Abstract
Background: The aim was to determine prevalence and age at seroconversion of thyroid autoimmunity in relation to islet autoantibodies, gender and HLA-DQ genotypes in children with increased risk for type 1 diabetes followed from birth.
Methods: In 10-year-old children (n = 1874), blood samples were analysed for autoantibodies against thyroid peroxidase (TPOAb), thyroglobulin (TGAb), glutamic acid decarboxylase 65 (GADA), Zink transporter 8 (ZnT8R/W/QA), insulinoma-associated protein-2 (IA-2A), insulin (IAA) and HLA-DQ genotypes. Prospectively collected samples from 2 years of age were next analysed for TPOAb, and TGAb and, finally, in confirming samples at 11–16 years of age along with TSH and FT4. Frequencies were tested with Chi-square or Fischer’s exact tests, autoantibody levels with Wilcoxon and correlations between autoantibody levels with Spearman’s rank correlation test.
Results: The prevalence of thyroid autoimmunity was 6.9%, overrepresented in girls (p < .001) also having higher TPOAb levels at 10 years (p = .049). TPOAb was associated with GADA (p = .002), ZnT8R/W/QA (p = .001) and IA-2A (p = .001) while TGAb were associated with ZnT8R/W/QA (p = .021). In boys only, TPOAb were associated with GADA (p = .002), IA-2A (p = .001), ZnT8R/W/QA (p = .001) and IAA (p = .009), and TGAb with GADA (p = .013), IA-2A (p = .005) and ZnT8R/W/QA (p = .003). Levels of IA-2A correlated to both TPOAb (p = .021) and to TGAb (p = .011). In boys only, levels of GADA and TGAb correlated (p = .009 as did levels of IA-2A and TPOAb (p = .013). The frequency and levels of thyroid autoantibodies increased with age. At follow-up, 22.3% had abnormal thyroid function or were treated with thyroxine.
Conclusions: Thyroid autoimmunity and high TPOAb levels were more common in girls. In contrast, in boys only, there was a strong association with as well as correlation between levels of thyroid and islet autoantibodies. It is concluded that while girls may develop autoimmune thyroid disease (AITD) independent of islet autoantibodies, the risk for thyroid disease in boys may be linked to concomitant islet autoimmunity.
Introduction
Autoimmune thyroid disease (AITD) is a common organ-specific autoimmune disorder that results from a dysregulation of the immune system, leading to an immune attack on the thyroid gland. AITD presents either as thyrotoxicosis, as in Graves’ disease (GD), or as hypothyroidism in the more common Hashimoto’s thyroiditis (HT) [Citation1]. The disease predominates for unknown reasons in females, reported in children and adolescents with a female-male ratio; 4:1 [Citation2]. As in many other autoimmune diseases, the aetiology is not completely defined, but the loss of immunological tolerance to unique thyroid autoantigens is assumed to develop in genetically susceptible individuals after exposure to environmental factors. The autoimmune condition is marked by autoantibodies against thyroid peroxidase (TPO) and thyroglobulin (TG) [Citation1].
TPO is a membrane-associated protein at the apical surface of the thyroid cell and the major enzyme involved in thyroid hormone synthesis [Citation3]. Autoantibodies against thyroid peroxidase (TPOAb) are complement-fixing in vitro and therefore, possibly directly cytotoxic [Citation4]. Not all studies agree on whether TPOAb mediates the cellular destruction or are just markers of the autoimmune process, but their existence correlates with thyroid damage and dysfunction [Citation5] as well as being predictive for AITD [Citation6].
TG is a protein produced in the thyroid follicular cells and the source for thyroxine and triiodothyronine production [Citation3]. Autoantibodies against TG (TGAb) do not activate complement and are, therefore, not thought to be pathogenic [Citation6]. TGAb has been found less predictive for later AITD than TPOAb in both an adult population [Citation7] and in a cohort of children and adolescents with type 1 diabetes [Citation8]. Both thyroid autoantibodies often occur together in patients with AITD but TGAb may be present in children and adolescents with AITD in the absence of TPOAb and vice versa [Citation9].
The aim of this study was to determine (1) the prevalence of TPOAb and TGAb, (2) the age at seroconversion, and (3) relations between thyroid autoimmunity, islet autoantibodies, gender, and HLA-DQ genotypes in the prospective Diabetes Prediction in Skåne (DiPiS) study of children at genetic risk for type 1 diabetes.
We hypothesized that (1) seroconversion of TPOAb and TGAb is rare before the age of 10 years, that (2) TPOAb and TGAb are associated with ZnT8A a relation previously described in children with newly diagnosed type 1 diabetes [Citation10], and (3) that gender differences are found in the association between thyroid and islet autoantibodies, since the female to male ratio of autoimmune hypothyroid disease differ in children with type 1 diabetes compared to those without type 1 diabetes.
Methods
Population
The children in this cohort participate in the DiPiS study, a prospective population-based study in the southern part of Sweden with 1.2 million inhabitants [Citation11]. The aim of the DiPiS study was to identify environmental factors that may trigger islet autoantibodies and determine the predictive value for type 1 diabetes of these autoantibodies, combined with genetic factors. Cord blood samples were obtained from 35,688 children between September 2000 and August 2004 and analysed for type 1 diabetes risk human leukocyte antigen (HLA) genotypes (). First, children in DiPiS were primarily selected based on HLA risk (Larson et al, Table 3) [Citation12]. Second, prior observations in population-based studies in new-born children indicated that (a) cord blood islet autoantibodies [Citation13], (b) ABO incompatibility, mother’s age and large for gestational age children [Citation14], (c) infection during pregnancy [Citation15] as well as (d) severe life event [Citation16] increased the risk for type 1 diabetes. The following score system for follow up was therefore used based on the parental questionnaire when the child was two months of age (participating parents were n = 21,842; ):
Figure 1. Flowchart of children screened at birth and followed in the DiPiS study to 10 years of age, and the distribution of thyroid autoantibodies in the population.
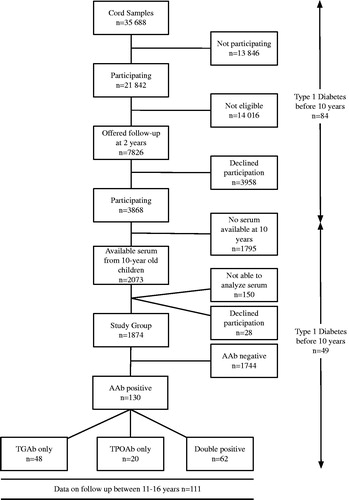
Table 3. Correlations between TPOAb and TGAb levels in thyroid autoantibody positive children.
Step 1. HLA DQ risk: High risk A1*05-B1*02/B1*03:02 (6 points), medium risk B1*03:02/B1*06:04, B1*03:02/X (4 points) and low risk A1*05-B1*02/B1*03:04, A1*05-B1*02/B1*06:04, A1*05-B1*02/X (3 points). Neutral (2 points) and negatively associated (0 points) HLA-DQ genotypes as listed [Citation12] (Larson et al, Table 3).
Step 2. Other risk factors were first-degree relatives (2 points), cord blood islet autoantibodies (2 points), ABO incompatibility (1 point), infection during pregnancy (1 point), severe life events (1 point), mother’s age (1 point) as well as large for gestational age (1 point). The parents were asked to participate if their child reached 3 points. The score system was used to ask 7826 parents to participate in the 2 year follow up resulting in 3868 participating children (). The HLA-DQ genotype distribution of the participating children in this study showed that 233 (12.4%) children were high risk, 562 (30%) medium risk, 488 (26%) low risk, 524 (30%) neutral and 67 (3.6%) negatively associated (Supplementary Table S1).
Study group
In 2015, blood samples from 1874 10-year-old DiPiS children, participating in DiPiS follow-up, were analysed for TPOAb and TGAb (). Children who had tested positive for thyroid autoantibodies at age 10 were asked to submit a confirmatory blood sample a year later when the children were 11–16 years old. This sample was analysed both for thyroid autoantibodies, thyroid-stimulating hormone (TSH) and free thyroxine (FT4). Additionally, in children positive for TPOAb or TGAb at 10 years of age, DiPiS samples collected during the prospective follow up from 2 years of age were tested for TPOAb and TGAb. Information on thyroxine treatment was gathered at the time of confirmatory sampling as well as 18 months later. Children who developed type 1 diabetes before 10 years of age were not included in the present study as samples from those children were unavailable.
The study was approved by the regional ethical review board in Lund.
HLA genotyping
HLA-DQ genotyping was carried out on dried blood spots on filters as described [Citation17]. HLA-type was then classified as HLA-DQA*0301-DQB1*0302 (DQ8) or HLA-DQA*0501-DQB1*0201(DQ2) and divided into 4 risk groups: DQ 8/X, DQ DQ2/X, DQ 2/8 or DQ Y/Y (Y is neither DQ8 nor DQ2).
Autoantibodies to GAD65 and IA-2A
Recombinant GAD65 and IA-2 were generated with 35S-methionine (PerkinElmer, Waltham, MA) by in vitro coupled transcription and translation using the TNT SP6 coupled reticulocyte lysate system (Promega, Madison, WI) as described [Citation18]. Full-length cDNA coding for human GAD65 in the pTNT vector (Promega) (pThGAD65) or the intracellular domain (amino acids 606-979) of IA-2 in the pSP64 Poly(A) vector (Promega) (IA-2ic) were used as templates [Citation19]. GADA and IA-2A were analysed in a radioligand binding assay (RBA) [Citation18]. Duplicate samples were incubated with radio-labelled antigen. The samples were transferred to filtration plates (Millipore, Solna, Sweden) and IgG antibodies precipitated with Protein A Sepharose (Zymed Laboratories Inc., San Francisco, CA). After washing to remove all unbound antigen supermix scintillation cocktail (Perkin Elmer) was added and the radioactivity counted in a Wallac Microbeta Trilux system (Perkin Elmer). GADA and IA2A levels were expressed as units per mL (U/mL) derived from the WHO standard 97/550. GADA levels >34 U/mL and IA-2A levels >5 U/mL were considered positive.
Autoantibodies to insulin
Insulin autoantibodies were analysed by RBA using insulin labelled with 125I (PerkinElmer) as described elsewhere [Citation20]. Samples were assessed under similar conditions as for GADA and IA-2A. All positive samples were analysed further in a competitive assay using non-radioactive human recombinant insulin (Actrapid® from Novo Nordisk, Bagsvaerd, Denmark) to verify false-positive binding. For both methods, levels were calculated as relative units related to positive controls. Positivity for IAA was set to >0.79 relative units. In 2010, the method was adapted as described previously [Citation21], a standard curve with seven different concentrations (3–358 U/mL), plotted against cpm values on a Log2scale, was used to calculate U/mL instead of the relative units.
Autoantibodies to zinc transporter 8 variants
Autoantibodies against the three variants of ZnT8, anti-ZnT8 arginine 325 (ZnT8RA), anti-ZnT8 tryptophan 325 (ZnT8WA), and anti-ZnT8 glutamine 325 (ZnT8QA) were analysed with an RBA as described previously [Citation22]. Duplicate samples were incubated with equal amounts of the three radio-labelled ZnT8 R/W/Q variants. Every sample >59 U/mL was considered positive and therefore, analysed for each of the three variants separately.
Autoantibodies to thyroid peroxidase and thyroglobulin
TPOAb and TGAb were determined in plasma samples using RIA kits supplied by RSR Limited (art. no. RS-TP/100 and RS-TG/100 respectively), according to the manufacturer’s instructions (RSR Limited, Cardiff, UK).
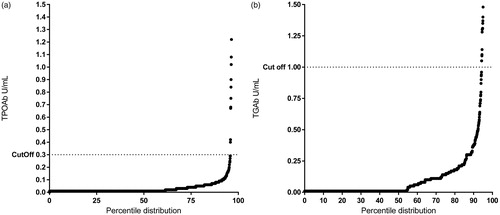
Levels of both TPOAb and TGAb were derived from the standard curve included in the kit and expressed as U/mL. For TPOAb, we used fit spline point to point curve fit and for TGAb cubic spline was used. Positivity for TGAb was set at >0.99 U/mL, defined after QQ plot analysis (). Cut off for positivity for TPOAb was >0.29 U/mL as suggested by the manufacturer and confirmed using QQ-plot analysis ().
TSH and FT4
TSH and FT4 in plasma were analysed in the clinical biochemical department in Malmö University hospital. Analyses were made using Electro-chemiluminescence immunoassay (ECLIA) according to the manufacturer’s instructions (Cobas, Roche Diagnostics Ltd., Rotkreuz, Switzerland). Reference value for TSH was 0.4–3.7 pmol/L and 12–22 pmol/L for FT4.
Statistical methods
Statistical analyses were performed using SPSS version 22.0 statistical software (IBM SPSS, Armonk, NY) and R 3.4.1.
Differences in proportions between groups were tested using the χ2 test, Fisher’s exact test when appropriate or Mann–Whitney U test depending on the variable. Difference in autoantibody levels was tested with Wilcoxon and correlations between autoantibody levels with Spearman’s rank correlation test. OR with 95% CI was calculated. p-Value <.05 was considered significant. QQ-plot analysis was performed using Microsoft Excel and GraphPad Prism 6.03 software (La Jolla, CA).
Results
The prevalence of thyroid autoimmunity in the 1874 10-year-old children was 6.9% (130/1874) distributed between TPOAb only (n = 48), TGAb only (n = 20) and double positive (n = 62) ().
Girls, 49.7% of the cohort (931/1874), showed an increased risk for TPOAb, TGAb and for having both (all three variables p < .001, ). Female to male ratios were 2.95:1 for TPOAb, 2.5:1 for TGAb and 3.13:1 for the double positives. In addition, TPOAb levels were higher in girls (p = .0049, Wilcoxon) while TGAb levels were not affected by gender ().
Figure 3. TPOAb and TGAb levels in females and males positive for thyroid autoimmunity at 10 years. Test; Wilcoxon.
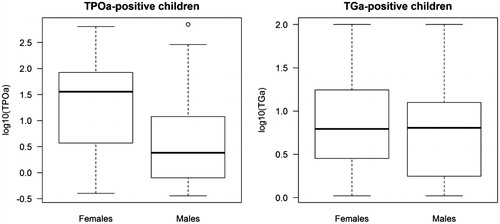
Table 1. Associations between islet autoantibodies and autoantibodies against TPO and TG at 10 years, χ2 test and fishers exact were used for analysis.
TPO and TG autoantibodies at 10 years of age in relation to islet autoantibodies
At 10 years of age, TPOAb was positively associated with GADA (p = .002), IA-2A (p = .001) and ZnT8AR/W/QA (p < .001), but not with IAA (p = .13).
In contrast, TGAb was positively associated only with ZnT8R/W/QA (p = .021) but not with GADA, IA-2A or IAA (). Children double positive for TPOAb and TGAb were more frequently also positive for ZnT8R/W/QA (p = .022) ().
We next analysed the correlation between levels of thyroid autoantibodies and GADA, IA-2A, and ZnT8R/W/QA (). There was a correlation between levels of IA-2A and TPOAb (p = .021) as well as with TGAb (p = .011) but not with GADA or ZnT8R/W/QA (). No correlation was found between levels of thyroid and islet autoantibodies in the thyroid autoantibody positive children (data not shown).
Table 2. Correlation between islet autoantibody and thyroid autoantibody levels in islet autoantibody positive children.
TPO and TG autoantibodies at 10 years of age in relation to islet autoantibodies in girls
The frequency of girls positive for any islet autoantibody at 10 years of age was 5.6% (52/931). No association was found between thyroid and any of the individual islet autoantibodies () nor was there any correlation between levels of any of the individual islet and thyroid autoantibodies ().
TPO and TG autoantibodies at 10 years of age in relation to islet autoantibodies in boys
At 10 years of age, 6.9% (65/943) of the boys were positive for any islet autoantibody. In contrast to the girls, TPOAb in boys were positively associated with all individual islet autoantibodies; GADA (p = .002), IA-2A (p = .001), ZnT8R/W/QA (p = .001), and IAA (p = .009) (). TGAb was found positively associated with GADA (p = .013), IA-2A (p = .005), ZnT8R/W/QA (p = .003) while no association was found with IAA (p = 1.0). Boys positive for both thyroid autoantibodies were more likely positive for either GADA (p = .03), IA-2A (p = .005), ZnT8R/W/QA (p = .003) or IAA (p = .038) ().
In addition, in GADA positive boys, levels of GADA correlated with that of TGAb (p = .009). In IA-2A positive boys, levels of IA-2A correlated with that of TPOAb (p = .013), while ZnT8R/W/QA positive boys showed no correlations to the thyroid autoantibodies ().
TPO and TG autoantibodies in relation to HLA-DQ genotypes
No association was found between thyroid autoimmunity and HLA-DQ genotypes (data not shown).
TPO and TG autoantibodies before 10 years of age
In children positive for thyroid autoantibodies at 10 years, we analysed TPOAb and TGAb in samples collected from two years of age and onwards (). Some (n = 7) children were found positive as young as 2 years of age. Overall, the two thyroid autoantibodies increased with increasing age in both frequency and levels.
Figure 4. The age of TPOAb and TGAb appearance in thyroid autoantibody positive children. Panel; (a) Children positive for TPOAb and TGAb, (b) Children positive for TPOAb or TGAb, (c) Children positive for TPOAb and (d) Children positive for TGAb.
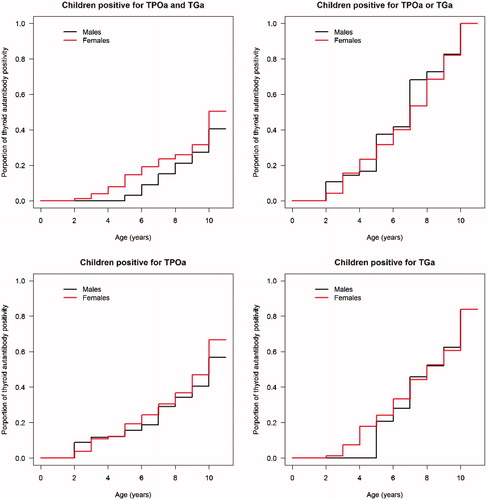
It was found that 2/12 developed a thyroid autoantibody before any islet autoantibody, 5/12 simultaneously while 5/12 developed islet autoantibodies before any thyroid autoantibody.
Thyroid autoantibody results in confirming analysis
Children positive for TPOAb or TGAb at 10 years of age, were asked to give a confirmatory sample at 11–16 years of age. A total of 102/130 agreed to this confirmatory test which also included a thyroid functional test. At confirmation, 96/102 (94%) where still thyroid autoantibody positive. All 6/102 children negative for thyroid autoimmunity had at 10 years of age been single positive for TGAb but negative for TPOAb.
Levels in children who were positive at 10 years of age as well as in the confirmation samples at age 11–16 years, and with at least one negative sample before seroconversion or who were positive already at 2 years of age, are shown in Supplementary Figure S1. TPOAb levels increased from the first positive sample to the next in 83.9% of the subjects’ positive for TPOAb. TGAb levels similarly increased in 60.6% of the TGAb positive children.
In TPOAb positive children, levels of TPOAb correlated with that of TGAb (p < .001) ().
Thyroid function and clinical thyroid disease
Among the 28/130 children who did not agree to a confirmatory test, nine were already diagnosed and treated for hypothyroidism (8/9) or hyperthyroidism (1/9). Among the 102 children who did agree to be tested, 15 had TSH above the reference (>3.8 mIE/L) and one below (<0.01 mIE/L). Additional follow up 18 months later revealed that 7 of the 16 children with abnormal thyroid function and 4 children with normal thyroid function at confirmatory sample developed hypothyroidism of the 102 autoantibody positive children. Taken together, 22.3% (29/130) of the children who were thyroid autoantibody positive at 10 years of age, either had an established diagnosis of hypo- or hyper-thyroidism, were diagnosed during follow-up, or had abnormal TSH levels at the confirmatory sample. No follow-up information at 11–16 years of age was available in 15% (21/130) of the children.
Discussion
This study is a large cross-sectional study of thyroid autoimmunity, both TPOAb and TGAb, at 10 years of age in a cohort of prospectively followed children at genetic risk for type 1 diabetes. In our study, we found that 6.9% of our 1874 children had thyroid autoimmunity, overrepresented in girls and positively related to GADA, ZnT8R/W/QA and IA-2A as well as to multiple islet autoantibodies.
Thyroid autoimmunity was, as expected, more common with increasing age although, interestingly, found in children as young as 2 years of age. It is also of interest that as many as 20/130 children tested positive for thyroid autoimmunity at 10 years of age, had been diagnosed with either hypo- or hyper-thyroidism at the end of follow-up. The quite high incidence of abnormal TSH and treatment for AITD in the cohort may be explained by the higher incidence of type 1 diabetes (1.9%) in the cohort compared to the Swedish population (0.8%) [Citation23] as the co-occurrence of the diseases and possible responsible genes is reported [Citation24–27].
When studying thyroid autoimmunity in children from the general population, frequencies vary between 0.4 and 3.7% in 8.8–12-year-old children, depending on population and which autoantibody that was tested [Citation28–30]. In a study screening for thyroid autoimmunity and coeliac disease in 12-year-old Swedish children, 2.8% were tested positive for TPOAb in the healthy control group [Citation30]. The frequency of TPOAb in our DiPiS children (6.9%) was therefore clearly increased similar to the 7.3% TPOAb positive children at 11 years of age in the BABYDIAB study [Citation31]. This similarity is interesting as the BABYDIAB children were born to parents with type 1 diabetes while no effort was made in DiPiS to specifically recruit children born in the 10% of families already affected by type 1 diabetes. We confirm the relationship between TPOAb and GADA reported in the BABYDIAB study but also report that TPOAb in our DiPiS children were associated with IA-2A, not found in the BABDIAB study [Citation31]. This difference may be due to power or a difference in overall HLA risk. For example, it is known that IA-2A are associated with HLA-DQ8 but negatively associated with HLA-DQ2 [Citation32]. It cannot be excluded that the difference between the two studies is that only IA-2A in the absence of GADA was analysed in the BABYDIAB study [Citation31]. It is also noted that ZnT8A was analysed in the present study, but not in BABYDIAB, to reveal an association with both TPOAb and TGAb ().
Studies on the seroconversion of thyroid autoimmunity are limited due to the lack of prospective studies. The strength of our study is the large cohort of children, followed from birth and sampled yearly from 2 years of age with full titrated analysis of all islet autoantibodies including ZnT8R/W/QA, and with the possibility to go back and evaluate previous samples from children positive for thyroid autoimmunity at 10 years of age. In prospective studies on islet autoimmunity, appearance of GADA peaked at 33 months, later than IAA which peaked at 18 months [Citation33]. Hence, the appearance of TPOAb in our cohort may appear later and in contrast to IAA seroconversion of TPOAb was more common with increasing age. However, two children developed thyroid autoimmunity before islet autoimmunity and five children simultaneously.
Another strength of the study is that children who tested positive for TPOAb or TGAb were retested revealing that 94% of the children were still positive at 11–16 years. Additionally, TSH and FT4 were analysed allowing information on thyroid function. Subclinical non-symptomatic thyroid autoimmune disease may be more common than hitherto known indicating a distinct need for screening and preventive therapies.
In our study, we were able to show that the association with islet autoantibodies differed between the two thyroid autoantibodies as well as gender. We could confirm the previously described association between TPOAb and GADA in children and adults screened for autoimmunity [Citation34], children and adolescents diagnosed with AITD [Citation35] as well as in patients with type 1 diabetes [Citation10,Citation36,Citation37] and GD [Citation38]. GADA in patients with AITD might not only indicate increased risk for type 1 diabetes, but also be a biomarker that reflects other endocrine autoimmunity like thyroid autoimmunity as GADA has been found associated to thyroid disease in patients with type 2 diabetes [Citation39]. The findings of GADA in patients with monogenic diabetes as well are interesting [Citation40], whether GADA in these patients reflects the risk for other autoimmune diseases remains to be studied. Interestingly, we previously reported that GADA in young children diagnosed with type 1 diabetes before 5 years of age was predictive of clinical AITD, while TPOAb was only predictive in older children [Citation8]. One explanation for the positive association between GADA and thyroid autoimmunity might be female gender, as thyroid autoimmunity and AITD are well known to be more common in females. GADA is likewise associated with female gender in children and young adults at type 1 diabetes diagnosis [Citation32]. Moreover, GADA has been shown positively associated with HLA-DQ2 irrespective of gender [Citation32], and an association between AITD and HLA-DQ2 has also been described [Citation41,Citation42]. However, in this study, although thyroid autoimmunity was more common in girls with a clear gender difference in TPOAb but not TGAb levels the observed association between GADA and thyroid autoimmunity was only seen in boys. Moreover, the positive relation found between thyroid autoantibodies and islet autoantibodies was only seen in boys with exception of girls with multiple islet autoantibodies. Therefore, although more common in girls, thyroid autoimmunity is more strongly associated with islet autoimmunity in boys, possibly explaining the different F:M ratio in AITD amongst patients with type 1 diabetes 1.94:1 [Citation8] versus non-diabetic 4:1 [Citation2]. This is further stressed by the fact that levels of GADA were associated with TGAb and levels of IA-2A to TPOAb in boys but not in girls. Therefore, boys with high levels of islet autoantibodies discovered before or after clinical diagnosis of type 1 diabetes, seem to be at high risk of thyroid autoimmunity. Interestingly, a recent study found genetic specificity to influence the levels of thyroid autoantibodies dependant on gender [Citation43]. The fact that we did not find an association between levels of thyroid autoantibodies and levels of islet autoimmunity in children with thyroid autoimmunity, may be due to the relatively low number of thyroid positive boys, but also implicate that thyroid autoimmunity per se does not increase the risk of islet autoimmunity, while islet autoimmunity is – at least in boys – a risk factor for thyroid autoimmunity.
ZnT8R/W/QA was the only islet autoantibody found to be positively associated with both TPOAb and TGAb. We previously described a novel discovery that ZnT8R/W/QA was associated with thyroid autoimmunity in children and adolescent with newly diagnosed type 1 diabetes [Citation10], and we could now confirm this finding in children without diabetes, while at increased risk for the disease. ZnT8 is expressed in the beta cells [Citation44] and autoantibodies against ZnT8 are common at the diagnosis of type 1 diabetes [Citation45]. As ZnT8 is strongly linked to secretory activity, studies have focussed on endocrine organs and demonstrated that ZnT8 is also expressed in the cubical cells lining the thyroid gland as well as in the adrenal gland cortical cells [Citation46]. The findings that ZnT8 is expressed in endocrine secretory cells other than the beta cells and the fact that the ZnT8R/W/QA are not found to be associated with the two most common type 1 diabetes HLA haplotypes [Citation45] but with DQB1*06:04 [Citation47] might indicate polyendocrine autoimmunity and not only islet autoimmunity. However, no association was found between ZnT8R/W/QA and later treatment for AITD in a cohort of children and adolescent with type 1 diabetes at follow up [Citation8].
In our study, we could not confirm the association between HLA-DQ2 and thyroid autoimmunity nor either the positive association to HLA-DQ8 [Citation48], which may be due to the selection of HLA-DQ genotypes in the cohort. Lack of resources precluded HLA typing by next-generation sequencing [Citation49], which should prove important to future investigations of the possible association between HLA-DR-DQ-DP and thyroid autoimmunity.
A limitation of our study is the type 1 diabetes centric HLA-DQ genotypes, loss of follow up from the children who developed type 1 diabetes before 10 years of age, as well as discontinuous analysis of children who either choose to withdraw at some age or not to participate annually. Likewise, we do not have information on time of seroconversion and type of first autoantibody in children developing autoimmunity early as the follow-up in the DiPiS study started at age 2.
In conclusion, in our study of prospectively followed children at risk for type 1 diabetes, thyroid autoimmunity and high TPOAb levels were more common in girls, while the relation between thyroid and islet autoimmunity as well as associations of levels of autoantibodies was only found in boys. This indicates that thyroid autoimmunity in boys may be dependent on a concomitant autoimmune condition, such as islet autoimmunity or type 1 diabetes, and that high levels of autoantibodies may increase the risk of AITD. Screening of thyroid autoimmunity in such children should be done, since a high proportion of the children develop clinical thyroid disease. Although some children developed thyroid autoantibodies early in life, we confirm that the incidence of thyroid autoimmunity increased with increasing age.
Acknowledgements
All the children participating in DiPiS and their families are gratefully acknowledged. We also thank Beata Felisiak and Rasmus Bennet, for technical assistance. We thank Åke Lernmark for support and critical revision of this article. BJ designed the study, collected, analysed and interpreted data and wrote and revised the manuscript, CL analysed and interpreted the data and edited the manuscript, IJ analysed data and revised the manuscript, ML collected data and revised the manuscript and HEL designed the study, interpreted data and revised this article.
The members of the DiPiS study group are: C. Andersson, R. Bennet, I. Jönsson, M. Ask, J. Bremer, C. Brundin, C. Cilio, C. Hansson, G. Hansson, S. Ivarsson, B. Jonsdottir, Å Lernmark, B. Lindberg, B. Lernmark, M. Lundgren, J Melin, A. Ramelius, I. Wigheden, U.-M. Carlsson, A. Svärd (Department of Clinical Sciences Malmö, Lund University, Sweden) A. Carlsson (Department of Clinical Sciences Lund, Lund university, Sweden), E. Cedervall (Department of Paediatrics, Ängelholm hospital, Sweden), B. Jönsson (Department of Paediatrics, Ystad Hospital, Sweden), K. Larsson (Department of Paediatrics, Kristianstad Central Hospital, Sweden) and J. Neiderud (Department of paediatrics, Helsingborg Hospital, Sweden).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340.
- de Vries L, Bulvik S, Phillip M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow-up. Arch Dis Child. 2009;94:33–37.
- Sinclair D. Analytical aspects of thyroid antibodies estimation. Autoimmunity. 2008;41:46–54.
- Blanchin S, Estienne V, Durand-Gorde JM, et al. Complement activation by direct C4 binding to thyroperoxidase in Hashimoto’s thyroiditis. Endocrinology. 2003;144:5422–5429.
- McLachlan SM, Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid. 2007;17:939–948.
- McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid. 2004;14:510–520.
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226.
- Jonsdottir B, Larsson C, Carlsson A, et al. Thyroid and islet autoantibodies predict autoimmune thyroid disease at type 1 diabetes diagnosis. J Clin Endocrinol Metab. 2017;102:1277–1285.
- Antonelli A, Ferrari SM, Corrado A, et al. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14:174–180.
- Jonsdottir B, Andersson C, Carlsson A, et al. Thyroid autoimmunity in relation to islet autoantibodies and HLA-DQ genotype in newly diagnosed type 1 diabetes in children and adolescents. Diabetologia. 2013;56:1735–1742.
- Larsson K, Elding-Larsson H, Cederwall E, et al. Genetic and perinatal factors as risk for childhood type 1 diabetes. Diabetes Metab Res Rev. 2004;20:429–437.
- Larsson HE, Lynch K, Lernmark B, et al. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50:1161–1169.
- Lindberg B, Ivarsson SA, Lernmark A. Islet autoantibodies in cord blood could be a risk factor for future diabetes. Diabetologia. 1999;42:1375.
- Dahlquist G, Källén B. Maternal-child blood group incompatibility and other perinatal events increase the risk for early-onset type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:671–675.
- Lynch KF, Lernmark B, Merlo J, et al. Cord blood islet autoantibodies and seasonal association with the type 1 diabetes high-risk genotype. J Perinatol. 2008;28:211–217.
- Lernmark B, Lynch K, Lernmark A. Cord blood islet autoantibodies are related to stress in the mother during pregnancy. Ann N Y Acad Sci. 2006;1079:345–349.
- Larsson HE, Lynch K, Lernmark B, et al. Diabetes-associated HLA genotypes affect birthweight in the general population. Diabetologia. 2005;48:1484–1491.
- Grubin CE, Daniels T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–350.
- Payton MA, Hawkes CJ, Christie MR. Relationship of the 37,000- and 40,000-M(r) tryptic fragments of islet antigens in insulin-dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA512). J Clin Invest. 1995;96:1506–1511.
- Delli AJ, Vaziri-Sani F, Lindblad B, et al. Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the better diabetes diagnosis study. Diabetes. 2012;61:2556–2564.
- Lundgren M, Sahlin A, Svensson C, et al. Reduced morbidity at diagnosis and improved glycemic control in children previously enrolled in DiPiS follow-up. Pediatr Diabetes. 2014;15:494–501.
- Vaziri-Sani F, Delli AJ, Elding-Larsson H, et al. A novel triple mix radiobinding assay for the three ZnT8 (ZnT8-RWQ) autoantibody variants in children with newly diagnosed diabetes. J Immunol Methods. 2011;371:25–37.
- Swediabkids. 2016 online. Availble from https://swediabkids.ndr.nu/Documents/NDR-Child/AnnualReport-2016.pdf.
- Shun CB, Donaghue KC, Phelan H, et al. Thyroid autoimmunity in type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31:126–135.
- Tomer Y, Dolan LM, Kahaly G, et al. Genome wide identification of new genes and pathways in patients with both autoimmune thyroiditis and type 1 diabetes. J Autoimmun. 2015;60:32–39.
- Kim EY, Shin CH, Yang SW. Polymorphisms of HLA class II predispose children and adolescents with type 1 diabetes mellitus to autoimmune thyroid disease. Autoimmunity 2003;36:177–181.
- Grubic Z, Rojnic Putarek N, Maskalan M, et al. Human leukocyte antigen class II polymorphisms among Croatian patients with type 1 diabetes and autoimmune polyglandular syndrome type 3 variant. Gene 2018;674:93–97.
- Kondrashova A, Viskari H, Haapala AM, et al. Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. J Clin Endocrinol Metab. 2008;93:729–734.
- Garcia-Garcia E, Vazquez-Lopez MA, Garcia-Fuentes E, et al. Thyroid function and thyroid autoimmunity in relation to weight status and cardiovascular risk factors in children and adolescents: a population-based study. J Clin Res Pediatr Endocrinol. 2016;8:157–162.
- van der Pals M, Ivarsson A, Norstrom F, et al. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis. 2014;2014:417356.
- Bonifacio E, Mayr A, Knopff A, et al. Endocrine autoimmunity in families with type 1 diabetes: frequent appearance of thyroid autoimmunity during late childhood and adolescence. Diabetologia. 2009;52:185–192.
- Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355.
- Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–987.
- Marwaha RK, MK, Garg N, Tandon R, et al. Glutamic acid decarboxylase (anti-GAD) & tissue transglutaminase (anti-TTG) antibodies in patients with thyroid autoimmunity. Indian J Med Res. 2013;137:82–86.
- Pilia S, Casini MR, Cambuli VM, et al. Prevalence of Type 1 diabetes autoantibodies (GAD and IA2) in Sardinian children and adolescents with autoimmune thyroiditis. Diabet Med. 2011;28:896–899.
- Kordonouri O, Charpentier N, Hartmann R. GADA positivity at onset of type 1 diabetes is a risk factor for the development of autoimmune thyroiditis. Pediatr Diabetes. 2011;12:31–33.
- Hwang GB, Yoon JS, Park KJ, et al. Prevalence of autoimmune thyroiditis in patients with type 1 diabetes: a long-term follow-up study. Ann Pediatr Endocrinol Metab. 2018;23:33–37.
- Moriguchi M, Noso S, Kawabata Y, et al. Clinical and genetic characteristics of patients with autoimmune thyroid disease with anti-islet autoimmunity. Metabolism. 2011;60:761–766.
- Haller-Kikkatalo K, Pruul K, Kisand K, et al. GADA and anti-ZnT8 complicate the outcome of phenotypic type 2 diabetes of adults. Eur J Clin Invest. 2015;45:255–262.
- Urbanova J, Rypackova B, Prochazkova Z, et al. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med. 2014;31:466–471.
- Zeitlin AA, Heward JM, Newby PR, et al. Analysis of HLA class II genes in Hashimoto’s thyroiditis reveals differences compared to Graves’ disease. Genes Immun. 2008;9:358–363.
- Levin L, Ban Y, Concepcion E, et al. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum Immunol. 2004;65:640–647.
- Matana AM, Popovic T, Boutin V, et al. Genome-wide meta-analysis identifies novel gender specific loci associated with thyroid antibodies level in Croatians. Genomics. 2018;pii: S0888–7543(18);30242–30248.
- Chimienti F, Devergnas S, Favier A, et al. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337.
- Andersson C, Larsson K, Vaziri-Sani F, et al. The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity. 2011;44:394–405.
- Murgia C, Devirgiliis C, Mancini E, et al. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis. 2009;19:431–439.
- Andersson C, Vaziri-Sani F, Delli A, et al. Triple specificity of ZnT8 autoantibodies in relation to HLA and other islet autoantibodies in childhood and adolescent type 1 diabetes. Pediatr Diabetes. 2013;14:97–105.
- Kahles H, Fain PR, Baker P, et al. Genetics of autoimmune thyroiditis in type 1 diabetes reveals a novel association with DPB1*0201: data from the type 1 diabetes genetics consortium. Dia Care. 2015;38:S21–S28.
- Zhao LP, Alshiekh S, Zhao M, et al. Next-generation sequencing reveals that HLA-DRB3, -DRB4, and -DRB5 may be associated with islet autoantibodies and risk for childhood type 1 diabetes. Diabetes. 2016;65:710–718.