020). We do not interpret any furrow as related to nasal salt glands in G. newtoni (see also ‘Orbit and rostrodorsal region of the cranium’).
Viewed laterally, the fused processus nasalis of the maxillare, processus maxillaris of the nasal, and lacrimal (post-narial bar) is aligned obliquely with respect to the dorsoventral plane. Fused laterally to the casque, the dorsal-most lacrimal appears to lack a distinct processus supraorbitalis, instead terminating caudally in line with the craniorostral hinge (see ‘Craniorostral hinge’). The processus orbitalis (also termed the descending or ventrocaudal process of the lacrimal; Cracraft Citation1968; De Mendoza et al. Citation2020) follows the alignment of processus maxillaris, mediolaterally thinning ventrally and projecting caudally as a distinct angular flange of bone at its ventral terminus, immediately dorsal and lateral to the co-ossified rostral-most processus jugalis of the maxillare (see below). In D. planei NTM P932–2, there is contact between the lacrimal component and the jugal arch, and a sulcus formed between them on the lateral side. Rostral of their intersection, an obvious fenestra is evident (), which has not formed in G. newtoni. The homological nature of the structural link between the descending orbital process of the lacrimal and the jugal arch in dromornithids is unknown, due to the completely fused morphology of the former part; whether it was derived from ossification of ligamentum jugolacrimale (e.g. os lacrimale communicans of Cariamiformes, see Degrange et al. Citation2015; Degrange Citation2021) or contributed to by other bones (Mayr Citation2022b). In anatids, the jugo-lacrimal ligament provides a strong connection between two well developed processes on both the lacrimal and jugal arch (Davids Citation1952c; Zweers Citation1974).
Palatum osseum
Following Murray and Vickers-Rich (Citation2004), we here, consider the dromornithid palate, desmognathous (as described by Huxley Citation1867). Desmognathy is typical for anseriforms, and some basal galliforms, including some cracids and megapodiids (Dzerzhinsky Citation1995; Zusi and Livezey Citation2006; Mayr Citation2018a), and is considered to have derived several times from a relatively plesiomorphic schizognathous palate (Hofer Citation1945), which is observed in some galliforms and Asteriornis maastrichtensis (see Field et al. Citation2020: SI, p. 22).
The ventral rostrum of Genyornis newtoni consists of a continuous, plate-like palatum osseum, comprising synostosed structures relating to the praemaxillaria, maxillaria, palatina, and vomer (see Huxley Citation1867; Zusi and Livezey Citation2006), as in species of Dromornis, gastornithids (Matthew and Granger Citation1917; Andors Citation1988), and sylviornithids (Worthy Citation2000; Mourer-Chauviré and Balouet Citation2005). Similarly, all anseriforms have extensive palatal development due to the central fusion of the paired processus maxillopalatinus of the maxillaria, although their palate retains a central fenestra. The development of a bony palate is variable in galliforms though restricted to the rostral-most region of the upper bill. In G. newtoni, the shallow nature of the palate suggests a large internal cavity within at least the caudal ¾ of the rostrum.
The palatal region of Dromornis planei was described from specimen NTM P.9464–107 (see Murray and Megirian Citation1998), although direct observation suggests the compression and subsequent damage to the specimen had obscured most features, and the palatines are not preserved. The preserved osseous plate present on the ventral aspect of the D. planei rostrum NTM P. 9973–2, and partially retained in NTM P.932–2, is not considered the palatum osseum; instead, we identify this as the ventral surface of the pila supranasalis (composed of the praemaxillary processes of the nasale and the frontal processes of the praemaxillare) which forms the roof of the internal nasal cavity. This is for several reasons: a) in the D. planei rostrum NTM P.932–2, the dorsolateral face of the praemaxillaria extend considerably further ventral than the roof; b) the external nares open internally within the rostrum, ventral to this osseous roof; and c) neither specimen retains any traces of the palatines or associated structures which would be expected should this be the palatum osseum.
Two sutures on the palatum osseum, at margo medialis palatini, are each representative of the intersection between the ventral processus maxillopalatinus of the maxillare (sensu Baumel and Witmer Citation1993: annot. 58; Zusi and Livezey Citation2006; Mayr Citation2018a, p. 3), and the more lateral, raised and synostosed structure formed of the processus praemaxillaris, processus rostralis of the palatines, and processus palatinus of the praemaxillary bone. These sutures traverse rostrad in parallel towards the apex rostri, and possibly supported a median palatal ridge (ruga palatina mediana) between them (see McLelland Citation1993). The medial fusion of the paired processus maxillopalatinus in G. newtoni, an anseriform feature (Baumel and Witmer Citation1993: annot. 58), creates a single suture rostral of the vomer (see below), contributing to the rostrocaudally continuous, shallowly concave, palatum osseum. Three small, oval fenestrae palatina (sensu Baumel and Witmer Citation1993: annot. 13) are mediolaterally aligned with this central suture and spaced caudorostrally upon the palatum osseum. The median depression is likely analogous to the ‘median suture’, that is described as dividing the palatines and maxillopalatine process in gastornithids (see Matthew and Granger Citation1917). The pits on the palatum osseum in the juvenile gastornithid specimens, identified as neurovascular canals by Louchart et al. (Citation2021), are absent in dromornithid specimens, although these authors associated the presence and size with the age of the individuals, which suggests that, even if they were present through the ontogenetic cycle of G. newtoni, they would be absent in the adult specimens available.
Most galloanserans and the gastornithids (see Andors Citation1988; excluding Omorhampus storchii, see Louchart et al. Citation2021: fig. 1 and G. parisiensis) have a variably thin and sharp crista tomialis. In G. newtoni, the crista tomialis is instead goose-like (e.g. Anser anser) with an angular junction between the dorsally ascending lateral plates of the rostrum (praemaxillaria). It is continued by a distinctly flattened tomial surface, which widens towards the rostral apex, and is characterised by rugose, osseus material. In some anatids, similar osseous material is observed on macerated skulls, which support the ventrally overlying rhamphothecal lamellae rostri on the tomium maxillare (e.g. Zweers et al. Citation1977). However, Murray and Vickers-Rich (Citation2004, p. 168) considered it unlikely that dromornithids ‘would have developed any but the most rudimentary lamellae’ on their tomia. The sulcus paratomialis (sensu Livezey and Zusi Citation2006) in G. newtoni shallowly depresses the rostrocaudal length of the tomial shelf, is confluent with concavitas palati, and becomes deepest caudally towards the processus jugalis of the maxillare, just lateral of the palatines. Unlike a typical anatid rostrum, the crista tomialis (and angulus tomialis) migrates dorsally in the caudal area, corresponding with this change in sulcus depth. This is notably similar to the morphology of Anhima cornuta in which a deep depression is present just rostral of the processus jugalis, lateral of the processus maxillaris of the palatines. Compared to G. newtoni, the sulcus upon the tomial surface in D. planei (see Murray and Megirian Citation1998) is more mediolaterally narrow in the rostral half, widening and becoming shallower laterocaudally in effect of the dorsal retreat of crista tomialis. Regardless, they are overall similar in the sense of relating to the overlapping caudal tomia of the articulated jaws, described by Murray and Vickers-Rich (Citation2004), which was functionally attributed to a sheering facet and differentiated from the more rostral tomia in species of Dromornis.
Maxillare, septum nasale osseum and arcus jugalis
The processus maxillopalatinus of the maxillare is not visibly trabeculated as in Anseranas semipalmata, however, the process is enlarged and shows extensive co-ossification, a feature common among anseriforms but not galliforms (Mayr Citation2018a). This region of the rostrum is best preserved on specimen SAMA P59521, and therefore, forms the basis of the following descriptions. As in anseriforms, the paired maxillopalatine processes of the maxillaria are fused to one another along the mediolateral midline of the rostrum, however, this is more extensive in G. newtoni and appears to continue dorsocaudally towards the craniorostral hinge to form an apparent supra-narial plate (sn.pl., ), notably similar to parrots. The plate appears to join the dorsal-most nasal bones caudodorsally although crushing is evident in this region, and so this may be a taphonomic feature. By comparing the morphology of the synostosed ventral nasal and praemaxillary bones (pila supranasalis) in specimens of Dromornis, it is likely that these plates were not entirely fused to the ventral casque-region, but instead formed a shelf which air could pass over and into a cavity within the rostrum. Caudally along the mediolateral midline, a thin ridge of bone traverses from the craniorostral hinge to the vomer; we interpret this ridge to be an incorporated septum nasale osseum, which also co-ossifies to the processus maxillopalatinus of the maxillare in parrots (Mayr Citation2018a). While damaged, the lack of any prominent caudal extension of the septum nasale osseum suggests that it was not connected to the more caudal mesethmoidale and the interorbital septum, thereby creating a hiatus craniofacialis septi ventrad of the craniorostral hinge (Jollie Citation1957; Baumel and Witmer Citation1993). This is entirely consistent with the high mobility of the rostrum relative to the cranium as predicted by the mobile craniorostral hinge (Craniorostral hinge Murray and Vickers-Rich Citation2004, p. 235).
Figure 9. Genyornis newtoni rostrum, specimen SAMA P59521, and comparison of palatine and jugal arch morphology: A. Ventral/Palatal view image and annotated outline; B. Dorsal view image and annotated outline; C. Caudal view image and annotated outline; D. Hypothetical reconstruction of the palatines of Genyornis newtoni (pale orange), pars lateralis palatini (dark orange), and the jugal arch (brown) from specimen SAMA P59521; E. the galliform condition, Ortalis canicollis (see Zusi and Livezey Citation2006: fig. 7A); F. the anseriform condition, Anser albifrons (see Zelenkov and Stidham Citation2018: fig. 2A). Annotations: ang., angulus tomialis; bulb., bulbous regions largely associated with each palatine including complete synostosis with the processus maxillopalatinus of the maxillare, the processus rostralis of the palatines, and the processus palatinus of the praemaxillary; ca., casque; cho.na., choana nasalis ossea; cr., craniorostral hinge; cr.tom., crista tomialis; cul., culmen; fen.pal., fenestrae palatina; fos.cho., fossa choanalis palatini; fov., foveae corpusculorum nervosorum; lac. – lacrimal; iam.d. – lamella dorsalis, pars choanalis palatini; margo.lat., margo lateralis palatini; os.pal., ossa palatina; pal., palatum osseum; pons.mj. – pons maxillaro-jugalis; pr.cho. – processus choanalis palatini; pr.jug., processus jugalis of the maxillare; pr.max., processus maxillopalatinus; sep.na., septum nasale osseum; sn.pl., supranarial plate (of processus maxillopalatinus); sul.para., sulcus paratomialis and associated depression; sut.Vm., sutura vomeromaxillaris; vom., vomer. Scale bars: 20 mm, D.-F. not to scale. Dark grey shading indicates regions where damage precludes morphological assessment, light grey indicates sulci, fenestrae, foveae, and aperturae, dotted lines follow the rostral continuation of the sulcus paratomialis, and the white arrows in B and C indicate the lateral articular ‘condyles’ (internal processes of the nasolacrimals, sensu Murray and Vickers-Rich Citation2004).
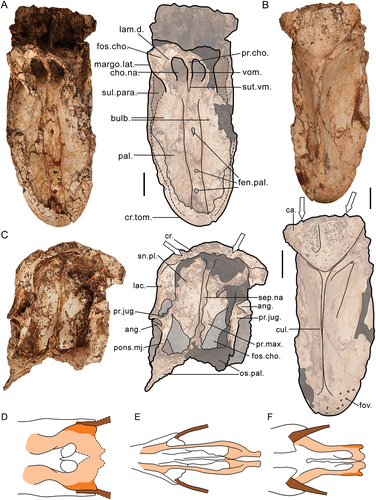
Laterally, this supra-narial plate of the processus maxillopalatinus contacts the caudomedial side of the combined processus maxillaris and processus nasalis for their complete dorsoventral length (see ). This forms a distinct crest which medially borders a dorsoventrally elongate and flat, caudally facing depression that is laterally delimited by the caudal edge of the orbital process of the lacrimal. The depression resembles that on the caudal lacrimal of Balaeniceps rex (Balaenicipitidae) which similarly has lacrimals that are solely fused to the caudal rostrum (Bühler Citation1980; see ‘Craniorostral hinge’). Notably, this region in anhimids is also flattened rostrocaudally (see Cracraft Citation1968). This depression is visible in rostrum specimens of D. planei, although it is shallower. The ventral-most part of the orbital process of the lacrimal (and associated structures, see above) meets the processus jugalis of the maxillare laterally and forms a caudal angular flange which demarks the ventrocaudal most extent of the depression. Immediately medial, the depression contacts a prominent and deep sulcus (see light grey filled outline, ) which travels mediorostrally beneath a bridge of the maxillare formed between the processus jugalis and the medially adjacent post-narial bar (possibly processus nasalis of the maxillare), onto the caudal surface of the supra-narial plate, just caudodorsal of the palatines. This sulcus on the supra-narial plate is delimited medially by a sharp crest and laterally by a sheet of processus maxillopalatinus which is co-ossified with the dorsomedial surface of lamella dorsalis of pars choanalis of the palatines, enclosing the choana nasalis ossea laterally (dashed outline, ). The caudolateral margin of this sheet is marked by a robust bar, the pons maxillaro-jugalis (sensu Livezey and Zusi Citation2006), which buttresses the palatines dorsally and is likely part of the maxillare. A deep cavity is present on the lateral surface of lamella dorsalis, confluent with the sulcus paratomialis (see above) and extends dorsocaudally onto a small triangular angulus tomialis (sensu Livezey and Zusi Citation2006). Although this flange could derive from the praemaxillare as in galliforms (see Livezey and Zusi Citation2006; Mayr Citation2018a), due to the similarities with anseriforms in this region, it appears more parsimonious to identify it as an extension of the maxillare.
The processus jugalis of the maxillare in SAMA P59521 is broken caudally, at the point where arcus jugalis becomes distinct with respect to the rostrum and lacks the more caudal region relating to fusion with the jugal bone. In anatids, and to a lesser degree anseranatids, the processus jugalis uniquely projects from the maxillary bone from a point ventral of, and coincident with, the rostrolateral margin of the mediolaterally expanded processus rostralis of the palatinum (see Zusi and Livezey Citation2006, p. 155; Zelenkov and Stidham Citation2018). Comparatively, in galliforms, gastornithids, and sylviornithids, the processus jugalis is positioned far lateral and more dorsal of the processus rostralis, so there is no interaction present between the two processes. Neither of these states are directly comparable to the rostrum of G. newtoni, which appears intermediate, i.e. the processus jugalis is more mediorostral of the galliform-position but not to the extent of the typical anatoid condition. Resultantly, processus jugalis extends from the maxillary bone just dorsolateral of the processus rostralis of the palatinum and contributes to the aforementioned depression associated with the sulcus paratomialis (see ‘The palate: palatum osseum’). This is very similar to the intermediate state also observed in Anhima cornuta.
Fragmented parts of the arcus jugalis of G. newtoni can be described from specimens SAMA P59516, SAMA P53830 (Appendix One: fig. A3a), and SAMA P10838, which corresponds to the jugale and quadratojugale bones. The caudal part of the arcus jugalis, which would have largely corresponded to the quadratojugale bone, has a slender, slightly mediolaterally compressed shaft which bows ventrally and then laterally towards the caudal region to participate in the articulatio quadrato-quadratojugalis. Caudal of the poorly developed condylus quadraticus, the terminus of the jugal arch is rounded caudally and flattened mediolaterally as per the description and figure of this region by Stirling (Citation1913).
Ventral of all other processus, including the processus jugalis, on palatum osseum, the maxillaria and praemaxillaria join the palatines through robust and complete synostosis of the processus praemaxillaris maxillaris, the processus rostralis of the palatinum, and the processus palatinus praemaxillaris. This region is defined rostrally by paired, rostrocaudally elongate, bulbous surfaces (see : bulb.; described as ‘bulges of spongey bone’ for D. planei by Murray and Megirian Citation1998) which lack a flexible zona flexoria palatina. We assume the absence of a processus palatinus of the maxillare (Zusi and Livezey Citation2006; sensu Mayr Citation2018a, SI 2) as this process is not common among neognaths (nor within Galloanserae, see Mayr Citation2018a: p. 7–8).
Palatinum et vomer
The pars choanalis et lateralis palatini of the palatine together form mediolaterally flattened, and largely dorsoventrally aligned, osseous wings. The pars lateralis palatini extends ventrolaterally from the ventral margin of lamella dorsalis, pars choanalis palatini (especially visible in G. newtoni SAMA P59517, P59516), ventral to the level of margo medialis palatini, a character shared with D. stirtoni (see Murray and Megirian Citation1998, p. 67 and 80, fig. 15). In anseriforms, the pars lateralis palatini is mediolaterally narrow along the ventral margin of lamella dorsalis, whereas in galliforms, it is absent or represented only by a thickened crista lateralis on the lateral margin of pars choanalis palatini (Zusi and Livezey Citation2000, p. 158, fig. 7). This variation has been utilised previously to support close association of fossil taxa to either lineage (Sylviornis neocaledoniae, see Mourer-Chauviré and Balouet Citation2005). The relatively mediolaterally widened structure of the ventrolateral palatines in G. newtoni is considered more anseriform-like although pars lateralis palatini appears to be proportionally greater in its ventrolateral extension (; e.g. compared with species of Anser), which may be functional (see Discussion) and/or related to an enlarged surface area associated with the origin of musculus pterygoideus (e.g. Lakjer Citation1926; Goodman and Fisher Citation1962; Zusi and Livezey Citation2006; Holliday and Witmer Citation2007). Unlike those of G. newtoni, the palatines of most extant taxa do not project far below the plane of the crista tomialis, if at all; only anhimids appear to have a palatine that extends noticeably ventrad of the crista tomialis (). Additionally, the angulus caudolateralis on pars lateralis of the palatine of anseriforms is also absent in galliforms (Zusi and Livezey Citation2006, p. 147, fig. 7). The palatines in G. newtoni specimens SAMA P59521, P59516, and P59517 are eroded at their ventrocaudal-most extremities, although appear to terminate in angulus caudolateralis palatini which clearly did not project much more caudally than what is preserved, based on the positions of other bones and articulatory surfaces (e.g. processus basipterygoideus, see ). The processus pterygoideus palatini, and the region of articulation with the pterygoids (facies articularis pterygopalatina), has been damaged or lost in all specimens and cannot be commented upon. The relatively short rostrocaudal length of the palatines in dromornithids, is entirely expected given the very short crania in these birds. That observed in specimens of G. newtoni, is more like grazing anseriforms than the filter feeding taxa (Marugán-Lobón and Buscalioni Citation2006; Pecsics et al. Citation2017). Regardless of the similarities in the dromornithid palatal structure to that of anseriforms, the general shape of the palatines appears to be unique among galloanserans, even when compared to gastornithids and sylviornithids (for comparison see Mourer-Chauviré and Balouet Citation2005: fig. 2).
The rostrocaudally short ossa vomeris are fused along their entire length, forming a single rod-like central element, here referred to as the vomer, that defines the medial margins of the bilaterally paired choana nasalis ossea. Caudally, the vomer is fused laterally to pars choanalis palatini, specifically processus choanalis palatini. A sutura vomeropalatina is not visible between the area corresponding to the vomer and the palatines, suggestive of complete synostosis between these two elements. This is reminiscent of an anseriform state, where the suture is indiscernible in adults. By contrast, no fusion occurs between these elements during maturation in galliforms and the vomer is comparatively easily disarticulated (Baumel and Witmer Citation1993: annot. 78; Zusi and Livezey Citation2006, p. 162). The margin of choana nasalis ossea is continued caudolaterally, and then rostrally by the margo choanalis palatini of the lamella dorsalis, pars choanalis of each palatinum, and defines the rostral limits of fossa choanalis palatini on each palatine (see Zusi and Livezey Citation2006). The vomer is connected to the processus maxillopalatinus of the maxillare dorsorostrally, where a narrow rostrally directed triangle identifies the sutura vomeromaxillaris. This marking is perhaps contributed to by the linea that demarks the synostosis of the left and right maxillopalatine processes, typical of anseriform birds (see above; Baumel and Witmer Citation1993: annot. 58). Unlike some galliforms, anhimids and anseranatids, there is no contact between processus choanalis palatini or the vomer with rostrum parasphenoidale (Zusi and Livezey Citation2000, p. 161–162); the terminus of the latter is far caudad in dromornithids. As in adult anatids (Zusi and Livezey Citation2000, p. 162), there is complete separation between palatum osseum and rostrum parasphenoidale in G. newtoni. The vomer of gastornithids (see Matthew and Granger Citation1917) and Sylviornis neocaledoniae (see Mourer-Chauviré and Balouet Citation2005) is small and not a major contributor to the palatal roof (Baumel and Witmer Citation1993: annot. 78).
Mandible
The partial mandible originally described by Stirling (Citation1913: pl. XXXVII, fig. 1–3; SAMA P.10788, ), differs a little from the mandible of the near-complete skull specimen, SAMA P59516. The former is longer from the rostral apex to the caudal-most point on the fossa articularis quadratica, and the symphysial region is proportionally shorter, 7% of length compared to 16%. This may be indicative of intraspecific variation and potentially sexual dimorphism. The mandible fragments of SAMA P59517 (Appendix One: ) represent the partes intermedia et caudalis, and have suffered much damage, rendering most measurements uninformative, although the pars caudalis and the fossa articularis quadratica are relatively intact. There are two additional isolated specimens that represent the rostral part of the mandibles, NMV P256893 and SAMA P59520, which preserve in good condition the left and right pars symphysialis, respectively.
The specimens are typically larger than that of the incomplete mandible attributed to Ilbandornis sp (NTM P2774–2; Worthy et al. Citation2016a), and dorsoventrally taller and more robust than that attributed to Barawertornis tedfordi (QM F57895; Worthy et al. Citation2016a); they are proportionally rostrocaudally longer with respect to maximum dorsoventral height than mandibles attributed to all species of Dromornis which are comparatively far taller. Mandibles of species of Dromornis are considered more massive and deeper than those of species of Gastornis (see Angst and Buffetaut Citation2013), although the opposite is true for all other dromornithid genera. Mandibular rami of anseriforms are generally dorsoventrally taller than those of galliform birds (Mayr et al. Citation2023), however, this character appears to be homoplasious when considering giant galloanserans (e.g. Sylviornis neocaledoniae).
Mandibles of G. newtoni are overall robust, with complete fusion of elements eliminating indications of the respective boundaries and areas of interactions between elements, even along the mandibular symphysis. The rostrocaudal length of the symphysis, 44.2 mm in specimen SAMA P59516, varies slightly between dromornithid species, although, all are far shorter than the extreme length of the gastornithids, that have a unique rostrocaudal elongation of the symphysial region accounting for 44% to 48.1% of total mandible length (Matthew and Granger Citation1917; Witmer and Rose Citation1991; Angst and Buffetaut Citation2013). A similarly great length was also noted for the Sylviornithidae (Worthy Citation2000, p. 357; Mourer-Chauviré and Balouet Citation2005: fig. 8; Worthy et al. Citation2016b). The apex rostri of the rostrum mandibulae in G. newtoni is curved with a notably blunt tip which approaches the shape of some anseriforms, e.g. Anseranas semipalmata, more so than in other dromornithids, although it lacks the distinct dorsoventral flattening of this region, notably extreme in Cygnus atratus and Melanitta perspicillata. The density of foveae corpusculorum nervosorum both dorsally and ventrally is low, relatively comparable to Anhima cornuta and galliforms.
The rostral mandible of G. newtoni, as evidenced by SAMA P10788, NMV P256893, and SAMA P59520, has two symmetrical depressions or broad sulci on the caudoventral part of the dorsal symphysis that extend caudally from this region along the medial side of their respective mandibular ramus ( D). They are divided at the midline by a low, wide, rugose elevation. Dromornis planei (specimen QVM:2000:GFV:440 and NTM P9464–112) has similar sulci. This morphology is likely associated with the gular and extrinsic lingual apparatus musculature, and adjoining fasciae and glands that contribute to the sublingual floor of the mouth cavity (see Homberger and Meyers Citation1989); the rostrolateral ridge that dorsally bounds each depression across their rostrocaudal length (dotted line, D) may indicate the origin of the m. mylohyoideus (m. mylohyoideus anterior, sensu Burggraaf Citation1954b; m. intermandibularis, sensu Fujioka Citation1963; Homberger and Meyers Citation1989). The m. genioglossus originates in the symphysial area in several avian taxa, as has been inferred for gastornithids (Homberger Citation1986, and references therein; Andors Citation1988; Angst and Buffetaut Citation2013: fig. 2; Jones et al. Citation2019), however, this muscle is absent in adult galloanserans, including Gallus, Aepypodius (see Fujioka Citation1963; Homberger and Meyers Citation1989; Weber Citation1996) and Anas (see Zweers et al. Citation1977). Instead, in addition to m. mylohyoideus, these sulci in G. newtoni/dromornithids are likely associated with the fascia intermandibularis and fascia sublingualis, and also potentially m. branchiomandibularis rostralis which arises from the rostral region of the medial mandible (Gadow and Selenka Citation1891; Goodman and Fisher Citation1962; Fujioka Citation1963; Zweers Citation1974; Homberger and Meyers Citation1989: p. 235–239, table 21, regarding clarification, synonymy and homology; Weber Citation1996: fig. 2). The sulci in G. newtoni and D. planei are, as in Anseres, rostrocaudally elongate and relatively flat compared to galliforms and anhimids. They are especially similar in form and position to that of Cereopsis novaehollandiae and to a lesser extent, Anseranas semipalmata, as other anseriforms generally possess less well-defined and mediolaterally narrower depressions and retain a dorsally overhanging medial plate (sensu Burggraaf Citation1954b), the latter of which is comparatively absent in dromornithids. In galliforms (Burggraaf Citation1954b: fig. 31) and anhimids, these sulci are restricted to the caudal margin of the symphysial region and meet along the midline, just ventral of the medial plate.
Paired foramen neurovasculare (Stirling Citation1913: p. 114; Baumel and Witmer Citation1993; sensu Livezey Citation1997: char. 16; lingual median symphysial foramina, sensu Stidham Citation1998) are located on the dorsal surface of the rostral mandible, either side of the symphysis, and sit at the rostral-most point of an associated shallow, predominately rostrocaudally aligned sulcus (potentially homologous with the canalis primordialis, see Burggraaf Citation1954b; and the longitudinal vascular groove, see Currie et al. Citation1993), as best seen in G. newtoni specimens NMV P256893 and SAMA P10788. Like in galliforms and anhimids, the foramina are each positioned within an elongate depression which extends caudally, whereas in the mandibles of D. planei and non-anhimid anseriforms, the foramen is located relatively further rostromedially on the rostrum mandibulae than these depressions and the associated sulci extend further caudally. In this region of the rostral mandible, the crista tomialis of G. newtoni is thin. Caudally a rostrocaudally long, wide, flat surface of bone, extends medially from the near indistinct crista tomialis, as described for Ilbandornis sp (see Worthy et al. Citation2016a). The medial margin of the shelf is truncated sharply as it drops ventrally towards the lateral margins of the aforementioned paired depressions which house the foramen neurovasculare. The shelf decreased in mediolateral width caudally (see also Stirling Citation1913). Such a flat tomial surface is also identified in the mandible of gastornithids, although the crista tomialis is more distinct, sharper and more blade-like across its entire length (Troxell Citation1931; Angst and Buffetaut Citation2013: fig. 2). Extant galloanserans appear to lack the shelf, and instead have a typically thin and sharp (as in many galliforms) or wide and rounded (i.e. Anseres) crista tomialis. A similar medial crest and associated tomial shelf is only present in the anhimids although it is far less developed, restricted in its rostral extension, and may not be homologous in origin (see Previatto Citation2012: fig. 21, 23).
The mandibular rami in G. newtoni and other dromornithids diverge caudally from the symphysis only slightly (fig. 1 g; Murray and Megirian Citation1998: figs. 11, 18, 20), at a much smaller angle than in mandibulae attributed to most galliform and gastornithid taxa (although see Stirling Citation1913, p. 115). The dorsally elevated angulus dorsalis mandibulae (ADM, sensu Livezey and Zusi Citation2006: characters 0662, 0679, 0680) is just rostral to the pars caudalis of the mandible and is coincident with the same ridge that hosts the processus coronoideus as in all dromornithids – the former marking the caudal-most limit of the rhamphothecal sheath (Baumel and Witmer Citation1993: annot. 43), and broadly correspondent with aponeurosis superficialis (sensu Dzerzhinsky and Potapova Citation1974; Dzerzhinsky Citation1982; see ; see also Weber Citation1996). In all dromornithids this appears dorsally raised, although it is confluent with, rather than distinctly dorsally produced, the dorsal edge of the rostrum mandibulae. Among extant galloanserans, the ADM is only clearly dorsally prominent with respect to the level of the more rostral tomium in Anseres, in most galliforms it is subtle. As in crown group Anseres, the dorsal elevation of angulus dorsalis mandibulae can be clearly distinguished from the more rostral crista tomialis in late Paleocene Anachronornis anhimops, whereas in early Eocene Danielsavis nazensis, there is comparatively no clear transitional change in dorsoventral height (as in galliforms, see Houde et al. Citation2023: figs. 1, 7; Mayr et al. Citation2023). The adjacency of the processus coronoideus and ADM in Anseres confines the insertions of the m. AME profundus partes zygomaticus et superficialis and m. AME superficialis to within a relatively caudal area (e.g. Davids Citation1952a, Citation1952b; Goodman and Fisher Citation1962; Zusi and Livezey Citation2000). Their similarly close proximity in dromornithids, likely results in the same musculature arrangement, as also interpreted for D. planei (see Murray and Vickers-Rich Citation2004: fig. 189). This contrasts with a typical galliform state, whereby the ADM is relatively far rostral, and the attachment surfaces for these external adductor muscles extend more rostrally on each mandibular ramus (relative to its total length, e.g. Kirikov Citation1944; Dzerzhinsky and Belokurova Citation1972; Dzerzhinsky Citation1982; Weber Citation1996; Zusi and Livezey Citation2000). Anhima cornuta appears intermediate between Anseres and galliforms with respect to proximity between processus coronoideus and ADM, and interrelated myology (see ; Dzerzhinsky Citation1982).
Figure 10. Genyornis newtoni mandible morphology: A. Caudal view of skull part NMV P256893, partially preserving ramus mandibulae, pars caudalis ventrally, which retains the medial process of the mandible; B. Image and annotated outline of SAMA P59516 in right lateral view, digitally removed from the image of the entire skull; C. Ventral view of the symphysial region of mandible specimen SAMA P59516; D. Caudal view of symphysial part of mandible specimen NMV P256893; E. Dorsal view of symphysial part of mandible specimen NMV P256893; F. Proposed arrangement of insertions of the adductor muscle complex on the lateral side of the mandible for dromornithids (drawn from specimen NTM P2774–2 of Ilbandornis sp. due to the limited distortion and conservative nature of dromornithid skull morphology) compared with re-drawn examples of extant galloanserans, Anseres – Anas platyrhynchos (Davids Citation1952a: fig. 3b), Anhimidae – Anhima cornuta (NMV B12574), Megapodiidae – Aepypodius arfakianus (Weber Citation1996: fig. 2a) and Phasianidae – Gallus gallus (Fujioka Citation1963: fig. 7). Annotations: c.lat., cotyla lateralis.; cr.par., crista paracoronoidea rostralis and crista paracoronoidea caudalis.; ADM, angulus dorsalis mandibulae; AVM, angulus ventralis mandibulae; exc., excavation associated with the region of recessus conicalis in anatids; f.neur., foramen neurovascularis.; m.AMEPc, m. AME profundus, pars coronoideus; m.AMEs, m. AME superficialis; m.AMEPs, m. AME profundus, pars superficialis; m.AMEPz, m. AME profundus, pars zygomaticus; m.AMP, m. adductor mandibulae posterior; m.ps., abraded prominence and approximate area for insertion of m. pseudotemporalis profundus; pr.cor., processus coronoideus (approximate position indicated, not visible as it is obscured by associated cranium); pr.lat.m., processus lateralis mandibulae; pr.med.m., processus medialis mandibulae; pr.ret., processus retroarticularis; sym., pars symphysialis, rostrum mandibulae; tom., crista tomialis; Scale bars: A., C.-E. 10 mm, B. 20 mm, F. not to scale. Dark grey shading indicates regions where damage precludes morphological assessment, and light grey indicates fenestra.
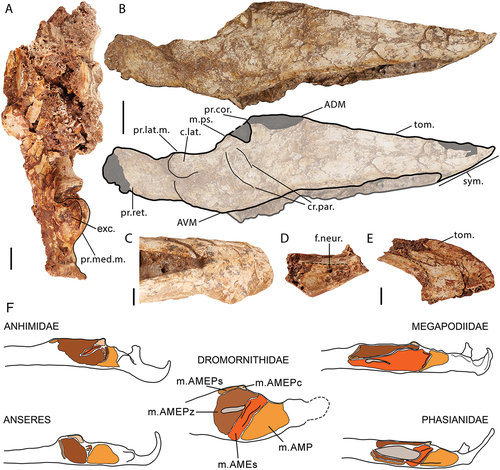
The gentle rostral transition from the ADM to the tomial margin of G. newtoni differs markedly from Anseres, in which the steep rostral incline of the ADM is comparatively near perpendicular, such as in Anser caerulescens, and even the fossil anseriform, Anachronornis anhimops which also has a relatively steep incline. The rounded rostral profile of the ADM in G. newtoni is largely reminiscent of that in D. murrayi (see Worthy et al. Citation2016a), and is less consistent with the more sloped form of Ilbandornis sp. NTM P2774–2. Both rami of Barawertornis tedfordi (QM F57895) are damaged in this area, although the left ramus mandibulae is better preserved in this region and does not appear to differ substantially from other dromornithids, although more detailed comparisons are not possible. The form of the ADM in transition to the tomial margin in G. newtoni is less hyperdeveloped than in species of Dromornis (see Murray and Megirian Citation1998).
A lack of symmetry in the rostrocaudal curvature of the ridge that hosts the ADM is evidenced in the G. newtoni mandible described by Stirling (Citation1913), whereby the more dorsocaudal angle on the caudal part of this ridge represents the processus coronoideus. This region is obscured (SAMA P59516) or damaged (SAMA P59517) in other specimens although the visible shape is consistent with this morphology. This is also consistent with other dromornithids of which G. newtoni is largely similar to that of D. murrayi (see Worthy et al. Citation2016a: fig. 3). In Ilbandornis sp., Barawertornis tedfordi (see Worthy et al. Citation2016a: fig. 3), and especially D. stirtoni and D. planei (larger in the latter taxon proportionally, see Murray and Vickers-Rich Citation2004: fig. 109), the processus coronoideus projects more sharply and prominently dorsocaudally. This may evidence a less well-developed aponeurosis coronoidea (sensu Iordansky Citation1970; aponeurosis rostralis, sensu Dzerzhinsky Citation1982; also following Weber Citation1996) in association with m. AME profundus coronoideus (Weber Citation1996; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007; Matsuoka et al. Citation2008) in both G. newtoni and D. murrayi, compared to other dromornithids. The processus coronoideus in dromornithids (except for species of Dromornis) is comparable to the small caudally protruding angle in Anhima cornuta or Oxyura australis, lacking the more extensive lateral flare of most Anseres (e.g. Cereopsis novaehollandiae), and differs from the prominent tubercle-like protrusion of most galliforms.
In other dromornithids, especially species of Dromornis, the ridge, just caudal of the processus coronoideus, shows greater attenuation in height towards the fossa articularis quadratica than in G. newtoni. Such variation may be associated with the attachments of corresponding musculature; m. adductor mandibulae posterior inserts on the lateral surface between the processus coronoideus and the fossa articularis quadratica in neognaths, a pattern galloanserans – and most likely dromornithids – also adhere to (e.g. Dzerzhinsky Citation1982; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007). Variable development of areas related to insertions of mm. pseudotemporalis superficialis et profundus and m. pterygoideus on the medial surface, and the origin of m. branchiomandibularis caudalis (m. geniohyoideus, lateral slip, sensu Goodman and Fisher Citation1962, only reported for Mergini among anatids) on the ventral edge and ventral half of the lateral caudal ramus (e.g. Goodman and Fisher Citation1962; Dzerzhinsky and Belokurova Citation1972; Homberger and Meyers Citation1989; Weber Citation1996), also likely contribute to this morphological disparity. Species of Dromornis additionally have a deeply depressed medial mandibular surface correlated with the large attachment site of the m. pterygoideus (Murray and Vickers-Rich Citation2004, p. 241, fig. 189), in association with their relatively dorsoventrally deep caudal mandible. A low, rounded, and subtle bulge on the dorsal part of the ramus mandibulae between the processus coronoideus and fossa articularis quadratica – likely homologous with the area for insertion of m. pseudotemporalis profundus in other galloanserans (Davids Citation1952a; Fujioka Citation1963; Zweers Citation1974; Weber Citation1996; Matsuoka et al. Citation2008) – can be observed in Ilbandornis sp., Dromornis murrayi, and is especially pronounced for B. tedfordi, however, its form is not accurately assessable in any specimens of G. newtoni. While similarly located in some birds, this bulge is not to be considered equivalent to the tuberculum praearticulare (sensu Weber Citation1996), for aponeurosis praearticularis caudalis (sensu Weber Citation1996) of m. pterygoideus dorsalis, which is instead positioned just rostral of the fossa articularis quadratica on the medial mandible in dromornithids, as is especially evident on the right caudal ramus of G. newtoni (SAMA P59517, see Appendix One: ) and Ilbandornis sp. NTM P2774–2 (see Weber Citation1996: fig. 26; Worthy et al. Citation2016a: fig. 3A).
The damaged lateral surfaces of all mandibles known for G. newtoni, restrict a confident examination of some structures pertinent to galloanseran systematics, such as the exact nature of the crista(e) paracoronoidea (lateral coronoid process, sensu Goodman and Fisher Citation1962; see also Weber Citation1996; terminology of Weber and Hesse Citation1995, p. 295; Matsuoka et al. Citation2008; Mourer-Chauviré and Bourdon Citation2020; SI 2), which is a laterally shifted homologue of the tuberculum paracoronoideum of many birds (see Weber Citation1996, p. 27). In galliforms and anhimids, the crista paracoronoidea is separated into two crests, distinguished as the crista paracoronoidea rostralis and caudalis, which are perpendicular to near parallel to one another and closely approximated, or touching, at their dorsocaudal-most points (sensu Weber and Hesse Citation1995; Weber Citation1996). In the Megapodiidae (including Alectura lathami) and Acryllium vulturinum (Numididae), these two cristae are more widely separated, and the rostral part is well-defined and rostrally extended into a process or flange (Weber and Hesse Citation1995, p. 295; Weber Citation1996, p. 27, 28). A similarly located, but undivided crista paracoronoidea forms a prominent processus paracoronoideus (sometimes mistakenly referred to as the processus coronoideus, see Weber and Hesse Citation1995; Weber Citation1996) in non-anhimid extant anseriforms (), and is analogous to the well-developed crista paracoronoidea rostralis in Megapodiidae (Weber Citation1996).
As far as can be discerned for G. newtoni, a distinct rostrocaudally elongated crista paracoronoidea rostralis is present, caudoventral of the processus coronoideus, corresponding to the aponeurosis paracoronoidea externa (sensu Weber Citation1996) and the associated insertion of m. AME superficialis (see ; also Dzerzhinsky and Potapova Citation1974; Dzerzhinsky Citation1982; Zusi and Livezey Citation2000). The crista paracoronoidea caudalis is less well-marked, yet is likely represented by a relatively swollen, dorsocaudally-ventrorostrally elongated ridge just caudoventral of its rostral counterpart. This is likely associated with the aponeurosis paracoronoidea interna (aponeurosis caudalis interna, sensu Dzerzhinsky and Potapova Citation1974; Dzerzhinsky Citation1982; sensu Weber Citation1996), which receives some fibres from, and caudally bounds, the insertion of m. AME superficialis (Weber Citation1996, p. 46, 53). The form and position of this structure may also be contributed to by the development of the caudally adjacent insertion of m. adductor mandibulae posterior (Weber and Hesse Citation1995, p. 58; Weber Citation1996; ‘adductor fossa’ Zusi and Livezey Citation2000; Murray and Vickers-Rich Citation2004: fig. 56; see above). A similar morphology is observed in Barawertornis tedfordi, with regards to both the rostral and caudal cristae. Comparatively, Ilbandornis sp. (NTM P2774–2) has distinct, lowly raised, narrowly separated, and parallel rostral and caudal paracoronoid crests (Murray and Megirian Citation1998: fig. 12; Worthy et al. Citation2016a). This also appears to be the case in well-preserved D. planei and D. stirtoni specimens (e.g. NTM P9464–112, P98107, see Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a). The nearly parallel orientation of the cristae in dromornithids appears to resemble that of the Anhimidae and Phasianidae, more than that of the Megapodiidae.
In contrast, the late Paleocene ‘screamer-like’ Anachronornis anhimops, Wyoming, preserves a laterally projected processus paracoronoideus, as is typical of Anseres. However, this is comparatively less laterally developed than crown group representatives (see Houde et al. Citation2023). The early Eocene Danielsavis nazensis has a well-developed, flange-like crista paracoronoidea rostralis that compares well with the aforementioned megapodiid condition (Houde et al. Citation2023; Mayr et al. Citation2023). In gastornithids, the crista paracoronoidea rostralis is potentially homologous with a long, predominately dorsoventrally orientated ridge which extends caudodorsally to meet the processus coronoideus. This ridge is further rostral of the processus coronoideus compared to the relatively caudal placement and rostrocaudal extension of this crest in dromornithids; a second faint ridge is evident in some gastornithid specimens, notably Ga. parisiensis MHNT.PAL.2012.1.1, which is caudally displaced from, but in near parallel dorsoventral orientation with the aforementioned crest, and may be homologous to the crista paracoronoidea caudalis in this taxon (see Matthew and Granger Citation1917, p. 312, pl. XXI; Angst and Buffetaut Citation2013: fig. 3C; Angst et al. Citation2014: fig. 4; Mourer-Chauviré and Bourdon Citation2020: figs. 1, 2).
The rounded angulus ventralis mandibulae (AVM; sensu Livezey Citation1998; Livezey and Zusi Citation2006) of G. newtoni is positioned at a point noticeably caudal to the processus coracoideus, as in B. tedfordi and D. stirtoni. In contrast, in Ilbandornis sp. NTM P2774–2, and specimens of D. murrayi, and especially D. planei, the AVM is relatively more rostrad. In D. planei and D. stirtoni (see Murray and Megirian Citation1998: fig. 12; Worthy et al. Citation2016a: fig. 3), the AVM is more pronounced ventrally, and more robust than in other species. This would support greater attachment regions for relevant musculature on medial and lateral sides (e.g. m. AME externus partes superficialis et profundus, m. adductor mandibulae posterior and m. pterygoideus ventralis, and m. branchiomandibularis caudalis, see , also Goodman and Fisher Citation1962; Vanden Berge and Zweers Citation1993; Murray and Vickers-Rich Citation2004; Holliday and Witmer Citation2007; Matsuoka et al. Citation2008; Holliday Citation2009). In contrast to mandibles attributed to D. stirtoni, D. planei and Ilbandornis sp., there is less of a dorsoventral shift from the AVM to the ventral edge of the rostral-most processus retroarticularis in both G. newtoni and B. tedfordi, although not as level as in D. murrayi. Although less accentuated than in the dromornithids, the AVM is only distinct as a rounded structure in goose-like anserines including Cereopsis novaehollandiae, Cygnus atratus, Branta canadensis, and Anser caerulescens. The AVM is indistinct in species of Gastornis.
The ventrocaudal edge of the AVM of G. newtoni continues as a low ridge dorsocaudally onto the lateral mandibular face and forms the caudal and ventral margins of a shallow depression just rostroventral to the processus lateralis mandibulae (lateral mandibular process), which likely corresponds to the bounds of the insertion of m. adductor mandibulae posterior ( F; Dzerzhinsky Citation1982; Weber Citation1996; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007). This fossa is deeper and more distinct in mandibles attributed to species of Dromornis (e.g. see ‘fossa for external adductors’, Murray and Megirian Citation1998: fig. 16; ‘adductor fossa’, Murray and Vickers-Rich Citation2004: fig. 56). Murray and Vickers-Rich (Citation2004: figs. 54, 56, 189) labelled an area near the ventral margin of the mandible of D. stirtoni, close to this fossa, as a ‘groove for m. mylohyoideus’ for ‘m. mylohyoideus posterior’ (similarly labelled by Davids Citation1952a, Citation1952c). In this sense, this more appropriately relates to the passage for m. serpihyoideus and m. stylohyoideus, passing across the ventral mandible towards the lingual apparatus, from the caudal processus retroarticularis, and is involved in retraction of the tongue and the raising of the tongue and trachea (see Davids Citation1952a, Citation1952c; Goodman and Fisher Citation1962; Fujioka Citation1963; Zweers Citation1974; sensu Homberger and Meyers Citation1989; Weber Citation1996; Matsuoka et al. Citation2008). This form of the ventral mandible may also be influenced by the origin of m. branchiomandibularis caudalis on this area of the caudal ramus (see above; Fujioka Citation1963; Homberger and Meyers Citation1989; Weber Citation1996; SI 2). The path of the former muscles on the ventral edge of the caudolateral mandible, especially, may contribute to the development of the low ridge that continues from the ventral margin onto the lateral face in G. newtoni and D. stirtoni.
As in B. tedfordi, D. stirtoni, and gastornithids (Angst and Buffetaut Citation2013; Worthy et al. Citation2016a), the mandible of G. newtoni is imperforate, lacking both fenestra caudalis mandibulae and fenestra rostralis mandibulae. Contrastingly, Ilbandornis sp. NTM P2774–2, D. planei, and D. murrayi all have a caudal fenestra (see Worthy et al. Citation2016a: fig. 3). Although considerable variation in the size and shape of the fenestra caudalis mandibulae is present within galloanserans, the presence and absence of the fenestra among some dromornithids is seemingly unusual. Only in anhimids, is this fenestra exclusively absent. Intercepting the caudal fenestra on the medial side of the mandible of Ilbandornis, an obvious ridge stretches rostrocaudally along pars caudalis towards the processus medialis mandibulae, representing the ventral edge of the fossa aditus canalis neurovascularis (see Murray and Megirian Citation1998: fig. 12; Worthy et al. Citation2016a: fig. 3). This is well-developed in mandibles attributed to Dromornis planei (e.g. NTM P9464–112), also present in B. tedfordi and to a far lesser extent, in G. newtoni.
A medial mandibular process of G. newtoni is well-preserved only in the fragmentary caudal ramus of SAMA P59517 and NMV P256893.4 and is partially visible on the skull of SAMA P59516. The medial process is larger than its lateral counterpart although proportionally to a far lesser extent than that of all extant galloanserans. In G. newtoni, like that of B. tedfordi, the process is short, truncated medially, and invaginated caudally as it curves to form a deep and mediolaterally wide depression on the caudal surface. The medioventral edge of this depression likely accommodated the attachment of ligamentum occipitomandibulare (Davids Citation1952a, Citation1952c; Goodman and Fisher Citation1962, p. 133; Bock Citation1964; Zweers Citation1974: fig. 14; Dzerzhinsky Citation1982: fig. 3; ligamentum neurocranio-mandibulare, sensu Baumel and Raikow Citation1993). Although there are no deep, depressed recessus conicalis as in anatids, nor an excavation as deep as that in Anseranas semipalmata, Anachronornis anhimops, Conflicto antarcticus or Presbyornis pervetus, the depression (: ‘exc’.) on the caudal surface of the process appears like the shallow depression in the region on mandibles of Anhima cornuta. This is perhaps also comparable to the mandible of galliform-like stem anseriform, or anseriform-like stem galliform, Danielsavis nazensis, which also lacks a distinct fossa caudalis and recessus conicalis (see Houde et al. Citation2023: fig. 5C; Mayr et al. Citation2023). Comparatively, this side of the process is dorsoventrally thinner and caudally rounded in most galliforms and has a horizontal mediolaterally oriented crest in some megapodes (e.g. Alectura lathami).
The rostromedial surface of the processus medialis mandibulae is gently sloped dorsorostrally-caudoventrally in G. newtoni, and would have primarily related to insertions of parts of m. pterygoideus ventralis (see following for references). Rostrally, a large, flattened, or shallow attachment surface likely primarily supported the insertion of m. pterygoideus dorsalis (Davids Citation1952a; Fuchs Citation1954a; Fujioka Citation1963; Zweers Citation1974; Dzerzhinsky Citation1982; Weber Citation1996; Holliday and Witmer Citation2007; Matsuoka et al. Citation2008; Bianki et al. Citation2013; see Appendix Three). The medial process is also well-preserved in Barawertornis tedfordi QM F57985 (Worthy et al. Citation2016a: fig. 3) and on the left ramus of Dromornis planei NTM P9464–112 (Murray and Megirian Citation1998: fig. 11–12). In these taxa, the process largely conforms with that of G. newtoni with regards to shape. Immediately dorsal of the m. pterygoideus dorsalis insertion area, an apparent rostral continuation of the processus medialis mandibulae as an obvious ridge or tubercle corresponds to tuberculum praearticulare for the attachment of aponeurosis praearticularis caudalis (see above; Weber Citation1996). The pattern of pterygoideus muscle attachments also appears similar across all dromornithids, although the surface for insertion of m. pterygoideus dorsalis, rostral of processus medialis mandibulae, is slightly more excavated in Ilbandornis sp. NTM P2774–2, and well-impressed in D. planei and D. stirtoni (see Murray and Vickers-Rich Citation2004, p. 241). Medially adjacent to cotyla medialis mandibulae, a foramen pneumaticum articulare is obvious dorsally on the medial process of D. planei (NTM P9464–112), Barawertornis tedfordi and Ilbandornis sp., although its presence is unknown for G. newtoni. Notably, the articulatory region differs considerably from those on gastornithid mandibles, the latter of which instead have greater morphological similarity to with S. neocaledoniae: the crista intercotylaris is sharper and more distinct, processus medialis mandibulae is proportionally larger and more medially elongate, and the processus lateralis mandibularis is also proportionally larger, as a prominent, convex tubercle (see Mourer-Chauviré and Balouet Citation2005: fig. 8; Angst and Buffetaut Citation2013: fig. 2).
The noticeable lateral overhang of the cotyla lateralis of processus lateralis mandibulae in G. newtoni appears more like that of some galliforms and anhimids than the relative lack of lateral projection in species of Dromornis, B. tedfordi, and Ilbandornis sp (Worthy et al. Citation2016a: fig. 3). In most non-anhimid anseriforms, the edge of the cotyla is more prominent, and in some derived forms, develops a lateral or rostral angularity (e.g. Melanitta perspicillata and Anas superciliosa). A caudally cambered ridge caudally borders the sloped rostral surface of the process; the morphology of this surface likely developed in relation to the insertion of the m. adductor mandibulae posterior (see above). In some galloanseran taxa where this muscle can be divided into distinct bellies, the lateral part consistently inserts on this area of the caudodorsal mandible (Holliday and Witmer Citation2007), and is associated with the attachment of a strong aponeurosis (e.g. aponévrose 15, Davids Citation1952a; m. quadratomandibularis ‘10A’ Fujioka Citation1963; ‘a. 11’ Dzerzhinsky and Belokurova Citation1972). A distinct lateral part of this muscle, the corresponding attachment to this area is absent in anhimids (Dzerzhinsky Citation1982; Zusi and Livezey Citation2000), but present in anseranatids and is typical of anatids (e.g. Goodman and Fisher Citation1962; Dzerzhinsky and Grintsevichene Citation2002; Bianki et al. Citation2013), consequently preventing a confident assessment of its presence in G. newtoni and other dromornithids (contra Murray and Vickers-Rich Citation2004: e.g. fig. 189). The potential lack of the ligamentum postorbitale in G. newtoni and the rest of the dromornithids, may be a factor in the variable development of the lateral mandibular process. Additionally, a small tubercle is variably developed just rostral of the lateral mandibular process where the ligamentum postorbitale attaches (Baumel and Raikow Citation1993: annot. 42) in galliforms and anseriforms but not in dromornithids. The ligamentum lacrimomandibulare also attaches in this region in most anseriforms and is associated with filter feeding in anatids due to its absence in non-filter feeders, e.g. Anseranas semipalmata (see Davids Citation1952c; Goodman and Fisher Citation1962, p. 131, 132; Baumel and Raikow Citation1993: annot. 41; Bout and Zweers Citation2001; Zelenkov and Stidham Citation2018). The lack of evidence supporting such specialised feeding in dromornithids suggests this particular ligament was absent in this family.
A feature common to all Galloanserae and present in dromornithids, is the bicondylar articulation with the quadrate (Dzerzhinsky Citation1995; Ericson Citation1996; Mayr et al. Citation2018; Field et al. Citation2020). This has assisted in supporting the galloanseran affinities of other fossil taxa including Conflicto antarcticus (see Tambussi et al. Citation2019). Of the articulatory region of G. newtoni, the caudal part of the right mandibular ramus is visible in SAMA P59517 and allows a dorsal view of the fossa articularis quadratica, while the cotyla lateralis is visible on the left side of SAMA P59516, and cotyla medialis is retained in specimen MNV P256893. These specimens show a similar morphology regarding the separation of the cotylae in other dromornithids and galloanserans (see Worthy et al. Citation2016a). Both cotylae are oval, angled obliquely to the main axis of the mandibular ramus, separated by a low crista intercotylaris although seeming to overlap for much of their long axis, and the cotyla medialis is deeper than cotyla lateralis (see also Worthy et al. Citation2016a). The cotylae in G. newtoni, Ilbandornis sp., and B. tedfordi are more rostrocaudally elongate compared to those in species of Dromornis (see Worthy et al. Citation2016a), although in all, the proportional sizes of the two cotylae to the surrounding pars caudalis mandibulae are more like those of Anhima cornuta and megapodes than to other galloanserans (in Anseres the medial cotyla is greatly expanded rostrocaudally and near equal in mediolateral width with the lateral cotyla, and in non-megapodiid galliforms, the lateral cotyla is larger mediolaterally).
The presence of a caudally prominent and laterally compressed processus retroarticularis is characteristic of Galloanserae (Baumel and Witmer Citation1993; Ericson Citation1996; Murray and Vickers-Rich Citation2004; Mayr Citation2017; Mayr et al. Citation2018; Tambussi et al. Citation2019; Field et al. Citation2020). While all caudal mandibles attributed to G. newtoni are incomplete, the processus retroarticularis is relatively visibly rostrocaudally short, lateromedially compressed, and robust with a caudal extension almost equal to its dorsoventral depth, similar to the morphology of other dromornithids (Worthy et al. Citation2016a) and Gastornis giganteus (Matthew and Granger Citation1917: pl. XXI; AMNH 6169; Angst and Buffetaut Citation2013: fig. 4). The process is directed caudally and has a slight dorsal upturn at its end creating a clear angle on the dorsal margin, immediately caudal to the fossa articularis quadratica. All dromornithid mandibles are somewhat damaged in this region, and so the extent of the dorsal upturn of the retroarticular process, and its caudal extremity, cannot be precisely ascertained. However, remnants of the rostral origin of the retroarticular process suggest some variation is present among dromornithids: D. planei has a steeply curved dorsal margin; D. stirtoni, a steeply curved ventral margin, yet a shallower dorsal curve; and D. murrayi and B. tedfordi are considered to lack dorsal deflection, as their retroarticular processes only project caudally. No mandible of species of Dromornis preserves a complete processus retroarticularis, yet Murray and Vickers-Rich (Citation2004, p. 81 and 105, fig. 57) speculated that D. stirtoni had a high, angular, caudodorsal tip. With new evidence from G. newtoni, if we are to assume D. stirtoni had similar morphology, such recurvature and caudodorsal apex would not be approximated. The processes on the mandibles of G. newtoni are dorsocaudally rounded and only have a small dorsal projection (SAMA P59516, right side). The process is caudally orientated as in most anseriforms (e.g. Anseranas semipalmata), not laterally deflected as in many galliforms (e.g. Pavo muticus and Gallus gallus).
Hyobranchial apparatus
Specimen SAMA P59516 retains, in association, two disarticulated, near complete ceratobranchials (ceratobranchiale), the rostral-most part of the basihyal (basihyale), broken just rostral of the articulatio ceratobasihyalis, and a partial, crushed paraglossum. Part of the left ceratobranchial is also preserved on the ventral-most caudal mandible of NMV P256893. These are the first osseous elements of the hyoid skeleton identified for any dromornithid.
The basihyal articular surface for the articulatio paraglosso-basihyalis in dorsal and lateral view, is similar to that of Anser caerulescens, where the basihyal thins bilaterally and expands dorsoventrally to form a curved saddle-type joint (see Baumel and Raikow Citation1993: annot. 51). The dorsal surface appears flat and ventrally the articular surface extends rostrally, to a blunt, taphonomically worn tip; this shape is present in Alectura lathami although proportionally much smaller than those of anseriforms that possess this structure, e.g. Anseranas semipalmata and Anser caerulescens. The body of the basihyal is rostrocaudally long and bilaterally narrow, proportionally more so than other anatid and anseranatid taxa (e.g. Anser caerulescens, pers obvs.), as is typical of Anhimidae and Galliformes, and lacks the wide form and wing-like projections of Danielsavis nazensis (see Mayr et al. Citation2023: fig. 3). The lateral expansion of the basihyal expected for the articulatio ceratohyalis (articulation with the ceratobranchials), including any indication of each process lateralis of the basihyal (sensu Zweers Citation1974), cannot be identified on the specimen. Additionally, no urohyal (urohyale) is preserved, fused (as in Anser caerulescens) or separate (Gallus gallus, see Homberger and Meyers Citation1989: fig. 1) to the basihyal.
The paraglossum () is present in specimen SAMA P59516, near the rostral end of the mandible, crushed between the medial aspect of the right mandibular rami, and the ventral surface of the attached rostrum. This bone is perforated by a large, obvious central foramen (for., ), and is overall a mediolaterally symmetrical, triangular, arrowhead shape. The paired cornua, or processus paraglossus caudalis (sensu Homberger and Meyers Citation1989), extend caudally away from the body of the bone but have lost much of their caudal length due to taphonomic damage. The general morphology of the paraglossum is similar to the form of most birds (see Baumel and Witmer Citation1993: annot. 80) including galliforms (see McLelland Citation1968; Homberger and Meyers Citation1989). The paraglossum of non-anhimids is comparatively rostrocaudally longer and more oval or rectangular (e.g. Zweers et al. Citation1977: fig. 6). The overall shape of this bone in Anhimidae is intermediate in form, being slightly rostrally tapered, but also bilaterally wide.
The ceratobranchials have a shaft minimally 115.7 mm in rostrocaudal length. They are displaced from the mandible and rostrum and disarticulated. Additionally, the articular ends are both damaged, preventing identification of either end. The shaft does not gently curve in one direction as in many galloanserans but instead curves twice to be slightly sigmoidal, potentially a result of taphonomic distortion.
Phylogenetic analyses
Parsimony analysis
The heuristic search resulted in 2 MPTs (Most Parsimonious Trees) with a length of 786 steps, where a total of 122,871,532 rearrangements were tested. Both MPTs (phylograms displayed in SI 5) have a Consistency Index of 0.2799, Homoplasy Index of 0.7201, Retention Index of 0.6939 and a Rescaled Consistency Index of 0.1942. The MPTs vary only in the exact topological relationship of Anas superciliosa (Anatini), Aythya australis (Aythini) and Melanitta perspicillata (Mergini) to one another, as evidenced by the associated formation of the polytomy in the strict consensus tree (). All other relationships, including those described below, were recovered in both MPTs.
Figure 11. The hyoid elements of Genyornis newtoni specimen SAMA P59516: A. Complete specimen SAMA P59516 in ventral view; B. paraglossum, arrows for orientation (dorsal, ventral); C. Right lateral view of the basihyal and both left and right ceratobranchials in situ, image rotated 90 degrees clockwise from A.; D. Annotated outline of hyoid elements, arrow for orientation of basihyal only (clockwise: dorsal, rostral, ventral, caudal). Annotations: a.cb., damaged region just rostral of the articulatio ceratobasihyalis; a.pb., surface corresponding to articulatio paraglosso-basihyalis; bh., basihyal; cb., ceratobranchials; cor., cornua (processus paraglossus caudalis); for., central foramen. Scale bars: A. 40 mm, B. 10 mm, C., D. 20 mm.
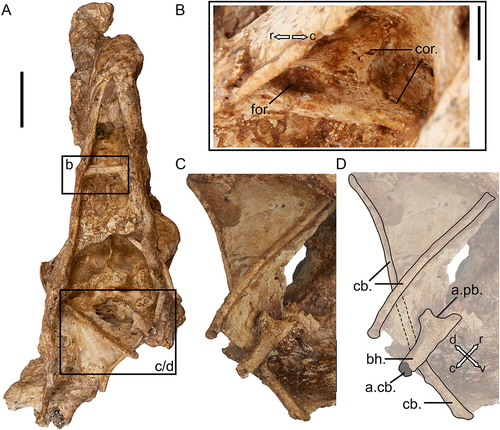
Figure 12. Galloanseran consensus trees derived from skull characters to assess the relationships of dromornithids: A. Parsimony strict consensus tree cladogram of 2 MPTs (length = 786, corresponding phylograms are displayed in SI 5), bootstrap support values are displayed below each corresponding branch; B. the consensus tree phylogram based on Bayesian inference (majority-rule, undated). The scale bar in B. relates to degree of morphological change across branch lengths. Posterior Probability values are specified next to respective nodes. The suborders Anhimae and Anseres are indicated, as is the superfamily Phasianoidea. Branches are differentially coloured corresponding to Galliformes (green) and Anseriformes (blue). The additional colour gradient across branches is indicative of support values in both consensus trees displayed (bootstrap values and posterior probabilities, respectively). Fossil taxa are shown in bold.
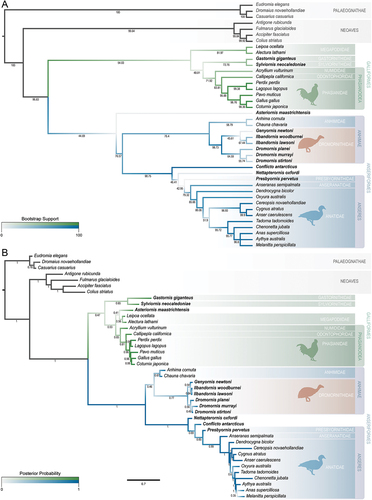
Since modern taxa were topologically constrained based on genetic data (see Materials and methods: Phylogenetic analyses), only the phylogenetic relationships of fossil taxa are described herein. A branch to the monophyletic clade containing all crown group Anseriformes, Asteriornis maastrichtensis, Conflicto antarcticus, Presbyornis pervetus, Nettapterornis oxfordi (Olson Citation1999) and Dromornithidae, was recovered in 44.69% of bootstrap replicates and is supported by two unambiguous (see SI 5 for all state changes discussed here) and 18 ambiguous character state changes. This clade to the exclusion of Asteriornis maastrichtensis was resolved in 76.57% of replicates and supported by three unambiguous and five ambiguous apomorphic state changes. A branch to the Anhimidae + Dromornithidae clade was found consistently across MPTs and received moderate bootstrap support (76.4%). The Anhimidae + Dromornithidae clade is supported by 13 unambiguous and 10 additional ambiguous character state changes. There are 24 character state changes that distinguish a monophyletic dromornithid clade with respect to the Anhimidae (20 unambiguous and four ambiguous), the branch to which received high bootstrap support (96.73%). The Genyornis newtoni + Ilbandornis species clade was supported by just one unambiguous and three ambiguous character state changes (bootstrap support for this branch is 45.61%), and the Dromornis clade distinguished by four unambiguous changes (bootstrap support is 64.59%).
A branch to the clade including all non-anhimid crown group anseriforms¸ Conflicto antarcticus, Presbyornis pervetus and Nettapterornis oxfordi was recovered sister to the Anhimidae + Dromornithidae clade in all MPTs, with high bootstrap support (98.75%), in association with 16 character state changes (11 unambiguous and five ambiguous). A sister-taxon relationship between Conflicto antarcticus and a clade including Nettapterornis oxfordi + Anseres was recovered in 46.41% of bootstrap replicates, related to nine character state transitions (three unambiguous and six ambiguous). The branch including Presbyornis pervetus and crown group Anseres, sister to Nettapterornis oxfordi, has low-moderate bootstrap support (42.95%), associated with two unambiguous and three ambiguous character state changes. The branch to crown group Anseres, sister to Presbyornis pervetus, is related to eight unambiguous and five ambiguous character changes and received moderate bootstrap support (79.32%).
A branch comprising a clade of crown group Galliformes, Gastornis giganteus, and Sylviornis neocaledoniae was recovered as sister to the Anseriformes clade in 54.03% of bootstrap replicates, supported by eight unambiguous and six ambiguous character transitions. A poorly supported (48.01%) branch that includes a clade of Gastornis giganteus, Sylviornis neocaledoniae, and all non-megapodiid galliforms is associated with three unambiguous character state changes and four ambiguous ones. A branch to a clade including the Gastornithidae and Sylviornithidae was resolved in 72.76% of bootstrap replicates; 13 unambiguous and two ambiguous character state changes support the monophyly of this clade as sister to a one comprising crown group Phasianoidea. The branch to the clade containing crown group Phasianoidea as sister to Gastornis giganteus and Sylviornis neocaledoniae received moderate bootstrap support of 71.92% and was distinguished by three unambiguous character state changes and five ambiguous ones.
Bayesian inference
MrBayes output was analysed in part using Tracer v. 1.7.2 (Rambaut et al. Citation2018). All four independent runs converged and achieved stationarity. All runs had a Potential Scale Reduction Factor (PSRF, Gelman and Rubin Citation1992) of 1.000. The Standard Deviation of Split Frequencies (SDSF) approached 0.0 as MCMC runs converged, with an average value across runs of 0.004799. After burn-in (20%), the mean log likelihood statistics (LnL) and Effective Sample Size (ESS) values for each run from one to four were −2505.3998 and 7601.4, −2505.2851 and 7750.2, −2505.33 and 8001, and −2505.4065 and 7817.2, respectively. Post-burn-in trees of the four independent runs were combined to produce a consensus tree, which is displayed with posterior probability (PP) support values (PP in ). As above, only the topological relationships of fossil taxa and the associated support for their resolved positions will be focused on here.
The topological relationships are similar to those recovered in parsimony analysis with respect to the clade that is associated with modern and fossil anseriforms. The most inclusive anseriform clade, formed of Anhimidae, Dromornithidae, Conflicto antarcticus, Nettapterornis oxfordi and Anseres, is robustly recovered with a posterior probability (PP) of 1. The posterior distribution supports the monophyly of a Anhimidae + Dromornithidae clade, as a distinct group sister to other anseriforms, but is poorly supported (PP of 0.46, resolved in 49.25% of post-burn-in trees). A clade including all dromornithid species is supported with PP values of 0.77 and recovered in 80.82% of post burn-in trees. This is sister to a poorly supported clade comprising of Anhimidae (PP of 0.67), resolved in 65.24% of post-burn-in trees; the low support and frequency for the Anhimidae clade may be linked to a clade comprising Dromornithidae + Chauna chavaria as sister to Anhima cornuta which occurred in 26.58% of post burn-in trees, but was not produced in the majority-rule consensus tree (). The exact interrelationships of dromornithid taxa within this clade receive low PP values for their resolved positions. A clade comprising Genyornis newtoni and species of Ilbandornis is resolved but is weakly supported (PP of 0.54). Other fossil and modern anseriforms are resolved in a strongly-supported clade (PP of 1). In contrast to parsimony analysis, the fossil taxon Nettapterornis oxfordi is resolved basal to Conflicto antarcticus, the latter of which is resolved in a monophyletic clade including the Anseres with weak support (PP of 0.69). The clade including Presbyornis pervetus and crown group Anseres is supported by a PP of 0.85, while the posterior probability of the clade comprising all non-anhimid crown Anseriformes receives strongly support (PP of 0.99).
All fossil and modern Galliformes, as well as Gastornis giganteus and Asteriornis maastrichtensis, are united in a poorly supported clade (PP of 0.47). The base of this clade is characterised by a clade including fossil taxa Gastornis giganteus and Sylviornis neocaledoniae, which is also weakly supported (PP of 0.65), and one comprising all crown group galliforms and Asteriornis maastrichtensis (in contrast to the position of A. maastrichtensis in the analysis parsimony optimality criterion) that is resolved with low support (PP of 0.53). The posterior distribution recovers fossil Asteriornis maastrichtensis as sister to the megapodiid taxa in a monophyletic clade (PP of 0.41). A monophyletic clade, sister to the aforementioned, comprising all non-megapodiid crown galliforms received weak support (PP of 0.65).
Remarks
Dromornithidae are phylogenetically resolved within Anseriformes, with close affinities to Anhimidae across both phylogenetic methods, as evidenced in both consensus trees (see ). Phylograms illustrate relatively little variation in branch lengths across dromornithid species, in the context of the wider Galloanserae, indicating only minor morphological change with regards to the characters assessed ( and SI ). It should be noted that while a clade of Anseriformes including dromornithids are strongly supported in Bayesian analyses (PP of 1), bootstrap support for this clade (excluding Asteriornis maastrichtensis) is only moderately supported (76.57%). This can likely be attributed to the nature of bootstrapping, where alternative topologies can be preferred in the random resampling of characters with replacement in each pseudoreplicate. This is due to the potential occasional exclusion of characters that are strong drivers of the resolved topology in the extensive heuristic search and may also represent characters multiple times in the ‘bootstrap matrix’ (Felsenstein Citation1985). All dromornithids are united in a monophyletic clade to the exclusion of non-dromornithids in all analyses, associated with high bootstrap support and low posterior probability values. The latter may be reflective of shared characteristics among dromornithids and anhimids, and resultant alternative branching arrangements within the wider clade comprising all of these taxa (such as a Dromornithidae + Chauna chavaria clade, sister to Anhima cornuta, see above). Similarly, the low posterior probability values estimated for the monophyly of a clade including dromornithids and anhimids can be associated with alternative topological placements of Dromornithidae and Anhimidae, variably as sister to a strongly supported clade (PP of 1) that includes Nettapterornis oxfordi, Conflicto antarcticus and Anseres (including Presbyornis pervetus). Specifically, the clade comprising Anhimidae + Nettapterornis oxfordi, Conflicto antarcticus and Anseres occurred in 32.87% of post burn-in trees estimated by the posterior distribution, compared to a frequency of 17.85% for a Dromornithidae + Nettapterornis oxfordi, Conflicto antarcticus and Anseres clade. These topological arrangements were comparatively associated with low bootstrap support values of 13.77% and 6.10% respectively, under parsimony optimality criterion.
Consensus trees derived from maximum parsimony and Bayesian phylogenetic approaches differ topologically in the positions of Nettapterornis oxfordi and Conflicto antarcticus, where the latter taxon is more basal in Anseriformes in all MPTs, whereas Bayesian phylogenetic inference resolved them in a relatively more derived position as sister to Anseres. Their close relationship, as supported in both analyses here, was inferred by the MPT presented by Tambussi et al. (Citation2019), although these authors specifically resolved these taxa as sister to one another within a monophyletic clade. These topological differences are likely highly contributed to by our focus on skull characters exclusively, where the additional incorporation of postcranial ones will likely better resolve their phylogenetic relationships.
Inconsistencies across parsimony and Bayesian inference methods are also evident in the phylogenetic positions of Asteriornis maastrichtensis and the clade containing Gastornis giganteus and Sylviornis neocaledoniae (see ). A clade comprising the latter two taxa is consistently resolved in a basal position among Galliformes, on a basal branch sister to a clade that includes all galliforms in Bayesian analysis, while under parsimony criterion it is resolved in a relatively more derived position, sister to a branch comprising Phasianoidea, as part of an inclusive clade that is itself, sister to Megapodiidae. The associated low branch support under both methods is illustrative of this topological inconsistency, likely contributed to by the presence of only three unambiguous character state changes supporting the resolution of these taxa in a clade sister to non-megapodiid crown galliforms in parsimony analysis. As for dromornithids, these results contrast with those presented by Worthy et al. (Citation2017b) and Tambussi et al. (Citation2019), whereas a sister taxon relationship between Gastornithidae and Dromornithidae is not supported by parsimony analysis or Bayesian phylogenetic inference using skull characters. Our results thus recover Gastornithiformes (sensu Worthy et al. Citation2017b) as a paraphyletic clade.
The poorly supported basal galliform affinities for Asteriornis maastrichtensis resulting from Bayesian inference, is comparable to relationships reported for an unconstrained parsimony analysis by Field et al. (Citation2020) which was associated with low bootstrap support values, as well as positions moderately and strongly supported by the posterior distribution in both tip-dated and undated Bayesian analyses (PP of 0.93 and 1.0, respectively), and favoured by their stepping-stone analyses. In contrast, Asteriornis maastrichtensis is resolved sister to the most inclusive crown group anseriform clade in parsimony analyses herein, yet the branch to the clade including this taxon and anseriforms only received low support values (42.98% bootstrap support). Field et al. (Citation2020) cited the lack of a postcranial skeleton in this taxon and the inability to appraise other fossil taxa for certain characteristics as contributing factors in it being resolved among stem galliforms in undated Bayesian analysis. The inability to consistently and strongly resolve the topological relationships of Asteriornis maastrichtensis in this study using exclusively skull characters, is similarly likely contributed to by limitations regarding the number of codable characters and the effects of missing data (e.g. Weins Citation2003, Citation2006), and notably a lack of information pertaining to the lateral and caudal cranium in this taxon. More extensive testing using a wider sample of modern and fossil taxa, and postcranial characters, is needed to better understand how Asteriornis maastrichtensis relates to modern and fossil Galloanserae.
In general, the differing topologies and inability to strongly resolve or support the placement of several taxa, especially near the base of Galliformes and Anseriformes, is likely reflected in the limited sample of characters (and to a lesser degree, taxa) used in these precursive analyses (skull features only; see Materials and methods: Phylogenetic analyses), missing data associated with fossil taxa, related taxon instability among trees, and conflicting signal of relationships between some taxa. The phylogenies presented herein are primarily presented as a means of phylogenetically testing the influence of morphological characters described and compared in this study on higher-level taxon associations and are not intended to represent an exhaustive test of phylogenetic relationships. We acknowledge the preliminary nature of these analyses, and the need to further test the phylogenetic placement of Dromornithidae among Galloanserae in a more focused phylogenetic study involving a more complete morphological character set (across the postcranial and cranial skeleton). We perceive benefits of further elaboration and comparison of Bayesian-inferred and parsimony-based phylogenetic methods, more consideration of their appropriateness in resolving the interrelationships of fossil taxa with regards to crown group Galloanserae, and a more complete evaluation of topological arrangements, however, these are outside of the scope of this study.
Discussion
The fossils of Genyornis newtoni described above, are nearly incomparable to those used to describe the skull of this species in 1913 (Stirling Citation1913; see Appendix One: ), 1998 (Murray and Megirian Citation1998), and again in 2004 (Murray and Vickers-Rich Citation2004). The new material is better preserved, less fragmentary, and provides a more complete basis for interpreting the skull morphology of G. newtoni and creating an updated reconstruction (). Some original interpretations of the skull are supported, e.g. the lack of the large dorsally convex shape of the rostrum described for species of Dromornis. However, the new specimens reveal different proportions of the cranium and rostrum and that the apparent narrow width of the rostrum is unsupported. We additionally illuminate further details of the morphology not previously recognised, reported on, and/or preserved.
Figure 13. An artistic reconstruction of the skull of Genyornis newtoni, based on all available fossil material, left lateral view. Illustration by Jacob C. Blokland. Scale bar is equal to 50 mm.
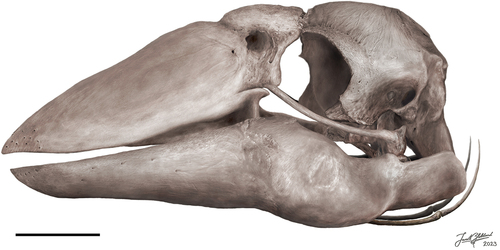
The Dromornithidae
Following the findings of Murray and Vickers-Rich (Citation2004, p. 328) and Worthy et al. (Citation2016a), the overall structure of the dromornithid skull is highly conserved and varies little amongst the species, G. newtoni included. The results of the phylogenetic analyses herein also support this (see above, and SI Fig, 1). Although no feature is necessarily unique to the family, together all features form a characteristic morphology. This is inclusive of, but not limited to: the structure of the synovial craniorostral hinge and its transection of the rostrodorsal portion of the orbit; a hemispherical dorsal cranial surface in caudal view; the reduction in cranium rostrocaudal length; the rostral extension of aponeurosis zygomatica ossificans along the ventrolateral postorbital process and the medial retreat of impressio m. AME profundus, pars coronoideus; the presence of an annulus tympanicus and a rostroventrally enclosed osseous meatus acusticus externus; the enlarged and ventrally elongate processus paroccipitalis; the fossa pseudotemporalis on the caudal wall of the orbit; the lacrimal fused to the caudal rostrum; the desmognathous palate; the fused elements of the mandible which eliminate flexion therein; the small medial mandibular processes; the oblique, sheering tomial margin of the caudal rostrum and corresponding part of the mandible; the broad shape of the retroarticular process; and the laterally arched and single-headed quadrate.
The skull morphology of G. newtoni appears to be most similar to that of Ilbandornis species, as supported by results of the phylogenetic analyses, and suggests a closer relationship to one another (and possibly to Barawertornis tedfordi), than to those within the monophyletic Dromornis clade (see ). Many features of the skulls of G. newtoni and species of Ilbandornis are more slender than those attributed to species of Dromornis. This is especially noticeable in the quadrates which seemingly show two forms: a robust Dromornis-type, and the slender G. newtoni/Ilbandornis-type with a more elongate pars otica, less lateral overhang of the pars otica by the capitulum squamoso-oticum, and a proportionally smaller pars mandibularis (see ; Worthy et al. Citation2016a: fig. 4). Additionally, although the foreshortening of the frontal and parietal bones of the cranium is shared by all dromornithids, the most extreme rostrocaudal compression of the cranium occurred only in species of Dromornis, with successive shortening from D. murrayi to D. planei and D. stirtoni, with the cranium of the latter twice as bilaterally wide and three times dorsoventrally taller than its rostrocaudal length (see Worthy et al. Citation2016a; Handley and Worthy Citation2021).
Regardless of such general similarities in morphology among dromornithids and further similarities to species of Ilbandornis, the skull of G. newtoni does show specific features which characterise the taxon and give support to its generic distinction. This includes the smaller, more enclosed osseous meatus acusticus externus and the especially long and slender processus paroccipitalis, both of which, may have relevance to functional adaptations for increased musculature attachment on these areas. Another feature, so far unique to G. newtoni, is the presence of the triangular casque on the rostrum, and the hypothesised extent of the rhamphotheca which covers most of the upper beak – apparently more than in species of Dromornis. This may suggest a potentially significant difference in communication and sexual displays, and there are several possibilities as to what the function of such a structure may be, similar features are known in other galloanserans such as Sylviornis neocaledoniae, and in curassow cracids (Mayr Citation2018b). Brightly coloured casques on the bill have a clear sexual display function. Furthermore, the rostrum of G. newtoni also differs from those of species of Dromornis in its wide, and rounded, more spatulate shape, with far shallower depth (see ‘Rostrum’). Although the upper bill morphology is unknown for species of Ilbandornis, it has a dorsoventrally shallow mandible compared to species of Dromornis, and given the similarities in the cranium, quadrate, and mandible with G. newtoni, we may expect the rostrum to also be similar in morphology.
Gastornithiformes
Previous phylogenetic analyses resulted in the weak association of dromornithids with the gastornithids in the Gastornithiformes Stejneger Citation1885 (Worthy et al. Citation2017b). The morphological descriptions and phylogenetic analyses presented herein, although preliminary, are not congruent with this hypothesis, warranting further discussion on the evolutionary relationships of these groups with larger datasets as more complete specimens become available
Skull morphology
Dromornithids and gastornithids share various aspects of cranial morphology but differ markedly in others. Some identified shared characters in both families are reflective of their galloanseran affinities, for example, the sessile processus basipterygoideus of the crania, the extensive processus retroarticularis of the mandible (see Baumel and Witmer Citation1993; Weber Citation1993; Dzerzhinsky Citation1995; Ericson Citation1996; Livezey Citation1997; Murray and Vickers-Rich Citation2004; Mayr Citation2017; Mayr et al. Citation2018; Tambussi et al. Citation2019; Field et al. Citation2020), the prokinetic skull, and holorhinal nares (Sibley and Ahlquist Citation1990; Dzerzhinsky Citation1995). Other morphological features conform to typical anseriform traits, such as the fused elements of the rostrum, the flat, enclosed palatal roof, caudally restricted external nares, shape and angle of the mandibular condyles on the quadrate, and the long processus paroccipitalis (see also Andors Citation1988, Citation1992). Notably, in the phylogenetic analyses, few, if any morphological character states shared by both gastornithids and dromornithids, are only found in galliforms (character 72, ambiguous for Anhimidae).
Other characters, such as the synovial craniorostral hinge, and the reduction of the anteorbital/supra-orbital region of the cranium, are less common in Galloanserae and yet are present in dromornithids, gastornithids and sylviornithids. These features likely inform more on a homoplasious functional adaptation to the robusticity and large size of both upper and lower jaws, rather than being representative of close relatedness phylogenetically. For sufficient mobility of the prokinetic upper bill and flexion capabilities of the zona flexoria craniofacialis to be maintained, the region must be non-pneumatic, very flat, and in larger species, composed of sheets of overlapping thin bone (Bühler Citation1980, p. 450, 451). This limits the strength of the region against feeding and movement stress, making it the weakest point of the prokinetic upper bill (Bock Citation1966). The transformation of a flexion zone to a synovial joint that is not spanned by any thin osseous tissues, allows greater flexion between the cranium and rostrum; as seen in parrots (Psittaciformes), for example, which have syndesmotic or synovial articulations with cartilaginous enclosures (Bühler Citation1980, p. 450, 451). Witmer and Rose (Citation1991) suggested the connective tissue within a synovial joint would provide the stability required to manage the stress associated with a large, heavy upper jaw, in reference to the form in gastornithids. As described above (‘Craniorostral hinge’), the dromornithids, especially the larger species, and S. neocaledoniae, have evolved a ball and socket arrangement to the hinge-like joint that confers much stability while allowing rotation on the occlusal plane (Murray and Megirian Citation1998; Murray and Vickers-Rich Citation2004; Mourer-Chauviré and Balouet Citation2005). The synovial hinge is then likely a size-scaling phenomenon, allowing, or deriving from, the development of larger skulls without the limitations associated with flexion zones. Should this be the case, gastornithids, dromornithids, and sylviornithids would have convergently evolved this hinge type as an adaptation to gigantism; a hypothesis supported by the restriction of this hinge type primarily to giant galloanserans. Reduction of the anteorbital region of the cranium could provide additional hinge support, as the increase in the robusticity and surface area available on the cranium for the rostrum to articulate with, would assist in managing and distributing a stress load. Alternatively, or in association, the synovial craniorostral hinge could have evolved as an essential functional adaptation to a specific feeding strategy (see 6.4). The various configurations between the lacrimals and the rostrum and cranium in these taxa, being fused to the rostrum in dromornithids, while they are totally fused to the frontals but articulate with the rostrum in gastornithids and sylviornithids (pers. observ.; Andors Citation1992; Mourer-Chauviré and Balouet Citation2005), possibly conferred an additional degree of lateral stabilisation in the latter families, as in large anatids (Bühler Citation1980, p. 452), compared to dromornithids (cf. Murray and Vickers-Rich Citation2004: fig. 184). The dromornithid state instead better resembles the less laterally confined hinge of parrots (except that the lacrimals exclusively articulate with the cranium rather than the rostrum in these birds, see Tokita Citation2003)
In Anatidae, and especially in species of Dendrocygna, the processus orbitalis of the lacrimal is ventrocaudally developed, coupled with a similarly orientated ligamentum lacrimomandibulare that likely prevents caudal displacement of the mandible during water expulsion in feeding (Zelenkov and Stidham Citation2018, and references therein). The fusion of the lacrimals to the frontal bones in many of these birds provides strength against caudal pulling, in the action of this ligament (Zelenkov and Stidham Citation2018). However, this ligament is absent in galliforms, anhimids, presbyornithids and anseranatids (although represented by extensive fascia in the latter, see Dzerzhinsky and Grintsevichene Citation2002; Zelenkov and Stidham Citation2018), most of which do not have lacrimals that are fused to the cranium or rostrum either (see ‘Craniorostral hinge’, character 32). This is paired with the absence of a strongly ventrocaudally directed orbital process in all the aforementioned taxa, which is also not observed in dromornithids, sylviornithids or gastornithids. Thus, the fusion of the lacrimals to the cranium in sylviornithids and gastornithids cannot be an adaptation to the feeding specialisations typical of Anatidae, in this regard. In anatids, however, the frontal-fused lacrimals articulate with a distinct notch on the caudal rostrum, which acts as a stop, supporting the mobility of the upper jaw, and reinforcing this joint against breaking and excessive upper bill retraction (Fisher Citation1955; Zelenkov and Stidham Citation2018). In sylviornithids and gastornithids, the strong and complete fusion of the lacrimals with the frontals, and their corresponding clear articulatory structure on the caudal rostrum (pers. observ., see Matthew and Granger Citation1917; Mourer-Chauviré and Balouet Citation2005) may have provided strength in a similar manner.
The ventrally short processus orbitalis of the lacrimal in sylviornithids and gastornithids also contrasts with the dromornithid condition (character 33). The ventral end of this process supports the ligamentum jugolacrimale which runs to the tuberculum lacrimale on the rostral arcus jugalis, which is well developed in anatids (Davids Citation1952c; Goodman and Fisher Citation1962; Bock Citation1964; Baumel and Raikow Citation1993), and may limit protraction of the rostrum (Fisher Citation1955). The processus orbitalis in anhimids is ventrally descending but not as robust as in anatids but is similarly coupled with a ligamentum jugolacrimale that is stronger than what is typically observed in galliforms (see Ghetie et al. Citation1976; Dzerzhinsky Citation1982, p. 1031). The ventrally long and narrow orbital process in presbyornithids is also hypothesised to have supported this ligamentous attachment to the jugal arch (Zelenkov and Stidham Citation2018). The poorly developed and ventrally short processus orbitalis on the lacrimals of sylviornithids and gastornithids probably did not host a well-formed ligamentum jugolacrimale if it was present. In contrast, the strut-like complete ossification between the processus orbitalis and the jugal arch in dromornithids presumably provided additional strength across the rostrum and arcus jugalis, and reduced bending at the rostral end of the latter (zona flexoria arcus jugalis); comparatively, this region is a more mobile synovial socket and joint articulation in sylviornithids and gastornithids (character 96, Matthew and Granger Citation1917; Mourer-Chauviré and Balouet Citation2005).
As discussed in the section ‘Temporal region’, the formation and structure of the lateral cranium (including the AZO) and the location of associated musculature (characters 7–12), is a major point of difference between the gastornithid and dromornithid skulls. This region is nearly identical between dromornithids and anhimids, with respect to homologous structures. Comparatively, that of gastornithids is more like the morphology of many cracids and phasianids, especially regarding the robust orbitozygomatic junction and resultant secondary temporal fenestra (see ). Elzanowski and Mayr (Citation2017) recognised cracids and phasianids to have independently evolved this morphology, because the closure of the orbitozygomatic junction is not so complete in the stem galliform S. neocaledoniae, and extant megapodiids. Therefore, independent evolution in the gastornithids would be possible.
Figure 14. Two select morphological features which show variation across Galloanserae, and, in isolation as single character studies, indicate different phylogenetic hypotheses for the evolutionary placement of the Dromornithidae: A. the presence of either the rostromedial or caudomedial foramen (dark orange) in relation to the crista medialis (light orange) on the quadrate, in medial view; B. the relationship between the aponeurosis zygomatica and processus postorbitalis, in addition to the presence of an ossified portion (dark orange) of the aponeurosis zygomatica (light orange). Dotted and dashed branches in B. illustrate alternative hypotheses discussed in text; the unresolved position illustrated by the dotted line denotes the uncertainty of the order of divergence between the anhimids and dromornithids. Positions of Conflicto antarcticus and the Presbyornithidae are based on Tambussi et al. (Citation2019: fig. 14) and Houde et al. (Citation2023: fig. 9B). Nodes do not correlate with time. Not all families within Galloanserae are displayed. Morphologies drawn from photos and figures in literature: A.: Anseres – Anseranas semipalmata (see Elzanowski and Stidham Citation2010: fig. 5C), Anhimidae – Anhima cornuta (see Elzanowski and Stidham Citation2010: fig. 8B), Presbyornithidae – (see Elzanowski and Stidham Citation2010: fig. 5B), Conflicto antarcticus – (Tambussi et al. Citation2019: fig. 6A), Dromornithidae – Genyornis newtoni (NMV P256893), Megapodiidae – Megapodius freycinet (see Elzanowski and Stidham Citation2010: fig. 5A), Phasianidae – Gallus gallus (FUR 119); B.: Anseres – Sarkidiornis melanotos (see Zusi and Livezey Citation2000: figs. 6D, 7D), Dromornithidae – Genyornis newtoni (SAMA P59516), Anhimidae – Chauna torquata (see Zusi and Livezey Citation2000: figs. 6C, 7B), Megapodiidae – Alectura lathami (SAMA B2439), Phasianidae – Meleagris gallopavo (see Zusi and Livezey Citation2000: fig. 4 G). Silhouettes (designed by PLM) are illustrative representations of species in the same family or appropriate clade, as indicated. Images are not to scale.
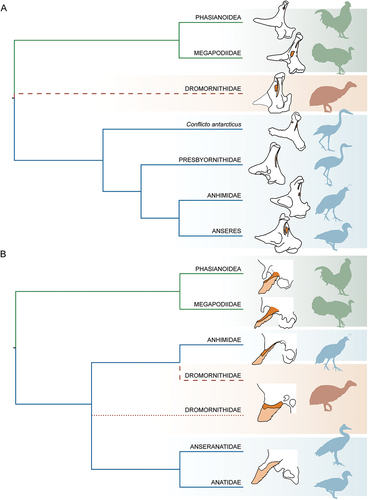
The extreme size of the ossified aponeurosis zygomatica and secondary temporal fenestra in gastornithids is linked to a second adaptation in this group for an increase in the surface area available for the external adductor muscles, primarily related to the origin of m. AME profundus, pars superficialis, on the lateral orbitozygomatic junction, but also m. AME profundus, pars zygomaticus, medially, and m. AME profundus, pars coronoideus, within the more medial, enclosed and well-excavated dorsotemporal fossa on the squamosum, and possibly some fibres related to m. AME superficialis dorsal of the quadrate articulation (see ‘Cranium’, also Witmer and Rose Citation1991, p. 102; Weber Citation1996; Zusi and Livezey Citation2000; Holliday and Witmer Citation2007). The rostrocaudally compressed cranium of species of Dromornis and the different morphology of the lateral cranium in dromornithids allows for a comparatively far smaller surface area for origin of m. AME profundus, pars coronoideus and would not have supported such a hyperdeveloped musculature. However, numerous well-developed osseous structures of the jaw apparatus in dromornithids show some emphasis on the m. AME superficialis (with some associated cristae on the dorsal or dorsocaudal margin of the osseous meatus acusticus externus), m. adductor mandibulae posterior, m. pterygoideus, and the m. pseudotemporalis superficialis (associated fossa and tubercle within the area muscularis aspera), indicative of considerable jaw power. The especially heavy and robust quadrates in species of Dromornis compared to that of G. newtoni and species of Ilbandornis, and the lack of a pneumatic foramen in this bone in D. stirtoni, may be further adaptions for supporting and maintaining large muscle mass in order to adduct the massive mandible. Functional implications of the dromornithid jaw apparatus are explored further in ‘Ecomorphology and niche adaptations’.
The contrasting conditions in both families appear to be driven by the need for well-developed musculature, in part to support large upper and lower jaws, but is limited by the variation in underlying cranium morphology (especially influenced by the development of the AZO). Further indications of large muscle size from analysis of the crania of Gastornis species are evident in the crista nuchalis sagittalis, prominentia exoccipitalis, and processus paroccipitalis. Such adaptations for enlarged muscle attachments have led to the suggestion that the adductors were unusually powerful (Andors Citation1988, p. 137) and were a driving factor in the carnivory hypothesis (see also Witmer and Rose Citation1991; Mayr Citation2022a, p. 52), although it also may support a folivorous diet including hard food items (Andors Citation1992; Mayr Citation2022b). It was not considered that the extensive musculature may be required to manipulate such a large rostrum and mandible without great force, a factor which further analysis of the skulls of these taxa may shed light on. Witmer and Rose (Citation1991) additionally linked the development of massive palatines in gastornithids to a requirement for large muscle attachments, specifically the m. pterygoideus, which they propose to be one of three important ways of increasing bite force and may explain the similarly massive size of the palatines of dromornithids. Unfortunately, confirmation of the area involved, and development and use of these muscles can only be made by assessing the formation of the separate cranial bones in chicks (e.g. Mayr and Manegold Citation2021) in association with myological dissections as per Zusi and Livezey (Citation2000), both of which are not possible for these fossil taxa (see also palaeomyological considerations as per Ostrom Citation1961, p. 88–89).
Aside from the dorsoventrally broad processus retroarticularis, and the large size—intermediate between that of species of Dromornis and G. newtoni—the mandible of all gastornithids varies considerably from that of dromornithids. Instead, several features of the mandible of Gastornis species bear more resemblance to that of S. neocaledoniae, including the great proportional rostrocaudal length of the symphysial region (character 59), and the morphology of pars caudalis of the mandibular ramus, specifically the articulatory region. Similarly, the rostra of both Gastornis species and S. neocaledoniae appear superficially like that of dromornithids, with large palatines, flat, fused, plate-like lateral surfaces and a casque structure (the latter only between S. neocaledoniae and G. newtoni). However, both differ from dromornithids in their maxillary articulation with the jugal arch, the small vomer, not likely contributing to the palatal roof, and the sharp tomial margin (Matthew and Granger Citation1917; Mourer-Chauviré and Balouet Citation2005). In the cranium, neither gastornithids, nor sylviornithids, have a rostrally enclosed osseous meatus acusticus externus (and cavum tympanicum), and they both retain distinct capitula on the head of the quadrate despite it being functionally single headed.
Phylogenetic relationships of the giant flightless galloanserans as informed by skull morphology
Among the Galloanserae, three extinct, basal lineages diversified to include giant terrestrial forms in the families Dromornithidae, Gastornithidae, and Sylviornithidae (Worthy et al. Citation2016b; Citation2017b). We exclude the Brontornithidae Moreno and Mercerat Citation1891, from the comparative and phylogenetic analyses following the findings on the phylogenetic affinities of the group by Worthy et al. (Citation2017b). The cranial material that can be associated, then only tentatively, with this taxon is restricted to a fragmented mandibular symphysis and a quadrate, which has a condylus mandibularis caudalis on pars mandibularis (for most recent analyses see Worthy et al. Citation2017b: fig. 5; Agnolín Citation2021: fig. 6). This feature is absent in all quadrates of all crown-group, and definitively placed fossil galloanserans which all have a bicondylar articulation (e.g. see Dzerzhinsky Citation1974, Citation1982, Citation1995; Mayr Citation2017, Citation2022a; Field et al. Citation2020).
Recent phylogenetic analyses have aligned the dromornithids with the gastornithids to include them in the order Gastornithiformes, in effect, excluding them from the orders Galliformes and Anseriformes, or being considered distinct at ordinal level. However, this relationship was weakly resolved in the phylogenetic analyses by Worthy et al. (Citation2017b), and several authors have since questioned it. Mayr (Citation2022a), one of such authors, listed several cranial and postcranial characteristics of dromornithids which distinguish them from gastornithids, and suggesting the relationship was supported only by convergent characters. Our morphological comparisons and phylogenetic assessments also find little support for a dromornithid-gastornithid clade (see above); several major differences in the morphology of the skulls are present, and many similarities can be explained by the galloanseran affinities of the lineages and convergent functional adaptation to large body and skull size. Thus, we consider enough evidence is present to warrant reconsideration of this relationship.
As an example, one of the most enticing similarities between the dromornithids and the gastornithids is the synovial craniorostral hinge. As aforementioned, there is a high likelihood that the shared hinge type convergently evolved as a functional adaptation to feeding or supporting large skull size. This suggests that the morphology of this region may not be as phylogenetically important in informing upon the higher-level evolutionary relationships of these taxa. The separation of dromornithids and sylviornithids in phylogenetic analyses, and the sister-group relationship between gastornithids and sylviornithids, further supports minimally two independent evolutions of the craniorostral hinge. Additionally, this supports our identification of morphological similarities between gastornithids and the convincingly galliform, S. neocaledoniae, showing the importance of recognising these similarities when reassessing gastornithid relationships.
Andors (Citation1988, Citation1992) recognised the mosaic structure of several elements in gastornithids as evidence of ‘intermediacy between the orders Galliformes and Anseriformes’ and considered them to be the ordinal-level sister-group to anseriforms, and more broadly related to galliforms. Similarities with galliforms, including cracids and phasianids were considered symplesiomorphic, and some shared features with anhimids were considered evidence of consanguinity with basal anseriform stock. Comparatively, Murray and Vickers-Rich (Citation2004, p. 167) concluded that any relationship between gastornithids and anseriforms is ‘undoubtedly remote’, noting that they appear to have more in common with the Megapodiidae. Our study of the skull morphology and phylogenetic analyses support a closer relationship of gastornithids with basal galliforms than anseriforms.
Dromornithids within Galloanserae
Bayesian and parsimony-based phylogenetic analyses by Worthy et al. (Citation2017b) variably placed Gastornithiformes at the ordinal level, as sister to galliforms and anseriforms respectively with no significant results favouring either placement. Ultimately, however, the slightly higher support and 13 identified unambiguous characters in their study, suggested the placement as sister to galliforms to be most likely. Handley and Worthy (Citation2021) additionally considered aspects of the morphology of the brain, several cranial nerves, and trigeminal ganglia to support this placement (see below). The alternative pull of dromornithids to galliform or anseriform lineages may be a function of the early diverging nature of the of this group, resulting in plesiomorphic characters confounding the results of analyses.
Galliformes
This study found little evidence to support the sister-group relationship of dromornithids and galliforms due to dromornithids having relatively few exclusively galliform skull characteristics. Typically crown group galliform characters which are present, include the apparent absence of a crista nuchalis sagittalis and fonticuli occipitalis (character 19), the short and ventrally directed processus postorbitalis of the cranium (character 7), the presence of paracoronoid crests on the lateral mandible (character 80), and the foramen pneumaticum rostromediale on the pars otica of the quadrate (character 38). Such characters could evidence galliform affinities for the dromornithids. However, many of these are also observed in anhimids (previously discussed by Dzerzhinsky Citation1982), concordant with their early divergence as basal anseriforms supported by all molecular phylogenetic analyses and most/all morphological ones (see ; for additional examples, see Worthy et al. Citation2017b; Kuhl et al. Citation2021). These traits may instead be plesiomorphic for Galloanserae and lost gradually associated with the evolution of Anseres and close relatives (see SI 5). Indeed, many similarities between Galliformes and Anseriformes are considered to be relatively primitive states (see Murray and Vickers-Rich Citation2004, p. 168–169). Therefore, we infer a priori polarisation for several characters.
For example, the paracoronoid crests on the lateral mandible that are present in dromornithids, and typical of galliform birds, are also present in anhimids (Weber and Hesse Citation1995; Weber Citation1996). Anhimids also have a processus postorbitalis that lacks the distinct rostral extension typical of other anseriforms (this is also true for Presbyornis pervertus, specimen USNM.VP.299846, pers. observ., see also Olson and Feduccia Citation1980; Zelenkov and Stidham Citation2018: fig. 3), and they lose the fonticuli occipitalis that are typical of waterfowl in adulthood (Ericson Citation1997). Additionally, a discernible, yet not prominent, crista nuchalis transversa is observed in anhimids, galliforms and dromornithids, in contrast with the hyper-developed form of many anseriforms. The location of the condylus occipitalis at near mid-dorsoventral height on the caudal surface of the cranium in most dromornithids (excluding D. murrayi in which it is more ventrally positioned), is also a trait shared with both galliforms and anhimids.
That several conditions considered typical of galliforms appear to be symplesiomorphic for anseriforms is especially important to consider with regards to the quadrate, as archaic fossil relatives such as the near-galloanseran Asteriornis maastrichtensis (see Field et al. Citation2020), and the basal anseriforms Presbyornis pervetus and Conflicto antarcticus, appear to share quadrate traits with basal galliforms (see Elzanowski and Stidham Citation2010; Elzanowski Citation2014; Zelenkov and Stidham Citation2018; Field et al. Citation2020: SI). The Upper Cretaceous A. maastrichtensis possesses a foramen pneumaticum rostromediale, typical of galliforms, although there is an apparent, deep depression on the caudomedial side of the medial crest, which is potentially a precursor to the state that characterises most anseriform taxa. Early Eocene Danielsavis nazensis appears to have neither foramina (Elzanowski Citation2014; Houde et al. Citation2023; Mayr et al. Citation2023), although it also forms a deep depression on the caudomedial surface (Houde et al. Citation2023: fig. 7; Mayr et al. Citation2023: fig. 3C). Despite clear anseriform affinities, Conflicto antarcticus has both a rostromedial and caudomedial pneumatic foramen (Tambussi et al. Citation2019: fig. 6), while species of Presbyornis often have a markedly deep depressio caudomedialis where a caudomedial pneumatic foramen is occasionally present (Elzanowski Citation2014). Similarly, late Paleocene Anachronornis anhimops has a deeply grooved depressio caudomedialis that terminates in a diminutive foramen pneumaticum caudomediale on one quadrate but may not be present on the other (Houde et al. Citation2023). Additionally, morphogenetic instability in the development of the pneumatic diverticula has been recognised for both anhimids (Elzanowski Citation2014) and presbyornithids (Elzanowski and Stidham Citation2010), as the caudomedial foramen is vestigial and often absent. In anhimids, their particularly erratic pneumatic variability has been correlated with their highly derived quadrate morphology and may be evidence of a transitionary state with respect to the more derived evolution of the caudomedial pneumatic foramen (Elzanowski and Stidham Citation2010). While some non-anhimid anseriforms possess the caudomedial foramen in the caudomedial position, in others it is rostrally displaced (e.g. species within Anserinae and Mergini, Elzanowski and Stidham Citation2010). This factor, in addition to the variable presence of the rostromedial foramen in individuals of G. newtoni and other dromornithids, demonstrates the diversity regarding the status of the pneumatic foramina within Galloanserae and challenges the weight of using the presence or absence of these foramina as polarising traits, with respect to assigning fossil taxa to galliform or anseriform clades. Regarding the dromornithids, this character may preclude close affinities with the danielsavids, presbyornithids, anachronornithids, anseranatids, and anatids, but has limited utility for definitively supporting or excluding a close relationship with any other galloanseran lineage (see ).
A similar case can be made for the foramen pneumaticum basiorbitale, which is typically present in crown group galliform birds, as well as Asteriornis maastrichtensis, Danielsavis nazensis, Conflicto antarcticus, Anachronornis anhimops, and Presbyornis pervetus (Elzanowski and Stidham Citation2010; Tambussi et al. Citation2019; Field et al. Citation2020; Houde et al. Citation2023; Mayr et al. Citation2023). However, this region of the quadrate is associated with considerable pneumatic variability in both anhimids and cracids (Elzanowski and Stidham Citation2010), and appears variably present in Dromornis planei, yet absent or unverified in other dromornithids. Homoplasy regarding this character in relatively basal galloanserans may additionally be affected by gigantism, considering the additional absence of a basiorbital pneumatic foramen in both gastornithids and sylviornithids.
Anseriformes
In recent decades, dromornithids have been considered to be basal anseriforms based predominantly on cranial material (Olson Citation1985; Vickers-Rich Citation1991; Murray and Megirian Citation1998). Murray and Vickers-Rich (Citation2004) provisionally refined this to a sister-group relationship with the Anhimidae to the exclusion of other anseriforms, although they suggested that a full revision of the systematic relationships of Anseriformes and anseriform-like birds, following identification of additional early Palaeogene fossil representatives, may result in dromornithids becoming an independent anseriform Suborder. From our independent assessment of the skull morphology of dromornithids, it is clear that the proposed anseriform affinities of these birds are well-founded; we find support for the skull synapomorphies identified by Murray and Vickers-Rich (Citation2004, p. 152–154) in addition to several more that further substantiate this relationship.
The goose-like rostrum of G. newtoni is a key factor supporting placement among basal anseriforms (5 character state changes in our phylogenetic analyses that support a clade containing crown group anseriforms, including dromornithids, are associated with the rostrum). The rostrum and mandible show morphological similarities with several taxa which are representatives of other independent occurrences of the polyphyletic ‘goose’ type (Li and Clarke Citation2016; Olsen and Gremillet Citation2017; Pecsics et al. Citation2017), including Cereopsis novaehollandiae. This is potentially linked to the convergent evolution of a primarily herbivorous diet and grazing behaviour (Olsen Citation2015), and the elevated rate of beak shape evolution compared to other bird clades (Cooney et al. Citation2017; Olsen and Gremillet Citation2017). Both have ultimately contributed to the evolution of diverse feeding ecologies among anseriforms (Li and Clarke Citation2016).
Of the cranium, the development of the paired exoccipital prominences (for characters see Appendix Four) and the tubercula basilaria, as well as the low, sessile basipterygoid processes are like that of anseriforms, with the shared states in dromornithids and anhimids unambiguously supporting their close relationship, as found in both phylogenetic analyses. For the mandible, the low angle of divergence of the rami, the presence and size of the dorsal and ventral mandibular angles, the caudally directed processus retroarticularis, and the inferred arrangement of the muscle attachments are all important anseriform characteristics. The prokinetic nature of the skull, the medial fusion of the paired maxillopalatine process of the maxillare, and the fusion of elements within the rostrum, with large, flat praemaxillary and maxillary plates broadly fused with the nasals, are additional characters dromornithids share with anseriforms. The desmognathous palate, which provides support (character 97) for the separation of these clades in the phylogenetic analyses, could further support anseriform affinities, although this has been proposed to have evolved several times independently from the schizognathous plesiomorphic condition in anseriforms and galliforms (Hofer Citation1945; Dzerzhinsky Citation1982, Citation1995; Zusi and Livezey Citation2006; Mayr Citation2018a; Field et al. Citation2020: SI).
Moreover, there are several similarities with the anseriforms in the quadrates of dromornithids. These include the angle and size of processus orbitalis compared to pars otica, the presence of the prominentia submeatica (albeit less inflated, see character 53), the deep fossa basiorbitalis, the angle of the medial condyle compared to the lateral one on pars mandibularis, and the adjacent or confluent condylus pterygoideus and facies articularis pterygoidea (see ‘Quadrate’). The latter differs from the condition in Presbyornis pervetus, Asteriornis maastrichtensis, megapodiids and all phasianids, which instead develop a separate facies articularis pterygoidei on the caudoventral base of the processus orbitalis (Elzanowski and Stidham Citation2010; Field et al. Citation2020). Dzerzhinsky (Citation1982) and Elzanowski and Stidham (Citation2010) concluded that this latter state was likely to be symplesiomorphic for galliforms and possibly all Galloanserae.
As discussed in sections ‘Temporal region’ and ‘Skull morphology’, the form of the AZO and the processus postorbitalis, and the related structural arrangement and osseous correlates for the mandibular adductor muscles in dromornithids, show compelling similarities with the anhimids. This morphology does not occur anywhere in the galloanseran radiation aside from anhimids and differs considerably from that of galliforms. However, dromornithids also share some similarities in the shape of this region with Anseranas semipalmata, as previously described. Concordant with the basal relationship of anhimids relative to other crown group anseriforms and several hypothesised character transformations (e.g. lamellae rostri, see Livezey Citation1997), the osteo-myological condition in Anseres is potentially derived from a state more similar to non-anserean Galloanserae (such as anhimids and dromornithids), whereby more extensive ossification of the zygomatic aponeurosis of the latter was secondarily lost in Anseres in association with structural modification of processus postorbitalis to support the adductor musculature (see Zusi and Livezey Citation2000). Fossil anseriforms such as Presbyornis pervetus and Conflicto antarcticus also appear to lack such ossification, resembling the state typical of Anseres, relative to the seemingly more plesiomorphic anhimid and dromornithid conditions. In this sense, the adductor complex morphology in birds like dromornithids and anhimids appears an appropriate precursor to the structure of the true waterfowl, Anseres. This may be a contributing factor to the low support values in the phylogenetic analyses for the clade comprising dromornithids and anhimids, as such morphology might instead suggest the Dromornithidae to be an independent basal anseriform lineage with morphologies somewhat intermediate between anhimids and anseranatids (as per the hypothesis shown in : dotted line; see ‘Remarks’). The homology of this region appears increasingly important regarding the evolution of the anseriforms and identification of character transitions across the Galloanserae radiation.
An aponeurosis zygomatica ossificans is observed in many adult crown group galliforms and may extend rostrad until near the level of processus postorbitalis, which is comparable to anhimids in this respect (Zusi and Livezey Citation2000). In some galliforms, such as megapodiids and cracids, the ossified aponeurosis is non-extensive and may not reach the postorbital process (Dzerzhinsky Citation1980; Zusi and Livezey Citation2000). Zusi and Livezey (Citation2000) documented similarities in the location of the m. AME profundus, pars coronoideus, between megapodes and anseriforms (i.e. the medial rotation and reduction of impressio m. AME coronoidea). They used this as evidence of the basal divergence of megapodes within the Galliformes, as supported by all phylogenetic analyses using molecular data (e.g. Ksepka Citation2009; Jetz et al. Citation2012; Worthy et al. Citation2017b; Kuhl et al. Citation2021), and to hypothesise the nature of the transformation to the anseriform-form from a hypothetical, common ancestor with galliforms. Considering the morphological similarities between species of Megapodiidae and Anhimidae for this adductor complex, the plesiomorphic state of this region may be intermediate between that of megapodes and anhimids, potentially similar to the morphology of the Eocene stem galliform Gallinuloides wyomingensis Eastman, Citation1900. No ossification is present in this latter taxon (see Mayr and Weidig Citation2004), which could be expected in stem Galloanserae given the osteo-myological structure present in basal galliforms is less hyper-specialised with regards to independent jaw joint mobility and rostral positioning of the adductor muscles (e.g. compared to phasianids, see Dzerzhinsky Citation1974, Citation1980, Citation1982; Zusi and Livezey Citation2000).
An alternative scenario then, is that the apparent lack of both a processus zygomaticus and ossification of the zygomatic aponeurosis in fossil anseriforms, such as Presbyornis pervetus and Conflicto antarcticus, is the plesiomorphic state, which is present in Anseres. This would resultantly infer the ossification of the zygomatic aponeurosis in anhimids, and, considering their close similarity, dromornithids, to be a derived state (see also Olson and Feduccia Citation1980; Ericson Citation1997; Zelenkov Citation2011, p. 909; as per the most parsimonious topology of Tambussi et al. Citation2019). The implication of this hypothesis would imply that the rostral movement of the adductor complex in Anseres was achieved through adaptation of the structure of processus postorbitalis only, without any antecedent contribution by AZO. In this case, a sister relationship between the Dromornithidae and Anhimidae would be best supported, as found in the phylogenetic analyses (; and illustrated in : dashed line) with the morphology of the AZO evolving early in their most recent common ancestor; similarities with anseranatids would then be convergent.
Both hypotheses interpret the independent evolution of the processus postorbitalis and AZO complex in galloanseran lineages as a sufficient explanation for the variation in the structure. Unfortunately, we do not yet have adequate cranial material for some fossil galloanseran lineages to better test such hypotheses; this adductor complex region is lacking in the skull of Asteriornis maastrichtensis (see Field et al. Citation2020), although considering the patterns of variation in this region within Galloanserae, and its basal positioning, it appears unlikely that this taxon would have developed an AZO (see above). Regardless of which hypothesis ultimately takes precedence (dotted line or dashed line: ), the shared state of this unique character between anhimids and dromornithids appears to be an important link evidencing their close evolutionary relationship.
Prior to their formal description, several anseriform fossils were historically reported on and described as anhimid-like, especially with reference to their non-spatulate (‘fowl-like’) bill shape, and contextually cited as pivotal in the understanding of anseriform evolution (e.g. Houde Citation1996; Ericson Citation1997; Boles Citation1999; Olson Citation1999; Mayr, Citation2022a). Of these, the recently described fossil anseriform family, Anachronornithidae, is represented by Anachronornis anhimops, of the latest Paleocene Willwood Formation, Wyoming, which possesses several synapomorphies characteristic of Anseres and anhimids, and was resolved ambiguously among Anseriformes in phylogenetic analyses (Houde et al. Citation2023; also see Mayr et al. Citation2023). A taxonomically unassigned, fossil from the early-middle Eocene Green River Formation, Wyoming, was considered more closely related to Anseres than anhimids. Concomitantly described fossils from the early Eocene London Clay Formation, England, including Danielsavis nazensis, were collectively considered more closely aligned with the Anhimae than Anseres (Houde et al. Citation2023; also see Mayr Citation2022a). Mayr et al. (Citation2023) recently refined attribution of material to Danielsavis nazensis and demonstrated that many shared characteristics with anseriforms were homoplasious, while a relatively greater number of morphological aspects aligned with galliforms, even considering that some may be plesiomorphic for the group. Part of this included the recognition that the presence of several characters which were used to exclude this taxon from galliform affinities may in fact be plesiomorphic for Galloanserae or Neognathae, rather than apomorphies exclusively relating to anseriforms. It was therefore considered as either the most early diverging anseriform, outside of the crown clade, or a stem group galliform with some anseriform characteristics, warranting family-level distinction (Danielsavidae, see Mayr et al. Citation2023). These fossils significantly preserve an important combination of characters for understanding the evolutionary trajectories of Anseriformes and Galliformes, which additionally extends to interpreting the relationship between Anhimae and Anseres, and character-state polarity. In particular, Ericson (Citation1997, p. 477) specifically noted the presence of a ‘laterally located coronoid process’ on the mandible of the Wyoming fossils. This observation was confirmed for Anachronornis anhimops and also reported for the unnamed Green River Formation taxon by Houde et al. (Citation2023) and appears representative of the paracoronoid process (see ‘Mandible’), which is characteristic of Anseres (processus paracoronoideus, see Weber and Hesse Citation1995; Weber Citation1996). The relatively less laterally produced, analogous structure on the lateral mandible of Danielsavis nazensis is more similar to that in megapodiids (Mayr et al. Citation2023), particularly, a well-developed crista paracoronoidea rostralis that is characteristic of this clade (Weber Citation1996). The presence of the former process in ‘screamer-like’ early birds has been used in support of the hypothesis that the modern anhimid skull is derived with respect to an ancestral condition that is typical of Anseres (including presbyornithids, Ericson Citation1997). However, current fossil evidence regarding the existence of superficially similar, but non-homologous structures in nearly contemporaneous fossil galloanserans, is in conflict with this proposed evolutionary trajectory. Furthermore, dromornithids, like the closely allied anhimids, and also galliform birds, also possess paracoronoid crests (crista paracoronoidea rostralis and crista paracoronoidea caudalis) rather than the singular process of Anseres. To be consistent with the aforementioned hypothesis, it is most parsimonious to assume that the dromornithid-anhimid condition was present in their most recent common ancestor, or alternatively, that both lineages independently evolved this following their divergence. The large dromornithid foot cast of (at least) Eocene age from Redbanks Plains Formation, Queensland, temporally constrains both these scenarios (Vickers-Rich and Molnar Citation1996).
More generally, the pattern of evolution whereby the anhimid skull is derived, is also considered less parsimonious or improbable and unsupported by our phylogenetic analyses (see also, Livezey Citation1997; Worthy et al. Citation2017b; Zelenkov and Stidham Citation2018; Houde et al. Citation2023). In consideration of the close relationship between presbyornithids and anatids found here and in cladistic analyses (Ericson Citation1997; Livezey Citation1997), their shared similar complex jaw and lingual morphology (see Olson and Feduccia Citation1980), and the ‘primitive’ presbyornithid postcranial osteology (see also De Pietri et al. Citation2016), it has been suggested that, rather than evidencing the basal position of Presbyornithidae in Anseriformes, the unique suite of morphological characters in this family may represent extreme specialisation (Mayr Citation2022a) or the retention of plesiomorphic postcranial characters with respect to Neognathae. The latter is perhaps evidenced by postcranial synapomorphies between presbyornithids and some fossils closely associated with anatids (Romainvillidae, e.g. Mayr Citation2008; De Pietri et al. Citation2016; Zelenkov Citation2018). In this case, the anserean-type adductor complex would not need to be plesiomorphic for all anseriforms and could have evolved from an anhimid- and/or galliform-like condition, as supported by character transitions across the phylogenetic trees (shown in ). This is further evidenced by poor-specialisation for filter feeding in Presbyornis pervetus, despite the indication of lamellae (Ericson Citation2000; Stidham Citation2001), which better supports Presbyornithidae as a possessing a transitional state to filter-feeding Anseres, retaining some plesiomorphic galliform-like characters, rather than being itself plesiomorphic to Anseriformes (Zelenkov and Stidham Citation2018; Houde et al. Citation2023). In consideration of skeletal characters in the early Wyoming anseriform fossils (above) that distinguished them from presbyornithids, and clearly represented dissimilar ecologies, Houde et al. (Citation2023) concluded that any shared character states must be plesiomorphies of Anseriformes. Furthermore, these authors draw comparisons in jaw apparatus morphology between Anachronornis anhimops, Presbyornis pervetus, and Conflicto antarcticus to suggest that the spatulate bill form was gradually evolutionarily derived from ancestors with more galliform-like jaws, consistent with our hypothesis and phylogenetic analyses. Better understanding of the phylogenetic polarities of morphological characters important in the early evolution of Anseriformes, and the exact relationship of these fossil clades among extant counterparts, awaits a more focused investigation (see also Field et al. Citation2020: SI).
In addition to those discussed in the section ‘Dromornithids within Galloanserae’, other compelling links between the dromornithids and anhimids are the absence of the fenestra caudalis mandibulae, and the apparent absence of the foramen temporale venosum. While pterygoids identified for G. newtoni are fragmentary, we find it pertinent here to note that the facies articularis pterygopalatina on the pterygoids of other dromornithids, appears comparable to that of anhimids as described by Zusi and Livezey (Citation2006). They seemingly lack the more complex articulation described for Anseres, e.g. the rostral development of the processus palatinus du ptérygoïde (sensu Davids Citation1952b). Instead, the surface is a concave, oblong facet, aligned nearly dorsoventrally along its main axis, as it is in species of Anhima and Chauna (see Zusi and Livezey Citation2006, p. 160). Unlike anhimids, however, the facies articularis pterygoidea is positioned in the rostral half of the pterygoid, and better compared to the condition in waterfowl (see Ericson Citation1997). Within anseriforms, the superficial shape of the mandible, and the convex dorsal profile of the rostrum are additional similarities that dromornithids share with anhimids and not Anseres (originally noted by Murray and Vickers-Rich Citation2004, p. 149, 158). Furthermore, in dromornithids and anhimids, the processus jugalis of the maxillare is located dorsal and slightly lateral of the palatines, which extend further rostral of the processus jugalis (). This contrasts with the state in Anseres in which the palatines are more dorsal of the processus jugalis and both converge rostrally. Conversely, the presence of a prominentia submeatica is better compared with several anatids, and Anseranas semipalmata (although in form it is projected, rather than bulge-like), while a lack of processus submeaticus contrasts with the anhimid condition (see ‘Quadrate’; Elzanowski and Stidham Citation2010; Elzanowski and Boles Citation2012).
Despite concluding basal galliform similarities from the dromornithid brain and associated nerves, the results of Handley and Worthy (Citation2021) could alternatively be interpreted as supporting a basal- or sister-anseriform relationship, as is consistent with our interpretation of the skull osteology and phylogenetic analyses. The features assessed are variable in their likeness to those of the two anseriforms and two galliforms included in the study, showing the dromornithid brain to have a hybrid character set reflective of their external skull morphology. Characters shared with the galliforms and basal anseriforms, Anhima cornuta and Anseranas semipalmata, include the separation of the glossopharyngeal and vagus nerves, the surface area ratios for the optic lobes, and the rostral location of the wulst structures on the dorsal endocast. Several characteristics were limited to dromornithids and Gallus gallus (e.g. the transmission of the maxillomandibular branch of the trigeminal ganglia through the cranium and the log length shape ratio for the caudal telencephalon module), although these are likely inconsequential phylogenetic indicators given Gallus gallus is a derived phasianid (in the context of all Galloanserae). Only morphological attributes which are shared between dromornithids and Leipoa ocellata (Megapodiidae, a basal galliform, i.e. the surface area ratio of the cerebellum module) could support galliform affinities for the dromornithids, yet these are limited.
Considering both the numerous shared characteristics between dromornithids and anhimids, and the discussed morphological transformations towards the evolution of early Anseres, we interpret the evolutionary placement of the Dromornithidae, within the Anseriformes, as likely sister to the Anhimidae, or alternatively an independent lineage branching more basal to anhimids and Anseres or between anhimids and anseranatids. The former-most hypothesis is resolved in both parsimony and Bayesian analyses and we thus, advocate for the inclusion of Dromornithidae along with Anhimidae, within the Suborder Anhimae (as per Nguyen et al. Citation2010). Both lineages then would together represent basal anseriforms in the gradual transition from galliform-like birds to those of more anatoid character. They may also have relatively close affinities to early anseriforms from Wyoming, particularly in consideration of their mosaic, apparently somewhat relatively derived morphology, seemingly intermediate between Anhimae and Anseres (see Houde et al. Citation2023). Whether the postcranial morphology of the dromornithids supports these inferences, is beyond the scope of our study, although Murray and Vickers-Rich (Citation2004) found postcranial evidence supporting anseriform affinities.
Palaeogeographical considerations
The long fossil record of dromornithids show the endemic Australian family obtained large size by the Eocene and essentially all its characteristic morphological features by the late Oligocene (Vickers-Rich and Molnar Citation1996; Worthy et al. Citation2016a). Gastornithids are only known from the Paleocene to middle Eocene of Europe, and the early Eocene of North America, and Asia (for review, see Mayr Citation2022a). This discord in the localities of the two groups has been proposed to be a limitation to the dromornithid-gastornithid clade hypothesis. Mayr (Citation2022a, p. 59) stated no current palaeogeographical reconstructions show potential dispersal routes in the early Cenozoic for large flightless birds between Eurasia and Australia. Regardless of our findings, this cannot be used to rule out a close relationship between the two families as there is currently no reason to assume gastornithids and dromornithids did not share a volant common ancestor and evolve flightlessness, large body size, and concomitant morphological adaptations independently. Given that the fossil record and several molecular-based phylogenetic approaches support a pre-KPg divergence estimate between Anseriformes and Galliformes (van Tuinen and Dyke Citation2004; Clarke et al. Citation2005; van Tuinen et al. Citation2006; Brown et al. Citation2008; Stein et al. Citation2015; Worthy et al. Citation2017b; Field et al. Citation2020; reviewed by Mayr Citation2022a), it is possible that the last common ancestor between the dromornithids and other Galloanserae diverged within the Upper Cretaceous. Additionally, fossil evidence not yet discovered, may provide evidence linking or further separating the two lineages geographically.
Regarding anseriforms, an apparently overrepresentation of basal lineages in Australasian faunas supports a Southern Hemisphere origin for the radiation (Worthy et al., Citation2023). This includes several extant crown group anseriform lineages such as Anseranas semipalmata (Anseranatidae) in Australia, and species of Chauna and Anhima (Anhimidae) in South America. Fossil anseriforms are comparatively widespread with an extensive record covering both the southern and northern Hemispheres (for a review, see Mayr Citation2022a, p. 59–68; also Houde et al. Citation2023). Despite limited terrestrial vertebrate fossil sites sampling the Eocene and Oligocene of Australia (Worthy et al. Citation2017b), several fossil anseriforms have been described, showing the lineage was present in Australia and the Southern Hemisphere throughout this time. For example, presbyornithids Wilaru tedfordi Boles et al. Citation2013 (late Oligocene: De Pietri et al. Citation2016) and Murgonornis archeri Worthy, De Pietri, Scofield, and Hand, Citation2023 (early Eocene: Worthy et al., Citation2023), the anseranatid Eoanseranas handae Worthy and Scanlon Citation2009 (late Oligocene/early Miocene: Worthy and Scanlon Citation2009), and several crown group anatids (Oligocene-Miocene: Worthy Citation2009). Additionally, a putative fossil quadrate of an anhimid-like taxon was described from the early Eocene Tingamarra Local Fauna by Elzanowski and Boles (Citation2012).
In a similar manner to the anseriforms, basal extant galliforms (the Megapodiidae) are also restricted to the Southern Hemisphere. However, conversely, the Southern Hemisphere fossil record for galliforms is far less extensive, being restricted to a single fossil megapodiid taxon Ngawupodius minya Boles and Ivison Citation1999, described from the late Oligocene of Australia (Boles and Ivison Citation1999), and Namaortyx sperrgebietensis Mourer-Chauviré et al. Citation2011, of unknown affinities from middle Eocene of Namibia, southern Africa (Mourer-Chauviré et al. Citation2011). Most stem galliform lineages, such as the Quercymegapodiidae, Paraortygidae, and Gallinuloididae, and potentially the Danielsavidae (see Mayr et al. Citation2023), are known from the Northern Hemisphere (see Mayr Citation2022a, 2022, and references therein).
The occurrence of latest Cretaceous Asteriornis maastrichtensis in Belgium challenges the hypothesis of a Gondwanan origin for crown birds (Claramunt and Cracraft Citation2015), as phylogenetic analyses suggest a phylogenetic position as a stem galloanseran, proximal to the divergence between Galliformes and Anseriformes, or a basal galliform (Field et al. Citation2020; see ). However, the early Paleocene anseriform Conflicto antarcticus, shows definitive anseriforms had already diversified and dispersed into the Southern Hemisphere shortly after this time (Tambussi et al. Citation2019). This presence of anseriforms in the Southern Hemisphere by the early Paleocene, and proposed austral origin of the anseriforms, contrasting that of galliforms (Mayr Citation2022a) and the gastornithids, provide appropriate support for our hypothesised anseriform affinities of the dromornithids.
Ecomorphology and niche adaptations
The kinetic abilities of the dromornithid skull, specifically of Dromornis species, have been explored previously (see Murray and Vickers-Rich Citation2004). Here we further elaborate on the ecology, cranial kinesis, and the functional application of dromornithid skull morphology, although, given limitations, we recommend dedicated kinetic and biomechanical analyses to appropriately test that which is discussed below.
Bite force and jaw opening
Recent studies on dromornithids have concluded that they had weak, or not especially powerful bite force capabilities due to the inferred reduction in surface area on the lateral cranium for attachment of the external adductor muscles (Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a). Furthermore, a negative relationship between bite force and body mass identified for birds may implicate proportionally low bite forces for dromornithids in consideration of their large size (Dickinson et al. Citation2022). However, assessment of biomechanical attributes associated with dromornithid jaws is multifaceted, and the interactions of all elements contributing to the potential force exerted throughout jaw closure is complex, requiring consideration of numerous factors. All galliforms and anseriforms share morphological adaptations which support powerful bite force, i.e. the lack of the ligamentum jugomandibulare laterale, and the variable rostral positioning of the processus postorbitalis and/or AZO complex (Dzerzhinsky Citation1974, Citation1980, Citation1982; Dzerzhinsky and Grintsevichene Citation2002), both explored in greater depth below. Considering the relatively definitive galloanseran affinities for dromornithids, specifically with anseriforms, and Dollo’s law, dromornithids are phylogenetically constrained with respect to the limitations imposed by inherited morphological adaptations, characteristic of this group; some of which, such as the loss of the aforementioned ligament, can be confidently assumed to have existed in Dromornithidae but cannot be observed by direct examination of the fossils alone.
In most birds, the relative non-elasticity of the ligamentum jugomandibulare laterale plays a role in locking the jaw joint and is intrinsically connected to the condylus mandibularis caudalis on pars mandibularis of the quadrate and the corresponding caudal cotyla on the mandible, that acts as a pivot for abduction. The loss of both the ligament and the condyle (and mandibular cotyla) in Galloanserae is therefore equivalently linked, to allow freedom of longitudinal sliding between the quadrate and mandible, with rostral movement of the mandible limited by the caudally attaching ligamentum occipitomandibulare (Dzerzhinsky Citation1974, Citation1980, Citation1982, Citation1995; Dzerzhinsky and Grintsevichene Citation2002). The loss of the lateral jugomandibular ligament removes constraints on the functionality of the pterygoid muscles, allowing them to effectively retract the bony palate and quadrate with respect to the cranium, and adduct the rostrum without causing automatic, responsive movement of the mandible (Dzerzhinsky Citation1974, Citation1995, and references therein). Concomitantly, adduction of the mandible is primarily and more efficiently performed by the external adductor muscles and the superficial pseudotemporal muscle (Dzerzhinsky Citation1974, Citation1995; Dzerzhinsky and Grintsevichene Citation2002). This then has major functional implications for the jaw closing mechanisms in Galloanserae, and by extension, inferences of dromornithid jaw biomechanics, due to the resultant relative independence of lower and upper jaw movement, and consequential specialisation of osteo-myological structures contributing to efficiency in their respective roles (Dzerzhinsky Citation1980, p. 155–156).
In galloanserans, powerful jaw movement is especially afforded by the rostral positioning of the processus postorbitalis and/or AZO complex, and associated adductor musculature (especially m. AME profundus, partes zygomatica et superficialis) from the point of jaw rotation (i.e. the area of articulation with the quadrate), resulting in a longer lever arm for efficient transmission of forces during lower jaw adduction (Dzerzhinsky Citation1974). This increased space is also associated with greater area for muscle attachment, particularly m. AME superficialis and m. adductor mandibulae posterior, interrelated with other shared biomechanical features (as discussed by Dzerzhinsky Citation1974, Citation1980, Citation1982; see also Zusi and Livezey Citation2000). Dromornithids have jaw adductor musculature emanating in association with a rostrally developed processus postorbitalis and AZO complex, especially comparable to anhimids (see above), and while their cranium is rostrocaudally compressed, this is primarily with relation to the supraorbital area, and does not appear to limit the available caudal attachment area for mandibular external adductor muscles. Neither does it reduce the distance between the quadrate and the orbit. This would likely somewhat similarly achieve the functional benefit of increased bite force produced from the aforementioned rostral positioning of the adductor musculature that occurs in other galloanserans (see Dzerzhinsky Citation1974). Accordingly, several structures corresponding to the origin of the m. AME superficialis on the cranium and quadrate of dromornithids (crista m. AME articularis, tuberculum subcapitulare) are well-developed. The site of origin of m. adductor mandibulae posterior on the quadrate is similarly well formed, interrelated with a vast insertion site on the lateral caudal mandible, and evidencing strong retraction power for adducting both the mandible and the rostrum (Goodman and Fisher Citation1962; Dzerzhinsky Citation1974). In close association with the origin of m. AME profundus, pars coronoideus (Murray and Vickers-Rich Citation2004, p. 240), the obviously excavated fossa, associated crests and tuberculum in the area of origin for m. pseudotemporalis superficialis in dromornithids marks an extensive and likely hypertrophied superficial pseudotemporalis muscle (Murray and Vickers-Rich Citation2004) which also participates in the adduction of the lower jaw (Dzerzhinsky Citation1974).
Furthermore, the large, dorsoventrally deep and wing-like palatines in dromornithids provide an expansive attachment surface for both dorsal and ventral bellies associated with m. pterygoideus muscles (e.g. Dzerzhinsky Citation1982; Vanden Berge and Zweers Citation1993: annot. 21; Carril et al. Citation2015). This has been considered one of several mechanisms for increasing bite force (see Witmer and Rose Citation1991, p. 100) as they provide a surface for powerful muscle leverage (Murray and Vickers-Rich Citation2004, p. 244). Their insertion area on the mandible also appears well developed in dromornithids (see ‘Rostrum’; Murray and Vickers-Rich Citation2004, p. 241, fig. 189), and so, in conjunction with the anchoring ligamentum occipitomandibulare, contraction of these muscles from their fixed point on the mandible provides a large force of retraction to caudoventrally pull the quadrate and bony palate, and efficiently adduct the rostrum, which is further facilitated by the mobility of the craniorostral hinge in its relatively caudal position (Dzerzhinsky Citation1995; see also Murray and Vickers-Rich Citation2004: fig. 190). The deep pseudotemporal muscle (m. pseudotemporalis profundus) also acts to retract the rostrum, with corresponding areas of origin on the rostrolateral processus orbitalis (crista orbitalis) of the quadrate, and its insertion the medial mandible, whereby the former, as a lever arm, is pulled caudoventrally to lower the rostrum (Dzerzhinsky Citation1974, Citation1980; Bühler Citation1980). This muscle also acts to adduct the mandible (Goodman and Fisher Citation1962; Zweers Citation1974; Buhler, 1980). The surface of origin is especially marked in dromornithid quadrates, when preserved, and infers strong emanating musculature. Similarly, in addition to the adduction of the lower jaw (see above), the m. adductor mandibulae posterior, which originates on the lateral quadrate and extends to the mandible, also participates in active retraction of the rostrum in an arc-like path (rather than purely ventrally, see Dzerzhinsky Citation1974: fig. 1A). This active lowering of the upper jaw thus assists in severing material between the jaws, compared to only vertically clasping them (Dzerzhinsky Citation1974, Citation1980). Such functional adaptations, coupled with specialisations for adducting the lower jaw (above), would have resulted in formidable closing power of the jaws, although not as proportionally extreme as those that may be inferred for gastornithids. Evidently, the lack of the lateral jugomandibular ligament, and resultant muscle specialisation with respect to function in the isolated movement of the upper and lower jaws (Dzerzhinsky Citation1980), likely translated to positively affecting potential bite force capabilities in dromornithids.
The consumption of fixed, resistant foods which place greater and differential stress loads on the upper or lower jaws, are related to the significant evolution of independent jaw mobility in Galloanserae (Dzerzhinsky and Grintsevichene Citation2002). In a bird that possesses a ligamentum jugomandibulare laterale, such as non-Galloanserae, upwards pressure exerted upon the upper bill would automatically impact the jaw joint and depress the lower bill; this is to be avoided during activities such as digging, as opening the beak would result in an influx of sediment or water into the mouth (Dzerzhinsky Citation1974). Isolation of the movement of each jaw relative to the other can therefore be considered advantageous as it restricts passive protraction, and is especially beneficial when feeding on attached, coarse vegetation, or in association with mediums that may exert resistance, such as digging through wet and dry sediment (Dzerzhinsky Citation1974, Citation1980, Citation1982). A morphological feature of note with regards to this, and jaw closing, are the mandibular cotylae in dromornithids which are oblique with respect to the rostrocaudal plane (as especially observed in Ilbandornis sp. NTM P2774–2 and Dromornis planei NTM P9464–112, and perhaps in Genyornis newtoni SAMA P59517), which can be compared with the orientation of those in Penelope jacucaca (Cracidae). The angle of the cotylae in this taxon, offset from the sagittal plane of the mandible, have been interpreted as contributing to efficiently locking the quadrate-mandible joint at the point of maximum retraction (Dzerzhinsky Citation1980, and references therein). The action of tearing off fixed vegetation that is clasped between these jaws creates additional forces of protraction, and so this joint locking is hypothesised as a mechanism of preventing passive opening of the jaws that is effective even with a relatively weak jaw apparatus, especially when additionally contributed to by dorsoventral compression placed under the taut caudally attaching ligamentum occipitomandibulare (Dzerzhinsky Citation1980). In conjunction with the oblique alignment of the cotylae, the transverse, lateromedial movement of the quadrate during contraction of the m. pseudotemporalis profundus and m. adductor mandibulae posterior, in adduction, provides reinforcement to this lock (Dzerzhinsky Citation1974). While dromornithids do not appear to have such a distinct mandibular crista intercotylaris as P. jacucaca, the inclination of the cotylae may indicate some similar adaptations for efficiently feeding on attached vegetation while mechanically minimising the use of musculature that would resist adduction. As P. jacucaca is a representative of a basal galliform with relatively weak jaw musculature, the advantage in foraging enabled by this structural specialisation has been considered an archaic adaptation, important in the successful radiation of Galloanserae (Dzerzhinsky Citation1980), and as such may be symplesiomorphic with respect to Dromornithidae.
Analysis of the skull morphology of dromornithids also allows some interpretations of other biomechanical features of the jaw apparatus, particularly related to finer adjustment in upper and lower jaw interaction. The aforementioned adaptations for increased mobility of the quadrate are associated with several additional related adaptations in the dromornithids. The low crista intercotylaris between the lateral and medial cotyla on the mandible of dromornithids allows for additional rotational movement of the quadrate upon this surface (Murray and Vickers-Rich Citation2004). In conjunction with forces exerted by m. protractor pterygoidei et quadrati, and the muscles involved in quadrate-mandible adduction (m. pseudotemporalis profundus and m. adductor mandibulae posterior), low intercotylar crests may have enabled the movement of the quadrate that thus shifted the fulcrum across the mandible surface during feeding, as is observed in species of Anas (see Goodman and Fisher Citation1962; Zweers et al. Citation1994, p. 269; Murray and Vickers-Rich Citation2004). The contraction of the m. protractor quadrati et pterygoidei, via quadrate and pterygoid movement relative to the cranium, is the primary driver of active protraction and elevation of the rostrum (Bühler Citation1980), and is likely facilitated by the mobility of the craniorostral hinge, however, the dorsal quadrate is further freed for greater movement by the functional single-headedness of the capitulum squamoso-oticum and supporting modifications to the quadratic cotyla of the cranium in which it sits. With no buttressing from a distinct otic capitulum, the quadrate can rotate mediolaterally around its dorsoventral axis, rather than being restricted to a primary rostrocaudal direction of movement (see Dzerzhinsky Citation1974, Citation1982; Zweers Citation1974; Murray and Vickers-Rich Citation2004, p. 244; Zelenkov and Stidham Citation2018). Notably, movement of the quadrate is further unhindered in dromornithids by the weak condition of interlocking articulation points between the quadrate and both the pterygoids and jugal arches (also see Samejima and Otsuka Citation1987). In most galliforms, the pterygoid articulates at two points on the quadrate, whereas in anseriforms (although to a lesser extent in anhimids, see Elzanowski and Stidham Citation2010), cracids and dromornithids, the simplicity of the single continuous quadrate-pterygoid articulation and the lack of a fortifying secondary articulation on the orbital process (see Dzerzhinsky Citation1982; Elzanowski and Stidham Citation2010; Zelenkov and Stidham Citation2018), permits rotation of the quadrate while maintaining contact with the pterygoid (Dzerzhinsky Citation1974; Murray and Vickers-Rich Citation2004). Effectively, this mobility in quadrate-pterygoid articulation would have limited the capacity for transferral of forces to stress and distort the palatal apparatus during adjustments of the quadrate (Zelenkov and Stidham Citation2018). Less potential for deformation is also facilitated by the very robust palatines of dromornithids, which, in conjunction with this mobile, continuous articulation, would have been able to effectively transmit protraction forces through rostral quadrate movement (Zelenkov and Stidham Citation2018). Furthermore, dromornithids lack a bowl-like fovea quadratojugalis for the articulation with the quadratojugale and instead only retain a shallow, plate-like articular facet. This weak articulation, in conjunction with the robust jugal arch that would have been resistant to deformation or lateral flexion, would have also been able to efficiently impart rostrocaudal forces during upwards movement (protraction) of the rostrum. This adaptation combined with the mediolateral rotational mobility afforded by the ball and socket-like quadrate-cranium joint morphology, may have thus been able to partly compensate for the lack of flexibility in the jugal arch. These quadratic adaptations are therefore vital for maintaining independent movement of the upper bill of dromornithids, while also enabling use of its robust and inflexible structure for other aspects of feeding. This is in contrast to other galloanserans, especially Anseres, wherein flexibility of the jugal arches particularly, is integral for feeding (see Zelenkov and Stidham Citation2018).
Rotational mobility in jaw use is also complimented by other aspects of the dromornithid skull. This importantly extends to the craniorostral hinge, whereby the uniting ligamentous attachments between the caudal rostrum and rostral cranium may have allowed some small degree of rotation on transverse, frontal and sagittal planes (Murray and Vickers-Rich Citation2004, p. 241). Given the obviously impressed and extensive attachment of m. pterygoideus, Murray and Vickers-Rich (Citation2004) also suggested that independent contraction of parts of this musculature may have been able to slightly reposition the mandibles on the frontal plane, and hypothesised that when supported by other musculature involved in adduction, this would allow dromornithids to control the edges of their jaws transversely when forces were applied to resistant objects. The deeply socketed origin of the superficial pseudotemporal muscle on the caudal orbit of all dromornithids, near the level of, or just dorsal to the tomial margins, indicates high levels of control over, and resistance of, axial rotation and mediolateral displacement of the mandible, but also maintenance of strong, stable adduction (see also Murray and Vickers-Rich Citation2004, p. 244). This would be supported and further enhanced by the aforementioned rostral positioning of the external adductor muscles, and the active lowering of the rostrum enabled by the m. adductor mandibulae posterior (Dzerzhinsky Citation1974; Dzerzhinsky and Grintsevichene Citation2002). Based on their considerable control over movement of the upper and lower jaws independently, and proposed vectors involved in muscle action, it has been suggested that dromornithids were well-adapted for holding and manipulating food held in the tip of the beak, and had relatively high static bite force in this region, as is achieved by other goose-type anseriforms (Murray and Vickers-Rich Citation2004; Olsen and Gremillet Citation2017; Pecsics et al. Citation2017). Considering these adaptations, as well as those corresponding to a highly developed lingual apparatus, Murray and Vickers-Rich (Citation2004, p. 245) concluded that the manipulative jaw abilities of dromornithids may have been approaching, or comparable to, those observed in parrots.
Dzerzhinsky (Citation1974) noted that the extensive retroarticular process of the caudal mandible in Galloanserae (specifically in reference to Tetrao urogallus) may be primarily related to the necessity for a more caudal origin of lingual musculature on its lateral surface (e.g. m. serpihyoideus and m. stylohyoideus). While a complete retroarticular process is not known for any dromornithid, preserved areas indicate that it was large, dorsoventrally deep, and extended well caudal of the fossa articularis quadratica, not unlike other galloanserans, and certainly also accommodated lingual musculature on its lateral surface. In Gallus gallus, the paraglossum is small and relatively broad, as it is in Genyornis newtoni, which, in the former taxon, supports a large, thick and somewhat flexible tongue in the context of a feeding apparatus specialised for pecking (Zweers et al. Citation1994). How this affected the function and manipulability of the lingual apparatus in dromornithids is unknown, however.
Because of the size and lever-like caudal extension of the retroarticular processes, and the consequent oblique nature of insertion of m. depressor mandibulae, contraction of this muscle produces a strong protraction force, and efficient abduction of the lower jaw (Dzerzhinsky Citation1974, Citation1980). In several galliforms, this morphology has been associated with optimised forceful gaping, against soil resistance or to overcome the friction of vegetation stuck between the upper and lower jaws (Dzerzhinsky Citation1980; Zweers et al. Citation1994), and has also been related to the rapid filter-feeding jaw movements in anatids (Bühler Citation1980). The plausibility of the latter in dromornithids is not well supported considering other aspects of their anatomy. The development of the processus retroarticularis is generally considered an adaptation to depress the lower jaw against resistance (Zweers et al. Citation1994, p. 246, 271), and the well-developed retroarticular process in dromornithids would then imply that forceful opening of the mandible was important in their feeding or other aspects of their ecological niche.
Consequently, considering the aforementioned adaptations, we do not support the hypothesis that dromornithid jaws had an especially weak bite force, despite rostrocaudal compression of the cranium. While this latter factor may have limited the potential force generated in the closing of the jaws, dromornithids have inherited adaptations such as independent rostrum-mandible mobility that are characteristic of other galloanserans, which has permitted associated subsequent osteological specialisation and hyper-development of musculature involved in feeding. This likely translated to powerful bite forces, jaw opening, and also notable manipulative abilities in relation to feeding on plant matter.
However, despite the shared characteristics or comparisons that can be drawn between dromornithids and other galloanserans, the unique combination of traits observed in the dromornithid skull do not present any close known analogue. The robust palatines and all associated components form a single functional structure in dromornithids, while another is formed by the adjacent articulations of the quadrate and pterygoid, indicated by the elongate mandibular condyles of the former that are longitudinally aligned with the axis of the latter. Together with the jugal arch as a third functional unit, dromornithids exhibit a structural condition of the palatal apparatus that is typical of anseriforms (Dzerzhinsky Citation1974, Citation1982; Zelenkov Citation2017; Zelenkov and Stidham Citation2018). In contrast, the dorsoventral separation of the palatines and jugal arches where they interact with the rest of the rostrum is unlike Anseres, nor especially comparable to galliforms (for comparative description of the functionality of the palate in Galloanserae, see Zelenkov and Stidham Citation2018: fig. 2). The implication of this is that the functionality of the dromornithid palatal apparatus is unique with respect to the condition of any one galloanseran order (Dzerzhinsky Citation1974, Citation1982; Zelenkov and Stidham Citation2018: fig. 2). However, aside from the quadrate-quadratojugal articulation, this distinctive character composition is in some ways intermediate between that which is characteristic of each of these galloanseran orders, as would be expected by a close evolutionary affinity to anhimids, which also appear somewhat intermediary in their morphology and functionality of the palatal apparatus (see Dzerzhinsky Citation1982).
Feeding and aquatic adaptations
The typical galliform skull morphological arrangements support feeding through digging, active tearing of attached and sometimes coarse vegetation, or grasping of fruits and grains, compared to the grazing, submerged dexterous manipulation of soft aquatic-based plant matter, and filter-feeding that is typical of many anseriforms (e.g. compare with Tetrao urogallus, see Kirikov Citation1944; Dzerzhinsky Citation1974, Citation1980, Citation1982, p. 1036–1039; Zweers Citation1974; Zusi and Livezey Citation2000). In anhimids, their structural arrangement combined with a galliform-like bill, likely supports their consumption of coarser foliage through grazing and aquatic macro-feeding, as well as foraging in mud – although it is unknown if they filter-feed while digging (Dzerzhinsky Citation1982; Naranjo Citation1986). Anseranas semipalmata also feeds predominately on water-based macro-vegetation, and filtration-feeds in mud (Davies Citation1963; Murray and Vickers-Rich Citation2004).
Dromornithids have long been known to be herbivorous (for a review, see Murray and Vickers-Rich Citation2004). Genyornis newtoni is no exception with the consistent presence of jasper, sandstone, claystone, and quartz (Rich and van Tets Citation1984) gastroliths, found associated with its skeletons, being a strong link to herbivory. These gastroliths are small in size (most in range 4–8 mm in diameter, with few over 12 mm) and overall mass ratio with respect to mean body mass, suggesting only soft foods are being consumed and not items such as twigs (Handley and Worthy Citation2021: SI, p. 36; see SI 6 for additional data of G. newtoni gizzard stones). This is consistent with the dromornithid diet as proposed by Murray and Vickers-Rich (Citation2004, p. 295–322), who extensively explored known dromornithid habitat and available sources of food, and Handley and Worthy (Citation2021), who found the well-developed somatosensory and sensorimotor capabilities of dromornithids to be indicative of a selective soft browse diet dominated by leaves, new growth, and fruit. The morphology of the skull of Genyornis newtoni as described above, further supports herbivorous niche occupation, in a manner not unlike that of geese or the Anhimidae. The lack of several filter-feeding adaptations that are contrastingly present in Anseres, notably the thin, flexible jugal arch, and the modified attachment of the jugal arch and palatines to the rostrum (see Zelenkov and Stidham Citation2018), and the more similar nature of these regions to galliforms and anhimids, supports an absence of specialised filter feeding in the diet of dromornithids.
Relationships have been identified linking relatively rostrocaudally short and dorsoventrally high skulls and rostrocaudally short palatines with grazing and omnivorous diets, in contrast to the more elongate, gracile skulls of filter feeding and piscivorous anseriforms (Goodman and Fisher Citation1962; Pecsics et al. Citation2017). In dromornithids, the rostrocaudal compression and rostral foreshortening of the cranium may then be associated with a capacity for grazing-type behaviour and niche occupation. As in species of Dromornis, Genyornis newtoni also possesses shearing planes at the caudal region of the upper and lower bill, created by overlapping of the lateral plate on each side of the rostrum and the corresponding tomial shelf of the lower jaws in articulation. The nearly vertical oriented planes of the interlocking jaws in this region may contribute to the efficiency of clamping and tearing fixed vegetation (as described for some galliforms, see above and Dzerzhinsky Citation1980), and is additionally considered to be functionally important for shearing of small and resistant objects (see Murray and Vickers-Rich Citation2004). This caudal tomium is differentiated from the more rostral crista tomialis in dromornithids, although the surface of the latter is comparatively flat in G. newtoni and contrasts with the more angular cristae in species of Dromornis, which probably relates to different feeding ecologies (see Murray and Vickers-Rich Citation2004; Worthy et al. Citation2016a). Dromornithids also appear to have well-developed neck musculature, to support their large (and heavy) heads. Their expansive, high attachment over the caudal cranium that reaches the dorsal apex of the calvaria, more similar to Anseranas semipalmata than that of anhimids, would have directed forces away from the occipito-atlas joint to be distributed further down the neck, likely in association with pulling or tugging at resistive vegetation, such as when browsing (see Murray and Vickers-Rich Citation2004). The difference in dorsoventral location of the foramen magnum between dromornithids (i.e. the dorsoventral middle of the skull in Genyornis newtoni and Dromornis planei, for example, compared to the more ventral position in D. murrayi) suggests variability in postural holding of the head relative to the neck, or in exact feeding behaviour between species.
The presence of the synovial craniorostral hinge in dromornithids likely permitted greater upwards movement of the rostrum, ultimately increasing the available gape width, and allowing for food of larger size to be consumed (see Bock Citation1960a). According to Murray and Vickers-Rich (Citation2004, p. 246), the rostrum of species of Dromornis was capable of much dorsoventral movement at the craniorostral hinge, contributed to by the proportionally dorsoventrally thin articular surface, and possibly some slight axial rotation. This is a key benefit of this hinge type for parrots in which it likely evolved in association with feeding on hard and large items (Tokita Citation2003). The desmognathous, and inflexible upper jaw would have structurally reinforced and strengthened the rostrum (Dzerzhinsky Citation1980). The enclosed bony palate, and interpretations on the tongue mobility of dromornithids that may have mirrored parrot-like manipulative abilities (see above, Murray and Vickers-Rich Citation2004, p. 245), allowed some capacity to crush food with their tongue on the palatal roof (as in some passerines, see Bock Citation1960a). It is unknown whether the dromornithids may have been better able to maintain the position of their eyes relative to their food, although the rotational movement of the rostrum about the craniorostral hinge would likely allow the cranium to maintain a constant food – eye position, as is important for many birds (see Bock Citation1960a).
Many of the features which distinguish anseriforms from galliforms, are considered derived transformations intrinsically linked to the transition to aquatic habitats (Dzerzhinsky Citation1982) early in the evolution of anseriforms; given aquatic habitat preference for anhimids (e.g. Naranjo Citation1986) and the aquatic-adapted morphology of other fossil anseriforms (see Mayr Citation2022a). These characteristic anseriform adaptations which are also present in dromornithids, are more parsimoniously symplesiomorphic and evolved prior to the divergence of the dromornithid lineage, rather than convergently. The closure of the osseous meatus acusticus externus ventrorostrally on the dromornithid skull (in association with annulus tympanicus) may additionally be associated with aquatic adaptations. This is an inferred adaptation for isolating the ear from the quadrate while the head is submerged in water, whereby movement of this bone could periodically deform the ear cavity, making it difficult to protect the ear from water ingress and create acoustic interference (Dzerzhinsky Citation1982, p. 1039). In G. newtoni, the osseous meatus acusticus externus is enclosed to an extreme degree and in turn well separated from the quadrate, compared to other dromornithids.
This then suggests two options for dromornithid habitat preference. First, that dromornithids were adapted to aquatic-associated habitats (e.g. edges of wetlands, large lakes, and flood plains, as discussed by Murray and Vickers-Rich Citation2004, p. 2, 157, 167; initially proposed by Stirling and Zietz Citation1900) and had a varied grazing folivorous and frugivorous diet with relation to foraging in the surrounding environment, not dissimilar to anhimids (see above). This is additionally supported by the common discovery of dromornithid fossils around former bodies of water, such as Lake Callabonna and Alcoota (see Worthy and Yates Citation2017), and the inferred tethering of G. newtoni to channel systems and floodplains (Stirling and Zietz Citation1900; Smith Citation2009). The second hypothesis is that dromornithids were constrained by or retained ancestral adaptations to the sub-aquatic environments yet did not necessarily utilise these in association with a sub-aquatic niche or environment.
Desmognathy in the rostrum, and the caudal position of the choana nasalis ossea in Genyornis newtoni, may have additionally offered protection against water ingress (achieved by fascia in Chauna torquata, see Dzerzhinsky Citation1982). Furthermore, the flat tomial surface of the rostrum in G. newtoni with associated rough osseous material similar to that which supports rhamphothecal lamellae rostri in some anseriforms, is consistent with the idea that dromornithids may have possessed very rudimentary lamellae on their tomia, most comparable to anhimids, to facilitate the successful seizing and tearing of slippery attached aquatic vegetation (see Murray and Vickers-Rich Citation2004). Murray and Vickers-Rich (Citation2004) additionally suggested that the sulcus paratomialis of dromornithids may have also allowed expelling of excess water in this method of feeding. Many anseriforms also have adaptations for straining water from the between their jaws in food acquisition (Dzerzhinsky Citation1982). Alternatively, simple, non-extensive rhamphothecal lamellae could be used in efficiently gripping and tearing other types of fixed plant matter with the jaws and considering the more blade-like rostral tomia in species of Dromornis, may have supported diversification with regards to other dietary preferences, including an inferred head-level browsing niche in this lineage (see Murray and Vickers-Rich Citation2004, p. 192). It is also worth noting here that we do not find convincing evidence for supraorbital ‘nasal salt glands’ (fossa glandulae nasalis) in any dromornithid (see ‘Cranium’ and ‘Rostrum’), which Murray and Vickers-Rich (Citation2004) suggested were adaptations to more arid environmental conditions, and against habitation of freshwater or rainforest regions.
The aquatically adapted skull may seem contradictory to purported terrestrial locomotion adaptations in the hindlimbs of G. newtoni and other dromornithids. The hind limbs (including the blunt, hoof-like, but mobile toes and inferred elastic recovery system of the ankle joint), as described by Murray and Vickers-Rich (Citation2004, p. 221–225), were hypothesised to be adaptations for terrestrial locomotion in dromornithids. In the context of the hypothesis that dromornithids were at some point adapted to exploiting aquatic-associated habitats, then such unusual morphology of the hind limb may be a result of large birds which rely on aquatic systems being forced to adapt to hard, dry ground. This would occur as they become less confined to water and travelled increasingly long distances between appropriate habitats due to dry environmental conditions, ephemeral lake systems, and increasing aridity in Australia since the middle Miocene (for reviews of environmental changes and their influence on the dromornithids, see Murray and Vickers-Rich Citation2004; Angst and Buffetaut Citation2017). In this way, forced range expansion may be related to diversification of their diet to include soft browse not exclusively associated with near-freshwater regions. Indeed, the variable hindlimb morphology of dromornithids (especially late Miocene forms) demonstrate adaptations to different modes along the cursorial-graviportal spectrum of locomotion and have been paired with contrasting capacity to expand their range, as well as potential for nomadic or migratory lifestyles (Murray and Vickers-Rich Citation2004; Worthy and Yates Citation2015).
Variation of rostrum shape within the Dromornithidae
Across dromornithids, beak shape varies considerably when comparing G. newtoni to species of Dromornis and suggests divergent evolutionary history and possibly niche adaptation. For example, the tomial margin of G. newtoni rostral of the palatines is bordered by a wide, flattened tomial surface more effective for crushing and gripping than the thinner, grooved margin of Dromornis (see Murray and Vickers-Rich Citation2004, p. 245–246). Additionally, the general shape of the bill varies, with G. newtoni lacking the extreme dorsoventral height and dorsal curvature of the rostrum noted for species of Dromornis (see ‘Rostrum’ and Murray and Megirian Citation1998); such height may have increased resistance to reaction forces from crushing seeds (as was identified in Darwin’s finches, see Herrel et al. Citation2005). The shorter rostrocaudal rostrum length relative to cranium length for G. newtoni, likely instead facilitated greater dexterity and proportionally increased power of the jaw musculature (see above, Goodman and Fisher Citation1962, p. 169), and may be associated with the wide shape and flattened tomial surface of the beak of Genyornis newtoni, absent in species of Dromornis. Goodman and Fisher (Citation1962, p. 170) noted that force is applied more effectively in birds with narrow bill tips (species of Dromornis) compared to those with wide bill tips (G. newtoni). There are currently no known rostra associated with species of Ilbandornis or Barawertornis, although their relatively slender and dorsoventrally narrow mandibles (see Worthy et al. Citation2016a), compared to those of Dromornis species, may suggest that their upper jaws were also not so dorsoventrally deep.
The synovial craniorostral hinge of dromornithids was supported by two lobes which would act to stabilise the joint and restrict excessive movement at high force loads (see ‘Craniorostral hinge’). As the lobes are proportionally larger in species of Dromornis than in G. newtoni, less movement in the former would have likely been possible, potentially driven by the large size of the rostrum requiring increased stability. Although the incorporation of the lacrimal into the rostrum would have increased robusticity of the caudal rostrum itself, other than providing some lateral strengthening and stabilisation at the hinge, this was likely limited since the dorsal lacrimal did not appear to have caudally crossed the hinge in dromornithids.
Beak shape evolution in anseriforms has both facilitated and been driven by expansion into new ecological niches (see Olsen and Gremillet Citation2017) and this may have played a role in this variation. Olsen and Gremillet (Citation2017) found that an increase in culmen angle led to an increase in stress resistance, and in finches, bite force has previously been positively correlated with depth of the beak (see Herrel et al. Citation2005); the latter a factor likely contributing to beak shape evolution in parrots. Such a driver may have been more dominant in the Dromornis group resulting in such disparate rostrum shapes relative to the shape of G. newtoni. Alternatively, the more goose-like shape of the rostrum of G. newtoni may be a derived morphology resulting from a transition towards a similar niche occupation as some geese. This could have been driven by millions of years of adaptation to changing environmental conditions and food resources, linked to the expansion of sclerophyll and grassland habitats across Australia, which Murray and Vickers-Rich (Citation2004, p. 295–322) consider an important factor in dromornithid evolution and extinction.
We thus propose two alternative hypotheses for such variation in beak shape among dromornithids. Genyornis newtoni displays a derived morphology driven by adaptations to Australia’s Quaternary environment. Alternatively, G. newtoni may have been descended from close relatives of Ilbandornis species and retained an Ilbandornis-type bill. The difference between the Genyornis- and Dromornis- type jaws then may have been driven by divergent niche occupation, allowing geographic and temporal overlap of species of Ilbandornis and Dromornis, of which two and one species, respectively, were sympatric in Alcoota and perhaps Bullock Creek local faunas. The two alternative hypotheses for adaptation to aquatic environments (see ‘Feeding and aquatic adaptations’), may both be applicable but to different dromornithid lineages, that is, one lineage retaining a water-based feeding habitat and the other, only constrained by historical aquatic adaptations. Having a large, heavy skull, as is especially characteristic of Dromornis species, is a large energy investment (estimated to have been between 5–8 kg in D. stirtoni, see Murray and Vickers-Rich Citation2004), and thus must have been integral to evolutionary fitness in this group of birds, such as feeding on large and/or resistant items. It is possible that the need for such large head size may have also been a factor that necessitated the evolution of correspondingly large body, since large body size alone is not a sufficient driver of increased head size (as exemplified by the skull to body proportions of flightless palaeognathous taxa). Irrespective of what drove large body and head size in dromornithids, compensatory adaptations for additional structural robusticity are required for supporting such a large structure, and to also enable functional capabilities at such extreme dimensions. As such, the large skull size of Dromornis species may itself predominately explain their mediolaterally narrow and dorsoventrally deep rostrum that has capacity for reducing vertical compression, torsion, and shear, to maintain rigidity in such a large bony structure which is only supported by a single suspension point (see Murray and Vickers-Rich Citation2004, p. 235). Other functional roles which may have significantly contributed to the variation includes sexual selection, and functions associated with vocalisation, preening, and thermoregulation, which can lead to trade-offs with feeding performance (e.g. Clayton et al. Citation2005; Bright et al. Citation2016; Olsen and Gremillet Citation2017, and references therein). As there is currently no rostrum attributed to any species of Ilbandornis, it is difficult to deduce the exact relationships between such factors and beak shape diversity among dromornithids. The discovery of further cranial material especially, will be vital in better elucidating the patterns of dromornithid evolution, and how interspecific differences in morphology relate to environmental changes across the Cenozoic of Australia.
Conclusion
The discovery of new skull fossils of the Pleistocene dromornithid Genyornis newtoni, and their description and phylogenetic analysis in this study, have considerably furthered the current understanding of this taxon, as well as the Dromornithidae. Their morphology, based on their osteology and inferred syndesmological and myological structures, likely facilitated a wide gape, fine and independent control over movement of the upper and lower bills, and adaptations for water-associated habitats, potentially retained from the early divergence of the anseriform lineage from stem Galloanserae. We support a varied soft-browse, folivorous and frugivorous diet, as previously proposed for dromornithids, although variation between Genyornis newtoni and species of Dromornis, suggest differences in niche occupation and beak functionality.
This study additionally provides a more complete morphological basis for addressing the relationships within this family, and with other galloanserans. We find support for the generic distinction of the genus Genyornis, and little evidence for close galliform or gastornithiform affinities. There are few features of the skull which could be inferred as confident indicators of such affinities and the differing approaches to the evolution of large, deep mandibles between dromornithids and gastornithids, appear to be driven by functional adaptations to their respective ecological niches, constrained by their contrasting galloanseran ancestry. Instead, a more compelling hypothesis, and that which we advocate for, is that the dromornithids have basal anseriform affinities, and are likely sister to the Anhimidae, within the Anhimae. Even among their diverse modern relatives within Galloanserae, and indeed all of Aves, only the anhimids appear to be a close morphological counterpart in skull anatomy, despite some notable divergences. However, the complexity surrounding the hybrid concoction of galliform and anseriform traits in the skull of dromornithids, presents an impediment in determining phylogenetic affinities using morphology. Identifying ancestral or plesiomorphic conditions is challenging for both observational and phylogenetic studies, especially considering the great age of the Galloanserae lineage, with only a fragmentary Upper Cretaceous and early Palaeogene fossil record. The early divergence of dromornithids likely contributes to the combination of galliform and anseriform characters which has produced conflicting findings, and unresolved placements in previous phylogenetic studies. Consequently, the dromornithid form provides an unforeseen opportunity to consider character polarities deep within the Galloanserae clade and indicates that future analyses of morphology in early diverging Anseriformes, especially, must be considered within the greater context of the entire Galloanserae radiation. Although we find phylogenetic support for several hypothesised character polarities in our preliminary phylogenetic study, there remains a need for a more extensive analyses, to aid in better understanding and resolving the interrelationships of all early diverging fossil galloanseran lineages.
Author contributions
PLM made the morphological observations, conducted the comparisons, and created the initial drafts of the manuscript. THW provided physical access to, and/or photographs of, many specimens, as well as the field work and funding required to conduct this research (Australian Research Council grant DP180101913, see below). THW and PLM conceived the project as part of the larger project this work has contributed to, namely investigating the biology of Genyornis newtoni, and forms a part of PLM’s PhD thesis. PLM, JCB and THW contributed to research and interpretations regarding relevant morphology and palaeobiology. JCB provided detailed and significant contributions to the understanding of myological and syndesmological correlates of osseous structures, and their homologues across the specimens, taxa and publications studied (Appendix Three). The assembly of morphological character descriptions and states, and subsequent coding of all taxa was undertaken by PLM, which was revised and edited by PLM and JCB. JCB ran the phylogenetic analyses, and JCB and PLM interpreted results and wrote associated sections. PLM created all figures aside from (and part of 14) which were produced by JCB. All authors participated in associated field work. All authors contributed to editing the manuscript and approved the submitted version.
Supplemental Material
Download Zip (866.6 KB)Acknowledgments
There are many people who contributed to this research, who we would like to acknowledge. Firstly, Adam Yates provided access to many MAGNT specimens, including NTM P932-2 (), and patiently allowed us to keep the specimens for longer than initially stated. Adam Yates and Sam Arman both assisted with answering questions on the specimens available from MAGNT. Mary-Anne Binnie (SAMA), Maya Penck (SAMA), Katie Date (NMV), Lisa Nink (FU), and Kristen Spring (QM) also loaned us material and/or gave us access to their collections. We thank Lawrence M. Witmer (Ohio University) for his generosity in providing a micro-CT scanned skull of Presbyornis pervetus (USNM 299846). We would additionally like to acknowledge Patricia Vickers-Rich and Peter Murray for their extensive monograph on the Dromornithidae (Murray and Vickers-Rich, 2004); Warren Handley (FU) contributed to data analysis on gastroliths; Jacob van Zoelen (FU) who provided guidance in using Blender v. 2.93.2 (CitationBlender Online Community, Citation2023); Michael S. Y. Lee for tutelage with regards to phylogenetic methodologies and photographs of dromornithid fossil from MAGNT; Peter Trusler for interesting and useful discussions; Mahala Fergusen for photographing specimen SAMA P10838; and Joseph Bevitt who kindly took time to scan the specimen SAMA P59516 using the neutron scanner at ANSTO which was a big contribution to understanding the internal structure of the fossils and the scanning potential for the Lake Callabonna Genyornis newtoni material. We also thank three anonymous reviewers who contributed to improving the manuscript. We would like to thank the other Chief Investigators for the Australian Research Council grant DP180101913 (Extricating extinction histories at Lake Callabonna’s megafauna necropolis) which funded the collection of material from Lake Callabonna, namely Lee Arnold and Anusuya Chinsamy-Turan. Additionally, all the people who conducted fieldwork at Lake Callabonna and contributed to preparing the fossils, primarily Carey Burke who spent many months cleaning and preparing the specimens use in this study, Aaron Camens, and Keith Cook (Maxim Foundation) who made large contributions to the field work at Lake Callabonna. Finally, thank you to Gerard and Karina Sheehan for the access to Lake Callabonna that allowed this work to transpire.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/08912963.2024.2308212.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are included in this published article, the appendices and supplementary information files.
Additional information
Funding
References
- Agnolín FL. 2021. Reappraisal on the phylogenetic relationships of the enigmatic flightless bird (Brontornis burmeisteri) Moreno and Mercerat, 1891. Diversity. 13(2):90. doi: 10.3390/d13020090.
- Andors AV. 1988. Giant groundbirds of North America (Aves, Diatrymidae) [ Ph.D. thesis]. Columbia University (unpubl.).
- Andors AV. 1992. Reappraisal of the Eocene groundbird Diatryma (Aves: Anserimorphae). Nat Hist Mus Los Angeles County Sci Seri. 36:109–125.
- Angst D, Buffetaut E. 2013. The first mandible of Gastornis Hébert, 1855 (Aves, Gastornithidae) from the Thanetian (Paleocene) of Mont-de-Berru (France). Rev de Paleobiologie. 32(2):423–432.
- Angst D, Buffetaut E. 2017. Palaeobiology of giant flightless birds. United Kingdom: ISTE Press Ltd and Elsevier Ltd .
- Angst D, Lécuyer C, Amiot R, Buffetaut E, Fourel F, Martineau F, Legendre S, Abourachid A, Herrel A. 2014. Isotopic and anatomical evidence of an herbivorous diet in the Early Tertiary giant bird Gastornis. Implications for the structure of Paleocene terrestrial ecosystems. Naturwissenschaften. 101(4):313–322. doi: 10.1007/s00114-014-1158-2.
- Bailleul AM, Witmer LM, Holliday CM. 2017. Cranial joint histology in the mallard duck (Anas platyrhynchos): new insights on avian cranial kinesis. J Anat. 230(3):444–460. doi: 10.1111/joa.12562.
- Baumel JJ, Raikow RJ. 1993. Arthrologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd ed. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 133–188.
- Baumel JJ, Witmer LM. 1993. Osteologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd Edn. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 45–132.
- Beddard FE. 1898. The structure and classification of birds. London: Longmans, Green, and Company.
- Bernhard F. 1924. Über den Einfluß der Muskulatur auf die Formgestaltung des Skelettes. Untersucht an der vorderen Tibiakante [About the influence of the muscles on the shape of the skeleton. Examined at the anterior edge of the tibia]. Archiv für mikroskopische Anatomie und Entwicklungsmechanik. 102(1–3):489–498. doi:10.1007/BF02107648.
- Bianki VV, Dzerzhinsky FY, Grintsevichene TI. 2013. Morfofunktsional’nyye osobennosti rotovogo apparata Lutka (Mergellus albellus), svyazannyye s yego troficheskimi adaptatsiyami [Morpho-functional peculiarities of the feeding apparatus of the Smew (Mergellus albellus) related to its trophic adaptations]. Zoologicheskiĭ Zhurnal. 92(5):577–587. doi:10.7868/S0044513413030045.
- Blender Online Community. 2023. Blender—a 3D modelling and rendering package. Amsterdam: Blender Foundation, Stichting Blender Foundation. http://www.blender.org.
- Boas JEV. 1929. Biologisch-anatomische studien ber den hals der vogel [Biological-anatomical studies on the necks of birds]. Kongelige Danske videnskabernes Selskabs Skrifter. Naturvidenskabelig og Mathematisk Afdeling. 9:101–222. in German.
- Bock WJ. 1960a. The palatine process of the premaxilla in the Passeres: A study of the variation, function, evolution and taxonomic value of a single character throughout an avian order. Bulletin Museum of Comp Zool. 122(8):361–488.
- Bock WJ. 1960b. Secondary articulation of the avian mandible. The Auk. 77(1):19–55. doi: 10.2307/4082382.
- Bock WJ. 1964. Kinetics of the avian skull. J Morphol. 114(1):1–41. doi: 10.1002/jmor.1051140102.
- Bock WJ. 1966. An approach to the functional analysis of bill shape. The Auk. 83(1):10–51. doi: 10.2307/4082976.
- Böhmer C, Prevoteau J, Duriez O, Abourachid A. 2020. Gulper, ripper and scrapper: anatomy of the neck in three species of vultures. J Anat. 236(4):701–723. doi: 10.1111/joa.13129.
- Boles WE. 1999. Early Eocene shorebirds (Aves: Charadriiformes) from the Tingamarra Local Fauna. Rec Western Australian Mus. 57:229–238.
- Boles WE, Finch MA, Hofheins RH, Vickers-Rich P, Walters M, Rich TH. 2013. A fossil stone-curlew (Aves: Burhinidae) from the Late Oligocene/Early Miocene of South Australia. In: Göhlich UB, Kroh A, editors. Paleornithological Research: Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution, Natural History Museum; 2012 June 11–16; Vienn: Naturhistorisches Museum, Wien. p. 43–61.
- Boles WE, Ivison TJ. 1999. A new genus of dwarf megapode (Galliformes: Megapodiidae) from the Late Oligocene of central Australia. In: Olson SL, editor. Avian Paleontology at the Close of the 20th Century: Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution; 4–7 June 1996; Washington, D.C.: Smithsonian Contributions to Palaebiology. 89:199–206.
- Bourdon E, Mourer-Chauviré C, Laurent Y. 2016. Early Eocene birds from La Borie, southern France. Acta Palaeontol Pol. 61(1):175–190.
- Bout RG, Zweers GA. 2001. The role of cranial kinesis in birds. Comp Biochem Physiol A Mol Integr Physiol. 131(1):197–205. doi: 10.1016/S1095-6433(01)00470-6.
- Bright JA, Marugán-Lobón J, Cobb SN, Rayfield EJ. 2016. The shapes of bird beaks are highly controlled by nondietary factors. PNAS. 113(19):5352–5357. doi: 10.1073/pnas.1602683113.
- Brown JW, Rest JS, García-Moreno J, Sorenson MD, Mindell DP. 2008. Strong mitochondrial DNA support for a Cretaceous origin of modern avian lineages. BMC Biol. 6(1):6. doi: 10.1186/1741-7007-6-6.
- Bryant HN, Seymour KL. 1990. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J Morphol. 206(1):109–117. doi: 10.1002/jmor.1052060111.
- Bubień-Waluszewska A 1980. The cranial nerves. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 2, London: Academic Press; p. 385–438.
- Bühler P 1980. The functional anatomy of the avian jaw apparatus. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 2, London: Academic Press; p. 439–468.
- Bühler P, Martin LD, Witmer LM. 1988. Cranial kinesis in the Late Cretaceous birds Hesperornis and Parahesperornis. The Auk. 105(1):111–122. doi: 10.1093/auk/105.1.111.
- Burggraaf PD. 1954a. On the correlation between the skull structure and the muscles in the male Phasianus colchicus L. II. The attachment of the musculus adductor mandibulae externus. Proc K Ned Akad Wet Series C, Biol Med Sci. 57:292–303.
- Burggraaf PD. 1954b. On the correlation between the skull structure and the muscles in the male Phasianus colchicus L. V. The attachment of the musculus mylohyoideus and of the musculus geniohyoideus. Proc K Ned Akad Wet Series C, Biol Med Sci. 57:673–677.
- Burleigh JG, Kimball RT, Braun EL. 2015. Building the avian tree of life using a large-scale, sparse supermatrix. Mol Phylogenet Evol. 84:53–63. doi: 10.1016/j.ympev.2014.12.003.
- Carril J, Degrange FJ, Tambussi CP. 2015. Jaw myology and bite force of the monk parakeet (Aves, Psittaciformes). J Anat. 227(1):34–44. doi: 10.1111/joa.12330.
- Chinsamy A, Worthy TH. 2021. Histovariability and palaeobiological implications of the bone histology of the dromornithid, Genyornis newtoni. Diversity. 13(5):219. doi: 10.3390/d13050219.
- Claramunt S, Cracraft J. 2015. A new time tree reveals Earths history’s imprint on the evolution of modern birds. Evol Ecol. 1(11):1–13. doi: 10.1126/sciadv.1501005.
- Clarke GA Jr. 1993. Anatomia topographica externa. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd Edn. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 7–16.
- Clarke JA, Tambussi CP, Noriega JI, Erikson GM, Ketcham RA. 2005. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 433(7023):305–308. doi: 10.1038/nature03150.
- Clayton DH, Moyer BR, Bush SE, Jones TG, Gardiner DW, Rhodes BB, Goller F. 2005. Adaptive significance of avian beak morphology for ectoparasite control. Proceedings of the Royal Society B. 272:811–817.
- Cooney CR, Bright JA, Capp EJR, Chira AM, Hughes EC, Moody CJA, Nouri LO, Varley ZK, Thomas GH. 2017. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature. 542(7641):344–347. doi: 10.1038/nature21074.
- Cope ED. 1876. On a gigantic bird from the Eocene of New Mexico. Proc Acad Nat Sci Philadelphia. 28(2):10–11.
- Cracraft J. 1968. The lacrimal-ectethmoid bone complex in birds: a single character analysis. Am Midl Nat. 80(2):316–359. doi: 10.2307/2423530.
- Currie PJ, Godfrey SJ, Nessov L. 1993. New caenagnathid (Dinosauria: Theropoda) specimens from the Upper Cretaceous of North America and Asia. Can J Earth Sci. 30(10):2255–2272. doi: 10.1139/e93-196.
- Davids JAG. 1952a. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.), I [Study on the attachments to the skull of the head and neck muscles in Anas platyrhyncha platyrhyncha (L.),I]. Proc K Ned Akad Wet Series C, Biol Med Sci. 55:81–94. (in French).
- Davids JAG. 1952b. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.). II [Study on the attachments to the skull of the head and neck muscles in Anas platyrhyncha platyrhyncha (L.)II]. Proc K Ned Akad Wet Series C, Biol Med Sci. 55:525–533. (in French).
- Davids JAG. 1952c. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.). III [Study on the attachments to the skull of the head and neck muscles in Anas platyrhyncha platyrhyncha (L.), III]. Proc K Ned Akad Wet Series C, Biol Med Sci. 55:533–540 (in French).
- Davies SJJF. 1963. Aspects of the behaviour of the Magpie Goose Anseranas semipalmata. Inter J Avian Sci. 105(1):76–98. doi: 10.1111/j.1474-919X.1963.tb02476.x.
- Degrange FJ. 2021. A revision of skull morphology in Phorusrhacidae (Aves, Cariamiformes). J Vertebr Paleontol. 40(6):e1848855. doi: 10.1080/02724634.2020.1848855.
- Degrange FJ, Tambussi CP, Taglioretti ML, Dondas A, Scaglia F. 2015. A new Mesembriornithinae (Aves, Phorusrhacidae) provides new insights into the phylogeny and sensory capabilities of terror birds. J Vertebr Paleontol. 35(2):e912656. doi: 10.1080/02724634.2014.912656.
- De Mendoza RS, Gómez RO, Tambussi CP. 2020. The lacrimal/ectethmoid region of waterfowl (Aves, Anseriformes): Phylogenetic signal and major evolutionary patterns. J Morphol. 281(11):1486–1500. doi: 10.1002/jmor.21265.
- De Pietri VL, Scofield P, Zelenkov NV, Boles WE, Worthy TH. 2016. The unexpected survival of an ancient lineage of anseriform birds into the Neogene of Australia: the youngest record of Presbyornithidae. R Soc Open Sci. 3(2):150635. doi: 10.1098/rsos.150635.
- Dickinson E, Young MW, Granatosky MC. 2022. In vivo bite force in lovebirds (Agapornis roseicollis, Psittaciformes) and their relative biting performance among birds. J Zool. 318(4):272–282. doi: 10.1111/jzo.13014.
- Dzerzhinsky FY. 1974. K Funktsionalnoy morfologii chelustnogo apparata glukharya [On functional morphology of the jaw apparatus in the capercaillie]. Ornitologia. 11:54–68. in Russian.
- Dzerzhinsky FY. 1980. Adaptivnyye preobrazovaniya chelyustnogo apparata v evolyutsii kurinykh [Adaptive transformation of the maxillary apparatus in the evolution of gallinaceous birds]. In: Sokolov VE, Lebedkina NS, editors. Morfologicheskiye Aspekty Evolyutsii [Morphological Aspects of Evolution]. Moscow: Moscow State University; p. 148–158. in Russian.
- Dzerzhinsky FY. 1982. Adaptivnʹіe čerty v stroenii čelyustnogo apparata nekotoryh guseobraznyh ptic i veroyatnye puti èvolyucii otrjada [Adaptive features in the construction of the jaw apparatus of some Anseriformes and probable evolutionary paths of the order]. Zoologicheskiĭ Zhurnal. 56:1030–1041. in Russian.
- Dzerzhinsky FY. 1995. Evidence for common ancestry of the Galliformes and Anseriformes. Courier Forschungsinstitut Senckenberg. 181:325–336.
- Dzerzhinsky FY, Belokurova IN. 1972. Sravnitelnoy anatomii chelustnoy muskul tury ptits. Chelustnyye myshtsy glucharya (Tetrao urogallus) [Comparative anatomy of the jaw muscles of birds. Jaw muscles of capercaillie (Tetrao urogallus)]. Zoologicheskiĭ Zhurnal. 51:555–564. in Russian.
- Dzerzhinsky FY, Grintsevichene TI. 2002. O vozmozhnykh filogeneticheskikh svyazyakh guseobraznykh ptits [On possible phylogenetic relationships of Anseriformes]. Kazarka. 8:19–39. in Russian.
- Dzerzhinsky FY, Potapova EG. 1974. Sistema sukhozhil’nykh obrazovaniy kak ob“yekt sravnitel’noy miologii chelyustnogo apparata ptits [The system of tendon formations as an object of comparative theology of the jaw apparatus of birds]. Zoologicheskiĭ Zhurnal. 53:1341–1351. in Russian.
- Eastman CR. 1900. New fossil bird and fish remains from the Middle Eocene of Wyoming. Geol Mag. 7(2):54–58. doi: 10.1017/S0016756800181701.
- Ellrott C, Schmitz G. 2010. Skull identification key for central European waterfowl (Aves: Anseriformes: Anatidae). Stuttg Beitr Naturkd A Neue Ser. 3:347–362.
- Elzanowski A. 1977. On the role of basipterygoid processes in some birds. Verh Anat Ges. 71:1303–1307.
- Elzanowski A. 1987. Cranial and eyelid muscles and ligaments of the tinamous (Aves: Tinaformes). Zool Jahrb Abt für Anatomie und Ontogenie der Tiere. 116(1):63–118.
- Elzanowski A. 2014. More evidence for plesiomorphy of the quadrate in the Eocene anseriform avian genus Presbyornis. Acta Palaeontol Pol. 59(4):821–825. doi: 10.4202/app.00027.2013.
- Elzanowski A, Boles WE. 2012. Australia’s oldest Anseriform fossil: a quadrate from the Early Eocene Tingamarra Fauna. Palaeontology. 55(4):903–911. doi: 10.1111/j.1475-4983.2012.01166.x.
- Elzanowski A, Mayr G. 2017. Multiple origins of secondary temporal fenestrae and orbitozygomatic junctions in birds. J Zool Syst Evol Res. 56(2):248–269. doi: 10.1111/jzs.12196.
- Elzanowski A, Paul GS, Stidham TA. 2001. An avian quadrate from the Late Cretaceous Lance formation of Wyoming. J Vertebr Paleontol. 20(4):712–719. doi: 10.1671/0272-4634(2000)020[0712:AAQFTL]2.0.CO;2.
- Elzanowski A, Stidham TA. 2010. Morphology of the quadrate in the Eocene anseriform Presbyornis and extant galloanserine birds. J Morphol. 271(3):305–323. doi: 10.1002/jmor.10799.
- Ericson PGP. 1996. The skeletal evidence for a sister-group relationship of anseriform and galliform birds: a critical evaluation. J Avian Biol. 27(93):195–202. doi: 10.2307/3677222.
- Ericson PGP. 1997. Systematic relationships of the Palaeogene family Presbyornithidae (Aves, Anseriformes). Zool J Linn Soc. 121(4):429–483. doi: 10.1111/j.1096-3642.1997.tb01286.x.
- Ericson PGP. 2000. Systematic revision, skeletal anatomy, and paleoecology of the New World early Tertiary Presbyornithidae (Aves: Anseriformes). PaleoBios. 20(3):1–23.
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 39(4):783–791. doi: 10.2307/2408678.
- Field DJ, Benito J, Chen A, Jagt JW, Ksepka DT. 2020. Late Cretaceous neornithine from Europe illuminates the origins of crown birds. Nature. 579(7799):397–401. doi: 10.1038/s41586-020-2096-0.
- Fisher HI. 1955. Some aspects of the kinetics in the jaws of birds. Wilson Bull. 67(3):175–188.
- Fisher HI, Goodman DC. 1955. The myology of the whooping crane, Grus americana. Illinois Biol Monogr. 24:1–127.
- Frazatta TH. 1962. A functional consideration of cranial kinesis in lizards. J Morphol. 111(3):287–319. doi: 10.1002/jmor.1051110306.
- Fuchs A. 1954a. On the correlation between the skull structure and the muscles in the male Phasianus colchicus L. III. The attachment of the musculus adductor mandibulae posterior and the musculus adductor mandibulae internus. Proc K Ned Akad Wet Series C, Biol Med Sci. 57:454–470.
- Fuchs A. 1954b. On the correlation between the skull structure and the muscles in the male Phasianus colchicus L. IV. The attachment of the musculus protractor quadrati et pterygoidei and of the musculus depressor mandibulae. Proc K Ned Akad Wet Series C, Biol Med Sci. 57:666–672.
- Fujioka T. 1963. Niwatori no hikaku kaibōgakuteki narabini kyokusho kaibōgakuteki kenkyū. IV. Niwatori no atama keibu ni okeru suji no kishi oyobi teishi ni tsuite sono. 1. Tōbu no suji [Comparative and topographical anatomy of the fowl. IV. Origins and insertions of muscles of the head and neck in the fowl. 1. Muscles of the head]. Nihon Juigaku Zasshi. 25(4): 207–226. in Japanese.
- Fürbringer M. 1888. Untersuchungen zur morphologie und systematik der vogel, zugleich ein beitrag zur anatomie der stutz-und bewegungsorgane [Investigations into the morphology and systematics of birds, at the same time a contribution to the anatomy of the supporting and locomotor organs]. I. Specieller Theil, II. Allgemeiner Theil. Verlag Von TJ. Van Holkema, Amsterdam.
- Gadow H. 1892. On the classification of birds. Proc Zool Soc Lond. 1892: 229–256.
- Gadow H, Selenka E 1891. Vögel. 1. Anatomischer Theil [Birds. 1. Anatomical part]. In: Bronn HG, editor. Klassen und Ordnungen des Thier-Reichs, wissenschaftlich dargestellt in Wort und Bild. Vol. 6, Leipzig: Winter’sche Verlagshandlung; p. 1–1008.
- Gelman A, Rubin DB. 1992. Inferences from iterative simulation using multiple sequences. Stat Sci. 7(4):457–511. doi: 10.1214/ss/1177011136.
- Genbrugge A, Herrel A, Boone M, Van Hoorebeke L, Podos J, Dirckx J, Aerts P, Dominique A. 2011. The head of the finch: the anatomy of the feeding system in two species of finches (Geospiza fortis and Padda oryzivora). J Anat. 219(6):676–695. doi: 10.1111/j.1469-7580.2011.01437.x.
- Ghetie V, Chitescu ST, Cotofan V, Hillebrand A. 1976. Atlas de anatomie a pasarilor domestice [Atlas of anatomy of domestic birds]. Romania: Editura Academiei Republicii Socialiste Romania.
- Goodman DC, Fisher HI. 1962. Functional anatomy of the feeding apparatus in waterfowl (Aves: Anatidae). Carbondale: Southern Illinois University Press.
- Gussekloo SWS, Vosselman MG, Bout RG. 2001. Three-dimensional kinematics of skeletal elements in avian prokinetic and rhynchokinetic skulls determined by roentgen stereophotogrammetry. J Exp Biol. 204(10):1735–1744. doi: 10.1242/jeb.204.10.1735.
- Handley WD, Worthy TH. 2021. Endocranial anatomy of the giant extinct Australian Mihirung birds (Aves, Dromornithidae). Diversity. 13(3):124. doi: 10.3390/d13030124.
- Herrel A, Podos J, Huber SK, Hendry AP. 2005. Bite performance and morphology in a population of Darwin’s finches: implications for the evolution of beak shape. Funct Ecol. 19(1):43–48. doi: 10.1111/j.0269-8463.2005.00923.x.
- Hofer H. 1945. Untersuchungen über den Bau des Vogelschädels, besonders über den der Spechte und Steißhühner [Investigations into the structure of the bird skull, especially that of woodpeckers and grouse]. Zool Jahrb Abt Anat Ontog Tiere. 69:1–158.
- Hofer H. 1949. Die Gaumenlücken der Vögel [The gaps between the palates of birds]. Acta Zool. 30(1–2):209–248. doi: 10.1111/j.1463-6395.1949.tb00507.x.
- Hofer H. 1950. Zur Morphologie der Kiefermuskulatur der Vögel Zoologische Jahrbücher [On the morphology of the jaw muscles of birds]. Abteilung für Anatomie und Ontogenie der Tiere. 70:427–556.
- Holliday CM. 2009. New insights into dinosaur jaw muscle anatomy. Anat Rec Adva Integr Anat Evol Biol Advan Integ. 292(9):1246–1265. doi: 10.1002/ar.20982.
- Holliday CM, Witmer LM. 2007. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 268(6):457–484. doi: 10.1002/jmor.10524.
- Homberger DG. 1986. The lingual apparatus of the African Grey Parrot, Psittacus erithacus Linné (Aves: Psittacidae): Description and theoretical mechanical analysis. Ornithol Monogr. 39(39):iii–233. doi: 10.2307/40166788.
- Homberger DG, Meyers RA. 1989. Morphology of the lingual apparatus of the domestic chicken, Gallus gallus, with special attention to the structure of the fasciae. Am J Anat. 186(3):217–257. doi: 10.1002/aja.1001860302.
- Houde P. 1996. A fossil screamer from the Eocene of Wyoming (Anseriformes: Anhimidae). In: Program and Abstracts, 4th International Meeting of the Society of Avian Palaeontology and Evolution; 4–7 June 1996; Washington, D.C. p. 8.
- Houde P, Dickson M, Camarena D. 2023. Basal Anseriformes from the Early Palaeogene of North America and Europe. Diversity. 15(2):233. doi: 10.3390/d15020233.
- Huxley TH. 1867. On the classification of birds: and on the taxonomic value of the modifications of certain of the cranial bones observable in that class. Proc Zool Soc Lond. 1867:415–471.
- Iordansky NN. 1970. Structure and biomechanical analysis of functions of the jaw muscles in the lizards. Anat Anz. 127(4):383–413.
- Jetz W, Thomas GH, Joy JB, Hartman K, Mooers AO. 2012. The global diversity of birds in space and time. Nature. 491(7424):444–448. doi: 10.1038/nature11631.
- Jollie MT. 1957. The head skeleton of the chicken and remarks on the anatomy of this region in other birds. J Morphol. 100(3):389–436. doi: 10.1002/jmor.1051000302.
- Jones ME, Button DJ, Barrett PM, Porro LB. 2019. Digital dissection of the head of the rock dove (Columba livia) using contrast-enhanced computed tomography. Zool Letter. 5(1):17. doi: 10.1186/s40851-019-0129-z.
- Kimball RT, Oliveros CH, Wang N, White ND, Barker K, Field DJ, Ksepka DT, Chesser RT, Moyle RG, Braun MJ, et al. 2019. A Phylogenomic Supertree of Birds. Diversity. 11(7):109. doi: 10.3390/d11070109.
- King AS. 1993. Apparatus Respiratorius [Systema Respiratorium]. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd Edn. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 257–300.
- Kirikov SV. 1944. Vozrastnyie izmeneniya zhevatelnoy muskulatury i cherepa u glukharey [Age-related changes in the chewing muscles and skull of wood grouse]. Zoologicheskiĭ Zhurnal. 23:16–25. in Russian.
- Ksepka DT. 2009. Broken gears in the avian molecular clock: new phylogenetic analyses support stem galliform status for Gallinuloides wyomingensis and rallid affinities for Amitabha urbsinterdictensis. Cladistics. 25(2):173–197. doi: 10.1111/j.1096-0031.2009.00250.x.
- Kuhl H, Frankl-Vilches C, Bakker A, Mayr G, Nikolaus G, Boerno ST, Klages S, Timmermann B, Gahr M. 2021. An unbiased molecular approach using 3-UTRs resolves the avian family-level tree of life. Mol Biol Evol. 38(1):108–127. doi: 10.1093/molbev/msaa191.
- Lakjer T. 1926. Studien über die Trigeminus-versorgte Kaumuskulatur der Sauropsiden [Studies on the trigeminal-supplied masticatory muscles of sauropods]. Reitzel, CA, Ed. Kopenhagen: Biango Lunos Buchdruckerei 154.
- Lambrecht K. 1933. Handbuch der palaeornithologie [Handbook of Palaeornithology] (1024). Berlin: Verlag von Gebrüder Borntraeger.
- Landolt R, Zweers G. 1985. Anatomy of the muscle-bone apparatus of the cervical system in the mallard (Anas platyrhynchos L.). Neth J Zool. 35(4):611–670. doi: 10.1163/002829685X00226.
- Lautenschlager S, Bright JA, Rayfield EJ. 2014. Digital dissection–using contrast‐enhanced computed tomography scanning to elucidate hard‐and soft‐tissue anatomy in the Common Buzzard Buteo buteo. J Anat. 224(4):412–431. doi: 10.1111/joa.12153.
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol. 50(6):913–925. doi: 10.1080/106351501753462876.
- Li Z, Clarke JA. 2016. The craniolingual morphology of waterfowl (Aves, Anseriformes) and its relationship with feeding mode revealed through contrast-enhanced X-ray computed tomography and 2D morphometrics. Evol Biol. 43(1):12–25. doi: 10.1007/s11692-015-9345-4.
- Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis [The system of nature through the three kingdoms of nature, according to classes, orders, genera, species with characters, differences, synonyms, places]. Stockholm: Tomus I. Editio decima, reformata. Laurentius Salvius; Holmiae.
- Livezey BC. 1997. A phylogenetic analysis of basal Anseriformes, the fossil Presbyornis, and the interordinal relationships of waterfowl. Zool J Linn Soc. 121(4):361–428. doi: 10.1111/j.1096-3642.1997.tb01285.x.
- Livezey BC. 1998. A phylogenetic analysis of the Gruiformes (Aves) based on morphological characters, with an emphasis on the rails (Rallidae). Philos Trans R Soc Lond B Biol Sci. 353(1378):2077–2151. doi: 10.1098/rstb.1998.0353.
- Livezey BC, Zusi RL. 2006. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. Methods and characters. Bull Am Mus Nat Hist. 37:1–544. doi: 10.2992/0145-9058(2006)37[1:PON]2.0.CO;2.
- Louchart A, Bhullar B, Riamon S, Field DJ. 2021. The true identity of putative tooth alveoli in a Cenozoic crown bird, the Gastornithid Omorhamphus. Front Earth Sci. 9:661699. doi: 10.3389/feart.2021.661699.
- Lowe PR. 1926. More notes on the quadrate as a factor in avian classification. Ibis. 68(1):152–188. doi: 10.1111/j.1474-919X.1926.tb07563.x.
- Marugán-Lobón J, Buscalioni ÁD. 2006. Avian skull morphological evolution: exploring exo-and endocranial covariation with two-block partial least squares. Zoology. 109(3):217–230. doi: 10.1016/j.zool.2006.03.005.
- Matsuoka H, Kurosu H, Inglis MP, Kitagawa H, Kusuhashi N, Hasegawa Y. 2008. Myology and osteology of the Whooper Swan Cygnus cygnus (Aves: Anatidae) part 2. Muscles of the jaws, tongue and anteriormost neck. Bull Gm Mus Nat Hist. 12:1–14.
- Matthew WD, Granger W. 1917. The skeleton of Diatryma, a gigantic bird from the Lower Eocene of Wyoming. Bull Am Mus Nat Hist. 37(11):307–354.
- Mayr G. 2008. Phylogenetic affinities and morphology of the late Eocene anseriform bird Romainvillia stehlini Lebedinsky, 1927. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen. 248(3):365–380. doi: 10.1127/0077-7749/2008/0248-0365.
- Mayr G. 2011. Cenozoic mystery birds – on the phylogenetic affinities of bony-toothed birds (Pelagornithidae). Zool Scr. 40(5):448–467. doi: 10.1111/j.1463-6409.2011.00484.x.
- Mayr G. 2017. Avian evolution: the fossil record of birds and its paleobiological significance. United Kingdom: John Wiley & Sons, Inc., West Sussex.
- Mayr G. 2018a. Comparative morphology of the avian maxillary bone (os maxillare) based on an examination of macerated juvenile skeletons. Acta Zool. 101(1):24–38. doi: 10.1111/azo.12268.
- Mayr G. 2018b. A survey of casques, frontal humps, and other extravagant bony cranial protuberances in birds. Zoomorphology. 137(3):457–472. doi: 10.1007/s00435-018-0410-2.
- Mayr G. 2020. The otic region of the skull of neognathous birds: on the homology and comparative morphology of some neurovascular and muscular foramina and other external skeletal structures. Vertebr Zool. 70(1):69–85.
- Mayr G. 2022a. Palaeogene fossil birds. 2nd Edn ed. Cham, Switzerland: Springer.
- Mayr G. 2022b. A survey of the uncinate bone and other poorly known ossicles associated with the lacrimal/ectethmoid complex of the avian skull. Anat Rec. 305(9):2312–2330. doi: 10.1002/ar.24869.
- Mayr G, Carrió V, Kitchener AC. 2023. One the “screamer-like” birds from the British London Clay: An archaic anseriform-galliform mosaic and a non-galloanserine “barb-necked” species of Perplexicervix. Palaeont Electr. 26(2):a33. doi: 10.26879/1301.
- Mayr G, De Pietri VL, Scofield RP, Worthy TH. 2018. On the taxonomic composition and phylogenetic affinities of the recently proposed clade Vegaviidae Agnolín et al., 2017‒neornithine birds from the Upper Cretaceous of the Southern Hemisphere. Cretaceous Res. 86:178–185. doi: 10.1016/j.cretres.2018.02.013.
- Mayr G, Goedert JL, Rabenstein R. 2021. Cranium of an Eocene/Oligocene pheasant-sized galliform bird from western North America, with the description of a vascular autapomorphy. J Ornithol. 163(1):315–326. doi: 10.1007/s10336-021-01935-4.
- Mayr G, Manegold A. 2021. On the comparative morphology of the juvenile avian skull: An assessment of squamosal shape across avian higher‐level taxa. Anat Rec. 304(4):845–859. doi: 10.1002/ar.24504.
- Mayr G, Weidig I. 2004. The Early Eocene bird Gallinuloides wyomingensis—a stem group representative of Galliformes. Acta Palaeontol Pol. 49(2):211–217.
- McInerney PL, Arnold LJ, Burke C, Camens AB, Worthy TH. 2022. Multiple occurrences of pathologies suggesting a common and severe bone infection in a population of the Australian Pleistocene giant, Genyornis newtoni (Aves, Dromornithidae). Pap Palaeontol. 8(1):e1415. doi: 10.1002/spp2.1415.
- McLelland J. 1968. The hyoid muscles of Gallus gallus. Acta Anat. 69(1):81–86. doi: 10.1159/000143065.
- McLelland J. 1993. Apparatus Diggestorius [Systema Alimentarium]. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd Edn. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 189–247.
- Miller GH, Fogle ML, Magee JW, Gagan MK, Clarke SJ, Johnson BJ. 2005. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science Reports. 309(5732):287–290. doi: 10.1126/science.1111288.
- Mivart SG. 1896. A monograph of the lories, or brush-tongued parrots, composing the family Loriidae. London: R. H. Porter.
- Moreno FP, Mercerat A. 1891. Paleontología Argentina I. Catalogo de los pajaros fosiles de la Republica Argentina conservados en el. Museo de La Plata. 1:8–71.
- Mourer-Chauviré C, Balouet J. 2005. Description of the skull of the genus Sylviornis Poplin, 1980 (Aves, Galliformes, Sylviornithidae new family), a giant extinct bird from the Holocene of New Caledonia. In: Alcover JA, Bover P, editors. Proceedings of the International Symposium “Insular Vertebrate Evolution: the Palaeontological Approach”; Sept 16–19; Mallorca. Monografies de la Societat d’Història Natural de les Balears. p. 205–218.
- Mourer-Chauviré C, Bourdon E. 2020. Description of a new species of Gastornis (Aves, Gastornithiformes) from the early Eocene of La Borie, southwestern France. Geobios. 63:39–46.
- Mourer-Chauviré C, Pickford M, Senut B. 2011. The first Palaeogene galliform from Africa. J Ornithol. 152(3):1–13. doi: 10.1007/s10336-010-0630-9.
- Murray PF, Megirian D. 1998. The skull of dromornithid birds: anatomical evidence for their relationships to Anseriformes. Rec South Aust Mus. 31(1):51–97.
- Murray PF, Vickers-Rich P. 2004. Magnificent mihirungs: the colossal flightless birds of the Australian dreamtime. Indiana: Indiana University Press.
- Naranjo LG. 1986. Aspects of the biology of the Horned Screamer in Southwestern Colombia. Wilson Bull. 98(2):243–256.
- Nguyen JM, Boles WE, Hand SJ. 2010. New material of Barawertornis tedfordi, a dromornithid bird from the Oligo-Miocene of Australia, and its phylogenetic implications. Rec Aust Mus. 62(1):45–60. doi: 10.3853/j.0067-1975.62.2010.1539.
- Olsen AM. 2015. Exceptional avian herbivores: multiple transitions toward herbivory in the bird order Anseriformes and its correlation with body mass. Ecol Evol. 5(21):5016–5032. doi: 10.1002/ece3.1787.
- Olsen AM, Gremillet D. 2017. Feeding ecology is the primary driver of beak shape diversification in waterfowl. Funct Ecol. 31(10):1985–1995. doi: 10.1111/1365-2435.12890.
- Olson SL 1985. The fossil record of birds. In: Farner DS, King JR, Parkes KC, editors. Avian biology. Vol. 8, Orlando: Academic Press; p. 79–252.
- Olson SL. 1999. The anseriform relationships of Anatalavis Olson and Paris (Anseranatidae), with a new species from the Lower Eocene London Clay. In: Olson SL, editor. Avian paleontology at the close of the 20th century: Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution; 1996 June 4–7; Washington, D.C.: Smithsonian Contribution to Palaeobiology. 89, p. 231–243.
- Olson SL, Feduccia A. 1980. Presbyornis and the origin of the Anseriformes (Aves: Charadriomorphae). Smithson contrib zool. 323:1–24.
- Ostrom JH. 1961. Cranial morphology of the hadrosaurian dinosaurs of North America. Bull AMNH. 122(2):33–186.
- Owen R. 1872. [Notice of his Nineteenth Memoir on the extinct birds of the genus Dinornis]. Proc Zool Soc Lond. 1872:682–683.
- Owen R. 1873. Description of the skull of a dentigerous bird (Odontopteryx toliapicus) from the London Clay of Sheppey. Qr J Geol Soc. 29(1–2):511–522. doi: 10.1144/GSL.JGS.1873.029.01-02.45.
- Owen R. 1874. On Dinornis (Part XIX.): containing a description of a femur indicative of a new genus of large wingless bird (Dromornis australis, Owen) from a post-tertiary deposit in Queensland, Australia. Trans Zoolog Soc London. 8(6):381–384. doi: 10.1111/j.1096-3642.1873.tb00563.x.
- Pecsics T, Laczi M, Nagy G, Csörgő T. 2017. The cranial morphometrics of the wildfowl (Anatidae). Ornis Hungarica. 25(1):44–57. doi: 10.1515/orhu-2017-0004.
- Poplin F. 1980. Sylviornis neocaledoniae, n. g., n. sp. (Aves), Ratite éteint de la Nouvelle Calédonie [Sylviornis neocaledoniae, n. g., s. sp. (Aves), extinct ratite from New Caledonia]. C R Hebd Séances Acad Sci. 290:691–694. in French.
- Previatto DM. 2012. Osteologia craniana da família Anhimidae (Aves: Anseriformes). Post-Graduate Program in Biological Sciences- Zoology. São Paulo State University.
- Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 526(7574):569–573. doi: 10.1038/nature15697.
- Pycraft WP. 1900. On the morphology and phylogeny of the Palaeognathae (Ratitae and Crypturi) and Neognathae (Carinatae). Trans Zoolog Soc London. 15(5):149–290. doi: 10.1111/j.1096-3642.1900.tb00023.x.
- Pycraft WP. 1902. Contributions to the osteology of birds. Part V. Falconiformes Proc Zool Soc Lond. 1902 1(2):277–320.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 67(5):901–904. doi: 10.1093/sysbio/syy032.
- Rich PV. 1979. The Dromornithidae, an extinct family of large ground birds endemic to Australia. Bull Miner Res Explor Geol Geophy. 184:1–196.
- Rich PV. 1980. The Australian Dromornithidae: A group of extinct large ratites. Contr Sci Los Angeles County Mus. 330:93–103. doi: 10.5962/p.226841.
- Rich PV, van Tets GF. 1984. What fossil birds contribute towards an understanding of origin and development of the Australian avifauna. In: Archer MC, Clayton G, editors. Vertebrate Zoogeography and Evolution in Australasia. Western Australia: Hesperian Press; p. 244–446.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19(12):1572–1574. doi: 10.1093/bioinformatics/btg180.
- Saltré F, Rodríguez-Rey M, Brook BW, Johnson CN, Turney CSM, Alroy J, Cooper A, Beeton N, Bird MI, Fordham DA. 2016. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat Commun. 7(1):1–7. doi: 10.1038/ncomms10511.
- Samejima M, Otsuka J-I. 1987. Observations on the quadrate of birds. Jap J Ornithol. 35(4):129–144. doi: 10.3838/jjo.35.129.
- Shufeldt RW. 1890. The myology of the raven (Corvus corax sinuatus.): a guide to the study of the muscular system in birds. London: Macmillan and Company.
- Sibley CG, Ahlquist JE. 1990. Phylogeny and classification of birds: a study in molecular evolution. Connecticut: Yale University Press.
- Sibley C G, Ahlquist J E, Monroe B L. 1988. A Classification of the Living Birds of the World Based on Dna-Dna Hybridization Studies. The Auk. 105(3):409–423. doi: 10.1093/auk/105.3.409.
- Smith MA. 2009. Genyornis: last of the dromornithids. In: Robin L, Heinsohn R, Joseph L, editors. Boom and bust: bird stories for a dry country. Collingwood, Victoria: CSIRO Publishing; p. 147–183.
- Smith-Paredes D, Bhullar B-AS. 2019. The Skull and Head Muscles of Archosauria. In: Ziermann JM, Diaz RE, Jr, Diogo R, editors. Heads, Jaws, and Muscles. Cham, Switzerland: Springer; p. 229–251.
- Starck D, Barnikol A. 1954. Beiträge zur Morphologie der Trigeminusmuskulatur der Vögel (besonders der Accipitres, Cathartidae, Striges und Anseres) [Contributions to the morphology of the trigeminal muscles of birds (especially Accipiters, Cathartidae, Striges, and Anseres]. Gegenbaurs Morphol Jahrb. 94:1–64. in German.
- Stein RW, Brown JW, Mooers AØ. 2015. A molecular genetic time scale demonstrates Cretaceous origins and multiple diversification rate shifts within the order Galliformes (Aves). Mol Phylogenet Evol. 92:155–164. doi: 10.1016/j.ympev.2015.06.005.
- Stejneger L. 1885. [Most articles on the individual avian families, although they are not separately signed]. In: King JS, editor. The Standard Natural History, Birds. Vol. 4. Boston: SE Cassino VII–558.
- Stellbogen E. 1930. Über das äussere und mittlere Ohr des Waldkauzes (Syrnium aluco L.) [About the outer and middle ear of the tawny owl (Syrnium aluso L.)]. Z Morphol Ökol Tiere. 19(4):686–731. in German. doi:10.1007/BF00406206.
- Stidham TA. 1998. A lower jaw from a Cretaceous parrot. Nature. 396(6706):29–30. doi: 10.1038/23841.
- Stidham TA. 2001. The origin and ecological diversification of modern birds: Evidence from the extinct wading ducks, Presbyornithidae (Neornithes: Anseriformes) [ Ph.D. thesis]. Berkeley (unpubl): University of California.
- Stirling EC. 1894. The recent discovery of fossil remains at Lake Callabonna, South Australia Part I. Nature. 50(1286):184–188.
- Stirling EC. 1913. Description of some further remains of Genyornis newtoni Stirling and Zietz. Mem Roy Soc South Australia. 1:111–126.
- Stirling EC, Zietz AHC. 1896. Preliminary notes on Genyornis newtoni- A fossil 5405 struthious bird from Lake Callabonna, South Australia: description of the bones of the leg and foot. Transactions and proceedings and report of the Royal Society of South Australia. 20 171–211.
- Stirling EC, Zietz AHC. 1900. Fossil remains of Lake Callabonna. Part II. I. Genyornis newtoni. A new genus and species of fossil struthious bird. Mem Roy Soc South Australia. 1:41–80.
- Stirling EC, Zietz AHC. 1905. Fossil remains of Lake Callabonna. Part III. Description of the vertebrae of Genyornis newtoni. Mem Roy Soc, South Australia. 1(3):81–110.
- Sun Z, Pan T, Hu C, Sun L, Ding H, Wang H, Zhang C, Jin H, Chang Q, Kan X, et al. 2017. Rapid and recent diversification patterns in Anseriformes birds: Inferred from molecular phylogeny and diversification analyses. PloS ONE. 12(9):e0184529. doi:10.1371/journal.pone.0184529.
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (* and other methods) [Computer software]. Version 4. Sunderland, Massachusetts: Sinauer Associates, Sinauer Associates. http://phylosolutions.com.
- Tambussi CP, Degrange FJ, De Mendoza RS, Sferco E, Santillana S. 2019. A stem anseriform from the early Palaeocene of Antarctica provides new key evidence in the early evolution of waterfowl. Zool J Linn Soc. 186(3):673–700. doi: 10.1093/zoolinnean/zly085.
- Tokita M. 2003. The skull development of parrots with special reference to the emergence of a morphologically unique cranio-facial hinge. Zoolog Sci. 20(6):749–758. doi: 10.2108/zsj.20.749.
- Troxell EL. 1931. Diatryma, a colossal heron. Am J Sci. 5-22(127):18–34. doi: 10.2475/ajs.s5-22.127.18.
- Vanden Berge JC, Zweers GA. 1993. Myologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: nomina Anatomica Avium. 2nd Edn. Cambridge, Massachusetts: Publications of the Nuttall Ornithological Club; p. 189–247.
- van Gennip EMSJ. 1986. The osteology, arthrology and myology of the jaw apparatus of the Pigeon (Columba livia L.). Neth J Zool. 36(1):1–46. doi: 10.1163/002829685X00398.
- van Tuinen M, Dyke GJ. 2004. Calibration of galliform molecular clocks using multiple fossils and genetic partitions. Mol Phylogenet Evol. 30(1):74–86. doi: 10.1016/S1055-7903(03)00164-7.
- van Tuinen M, Stidham TA, Hadly EA. 2006. Tempo and mode of modern bird evolution observed with large-scale taxonomic sampling. Hist Biol. 18(2):209–225. doi: 10.1080/08912960600641174.
- Vickers-Rich P. 1991. The Mesozoic and Tertiary history of birds on the Australian Plate. In: Vickers-Rich P, Monaghan JM, Baird RF, Rich TH, editors. Vertebrate palaeontology of Australasia. Melbourne: Pioneer Design Studio and Monash University Publications Committee; p. 721–808.
- Vickers-Rich P, Molnar RE. 1996. The foot of a bird from the Eocene Redbanks Plains Formation of Queensland, Australia. Alcheringa. 20(1):21–29. doi: 10.1080/03115519608619220.
- Wang N, Kimball RT, Braun EL, Liang B, Zhang Z, Janke A. 2013. Assessing phylogenetic relationships among Galliformes: A multigene phylogeny with expanded taxon sampling in Phasianidae. PloS ONE. 8(5):e64312. doi: 10.1371/journal.pone.0064312.
- Weber E. 1993. Zur Evolution basicranialer Gelenke bei Vögeln, insbesondere bei Hühner‐und Entenvögeln (Galloanseres) [On the evolution of basicranial joints in birds, especially in chickens and ducks (Galloanseres)]. Zeitschrift für Zoologische Systematik und Evolutionsforschung. 31(4):300–317. in German. doi:10.1111/j.1439-0469.1993.tb00198.x.
- Weber E. 1996. Das skelet-muskel-system des kieferapparates von Aepypodius arfakianus (Salvidori, 1877) (Aves, Megapodiidae) [The skeletal-musculature system of the jaw apparatus of Aepypodius arfakianus (Salvidori, 1877) (Aves, Megapodiidae)]. Courier Forschungsinstitut Senckenberg. 189:1–132. in German.
- Weber E, Hesse A. 1995. The systematic position of Aptornis, a flightless bird from New Zealand. Courier Forschungsinstitut Senckenberg. 181:293–301.
- Weins JJ. 2003. Missing data, incomplete taxa, and phylogenetic accuracy. Syst Biol. 52(4):528–538. doi: 10.1080/10635150390218330.
- Weins JJ. 2006. Missing data and the design of phylogenetic analyses. J Biomed Inform. 39(1):34–42. doi: 10.1016/j.jbi.2005.04.001.
- Wells RT, Tedford RH. 1995. Sthenurus (Macropodidae, Marsupialia) from the Pleistocene of Lake Callabonna, South Australia. Bull Am Mus Nat Hist. 225:1–112.
- Wetmore A. 1926. Fossil birds from the Green River deposits of eastern Utah. Ann Carnegie Mus. 16(3):391–402. doi: 10.5962/p.231090.
- Witmer LM. 1990. The craniofacial air sac system of Mesozoic birds (Aves). Zool J Linn Soc. 100(4):327–378. doi: 10.1111/j.1096-3642.1990.tb01865.x.
- Witmer LM, Rose KD. 1991. Biomechanics of the jaw apparatus of the gigantic Eocene bird Diatryma: implications for diet and mode of life. Paleobiology. 17(2):95–120. doi: 10.1017/S0094837300010435.
- Worthy TH. 2000. The fossil megapodes (Aves: Megapodiidae) of Fiji with descriptions of a new genus and two new species. J Roy Soc New Zeal. 30(4):337–364. doi: 10.1080/03014223.2000.9517627.
- Worthy TH. 2009. Descriptions and phylogenetic relationships of two new genera and four new species of Oligo-Miocene waterfowl (Aves: Anatidae) from Australia. Zool J Linn Soc. 156(2):411–454. doi: 10.1111/j.1096-3642.2008.00483.x.
- Worthy TH, Degrange FJ, Handley WD, Lee MSY. 2017a. Correction to ‘The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres)’. R Soc Open Sci. 4(11):171621. doi: 10.1098/rsos.171621.
- Worthy TH, Degrange FJ, Handley WD, Lee MSY. 2017b. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). R Soc Open Sci. 4(10):170975. doi: 10.1098/rsos.170975.
- Worthy T H, De Pietri V L, Scofield R Paul and Hand S J. (2023). A new Eocene species of presbyornithid (Aves, Anseriformes) from Murgon, Australia. Alcheringa: An Australasian Journal of Palaeontology, 47(4), 416–430. 10.1080/03115518.2023.2184491
- Worthy TH, Handley WD, Archer M, Hand SJ. 2016a. The extinct flightless mihirungs (Aves, Dromornithidae): cranial anatomy, a new species, and assessment of Oligo-Miocene lineage diversity. J Vertebr Paleontol. 36(3):e1031345. doi: 10.1080/02724634.2015.1031345.
- Worthy TH, Holdaway RN, Sorenson MD, Cooper AC. 1997. Description of the first complete skeleton of the extinct New Zealand goose Cnemiornis calcitrans (Aves: Anatidae), and a reassessment of the relationships of Cnemiornis. J Zool. 243(4):695–718. doi: 10.1111/j.1469-7998.1997.tb01971.x.
- Worthy TH, Mitri M, Handley WD, Lee MSY, Anderson A. 2016b. Osteology supports a stem-galliform affinity for the giant extinct flightless bird Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PloS ONE. 11(3):e0150871. doi: 10.1371/journal.pone.0150871.
- Worthy TH, Scanlon JD. 2009. An Oligo-Miocene magpie goose (Aves: Anseranatidae) from Riversleigh, northwestern Queensland, Australia. J Vertebr Paleontol. 29(1):205–211. doi: 10.1671/039.029.0103.
- Worthy TH, Scofield RP. 2012. Twenty-first century advances in knowledge of the biology of moa (Aves: Dinornithiformes): a new morphological analysis and moa diagnoses revised. N Z J Zool. 39(2):87–153. doi: 10.1080/03014223.2012.665060.
- Worthy TH, Scofield RP, Salisbury SW, Hand SJ, De Pietri VL, Blokland JC, Archer M. 2022. A new species of Manuherikia (Aves: Anatidae) provides evidence of faunal turnover in the St Bathans Fauna, New Zealand. Geobios. 70:87–107. doi: 10.1016/j.geobios.2021.08.002.
- Worthy TH, Yates AM. 2015. Connecting the thigh and foot: resolving the association of post-cranial elements in the species of Ilbandornis (Aves: Dromornithidae). Alcheringa An Australas J Palaeontol. 39(3):407–427. doi: 10.1080/03115518.2015.1015818.
- Worthy TH, Yates AM. 2017. A review of the smaller birds from the late Miocene Alcoota local faunas of Australia with a description of a new anatid species. Contribuciones del MACN. 7:221–252.
- Zelenkov NV. 2011. Morphological homoplasies in cladistic studies of phylogeny, with examples from birds. Biol Bull. 38(9):905–911. doi: 10.1134/S106235901109010X.
- Zelenkov NV. 2017. Bylo li fil’tratsionnoye pitaniye u drevneyshikh guseobraznykh Presbyornithidae? [Were Presbyornithidae, the oldest Anseriformes, filter-feeders?]. In: Popovkina AB, Potapova EG, Kryukova NV, editors. Evolyutsionnaya i funktsional’naya morfologiya pozvonochnykh: materialy Vserossiyskoy konferentsii i shkoly dlya molodykh uchenykh pamyati Feliksa Yanovicha Dzerzhinskogo, Zvenigorod Biological Station, Moscow State University, Moscow, 28 September–2 October 2017. Moscow: KMK Scientific Press Ltd; p. 115–123. in Russian.
- Zelenkov NV. 2018. The earliest asian duck (Anseriformes: Romainvillia) and the origin of Anatidae. Doklady Biol Sci. 483(2):225–227. doi: 10.1134/S0012496618060030.
- Zelenkov NV, Stidham TA. 2018. Possible filter-feeding in the extinct Presbyornis and the evolution of Anseriformes (Aves). Zoologicheskiĭ Zhurnal. 97(8):943–956. doi: 10.1134/S0044513418080159.
- Zusi RL. 1967. The role of the depressor mandibulae muscle in kinesis of the avian skull. Proc US Natl Mus. 123(3607):1–28. doi: 10.5479/si.00963801.123-3607.1.
- Zusi RL. 1984. A functional and evolutionary analysis of rhynchokinesis in birds. Smithson contrib zool. 395():1–40. doi: 10.5479/si.00810282.395.
- Zusi RL. 1993. Patterns of diversity in the avian skull. In: Hanken J, Hall B, editors. The Skull, Volume 2 - Patterns of Structural and Systematic Diversity. Chicago: University of Chicago Press; p. 391–437.
- Zusi RL, Livezey BC. 2000. Homology and phylogenetic implications of some enigmatic cranial features in galliform and anseriform birds. Ann Carnegie Mus. 69(3):157–193. doi: 10.5962/p.330539.
- Zusi RL, Livezey BC. 2006. Variation in the os palatinum and its structural relation to the palatum osseum of birds (Aves). Ann Carnegie Mus. 75(3):137–180. doi: 10.2992/0097-4463(2006)75[137:VITOPA]2.0.CO;2.
- Zusi RL, Storer RW. 1969. Osteology and myology of the head and neck of the Pied-billed Grebes (Podilymbus). Vol. 39. Michigan: Miscellaneous Publications Museum of Zoology, University of Michigan; p. 1–49.
- Zweers GA. 1974. Structure, movement, and myography of the feeding apparatus of the mallard (Anas platyrhynchos L.) A study in functional anatomy. Neth J Zool. 24(4):323–467. doi: 10.1163/002829674X00192.
- Zweers GA, Berkhoudt H, Vanden Berge JC. 1994. Behavioural mechanisms of avian feeding. In: Bels VL, Chardon M, Vandewalle P, editors. Biomechanics of Feeding in Vertebrates. Berlin, Heidelberg: Springer; p. 241–279.
- Zweers GA, Gerritsen AFC, Van Kranenburg-Voogd JP. 1977. Mechanics of the feeding of the Mallard (Anas platyrhynchos, L.; Aves, Anseriformes): the lingual apparatus and the suction-pressure pump mechanism of straining. In: Hecht MK, Szalay FS, editors. Contributions to Vertebrate Evolution. Vol. 3. Basel: Karger.
- Zweers GA, Vanden Berge JC, Koppendraier R. 1987. Avian cranio-cervical systems. Part I: Anatomy of the cervical column in the chicken (Gallus gallus L.). Acta Morphol Neerl Scand. 25(3):131–155.
Appendix 1: Additional figures of original and new fossils assigned to Genyornis newtoni
Figure A1. SAMA P 10838, Stirling (Citation1913) original skull of Genyornis newtoni as it is currently: A. lateral view of the skull; B. dorsal view of the skull, showing the block in which the skull has been set. Annotations: ap.zyg.oss., aponeurosis zygomatica ossificans; cond.oc, condylus occipitalis; cr.or., crista orbitalis; pr.post., processus postorbitalis. Image a credit: Mahala Fergusen. Scale bar: 2 cm.

Figure A2. Laterally compressed rostrum, specimen SAMA P59517, transected by a fault in the clay matrix which has offset the caudal part from that more rostral: A. right lateral; B. left lateral; C. dorsal; D. ventral. Annotations: ang.cl., angulus caudolateralis; ca., casque; cr.tom., crista tomialis; cul., culmen; margo.lat., margo lateralis; nas., apertura nasi ossea; pala., os palatinum. Scale bars: 20 mm.

Figure A3. Additional skull specimens of Genyornis newtoni: A. NMV P256893, partial dorsal calvaria in dorsal and ventral views; B. SAMA P53830 jugal arch, right side, medial, lateral, and dorsal views; C. SAMA P53830, right quadrate; D. SAMA P53830 partial condylus occipitalis and occipital region of the cranium; E. SAMA P59520, ramus mandibulae, pars symphysialis, right side, dorsal and ventral views; F. SAMA P59520, partial left lateral cranium in medial (left) and lateral (right) views. Annotations: an.tymp., annulus tympanicus; c. quad, condylus quadratica; cap.s-o., capitulum squamoso-otica; cond.oc., condylus occipitalis; cr.med., crista medialis; cra., cranium; d.rost., depressio rostromedialis; m.a.e., osseous meatus acusticus externus; mand., mandible; quad., quadrate. Scale bars: 10 mm.
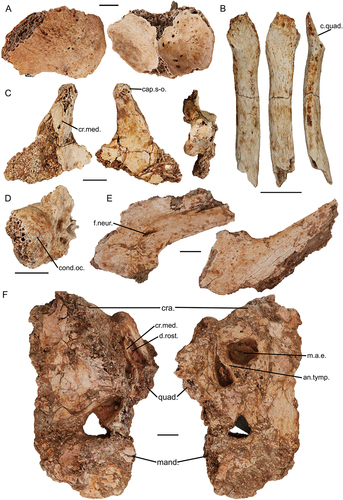
Figure A4. Additional specimens of Genyornis newtoni: A. NMV P256893 partial left skull medial view; B. – G. SAMA P59517, fragmentary mandibular rami, B. right ramus mandibulae, pars caudalis and pars intermedia in lateral view; C. and medial view; D. left ramus mandibulae, pars caudalis et intermedius, lateral view; E. and medial view; F. fragmentary right ramus mandibulae, pars caudalis, dorsal view; G. and rostral view. Abbreviations: c.med., cotyla medialis mandibulae; cer., ceratobranchial; d.rost., depressio rostromedialis; imp.cor., impressio m. AME profundus, pars coronoideus; m.pt.dor., insertion area of m. pterygoideus dorsalis; p.lat., processus lateralis mandibulae; p.med., processus medialis mandibulae; t.prae., tuberculum praearticulare. Scale bar: 10 mm.

Appendix 2: Measurements of skull elements assigned to Genyornis newtoni
Table A1. Genyornis newtoni skull measurements. Rounded to the first decimal point.
Appendix 3: Homological correlations for the terminology associated with the adductor muscles of the mandible across relevant literature
Table A2. (Part 1) Homological correlations for the terminology associated with musculus adductor mandibulae across examples of relevant literature.
References
Baumel JJ, Witmer LM. 1993. Osteologia. In: Baumel JJ King AS, Breazile JE, Evans HE, Vanden Berge JC (Eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edn (pp. 45–132). Publications of the Nuttall Ornithological Club, Cambridge, Massachusetts.
Davids JAG. 1952a. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.), I. Proceedings of The Koninklijke Nederlandse Akademie Van Wetenschappen. Series C. Biological and Medical Sciences. 55:81–94.
Davids JAG. 1952b. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.). II. Proceedings Of The Koninklijke Nederlandse Akademie Van Wetenschappen. Series C. Biological and Medical Sciences. 55:525–533.
Davids JAG. 1952c. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.). III. Proceedings Of The Koninklijke Nederlandse Akademie Van Wetenschappen. Series C. Biological and Medical Sciences. 55:533–540.
Dzerzhinsky FYa. 1982. Adaptivnʹіe čerty v stroenii čelyustnogo apparata nekotoryh guseobraznyh ptic i veroyatnye puti èvolyucii otrjada [Adaptive features in the construction of the jaw apparatus of some Anseriformes and probable evolutionary paths of the order]. Zoologichesky Zhurnal. 56:1030–1041.
Dzerzhinsky FYa, Belokurova IN. 1972. Sravnitelnoy anatomii chelustnoy muskul tury ptits. Chelustnyye myshtsy glucharya (Tetrao urogallus) [Comparative anatomy of the jaw muscles of birds. Jaw muscles of capercaillie (Tetrao urogallus)]. Zoologicheskiĭ Zhurnal. 51:555–564.
Dzerzhinsky FYa, Grintsevichene TI. 2002. O vozmozhnykh filogeneticheskikh svyazyakh guseobraznykh ptits [On possible phylogenetic relationships of Anseriformes]. Kazarka. 8:19–39.
Dzerzhinsky FYa, Potapova EG. 1974. Sistema sukhozhil’nykh obrazovaniy kak ob’yekt sravnitel’noy miologii chelyustnogo apparata ptits [The system of tendon formations as an object of comparative theology of the jaw apparatus of birds]. Zoologicheskiĭ Zhurnal. 53:1341–1351.
Fujioka T. 1963. Niwatori no hikaku kaibōgakuteki narabini kyokusho kaibōgakuteki kenkyū. IV. Niwatori no atama keibu ni okeru suji no kishi oyobi teishi ni tsuite sono. 1. Tōbu no suji [Comparative and topographical anatomy of the fowl. IV. Origins and insertions of muscles of the head and neck in the fowl. 1. Muscles of the head]. Nihon juigaku zasshi. The Japanese journal of veterinary science. 25(4):207–226.
Goodman DC, Fisher HI. 1962. Functional anatomy of the feeding apparatus in waterfowl (Aves: Anatidae). Southern Illinois University Press, Carbondale.
Hofer H. 1950. Zur Morphologie der Kiefermuskulatur der Vögel Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere. 70:427–556.
Holliday CM, Witmer LM. 2007. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. Journal of Morphology. 268(6):457–484.
Lakjer T. 1926. Studien über die Trigeminus-versorgte Kaumuskulatur der Sauropsiden. Reitzel, C.A. (Ed.), Buchhandlung, Kopenhagen. 154.
Matsuoka H, Kurosu H, Inglis MP, Kitagawa H, Kusuhashi N, Hasegawa Y. 2008. Myology and osteology of the Whooper Swan Cygnus cygnus (Aves: Anatidae) part 2. Muscles of the jaws, tongue and anteriormost neck. Bulletin of the Gunma Museum of Natural History. 12:1–14.
Vanden Berge JC, Zweers GA. 1993. Myologia. In: Baumel, J.J. King, A.S., Breazile, J.E., Evans, H.E., and Vanden Berge, J.C. (Eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edn. Publications of the Nuttall Ornithological Club, Cambridge, Massachusetts, pp. 189–247.
Weber E. 1996. Das skelet-muskel-system des kieferapparates von Aepypodius arfakianus (Salvidori, 1877) (Aves, Megapodiidae). Courier Forschungsinstitut Senckenberg. 189:1–132.
Zusi RL, Livezey BC. 2000. Homology and phylogenetic implications of some enigmatic cranial features in galliform and anseriform birds. Annals of Carnegie Museum. 69(3):157–193.
Zusi RL, Storer RW. 1969. Osteology and myology of the head and neck of the Pied-billed Grebes (Podilymbus). Miscellaneous Publications Museum of Zoology, University of Michigan. 39:1–49.
Zweers GA. 1974. Structure, movement, and myography of the feeding apparatus of the mallard (Anas platyrhynchos L.) A study in functional anatomy. Netherlands Journal of Zoology. 24(4):323–467.
Appendix 4: Morphological character list
Characters for the phylogenetic analysis. Original character list derived from Worthy et al. (Citation2017). Reassessment, modifications, and additions made to characters, states and coding based off personal observations and assessment of other matrices from Andors (Citation1988), Bourdon (Citation2005; 2011), Clarke (Citation2002), Cracraft and Clarke (Citation2001), Dyke et al. (2003), Elzanowski and Stidham (Citation2010: char. 1), Ericson (Citation1997), Field et al. (Citation2020), Ksepka (Citation2009), Livezey (Citation1986; 1996; 1997), Livezey and Zusi (Citation2006), Mayr and Clarke (Citation2003), Murray and Vickers-Rich (Citation2004), Stidham (Citation2001), Worthy and Lee (Citation2008), Worthy and Scofield (Citation2012), Worthy et al. (Citation2017), Zusi and Livezey (Citation2000).
Note: Here we use the name Nettapterornis oxfordi for the taxon previously called Anatalavis oxfordi following recommendations in Houde et al. (Citation2023).
Note: As we, and others (e.g., Worthy et al. Citation2016), are unable to assign the quadrates attributed to the genus Ilbandornis to species level, and since character state coding across all Ilbandornis sp. quadrates observed does not vary, we have opted to code the quadrate for both species identically. We treat the mandible similarly.
Note: We do not code the retroarticular process for Asteriornis maastrichtensis, following changes to specimen interpretation as reported on at the 10th International Meeting of the Society of Avian Palaeontology and Evolution by Crane (2023).
Ordered Characters:
1, 4, 7, 10, 12, 13, 14, 15, 16, 17, 18, 21, 24, 26, 30, 31, 33, 34, 35, 36, 37, 38, 41, 42, 44, 45, 48, 50, 52, 54, 55, 57, 58, 60, 61, 67, 69, 70, 73, 74, 76, 77, 79, 81, 85, 87, 88, 90, 92, 95, 98.
The above characters are ordered in recognition that the states in these characters represent a morphocline where the transition between relative extremes (i.e., states 0 to 2 or greater number) must pass through an intermediate state (or states). Assuming an unordered system of character state changes for such characters would not be accurate to the morphology observed and would often be biologically implausible.
Complete skull
Skull, lateral and dorsal perspectives, rostrocaudal proportions, rostrocaudal length of the rostrum (as measured between the rostral apex of the rostrum and the craniofacial hinge, in line with the rostral-most extension of the os frontale) relative to the rostrocaudal length of the cranium (as measured between the craniofacial hinge, in line with the rostral-most extension of the os frontale, to the most caudal point on the occipital region of the cranium): 0. Rostrum rostrocaudally shorter than the cranium; 1. Rostrum near equal to or up to 1.5 times the rostrocaudal length of cranium; 2. Rostrum is between 1.5 to 2 times the rostrocaudal length of cranium; 3. Rostrum is greater than 2 times rostrocaudal length of the cranium. Modified from Worthy et al. (Citation2017: char. 1) and Field et al. (Citation2020: char. 1). Ordered.
Skull, craniorostral hinge, formation, and hinge type: 0. Flexion zone absent or indistinct, no transverse sulcus, limited flexion; 1. Well-developed flexion zone present and distinct; 2. A synovial joint, essentially a hinge. Modified from Worthy et al. (Citation2017: char. 8), see also Mayr & Clarke (Citation2003: char. 5).
Skull, presence of an osseous casque or other elaborate osseous structure: 0. Absent; 1. Present on rostrum; 2. Present on cranium.
Cranium
(4)Cranium, lateral and dorsal perspectives, frontals, rostral truncation or rostrocaudal shortening, location of the craniorostral hinge (rostral termination of the frontal bones) in relation to the orbital area: 0. Frontals are extended rostrally, craniorostral hinge is far rostral of the orbits; 1. Craniorostral hinge intersects the most rostral point of the orbits; 2. Frontal bones are rostrally truncated, craniorostral hinge transects the dorsal-most point, at the supraorbital area of the orbits; 3. Frontal bones are substantially rostrally truncated, craniorostral hinge transects the orbits in near rostrocaudal alignment with, or caudal of, the processus postorbitalis. Ordered.
(5)Cranium, lateral and rostral perspectives, caudal orbit (and/or lateral cranium), area muscularis aspera, origin of the m. pseudotemporalis superficialis and presence of fossa pseudotemporalis, status and form: 0. Not distinct, area muscularis aspera is nearly flat; 1. Distinct, a shallow depression demarked dorsally by a ridge; 2. Distinct, fossa pseudotemporalis present as a deep fossa within a larger depression; 3. Homologous origin of the m. pseudotemporalis superficialis is not restricted to caudal orbit, present on lateral cranium. State 3 is typical of Palaeognathae (see Dzerzhinsky, Citation1983; Elzanowski, Citation1987).
(6)Cranium, lateral and rostral perspectives, caudal orbit (and/or lateral cranium), area muscularis aspera, tuberculum for origin of m. pseudotemporalis (superficialis): 0. Absent; 1. Present and projected; 2. Low osseous crest; 3. Lowly-raised rugose region. Modified from Dyke et al. (2003: char. 4). Note: Although Picasso et al. (2023) identified this crest as the origin of the m. pseudotemporalis superficialis in the Rhea, for tinamous, Elzanowski (Citation1987) noted that the tubercle is the origin of the ligamentum orbitoquadratum to which the m. pseudotemporalis is attached. As in either case, the crest is associated with the m. pseudotemporalis, we opt to treat the crest as the same across Palaeognathae and code its presence in palaeognaths accordingly.
(7)Cranium, lateral perspective, postorbital temporal region, processus postorbitalis, orientation: 0. Projects ventrally; 1. Directed rostroventrally; 2. Projected strongly rostrally. Modified from Worthy et al. (Citation2017: char. 46). Also see Bourdon (Citation2011, char. 57), Ksepka (Citation2009, char. 11), Zusi & Livezey (Citation2000: p. 175). Ordered.
(8)Cranium, lateral perspective, temporal region, relationship between areas of origin of m. AME profundus and m. AME superficialis, associated aponeuroses and their respective osteological structures (e.g., crista zygomatica, crista m. AME superficialis): 0. Origin area for m. AME profundus and associated structures are wholly dorsal or rostrodorsal of those corresponding to m. AME superficialis; 1. Origin area for m. AME profundus and associated structures are rostral or slightly rostroventral of those of m. AME superficialis; 2. Origin area for m. AME profundus and associated structures are clearly rostroventral of those of m. AME superficialis, where the latter extends substantially dorsal of the former; 3. Not comparable, the deep m. AME is not well differentiated or clearly subdivided with respect rostral (m. AME profundus, pars coronoideus) and caudal parts (m. AME superficialis). Note: In Palaeognathae, the combined regions of origin associated with the m. AME occupies areas of processus postorbitalis, processus zygomaticus (including crista temporalis), processus orbitalis of the quadrate, directly or via related membranes, ligaments or fascia (see Dzerzhinsky, Citation1983); Elzanowski, Citation1987). Interpretation of states for neoavians follows the array of literature available for various taxa (e.g., Dzerzhinsky & Ladygin, 2004; Dzerzhinsky & Potapova, Citation1974; Dzerzhinsky & Yudin, 1974; Fisher & Goodman, Citation1955; Korzun, 1998; Kuular, 2002; Sokolov, Citation1995).
(9)Cranium, lateral perspective, temporal region, processus zygomaticus, status: 0. Absent, osseous projection in area (if any) is homologous with aponeurosis zygomatica ossificans; 1. Present. See Zusi & Livezey (Citation2000) for details regarding this subject. Sylviornis neocaledoniae is coded as present following Mourer-Chauviré & Balouet (Citation2005). Despite the lack of juvenile skull material preserving this region for Gastornis giganteus, a processus zygomatica is also coded as present considering the similarities in the morphology of the lateral cranium to Galliformes (e.g., secondary temporal fenestra formation) which is inconsistent with the absence of this feature (see Zusi & Livezey, Citation2000).
(10)Cranium, lateral and ventral perspectives, temporal region, aponeurosis zygomatica ossificans, status and rostral development: 0. Absent or minor ossification; 1. Variably ossified and rostrally developed; 2. Extensively ossified, rostrally developed to intersect or exceed the processus postorbitalis. Modified from Worthy et al. (Citation2017: char. 18), see also Bourdon (Citation2011, char. 88), Dyke et al. (2003: char. 19), Ksepka (Citation2009, char. 12 and 13), Livezey (Citation1997: char. 8), Murray & Vickers-Rich (Citation2004: char. 8, table 8), Zusi & Livezey (Citation2000: p. 160, 175-177). Note: Numididae are coded as 0 as there is no ossification of the aponeurosis present, see Hofer (Citation1950) and Zusi & Livezey (Citation2000: p. 160). Ordered.
(11)Cranium, lateral perspective, processus postorbitalis and aponeurosis zygomatica ossificans, formation of a secondary temporal fenestra, status: 0. Absent; 1. Present. See Zusi & Livezey (Citation2000) and Elzanowski & Mayr (Citation2017).
(12)Cranium, lateral and ventral perspectives, temporal region, processus postorbitalis and/or aponeurosis zygomatica ossificans, location of the impression for the origin of m. AME profundus, pars coronoideus: 0. M. AME profundus, pars coronoideus does not form a distinct belly of the m. AME, associated fibres and interrelated parts of the m. AME originate on the lateral cranium; 1. Located laterally on the cranium, in a clear impression between the processus postorbitalis and processus zygomatica and/or aponeurosis zygomatica ossificans, may additionally originate ventrolaterally on the medial aspect of the aponeurosis zygomatica ossificans, if present; 2. Restricted to a medioventral location on the lateral cranium, lacking the significant dorsocaudal expansion onto the lateral side of the cranium; 3. Originates ventrally or medioventrally on the aponeurosis zygomatica ossificans, mediolaterally narrow; 4. Located medioventrally, medially expansive but confined laterally; 5. Located medioventrally, expansive both medially and laterally, often slightly visible from lateral perspective. Associated with Bourdon (Citation2005: char. 27, 44; 2011: char. 59), Ericson (Citation1997: char. 4), and Worthy et al. (Citation2017: char. 19). Note: see Hofer (Citation1950), Fujioka (Citation1963), Dzerzhinsky & Belokurova (Citation1972), Dzerzhinsky (Citation1983), Elzanowski (Citation1987), Weber (Citation1996), and Zusi & Livezey (Citation2000). Ordered – specific ordering of states is imposed following observations of evolutionary transitions (i.e., medial movement of the m. AME profundus, pars coronoideus, among galliforms and anseriforms) by Zusi and Livezey (Citation2000) as also discussed in the main text. An unordered model is additionally not evolutionarily plausible (e.g., state 4 to state 0 in one step).
(13)Cranium, lateral and ventral perspectives, otic region, osseus meatus acusticus externus, annulus tympanicus bridges the ala parasphenoidalis and processus suprameaticus to form a complete ring, enclosing the osseus meatus acusticus externus ventrorostrally, status: 0. Absent, no tympanic enclosure; 1. Partial or complete tympanic enclosure, thin and fragile; 2. Complete closure, robust and obvious. Modified from Livezey (Citation1997: char. 4) and Worthy et al. (Citation2017: char. 16), see also Bourdon (Citation2005: char. 28), Livezey and Zusi (Citation2006: char. 0019), and Worthy and Lee (Citation2008: char 17). Ordered.
(14)Cranium, lateral and ventral perspectives, otic region, processus suprameaticus, osseus meatus acusticus externus, cotyla quadratica otici (or otic part of the cotyla quadratica squamoso-otica), location relative to the more laterally positioned processus suprameaticus: 0. Cotyla is located caudal or caudoventral of the processus suprameaticus, the rostral bounds of osseus meatus acusticus externus are caudally located; 1. Cotyla is rostrocaudally aligned with the processus suprameaticus; 2. Cotyla is rostral of processus suprameaticus, the rostral bounds of osseus meatus acusticus externus are rostrally located. See Mayr (Citation2020: p. 80). Ordered.
(15)Cranium, ventral perspective, otic region, separation of the cotyla quadratica otici and cotyla quadratica squamosi, development: 0. Cotylae widely separated; 1. Cotylae are close together medially, width of separation increased laterocaudally; 2. Cotylae close together for entire border, narrowly separated; 3. Two cotyla adjacent, separated only by a thin ridge; 4. Cotylae form a single articular surface (cotylae quadratica-squamosi-otica); Modified from Bourdon (Citation2005, char. 50; 2011, char. 72), Livezey and Zusi (Citation2006: char. 0150). Ordered.
(16)Cranium, lateral perspective, os exoccipitale, processus paroccipitalis, length of ventral extension from the cranium and placement relative to the os parasphenoidale, lamina basisphenoidalis: 0. Processus paroccipitalis is near absent or short, terminates dorsad of, or in dorsoventral alignment with, the lamina basisphenoidalis; 1. Processus paroccipitalis is short, terminates just ventral of the lamina parasphenoidalis; 2. Processus paroccipitalis has minor elongation, terminates noticeably ventral of lamina parasphenoidalis; 3. Processus paroccipitalis is conspicuously elongate, extends considerably further ventrally past the lamina parasphenoidalis. Modified from Bourdon (Citation2005: char. 43, 108; 2011, char. 56), Livezey (Citation1997: char. 3), and Worthy et al. (Citation2017: char. 45). Ordered.
(17)Cranium, lateral, caudal and ventral perspectives, os exoccipitale, processus paroccipitalis, position of the connection of the ala parasphenoidalis to the processus paroccipitalis: 0. Connects to the ventral-most processus paroccipitalis; 1. Connects just dorsal to the ventral apex of processus paroccipitalis; 2. The ventral apex of processus paroccipitalis extends considerably ventral of its connection with ala parasphenoidalis. Ordered.
(18)Cranium, lateral and caudal perspectives, caudal cranium, crista nuchalis transversa and areas associated with insertions of m. complexus, m. biventer cervicis, and m. splenius capitis, form: 0. Crista nuchalis transversa is visible yet relatively flat and confluent with curvature of cranium; 1. Crista nuchalis transversa is a partially distinct ridge, accentuated caudally by paired depressions of muscle insertions in dorsal area; 2. Crista nuchalis transversa is a distinct ridge or flange for its complete dorsoventral length due to expanded and ventrally undercutting depressions of muscle insertions along caudal margins. Modified from Livezey & Zusi (Citation2006: char. 0033), see also, Bourdon (Citation2005: char. 90). Ordered.
(19)Cranium, caudal perspective, os supraoccipitale, fonticuli occipitalis, presence in adult condition: 0. Absent, 1. Present. Livezey (Citation1997: char. 5), see also Bourdon (Citation2005: char. 45), Ericson (Citation1997: char. 1), Livezey (Citation1986: char. 9), Mayr & Clarke (Citation2003: char. 27), Murray & Vickers-Rich (Citation2004: table 8: char. 9), Worthy & Lee (Citation2008: char. 8), Worthy et al. (Citation2017: char. 30). Note: In Anhimidae, the fontanelles are lost in adults (Ericson Citation1997). We code Nettapterornis as 1 considering the relevant statement in Olson (Citation1999) and Conflicto as 1 due to Tambussi et al. (Citation2019) stating the presence of fonticuli despite it not being clear in the figure.
(20)Cranium, lateral and caudal perspectives, occipital region, prominentia exoccipitalis, status and form: 0. Absent or indistinct from processus paroccipitalis; 1. Present, lowly raised caudal prominences; 2. Present, well-developed caudal prominences with distinct bulbous appearance.
(21)Cranium, caudal and ventral perspectives, occipital region, area encompassing the occipital region, ventral of the crista ventralis, degree of convexity or concavity: 0. Flat or convex; 1. Slightly depressed; 2. Noticeably concave; 3. Noticeably concave, substantially accentuated by the extreme caudolateral development of the exoccipital prominences. Ordered.
(22)Cranium, ventral perspective, occipital region, lamina parasphenoidalis, caudal region, tuberculum basilare, form: 0. Low, ridge-like tubercles, undifferentiated from the crista basilaris lateralis and/or crista basilaris transversa; 1. Prominent, ventrally raised tubercles joined by crests aligned with the crista basilaris lateralis and transversa, often right-angled in shape or profile; 2. Prominent, ventrally raised, rounded tubercle in lateral area; 3. Developed in association with, and coincident with, the processus medialis parasphenoidalis. Modified from Worthy et al. (Citation2017: char. 25). Note: see also Livezey & Zusi (Citation2006: chars. 0065 and 0123), Parker (1895), Pycraft (Citation1900: 172), Mayr & Clarke (Citation2003: char. 30), Worthy & Scofield (Citation2012: char 18).
(23)Cranium, caudal and ventral perspectives, occipital region, foramen magnum, shape: 0. Rounded, oval or near circular; 1. Rectangular, both dorsal and ventral margins near perpendicular to lateral sides; 2. Triangular, dorsal margin distinctly apexed dorsally. See Livezey & Zusi (Citation2006: char. 0027, 0028).
(24)Cranium, caudal and ventral perspectives, os parasphenoidale, lamina parasphenoidalis, bulla basitemporalis (sensu Davids Citation1952a, b): 0. Absent or indistinct, 1. Present, not well developed; 2. Present, well-developed. Ordered.
(25)Cranium, caudal and ventral perspectives, os parasphenoidale, lamina parasphenoidalis, degree of overall ventral convexity: 0. Flat; 1. Rounded or inflated. From Livezey and Zusi (Citation2006: char. 117).
(26)Cranium, lateral perspective, os parasphenoidale, ala parasphenoidalis, shape or profile in lateral view: 0. Dorsally arced or concave lateral margin, often directed rostrodorsally immediately rostral of processus paroccipitalis, proceeds rostrally on the horizontal (dorsoventral) plane; 1. Linear lateral margin directed rostrally or rostroventrally from processus paroccipitalis; 2. Ventrally curved or convex, rounded flange. See also Worthy & Lee (Citation2008: char. 12), and Worthy et al. (Citation2017: char. 20). Ordered.
(27)Cranium, ventral perspective, rostrum parasphenoidale, processus basipterygoideus, presence as distinct and developed process: 0. Absent; 1. Present. Modified from Worthy et al. (Citation2017: char. 27). Note: The presence of a process is assessed regardless of the presence of an articulatory surface (see below).
(28)Cranium, ventral perspective, rostrum parasphenoidale, articulatio pterygobasipterygoidea and facies articularis pterygoidea, articulation and contact between pterygoid and rostrum parasphenoidale (through facies articularis pterygoidea), status: 0. Absent; 1. Present. Note: Some birds (e.g., Accipiter fasciatus) possess a relatively vestigial processus basipterygoideus that is clearly present yet does not articulate with the pterygoid (coded as 0 here).
(29)Cranium, ventral perspective, rostrum parasphenoidale, articulatio pterygobasipterygoidea, facies articularis pterygoidea, shape and development of articular surface: 0. Facies articularis pterygoidea is absent or vestigial; 1. Present, large and clearly ovoid articulatory facet, may be slightly tapered. 2. Facies articularis pterygoidea is irregularly shaped and not flattened. Modified from Mayr & Clarke (Citation2003: char. 24).
(30)Cranium, ventral and lateral perspectives, rostrum parasphenoidale, facies articularis pterygoidea, degree of relief with respect to rostrum parasphenoidale: 0. Facies articularis pterygoidea is absent or vestigial; 1. Facies articularis pterygoidea is sessile and confluent with the rostrum parasphenoidale; 2. Facies articularis pterygoidea is sessile and slightly raised with respect to the rostrum parasphenoidale, well defined; 3. Facies articularis pterygoidea is present and slightly pedestalled upon a short processus basipterygoideus; 4. Facies articularis pterygoidea is present upon a pedicellate (stalked) processus basipterygoideus, elongate. Modified from Worthy et al. (Citation2017: char. 29), see also Murray & Vickers-Rich (Citation2004: char. 13, table 8) and Mayr (Citation2022). Ordered.
(31)Cranium, ventral perspective, rostrum parasphenoidale, facies articularis pterygoidea and/or the processus basipterygoideus, position: 0. Facies articularis pterygoidea is rostrolaterally adjacent to lamina parasphenoidalis, caudal to rostrum parasphenoidale; 1. Facies articularis pterygoidea is just rostral of, and distinctly separated from the rostral lamina parasphenoidalis on caudal-most rostrum parasphenoidale; 2. Facies articularis pterygoidea is located far rostrally from the lamina parasphenoidalis, and rostral on rostrum parasphenoidale. Modified from Worthy et al. (Citation2017: char. 28). Note: based on embryological evidence, Weber (Citation1993) interpreted that the processus basipterygoideus of Galloanserae was non-homologous with that of other Neornithes. However, further assessment using a larger range of taxa is deemed necessary to more adequately test this (see Dzerzhinsky, Citation1995; Ericson, Citation1997; Zusi & Livezey, Citation2006), and were considered conversely homologous by Worthy et al (Citation2017: char. 28). Ordered.
Lacrimal
(32)Lacrimal, dorsal perspective, synostosis with frontale and/or nasale: 0. Lacking; 1. Synostosed to frontale, caudal of craniorostral hinge; 2. Synostosed to nasale, rostral of craniorostral hinge; 3. Synostosed to both nasale and frontale, extends across the craniorostral hinge. Modified from Worthy et al. (Citation2017: char. 9), see also Ericson (Citation1997: char. 3), Livezey (Citation1986, char. 10; 1996, char. 6; 1997: char. 15), Murray & Vickers-Rich (Citation2004: char. 5, table 8), Stidham (Citation2001: char. 21), Worthy & Lee (Citation2008, char. 4).
(33)Lacrimal, lateral perspective, processus orbitalis and processus supraorbitalis, relative dorsoventral and rostrocaudal length, respectively: 0. Processus orbitalis is dorsoventrally shorter than the rostrocaudal length of processus supraorbitalis; 1. Respective lengths are near equal or processus orbitalis is slightly longer; 2. The dorsoventral length of processus orbitalis far exceeds the rostrocaudal length of the processus supraorbitalis. Ordered.
(34)Lacrimal, lateral perspective, processus orbitalis, dorsoventral length of the processus orbitalis relative to the dorsoventral height of the rostrum: 0. Short, especially so, does not approach the dorsoventral midline of the rostrum height; 1. Intermediate, approaches the dorsoventral midline of the rostrum height; 2. Long, length approaches the dorsoventral height of the rostrum, approaches or overlaps the jugal arch. Ordered.
Quadrate
(35)Quadrate, dorsal and caudal perspectives, pars otica, capitulum oticum and capitulum squamosum, separation of the capitulum by vallecula intercapitularis: 0. Absent, the two capitulae are fused to form a single articular head, the capitulum squamoso-otica, vallecula intercapitularis is absent, vestigial or indistinct; 1. Cotylae are close together and the vallecula intercapitularis is mediolaterally narrow; 2. A significant space between cotylae exists, the vallecula intercapitularis is mediolaterally wide. Modified from Worthy et al. (Citation2017: char. 49), see also Bourdon (Citation2011: char. 30, 35), Elzanowski & Stidham (Citation2010: char. 7), Mayr & Clarke (Citation2003: char. 34), Murray & Vickers-Rich (Citation2004: char. 14, table 8). Ordered.
(36)Quadrate, lateral perspective, pars otica, capitulum squamosum, degree to which articulatory surface of capitulum squamosum extends ventrally onto the lateral surface of the pars otica, status: 0. Absent, dorsally confined; 1. Present, slight curve of articular surface onto lateral side; 2. Present, significant ventral extension of the articular surface. Modified from Worthy & Lee (Citation2008: char. 21) and Worthy et al. (Citation2017: char. 57). Ordered.
(37)Quadrate, medial perspective, pars otica, dorsorostral of crista medialis, sulcus pneumaticus, status: 0. Nondescript surface, absent, or indeterminate; 1. Sulcus pneumaticus is present, but indistinct; 2. Sulcus pneumaticus is present, and distinctly depressed. May be associated with Stidham (Citation2001: char. 14). Note: see Elzanowski & Stidham (Citation2010: figure 5) regarding the presence of the sulcus pneumaticus with regards to a rostromedially positioned foramen pneumaticum. The sulcus pneumaticus appears indeterminant or absent in all non-anhimid Anseriformes because the pneumatic foramen is positioned caudal of the crista medialis, which contrastingly corresponds to depressio caudomedialis. Ordered.
(38)Quadrate, medial and caudal perspectives, pars otica, foramen pneumaticum caudomediale and foramen pneumaticum rostromediale, presence and position: 0. Both foramina are absent; 1. Foramen pneumaticum rostromediale is present only; 2. Both foramina are present; 3. Foramen pneumaticum caudomediale is present only; 4. Foramen pneumaticum caudomediale is present only, but is rostrally positioned. See Elzanowski & Stidham (Citation2010), Worthy & Scofield (Citation2012: char. 50 and 52), and Worthy et al. (Citation2017: char. 51 and 52). Note: we follow Worthy et al. (Citation2017) for the coding of Anhima cornuta, although differ from their coding for Cereopsis novaehollandiae following Elzanowski & Stidham (Citation2010: p. 315) who recognised that some taxa within the Anserinae sub-family and Mergini tribe show a rostral displacement of the caudomedial pneumatic foramen. Ordered.
(39)Quadrate, lateral perspective, pars otica, tuberculum subcapitulare associated with the m. AME superficialis (and aponeurosis articularis), development: 0. Not applicable, no visible corresponding feature on the quadrate; 1. Crested, follows the dorsoventral long axis of the pars otica; 2. Well-developed conical-shaped tubercle; 3. Large tubercle, extremely well developed, dorsoventrally expanded. See Bourdon (Citation2005: char. 19; 2011: char. 53), Livezey (Citation1997: char. 49), Mayr & Clarke (Citation2003: char. 35), Stidham (Citation2001: char. 17), and Worthy et al. (Citation2017: char. 50). Note: The m. AME superficialis does not originate on the tuberculum subcapitulare in Anseres. Due to differences in positions and spans of muscle origins, the superficially similar feature on the pars otica in the latter taxa instead corresponds to m. AM posterior, and thus should be treated separately (see char. 39, below).
(40)Quadrate, lateral perspective, pars otica and processus orbitalis, position of origin for m. AM posterior: 0. On, or close to, processus orbitalis; 1. Pars otica. Note: The origin of m. AME superficialis is associated with the tuberculum subcapitulare on the pars otica of the quadrate in most Neornithes, however, this muscle originates more dorsally on the lateral cranium in Anatidae and does not have this same attachment to the quadrate. Instead, a superficially similar tubercle is related to the origin of m. AM posterior in anatids, which is comparatively more ventral on the quadrate in other birds (e.g., see Davids, Citation1952a; Goodman & Fisher, Citation1962; Zusi & Livezey, Citation2000).
(41)Quadrate, lateral perspective, pars otica and corpus quadrati, tuberculum or prominence associated with the m. AM posterior on the pars otica, status and development: 0. Non-descript surface; 1. Present, low ridge; 2. Present, clearly crested eminence; 3. Present, well-developed and expansive tubercle. See Bourdon (Citation2005: char. 19; 2011: char. 53), Livezey (Citation1997: char. 49), Mayr & Clarke (Citation2003: char. 35), Stidham (Citation2001: char. 17), and Worthy et al. (Citation2017: char. 50). Note: The conspicuous eminence associated with m. AM posterior on the processus orbitalis corresponds to the medial part of m. AM posterior (m. AM posterior medialis), whereas attachment on the quadrate body or pars otica is related to the lateral part (m. AM posterior lateralis), in taxa which have clear differentiation of this muscle into medial and lateral parts (e.g., see Fujioka, Citation1963; Goodman & Fisher, Citation1962; Weber, Citation1996; Zusi & Livezey, Citation2000; Holliday & Witmer, Citation2007). While overall large and well developed, this differentiation is not observed in anhimid species, which possess an m. AM posterior that is characteristic of the medial part only (Dzerzhinsky, Citation1982: 1034). Comparisons here should strictly observe the influence of the origin of m. AM posterior upon the corpus quadrati and pars otica (most often related to m. AM posterior lateralis), rather than any corresponding to the more rostral origin on processus orbitalis (m. AM posterior medialis or m. AM posterior). Ordered.
(42)Quadrate, lateral and medial perspectives, pars otica and processus orbitalis, relative lengths: 0. Pars otica is longer than processus orbitalis; 1. Pars otica and processus orbitalis are subequal in length; 2. Pars otica is shorter than processus orbitalis. Ordered.
(43)Quadrate, lateral perspective, pars otica and processus orbitalis, orbital angle (the angle between the long axes of pars otica and processus orbitalis, sensu Elzanowski & Stidham Citation2010: p. 310): 0. Obtuse, greater than 100 degrees; 1. Right or acute angle, 90 degrees or less. From Elzanowski & Stidham (Citation2010: char. 6). Note: This does not refer to the curvature of the dorsal profile between pars otica and processus orbitalis, but rather the orientation of the long axes of pars otica and processus orbitalis).
(44)Quadrate, lateral perspective, processus orbitalis and pars otica, dorsal profile, curvature of the dorsal margin of the processus orbitalis as it intersects with the rostral side of pars otica: 0. Deep curvature of the dorsal profile; 1. Marked, but weak, curvature of dorsal profile; 2. Minor curvature of the dorsal profile, little distinction between dorsal margin of processus orbitalis and rostral margin of pars otica. Related to Livezey (Citation1986: character 15), Ericson (Citation1997: char. 11), Worthy & Lee (Citation2008: char. 22), Worthy et al. (Citation2017: char. 58). Ordered.
(45)Quadrate, lateral perspective, processus orbitalis, shape: 0. Processus orbitalis is dorsoventrally broad across its rostrocaudal length (superficially rectangular); 1. Processus is triangular, dorsoventrally wider caudally and tapering rostrally to the apex; 2. Processus orbitalis is dorsoventrally narrow across its rostrocaudal length. Related to Bourdon (Citation2005: char. 94, 112), Dyke et al. (2003: char. 22), Ericson (Citation1997: char. 11). Ordered.
(46)Quadrate, medial perspective, corpus quadrati, foramen pneumaticum basiorbitale, presence: 0. Absent or vestigial; 1. Present. Modified from Stidham (Citation2001: char. 13), Elzanowski and Stidham (Citation2010: char. 1), and Worthy et al. (Citation2017: char. 54). Note: some Chauna torquata have a vestigial foramen, see Elzanowski and Stidham (Citation2010: table. 4).
(47)Quadrate, rostral and medial perspectives, processus orbitalis and pars mandibularis, condylus pterygoideus and facies articularis pterygoidea, association: 0. Facies articularis pterygoidea is separated from the condylus pterygoideus, a narrow articular surface or ridge between is variably present; 1. Facies articularis pterygoidea and condylus pterygoideus are adjacent. Modified from Livezey and Zusi (Citation2006: char. 600), Elzanowski and Stidham (Citation2010: char. 4) and Worthy et al. (Citation2017: char. 56). Note: Elzanowski and Stidham (Citation2010) suggested that the condition where the articular surfaces are adjacent is the plesiomorphic condition (see also Worthy et al. Citation2017: char. 56).
(48)Quadrate, medial perspective, processus orbitalis and pars mandibularis, facies articularis pterygoidea, position: 0. Facies articularis pterygoidea is conspicuous on the medial face; 1. Facies articularis pterygoidea is on the ventromedial margin of the ventral processus orbitalis, visible in medial perspective; 2. Facies articularis pterygoidea is ventrally or ventrolaterally located on rostral pars mandibularis and partially on the ventral margin of processus orbitalis, not visible in medial perspective. Related to Livezey and Zusi (Citation2006: char. 600). Ordered.
(49)Quadrate, rostral perspective, pars mandibularis, condylus pterygoideus, prominence: 0. Flat, not developed; 1. Bulbous, broadly rounded tubercle; 2. Sharply defined, subangular or conical tubercle. Modified from Bourdon (Citation2011: char. 34) and Worthy et al. (Citation2017: char. 56).
(50)Quadrate, rostral perspective, pars mandibularis, processus medialis, condylus pterygoideus and condylus mandibularis medialis, distinction and dorsoventral separation between the ventral margin of condylus pterygoideus and the rostral margin of condylus mandibularis medialis: 0. Ventral margin of the condylus pterygoideus is confluent with the rostral margin of the condylus mandibularis medialis (inseparable); 1. The two condyles are dorsoventrally separated by a narrow furrow on the rostral pars mandibularis; 2. Dorsoventrally wide separation of the two condyles, condylus pterygoideus is located markedly dorsally. Ordered.
(51)Quadrate, ventral perspective, pars mandibularis, mandibular condyles, number and presence of condylus mandibularis caudalis: 0. Three mandibular condyles, condylus caudalis is present; 1. Bicondylar mandibular articulation, only condylus mandibularis lateralis and condylus mandibularis medialis are present. See Bourdon (Citation2005: char. 25; 2011: char. 1), Livezey (Citation1997: char. 51), Murray & Vickers-Rich (Citation2004: char. 16, table 8).
(52)Quadrate, ventral perspective, pars mandibularis, condylus mandibularis lateralis and processus orbitalis, orientation of the long axis of condylus mandibularis lateralis relative to that of the processus orbitalis (and/or the main rostrocaudal alignment of the quadrate): 0. The long axes of the lateral mandibular condyle and processus orbitalis are approximately aligned and parallel; 1. The caudal part of the lateral mandibular condyle is slightly rotated laterally, with its rostral terminus angled relatively rostromedially, compared to the orientation orbital process (less than 180 degree angle formed); 2. The lateral mandibular condyle has its long axis near perpendicular to that of the orbital process, producing a near 90 degree angle with respect to the main rostrocaudal alignment of the quadrate. See also Livezey (Citation1997: char. 52) and Murray & Vickers-Rich (Citation2004: char. 17, table 8). Ordered.
(53)Quadrate, lateral perspective, pars mandibularis, processus lateralis, processus submeaticus and prominentia submeatica, presence: 0. Both the process and the prominence are absent; 1. Only the prominentia submeatica is present; 2. Both the prominentia submeatica and processus submeaticus are present; 3. Only the processus submeaticus is present. From Elzanowski & Stidham (Citation2010: char. 3), see also Ericson (Citation1997: char. 12) and Worthy et al. (Citation2017: char. 48). Presbyornis pervetus is coded as 0. Note: Following Elzanowski & Stidham (Citation2010: fig. 8), we code anhimids as 2, despite whether the process is homologous with the prominentia submeatica, as noted by Worthy et al. (Citation2017: char. 48).
(54)Quadrate, lateral perspective, pars mandibularis, processus lateralis, fovea quadratojugalis: 0. A flat articular surface; 1. A shallow depression; 2. Distinctly depressed; 3. A very deep fovea. Ordered.
(55)Quadrate, lateral and caudal perspective, pars mandibularis, processus lateralis, cotyla quadratojugalis, development of caudal or caudoventral margin: 0. Margin is absent, fovea quadratojugalis is completely open caudoventrally, conspicuously notched appearance as a result; 1. Fovea quadratojugalis is partially closed caudoventrally at the base of the fovea (medially) by a notched caudoventral rim; 2. Fovea quadratojugalis is completely enclosed caudoventrally by a thin osseous rim; 3. Fovea quadratojugalis is completely enclosed, rim is uniformly well developed around fovea quadratojugalis. Modified from Worthy et al. (Citation2017: char. 59). For a discussion on this character, see Elzanowski and Stidham (Citation2010). Ordered.
(56)Quadrate, lateral perspective, pars mandibularis, processus lateralis, cotyla quadratojugalis, facies articularis quadratojugalis dorsalis and facies articularis quadratojugalis ventralis, presence: 0. Absent or indistinct; 1. Present, distinct.
Mandible
(57)Mandible, dorsal and ventral perspectives, rostrum mandibulae, ramus mandibulae, pars symphysialis, apex rostri, shape: 0. Rounded and bulbous rostral apex, bilaterally broad and flared with respect to the more caudal mandibular rami, producing a conspicuously spatulate shape; 1. Rounded and spatulate at the rostral apex, but not bilaterally widened relative to more caudal mandibular rami; 2. Mandibular rami are tapered to a blunt rostral apex; 3. Mandibular rami are tapered to a pointed rostral apex. See also Livezey (Citation1997: char. 22). Ordered.
(58)Mandible, rostral perspective, rostrum mandibulae, ramus mandibulae, pars symphysialis, apex rostri, degree of ventral convexity or curvature of dorsal surface: 0. Dorsal surface is deeply ventrally convex, lateral sides curve steeply dorsally; 1. Shallowly centrally convex, with weak lateral curvature; 2. The dorsal surface of the symphysial region is almost flat. Modified from Clarke (Citation2002: char. 44), Mayr & Clarke (Citation2003: char. 43). Ordered.
(59)Mandible, dorsal perspective, rostrum mandibulae, ramus mandibulae, pars symphysialis, rostrocaudal length of the symphysis relative to complete mandible rostrocaudal length: 0. Short, restricted to within the most rostral quarter of the mandible; 1. Extends significantly caudally, greater than one quarter the rostrocaudal length of the entire mandible.
(60)Mandible, dorsal and ventral perspectives, ramus mandibulae, lateral divergence of the mandibular rami as continued caudally from pars symphysialis: 0. None or very little, mandibular rami are near parallel, inter-ramal width is consistently narrow; 1. Minor, slight diverging, inter-ramal width is narrow but gradually increases caudally; 2. Marked, inter-ramal width significantly increases caudally. Ordered.
(61)Mandible, lateral perspective, ramus mandibulae, curvature of the mandibular rami: 0. Mandibular rami are arched (convex) dorsally; 1. Mandibular rami are not substantially curved (relatively straight); 2. Mandibular rami are arched (convex) ventrally. Modified from Livezey (Citation1996: char. 4; 1997: char. 17), Worthy & Lee (Citation2008: char. 23), and Worthy et al. (Citation2017: char. 61). Ordered.
(62)Mandible, dorsal perspective, rostrum mandibulae, ramus mandibulae, pars intermedia and pars symphysialis, mediolateral width: 0. Mediolaterally narrow, thin; 1. Mediolaterally wide, robust mandibular rami.
(63)Mandible, dorsal, medial and lateral perspectives, rostrum mandibulae, pars intermedia, margo tomialis and crista tomialis, form: 0. Margo tomialis is not expanded, often sharp and crest-like; 1. Margo tomialis is slightly mediolaterally expansive relative to the more ventral ramus, producing a robust, rounded edge; 2. Margo tomialis is conspicuously lateroventrally expanded and deflected, often rounded; 3. Margo tomialis is medioventrally expanded and deflected, producing a flat and obliquely sloped tomial surface; 4. Margo tomialis is laterally expanded as nearing pars symphysialis to create a flattened surface. Note: a marked crest is observed caudally, medioventral of the tomial margin in both Anhima cornuta and Chauna chavaria specimens, absent in other taxa examined. However, it does bear some resemblance to the medioventrally expanded tomial margin that is characteristic of dromornithids, and may represent an less well-developed form of such a structure.
(64)Mandible, lateral and ventral perspectives, rostrum mandibulae, ramus mandibulae, pars intermedia, mediolateral slant across the dorsoventral height of the rami, ventral of tomial margin: 0. Mandibular rami are slanted medially as continued ventrally; 1. Mandibular rami have no mediolateral slant as continued ventrally.
(65)Mandible, lateral perspective, rostrum mandibulae, ramus mandibulae, pars intermedia, groove that extends rostrocaudally along each lateral mandibular ramus, ventral of margo tomialis, status and form: 0. Absent or only a slight rostrocaudally elongate depression; 1. Present, groove is dorsoventrally thin, and often not well-defined; 2. Present, groove is dorsoventrally thin and well-defined for the complete rostrocaudal length of ramus mandibulae, pars intermedia; 3. Present, groove is dorsoventrally broad. Modified from Ericson (Citation1997: char. 17).
(66)Mandible, medial and lateral perspectives, pars intermedia, fenestra rostralis mandibulae, presence and form: 0. Absent; 1. Fenestra rostralis mandibulae is open medially although completely covered laterally; 2. Fenestra rostralis mandibulae is open and ovoid laterally although completely covered medially; 3. Fenestra rostralis mandibulae is open and ovoid laterally and open as a thin slit medially; 4. Fenestra rostralis mandibulae is open as a thin slit both medially and laterally; 5. Fenestra rostralis mandibulae is open and conspicuously ovoid both medially and laterally. Note: degree of medial and lateral opening is related to the interaction between the os dentale, os spleniale, os praearticulare and os suprangulare.
(67)Mandible, lateral perspective, ramus mandibulae, pars caudalis and pars intermedia, angulus dorsalis mandibulae and rostrum mandibulae, relationship between angulus dorsalis mandibulae and the caudal margo tomialis: 0. Confluent, a gradual slope connects angulus dorsalis mandibulae and margo tomialis; 1. Abrupt, a distinct, dorsoventrally short and variously rounded step is observed at the transition between margo tomialis and angulus dorsalis mandibulae; 2. Strongly abrupt, considerable dorsoventral displacement is observed at the transition between margo tomialis and angulus dorsalis mandibulae. Ordered.
(68)Mandible, lateral perspective, ramus mandibulae, pars caudalis, angulus dorsalis mandibulae and processus coronoideus, relative dorsal elevation: 0. The dorsal apex of processus coronoideus is dorsal to angulus dorsalis mandibulae; 1. The dorsal apex of processus coronoideus and that of angulus dorsalis mandibulae exist on approximately the same dorsoventral plane; 2. The dorsal apex of processus coronoideus is ventral to that of angulus dorsalis mandibulae.
(69)Mandible, lateral perspective, ramus mandibulae, pars caudalis and pars intermedia, processus coronoideus, angulus dorsalis mandibulae and angulus ventralis mandibulae, rostrocaudal positioning of angulus ventralis mandibulae: 0. Positioned markedly caudal to processus coronoideus; 1. Positioned slightly caudal or rostrocaudally aligned with processus coronoideus; 2. Positioned rostral of processus coronoideus and caudal of angulus dorsalis mandibulae; 3. Positioned noticeably rostral of angulus dorsalis mandibulae. Note: taxa that do not possess an unambiguous angulus ventralis mandibulae (see character below) should be coded as ‘?”. Ordered.
(70)Mandible, lateral perspective, ramus mandibulae, pars caudalis and pars intermedia, angulus dorsalis ventralis, prominence: 0. Ambiguous and not defined; 1. Variably rounded; 2. Well defined, prominent. Ordered.
(71)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, fossa articularis quadratica, cotylae fossae articularis, number of cotylae: 0. Three cotylae; 1. Two cotylae. Modified from Ericson (Citation1997, char. 13), and Worthy et al. (Citation2017: char. 60).
(72)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, fossa articularis quadratica, cotylae fossae articularis, orientation of cotylae medialis mandibulae and cotylae lateralis mandibulae: 0. Cotylae are parallel, both rostrocaudally aligned; 1. Cotylae are nearly perpendicular, cotylae medialis mandibulae oriented rostrocaudally; 2. Cotylae parallel, cotylae medialis mandibulae oriented mediolaterally. Modified from Andors (Citation1988: char. 20) and Mayr & Clarke (Citation2003: char. 38).
(73)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, fossa articularis quadratica, cotylae fossae articularis, cotyla medialis, shape: 0. Mediolaterally wide, rounded oval; 1. Relatively equal mediolateral and rostrocaudal widths; 2. Rostrocaudally elongate. Ordered.
(74)Mandible, dorsal, rostral and caudal perspectives, ramus mandibulae, pars caudalis, processus medialis mandibulae and fossa articularis quadratica, rostromedial projection of cotyla medialis with respect to the profile of the rostral processus medialis mandibulae and the more ventral ramus mandibulae: 0. Absent, not defined rostromedially; 1. Rostromedial margin of the cotyla medialis is marked in dorsal view, but indistinct with respect to the more ventral ramus mandibulae; 2. Rostromedial margin of the cotyla medialis is conspicuous in dorsal view, and distinctly projects to overhang the more ventral ramus mandibulae. Ordered.
(75)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, processus medialis mandibulae, shape of rostromedial-most cotyla medialis mandibulae: 0. Not arced to hemispherical or broadly rounded; 1. Subangular, almost right-angled.
(76)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, processus medialis mandibulae, orientation of medial-most area: 0. Caudally oriented; 1. Completely medially projected; 2. Rostrally oriented. Ordered.
(77)Mandible, rostral and caudal perspectives, ramus mandibulae, pars caudalis, processus medialis mandibulae, degree of dorsal orientation as continued medially: 0. Level, projects medially, does not project dorsally; 1. Projects mediodorsally; 2. Projects strongly dorsally. Note: A long, narrow, and dorsally oriented processus was listed as a synapomorphy of Galloanserae by Cracraft & Clarke (Citation2001, char. 41). Mayr & Clarke (Citation2003, char. 45) found this state to be more widely distributed, to the exclusion of Palaeognathae (Worthy et al. Citation2017: char. 68). Also modified from Bourdon (Citation2011: char. 51). Also see character below. Ordered.
(78)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, processus medialis mandibulae, length of the process medial of cotyla medialis mandibulae: 0. Short; 1. Long.
(79)Mandible, dorsal, rostral and caudal perspectives, ramus mandibulae, pars caudalis, processus lateralis mandibularis, form: 0. Absent, the cotyla lateralis does not project laterally; 1. Distinctly projected laterally, but confluent with the more ventral and rostral mandibular ramus; 2. Well-developed laterally and distinct from the more rostral mandibular ramus. Modified from Ericson (Citation1997: char. 16), Livezey (Citation1997: char. 24), Murray & Vickers-Rich (Citation2004: table 8, character 20). Ordered.
(80)Mandible, lateral and dorsal perspectives, ramus mandibulae, pars caudalis, insertion of m. AME superficialis, aponeurosis paracoronoidea and corresponding ossified structures, status and form: 0. Aponeurosis paracoronoidea is absent and therefore, so is any associated paracoronoid tubercle or crest; 1. Tuberculum paracoronoideum is present on the dorsal edge of the mandible, caudal of processus coronoideus, and may extend somewhat laterally onto the lateral mandible; 2. Located on the lateral mandible as separate rostral (crista paracoronoidea rostralis) and caudal (crista paracoronoidea caudalis) crests, crista paracoronoidea is rostrally extended and process-like in its development; 3. Located on the lateral mandible, crista paracoronoidea rostralis and caudalis are close together dorsally, or touching at their dorsocaudal-most points to form an acute angle, respective rostral and caudal crests are perpendicular to near parallel to one another; 4. Crista paracoronoidea is undivided, forms a prominent processus paracoronoideus. Note: see Weber & Hesse (Citation1995) and Weber (Citation1996) for details regarding the aponeurosis paracoronoidea and associated crests or tubercula. The prominent processus paracoronoideus in anatids is analogous to the prominently developed crista paracoronoideus rostralis of megapodiids (Weber & Hesse, Citation1995; Weber, Citation1996). The aponeurosis paracoronoidea is primarily related to the caudally located m. AME superficialis. This part of the external adductor musculature is not distinct in Palaeognathae to the same degree it is in Neognathae, where it is instead interrelated with other portions of the musculature (specifically the fibres associated with the more rostral deep part, m. AME profundus, pars coronoideus); the lack of this distinct subdivision equates to a lack of any osseous structure on the mandible associated with it (see Dzerzhinsky, Citation1983; Elzanowski, Citation1987; Weber & Hesse, Citation1995; Weber, Citation1996).
(81)Mandible, lateral perspective, ramus mandibulae, pars caudalis, processus retroarticularis, caudal development and projection: 0. Absent or present only as a rostrocaudally short, dorsoventrally aligned crest; 1. Present as a rostrocaudally short projection which extends caudally no more than its dorsoventral height at its rostral origin; 2. Present as a caudally long osseous projection, where total caudal length is greater than the dorsoventral height at its rostral origin but less than twice this height; 3. Present as a caudally long osseous projection, where total caudal length is greater than twice the dorsoventral height at its rostral origin. Modified from Worthy et al. (Citation2017: char. 64). Ordered. Note: Because of the wide taxonomic range being treated here, the lack of a processus retroarticularis must be a considered state for the following characters related to this structure.
(82)Mandible, lateral perspective, ramus mandibulae, pars caudalis, processus retroarticularis, degree of dorsal margin curvature: 0. Not applicable, processus retroarticularis is absent; 1. The dorsal margin is not curved dorsally as continued caudally; 2. The dorsal margin broadly or subtly curves dorsally as continued caudally; 3. The dorsal margin is conspicuously curved dorsally as continued caudally, particularly towards the caudal tip. Modified from Bourdon (Citation2011: char. 52), Livezey (Citation1997: char. 20), Mayr & Clarke (Citation2003: char. 44), Murray & Vickers-Rich (Citation2004: table 8), Worthy & Lee (Citation2008: char. 25). Note: observations should be restricted to the dorsal margin of the retroarticular process for this character, and not the ventral margin.
(83)Mandible, lateral perspective, ramus mandibulae, pars caudalis, processus retroarticularis, shape: 0. Processus retroarticularis is absent; 1. Dorsoventrally narrow and acicular (needle-like); 2. Dorsoventrally splayed and broad.
(84)Mandible, dorsal perspective, ramus mandibulae, pars caudalis, processus retroarticularis, direction/angle from which the processus retroarticularis extends caudally: 0. Process retroarticularis is absent; 1. Directed laterocaudally; 2. Extends caudally in line with the angle of the mandibular rami. Modified from Murray & Vickers-Rich (Citation2004: table 8) and Worthy & Lee (Citation2008: char. 25).
(85)Mandible, caudal perspective, ramus mandibulae, pars caudalis, processus medialis mandibularis, form of caudal surface, status of fossa caudalis and development of recessus conicalis: 0. Fossa caudalis is absent, the caudal processus medialis mandibularis is flat, dorsoventrally narrow; 1. Fossa caudalis absent, caudal processus medialis mandibularis is flat, dorsoventrally expansive; 2. Fossa caudalis is present and shallow, dorsoventrally expansive; 3. Fossa caudalis is present and deep, dorsoventrally expansive; 4. Fossa caudalis is significantly depressed, a large and deep recessus conicalis is present, dorsoventrally expansive. Modified from Ericson (Citation1997: char. 14), Livezey (Citation1997: char. 21), Stidham (Citation2001: char. 22), Worthy & Lee (Citation2008: char. 26), Worthy et al. (Citation2017: char. 65). Note: we code Presbyornis pervetus and Nettapterornis oxfordi based on descriptions by Olson (Citation1999). Ordered.
Rostrum
(86)Rostrum, co-ossification of rostral elements (particularly, maxillaria, nasalia and praemaxillaria): 0. Absent or partial; 1. Present.
(87)Rostrum, dorsal perspective, rostrum maxillae, apex rostri, shape: 0. Rostral end is broadly rounded, spatulate; 1. Rostral end is laterally spatulate or rounded, with a pointed rostral apex; 2. Tapered to a point. Modified from Livezey (Citation1997: char. 32), Bourdon (Citation2005: char. 46), and Ericson (Citation1997: char. 10). Ordered.
(88)Rostrum, lateral perspective, culmen, dorsal profile of caudal two-thirds of rostrum (caudal of rostrum maxillae): 0. Non-arced or linear to very subtly dorsally convex; 1. Dorsally arced throughout the rostrocaudal length; 2. Strongly dorsally convex throughout the rostrocaudal length. Ordered.
(89)Rostrum, lateral perspective, rostrum maxillae, profile of the dorsal rostrum maxillae towards its rostral apex: 0. Dorsally convex, uniform with respect to the more caudal rostrum; 1. Linear, not arced, uniform with respect to the more caudal rostrum; 2. Flattens and dorsoventrally levels with respect to more caudal rostrum, may appear ventrally arced; 3. Conspicuously dorsally inflated at the rostral apex.
(90)Rostrum, lateral perspective, apertura nasi ossea, development and span: 0. Very large, comprises most of, or a large proportion of, the lateral sides of the rostrum; 1. Large, comprises nearly two-thirds of the lateral side of the rostrum, the rostrocaudal length of the rostrum rostral of the apertura nasi ossea is no more than half the rostrocaudal length of the apertura nasi ossea itself; 2. Comprises approximately one-half of the lateral side of the rostrum, the rostrocaudal length of the rostrum rostral of the apertura nasi ossea is equal to or slightly greater than the rostrocaudal length of the apertura nasi ossea itself; 3. Small, caudally restricted, approximately one-third to one-quarter the rostrocaudal length of the rostrum; 4. Very small, well restricted to within the caudal half of the rostrum, less than one-fifth of the rostrocaudal length of the rostrum. Modified from Murray & Vickers-Rich (Citation2004: char. 2, table 8); Bourdon (Citation2005: char. 101). Ordered.
(91)Rostrum, lateral perspective, apertura nasi ossea, positioned significantly dorsally on rostrum: 0. Absent, centrally positioned dorsoventrally; 1. Present.
(92)Rostrum, ventral perspective, palatal surface, palatum osseum, os palatinum, degree of palatal ossification and closure of the fenestra palatina: 0. Very little to no ossification, the fenestra palatina is large; 1. Partial but marked ossification, fenestra palatina is large; 2. Near complete palatal ossification, the fenestra palatina is slit-like or reduced to a small opening. Modified from Worthy et al. (Citation2017: char. 7). Ordered.
(93)Rostrum, ventral perspective, palatal surface, palatum osseum, fenestra palatina, closure caudally: 0. Absent, fenestra palatina is open caudally, continuous with choana nasalis ossea; 1. Present, fenestra palatina is caudally closed through fusion of the vomer, palatines, and/or praemaxillary bones; 2. Present, fenestra palatina is caudally closed through medial fusion of the processus maxillopalatinus of the maxillary bones.
(94)Rostrum, lateral and ventral perspectives, ossa palatina and ossa vomeris, fusion along the mediolateral midline caudally (synostosis interpalatina, sensu Zusi & Livezey, Citation2006), with or without the vomer: 0. Absent; 1. Present, medial palatines are directly fused to one another or fused to, and separated by, a mediolaterally thin vomer; 2. Present, medial palatines are fused to the vomer, which widely mediolaterally separates the palatines. Note: medial fusion is characteristic of anseriform taxa, although not universally, to the exclusion of galliforms (see Zusi & Livezey, Citation2006). Modified from Worthy et al. (Citation2017: char. 36).
(95)Rostrum, ventral and lateral perspectives, os palatinum and os maxillare, position of the interaction between the processus rostralis of the palatinum and processus palatinus of the maxillare relative to the processus jugalis of the maxillare: 0. The rostral processus rostralis palatini is medially separated and ventral of the rostral processus jugalis of the maxillare; 1. The rostral processus rostralis palatini is medially separated and slightly ventral of the rostral processus jugalis of the maxillare; 2. The rostral processus rostralis palatini converges with the rostral processus jugalis of the maxillare, and is slightly ventral to it; 3. The rostral processus rostralis palatini converges with the rostral processus jugalis of the maxillare, and is dorsal to it. Modified from Ericson (Citation1997: char. 9), Worthy et al. (Citation2017: char. 33). Note: This character is derived from observations of Zelenkov & Stidham (Citation2018). State 2 can also be associated with a concavity that forms lateral of the processus rostralis palatini. Ordered.
(96)Rostrum, lateral perspective, arcus jugalis, os quadratojugale and processus jugalis of the os maxillare, arcus jugalis forms a synovial articulation with the rostrum: 0. Absent, the elements are fused; 1. Present.
(97)Rostrum, palatum osseum, palatal type: 0. Desmognathous; 1. Schizognathous; 2. Other. See Mayr & Clarke (Citation2003: char. 11), Murray & Vickers-Rich (Citation2004: char. 10, table 8), and Zusi & Livezey (Citation2006).
(98)Rostrum, ventral and lateral perspectives, os palatinum, pars lateralis, form and development: 0. Pars lateralis absent, a slightly enlarged lateral margin (crista lateralis) may exist; 1. Pars lateralis is poorly differentiated; 2. Pars lateralis is present but not well developed, does not extend far laterally or ventrally; 3. Pars lateralis is lateroventrally or ventrally flared, does not extend ventrally past the level of the tomial margin; 4. Pars lateralis extends considerably ventrally beyond the level of the tomial margin. See Livezey & Zusi (Citation2006: p. 158); also relevant is Bourdon (Citation2005: char. 68), Livezey (Citation1997: char. 41, 42), Mayr & Clarke (Citation2003: char. 16), and Worthy et al. (Citation2017: char. 34). Ordered.
(99)Rostrum, ventral and lateral perspectives, os palatinum, pars lateralis, formation of angulus caudolateralis palatini: 0. Absent; 1. Present.
(100)Rostrum, lateral and ventral perspectives, os palatinum, fusion to os pterygoideum (in adult birds): 0. Absent, 1. Present. From Worthy et al. (Citation2017: char. 41). Also see Worthy & Scofield (Citation2012), where the pterygoid is unfused or incompletely fused to the palatines in osteological immature dinornithiforms, and Benito et al. (Citation2022).
References
Andors AV. 1988. Giant groundbirds of North America (Aves, Diatrymidae). Columbia University [PhD thesis].
Benito J, Kou P, Widrig KE, Jagt JWM, Field DJ. 2022. Cretaceous ornithurine supports a neognathous crown bird ancestor. Nature. 612:100–105.
Bourdon E, 2005. Osteological evidence for sister group relationship between pseudo-toothed birds (Aves, Odontopterygiformes) and waterfowls (Anseriformes). Naturwissenschaften. 92:586–591.
Bourdon E. 2011. The pseudo-toothed birds (Aves, Odontopterygiformes) and their bearing on the early evolution of modern birds. In: Dyke, G. and Kaiser, G. (Eds.), Living dinosaurs: the evolutionary history of modern birds. John Wiley and Sons, Ltd, pp. 209–233.
Clarke JA. 2002. The morphology and systematic position of Ichthyornis Marsh 1872 and the phylogenetic relationships of basal Ornithurae. Yale University, Connecticut, [PhD Thesis].
Cracraft J, Clarke JA. 2001. The basal clades of modern birds. In: Gauthier, J., Gall, L.F. (Eds.), New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, pp. 143–156.
Crane A. 2023. Comparative morphology of the avian mandible: Phylogenetic implications for the fossil record of early crown group birds. 10th International Meeting of the Society of Avian Palaeontology and Evolution. 8-12 May 2023, Malaga, Spain.
Davids JAG. 1952a. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.), I. Proceedings Of The Koninklijke Nederlandse Akademie Van Wetenschappen. Series C. Biological and Medical Sciences. 55:81–94.
Davids JAG. 1952b. Etude sur les attaches au crâne des muscles de la tête et du cou chez Anas platyrhyncha platyrhyncha (L.). II. Proceedings Of The Koninklijke Nederlandse Akademie Van Wetenschappen. Series C. Biological and Medical Sciences. 55:525–533.
Dyke GJ. Gulas BE, Crowe TM, 2003. Suprageneric relationships of galliform birds (Aves, Galliformes): a cladistic analysis of morphological characters. Zoological Journal of the Linnean Society. 137:227–244.
Dzerzhinsky FYa. 1983. Chelyustnoy apparat tinamu Eudromia elegans. K voprosu o morfobiologicheskoy spetsifike chelyustnogo apparata paleognat [The jaw apparatus of the tinamou Eudromia elegans. On the morphobiological specificity of the palaeognath jaw apparatus]. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR. 116:12–33.
Dzerzhinsky FYa, Belokurova IN. 1972. Sravnitelnoy anatomii chelustnoy muskul tury ptits. Chelustnyye myshtsy glucharya (Tetrao urogallus) [Comparative anatomy of the jaw muscles of birds. Jaw muscles of capercaillie (Tetrao urogallus)]. Zoologicheskiĭ Zhurnal. 51:555–564.
Dzerzhinsky FYa, Grintsevichene TI. 2002. O vozmozhnykh filogeneticheskikh svyazyakh guseobraznykh ptits [On possible phylogenetic relationships of Anseriformes]. Kazarka. 8:19–39.
Dzerzhinsky FYa, Ladygin AV. 2004. Morfofunktsional’nyye razlichiya chelyustnogo apparata sokolinykh (Falconiformes, Falconidae) i yastrebiny (Accipitridae) kak istochnik materialov po ikh filogenii [form-functional peculiarities of the jaw apparatus in falconids (Falconiformes, Falconidae) and accipitrids (Accipitridae) as a source of information on their evolution]. Zoologicheskiĭ Zhurnal. 83(8):983–994.
Dzerzhinsky FYa, Potapova EG. 1974. Sistema sukhozhil’nykh obrazovaniy kak ob’yekt sravnitel’noy miologii chelyustnogo apparata ptits [The system of tendon formations as an object of comparative theology of the jaw apparatus of birds]. Zoologicheskiĭ Zhurnal. 53:1341–1351.
Dzershinsky FYa, Yudin KA. 1979. O gomologii chelyustnykh muskulov gatterii i ptits [One the homology of the jaw muscles in tuatara and birds]. Ornitologiâ. 14:14–34.
Elzanowski A. 1987. Cranial and eyelid muscles and ligaments of the tinamous (Aves: Tinaformes). Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere. 116(1):63–118.
Elzanowski A, Stidham TA. 2010. Morphology of the quadrate in the Eocene anseriform Presbyornis and extant galloanserine birds. Journal of Morphology. 271(3):305–323. DOI: 10.1002/jmor.10799
Ericson PGP. 1997. Systematic relationships of the Palaeogene family Presbyornithidae (Aves, Anseriformes). Zoological Journal of the Linnean Society. 121:429–483.
Field DJ, Benito J, Chen A, Jagt JW, Ksepka DT. 2020. Late Cretaceous neornithine from Europe illuminates the origins of crown birds. Nature. 579(7799):397–401.
Fisher HI, Goodman DC. 1955. The myology of the whooping crane, Grus americana. Illinois Biological Monographs. 24:1–127.
Fujioka T. 1963. Niwatori no hikaku kaibōgakuteki narabini kyokusho kaibōgakuteki kenkyū. IV. Niwatori no atama keibu ni okeru suji no kishi oyobi teishi ni tsuite sono. 1. Tōbu no suji [Comparative and topographical anatomy of the fowl. IV. Origins and insertions of muscles of the head and neck in the fowl. 1. Muscles of the head]. Nihon juigaku zasshi. The Japanese Journal of Veterinary Science. 25(4):207–226.
Hofer H. 1950. Zur Morphologie der Kiefermuskulatur der Vögel Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere. 70:427–556.
Houde P, Dickson M, Camarena D. 2023. Basal Anseriformes from the Early Palaeogene of North America and Europe. Diversity. 15(233). DOI: 10.3390/d15020233
Korzun LP. 1998. Evolution of trophic adaptations of forest wood birds. Moscow State University [PhD thesis].
Ksepka DT. 2009. Broken gears in the avian molecular clock: new phylogenetic analyses support stem galliform status for Gallinuloides wyomingensis and rallid affinities for Amitabha urbsinterdictensis. Cladistics. 25:173–197. DOI: 10.1111/j.1096-0031.2009.00250.x
Kuular USh. 2002. Adaptatsii chelyustnogo apparata zhuravley [Adaptation of crane mandible apparatus]. Zhuravli Yevrazii (raspredeleniye, chislennost’, biologiya) [Cranes of Eurasia (distribution, number, biology)]. 2002:244–271.
Livezey BC. 1986. A phylogenetic analysis of recent anseriform genera using morphological characters. The Auk. 103:737–754.
Livezey BC. 1996. A phylogenetic analysis of geese and swans (Anseriformes: Anserinae), including selected fossil species. Systematic Biology. 45(4):415–450.
Livezey BC. 1997. A phylogenetic analysis of basal Anseriformes, the fossil Presbyornis, and the interordinal relationships of waterfowl. Transactions of the Zoological Journal of the Linnean Society. 121(4):361–428.
Livezey BC, Zusi RL. 2006. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. Methods and characters. Bulletin of Carnegie Museum of Natural History. 37:1–544.
Mayr G. 2020. The otic region of the skull of neognathous birds: on the homology and comparative morphology of some neurovascular and muscular foramina and other external skeletal structures. Vertebrate Zoology. 70(1):69–85.
Mayr G, Clarke JA. 2003. The deep divergences of neornithine birds: a phylogenetic analysis of morphological characters. Cladistics. 19:527–553.
Murray PF, Vickers-Rich P. 2004. Magnificent mihirungs: the colossal flightless birds of the Australian dreamtime. Indiana University Press, USA.
Olson SL. 1999. The anseriform relationships of Anatalavis Olson and Paris (Anseranatidae), with a new species from the Lower Eocene London Clay. In: Olson, S.L. (Ed.), Avian paleontology at the close of the 20th century: Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution, Washington, D.C., 4–7 June 1996. Smithsonian Contribution to Palaeobiology. 89:231–243.
Parker TJ. 1895. XV. On the cranial osteology, classification, and phylogeny of the Dinornithidae. Zoological Society of London. 13(11):373–445.
Picasso MBJ, Mosto C, Tudisca AM. 2023. The feeding apparatus of Rhea americana (Aves, Palaeognathae): Jaw myology and ontogenetic allometry. Journal of Morphology. 284:e21596.
Pycraft WP. 1900. On the morphology and phylogeny of the Palaeognathae (Ratitae and Crypturi) and Neognathae (Carinatae). The Transactions of the Zoological Society of London. 15(5):149–290.
Sokolov AM. 1995. The interspecific relationships of Fulmar (Fulmarus glacialis) and Southern Fulmar (Fulmarus glacialoides) (Aves: Procellariformes). Zoosystematica rossica. 4(1):183–200.
Stidham TA. 2001. The origin and ecological diversification of modern birds: Evidence from the extinct wading ducks, Presbyornithidae (Neornithes: Anseriformes). University of California, Berkeley [PhD Thesis].
Tambussi CP, Degrange FJ, De Mendoza RS, Sferco E, Santillana S. 2019. A stem anseriform from the early Palaeocene of Antarctica provides new key evidence in the early evolution of waterfowl. Zoological Journal of the Linnean Society. 186(3):673–700.
Weber E. 1993. Zur Evolution basicranialer Gelenke bei Vögeln, insbesondere bei Hühner‐und Entenvögeln (Galloanseres). Zeitschrift für Zoologische Systematik und Evolutionsforschung. 31(4):300–317.
Weber E. 1996. Das skelet-muskel-system des kieferapparates von Aepypodius arfakianus (Salvidori, 1877) (Aves, Megapodiidae). Courier Forschungsinstitut Senckenberg. 189:1–132.
Worthy TH, Degrange FJ, Handley WD, Lee MSY. 2017. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). Royal Society Open Science. 4(10):170975. DOI: 10.1098/rsos.170975
Worthy TH, Lee MSY. 2008. Affinities of Miocene waterfowl (Anatidae: Manuherikia, Dunstanetta and Miotadorna) from the St Bathans Fauna, New Zealand. Palaeontology. 51(3):677–708.
Worthy TH, Scofield RP. 2012. Twenty-first century advances in knowledge of the biology of moa (Aves: Dinornithiformes): a new morphological analysis and moa diagnoses revised. New Zealand Journal of Zoology. 39(2):87–153. DOI: 10.1080/03014223.2012.665060
Zelenkov NV, Stidham TA. 2018. Possible filter-feeding in the extinct Presbyornis and the evolution of Anseriformes (Aves). Zoologicheskiĭ Zhurnal. 97(8):943–956.
Zusi RL, Livezey BC. 2000. Homology and phylogenetic implications of some enigmatic cranial features in galliform and anseriform birds. Annals of Carnegie Museum. 69(3):157–193.
Zusi RL, Livezey BC. 2006. Variation in the os palatinum and its structural relation to the palatum osseum of birds (Aves). Annals of Carnegie Museum. 75(3):137–180.
