Introduction
Originally, neutrophil granulocytes were described to phagocytose and kill bacteria. In 2004, a second mechanism in which neutrophils trap bacteria with extracellular DNA (NETs) was described by Brinkmann et al. [Citation1]. They reported that microbial agents can be ensnared in decondensed chromatin released together with granule proteins. NETs are not just binding the foe [Citation1], but also degrade virulence factors with NET-borne active enzymes [Citation2]. Since then, a plethora of articles emerged linking NETs to host defense but also to a number of diseases – often in ambivalent roles. Literature of NET formation can be confusing as evidence often suggests both, pathognomonic or protective roles of NETs. It cannot be ignored that NETs may play a pivotal role in inflammatory diseases. This editorial summarizes a collection of articles investigating the role of NETs in autoimmune diseases.
NETs as source of autoantigens in autoimmune diseases
Anti-citrullinated protein autoantibodies (ACPAs) are often generated in chronic inflammatory diseases, for example, rheumatoid arthritis (RA), chronic obstructive pulmonary disease, systemic lupus erythematosus (SLE), or periodontitis (PD). ACPAs often precede the onset of clinical and subclinical disease. Citrullination of proteins and systemic autoimmunity against these novel autoantigens occurs via enzymes like peptidylarginine deiminase 4 (PAD4), which can exemplarily be induced by cigarette smoke, partly explaining the elevated risk of smokers to develop RA. Vitkov et al. [Citation3] investigated PD, as it exhibits two sources of citrullination, PAD4 of periodontal neutrophils and neutrophil extracellular traps (NETs) as well as the PAD of Porphyromonas gingivalis (PPAD). They hypothesize that PD leads to an increased responsiveness to autoantigens via TLR4 activation participating in the development and propagation of RA. Furthermore, circulating PD-derived bacterial DNA could be taken up by phagocytes, activate TLR9, and thus increase the responsiveness to autoantigens ().
Figure 1. Implication of NETs in Autoimmune diseases. (A) NETs serve as Neo-Epitopes in a Number of Autoimmune Diseases. Vitkov et al. [Citation3] linked PD to NET formation and ACPA generation. Applegren et al. [Citation4] found NETs on mitral valves in a patient with severe SLE. Roitsch et al. [Citation5] detected ANCAs on NETs. (B) NETs can be utilized to detect ANCAs on NET-covered microbeads in flurocytometric assays (Roitsch et al. [Citation5]). (C) The modification of autoantibodies in MS by NET proteases alters glycan accessibility (Paryzhak et al. [Citation8]). (D) NET Formation has vast implications in autoimmune diseases. Podolska et al. [Citation6] and Dwivedi et al. [Citation7] take a closer look into the Literature surrounding the contribution of NETs to autoimmune diseases. (E) Exemplifies NET-driven pathologies.
![Figure 1. Implication of NETs in Autoimmune diseases. (A) NETs serve as Neo-Epitopes in a Number of Autoimmune Diseases. Vitkov et al. [Citation3] linked PD to NET formation and ACPA generation. Applegren et al. [Citation4] found NETs on mitral valves in a patient with severe SLE. Roitsch et al. [Citation5] detected ANCAs on NETs. (B) NETs can be utilized to detect ANCAs on NET-covered microbeads in flurocytometric assays (Roitsch et al. [Citation5]). (C) The modification of autoantibodies in MS by NET proteases alters glycan accessibility (Paryzhak et al. [Citation8]). (D) NET Formation has vast implications in autoimmune diseases. Podolska et al. [Citation6] and Dwivedi et al. [Citation7] take a closer look into the Literature surrounding the contribution of NETs to autoimmune diseases. (E) Exemplifies NET-driven pathologies.](/cms/asset/635baa25-a180-41a4-a6af-2ec57dd96549/iaut_a_1539839_f0001_c.jpg)
The case study by Appelgren et al. [Citation4] illustrates that different disease mechanisms mediated via autoantibodies may occur simultaneously in SLE. They describe a dramatic onset of SLE with clear-cut pathogenic implications for neutrophils and NET formation in a young woman with cardiac (Libman–Sacks endocarditis) and central nervous system (psychosis and seizures) involvement. They observed active NET formation in the tissue of the mitral valve, as well as in the circulation and discuss the implications of NET formation in the development of SLE.
Roitsch et al. [Citation5] analyzed the presence of p-ANCA (anti-neutrophil cytoplasmic antibodies), c-ANCA, and a-ANCA on NETs ().
NET involvement in disease onset, progression, and severity
In many rheumatic diseases, autoantibodies develop that react with molecules/structures commonly found hidden in neutrophils, but can also be expelled on NETs. Podolska et al. [Citation6] took a closer look at what happens if NETs are not removed in a timely manner. They investigate the involvement of NETs in chronic inflammatory diseases.
The contributions of NETs to disease onset or progression of autoimmune diseases often seam controversial. Dwivedi et al. [Citation7] focused on the current literature and highlight gaps and inconsistencies in published work and review NETs and their role in health and disease. In this article, they contrast NET formation with other types of cell death pathways that may affect autoimmunity ().
Utilizing NETs to measure autoantibodies
Current diagnostic tools for the detection of ANCAs are often time-consuming, expensive, and require highly trained personnel for significant test results. Roitsch et al. [Citation5] created NET-coated microbeads. They reliably detect p-ANCA, c-ANCA, and a-ANCA in analyses by flow cytometry of patient sera ().
Modifying autoantibodies with NETs
NETs are decorated with active enzymes like neutrophil proteases and can degrade cytokines. Paryzhak et al. [Citation8] wondered whether glycosidases are active on NETs. They searched for the occurrence of NET-related changes in glycan composition on circulating IgG molecules and IgG–IgM immune complexes in patients suffering from multiple sclerosis. Their data indicate the potential of neutrophil-derived proteases to partially degrade circulating immune complexes leading to the exposure of hidden, usually internal glycoepitopes ( and ).
Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) and Universitätsklinikum Erlangen, Erlangen, Germany
[email protected]
Disclosure statement
All authors report no conflict of interest – financial or otherwise – that may directly or indirectly influence the content of the manuscript submitted.
Additional information
Funding
References
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535.
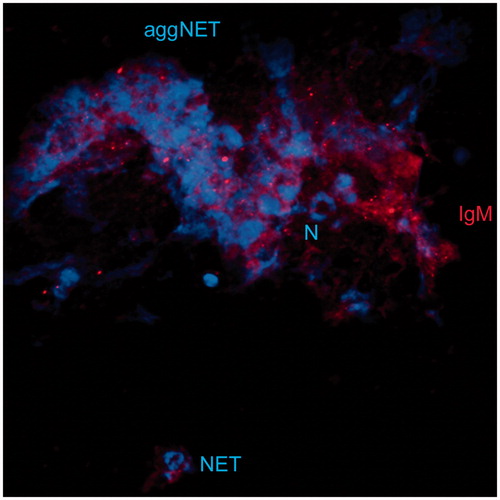
- Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517.
- Vitkov L, Hannig M, Minnich B, et al. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity. 2008;51:302–307.
- Appelgren D, Dahle C, Knopf J, et al. Active NET formation in Libman–Sacks endocarditis without antiphospholipid antibodies: A dramatic onset of systemic lupus erythematosus. Autoimmunity. 2008;51:308–316.
- Roitsch S, Goßwein S, Neurath MF, et al. Detection by flow cytometry of anti-neutrophil cytoplasmic antibodies in a novel approach based on neutrophil extracellular traps. Autoimmunity. 2008;51:286–294.
- Podolska MJ, Mahajan A, Knopf J, et al. Autoimmune, rheumatic, chronic inflammatory diseases: Neutrophil extracellula traps on parade. Autoimmunity. 2008;51:279–285.
- Dwivedi N and Radic M. Burning controversies in NETs and autoimmunity: The mysteries of cell death and autoimmune disease. Autoimmunity. 2008;51:265–278.
- Paryzhak S, Dumych T, Mahorivska I, et al. Neutrophil-released enzymes can influence composition of circulating immune complexes in multiple sclerosis. Autoimmunity. 2008;51:295–301.