Abstract
The use of high-quality antigen-specific immunoassays for detecting anti-neutrophil cytoplasmic antibodies (ANCA) and anti-glomerular basement membrane (GBM) autoantibodies is recommended in patients with suspected ANCA vasculitis and/or anti-GBM disease. We analysed the diagnostic performance of a semi-quantitative and rapid immunoblot (EUROIMMUN AG, Lübeck, Germany) in two settings. Patient sera from different cohorts (ANCA vasculitis n = 187, anti-GBM disease n = 19, and disease controls n = 51) were used. The diagnostic performance of the immunoblot was assessed when used as a confirmatory test for the presence of ANCA in suspected ANCA vasculitis and when evaluating the presence of ANCA and/or anti-GBM antibodies in AAV and/or anti-GBM disease patients with a rapidly progressive glomerulonephritis (RPGN). In a confirmatory test setting, the immunoblot had an optimal sensitivity and specificity of 97.4 and 98.1% for PR3-ANCA and 98.5 and 96.4% for MPO-ANCA, respectively. With increasing test result ranges, a higher interval likelihood ratio (LR) was found for both ANCA entities. When evaluating for ANCA in patients with RPGN, the highest diagnostic accuracy (sensitivity 92.9% and specificity 100%) was obtained by using different cut-off values of positivity for PR3- (>5) and MPO-ANCA (>10). Also, the diagnostic performance for detecting anti-GBM was good (sensitivity 100% and specificity 100%). There are advantages over other assays in terms of time, costs, and interpretation of results. The immunoblot is a useful addition to current guidelines, particularly when a rapid diagnosis is necessary.
Introduction
Anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) consists of an entity of immune-mediated, predominantly small-vessel vasculitides, such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA), in presence of antibodies against proteinase-3 (PR3) or myeloperoxidase (MPO) in most patients [Citation1,Citation2]. Renal involvement is found in 58.8% of patients with AAV with rapidly progressive glomerulonephritis (RPGN) as a severe manifestation [Citation3], requiring prompt initiation of immunosuppressive therapy to prevent end-organ damage. Interestingly, up to 10% of ANCA patients, predominantly MPO-ANCA, are also positive for anti-glomerular basement membrane (GBM) antibodies [Citation4]. Anti-GBM disease is a rare form of small-vessel vasculitis leading to immune-complex mediated RPGN, pulmonary capillaritis or both [Citation5,Citation6]. Serological detection of ANCA and anti-GBM antibodies should therefore be performed immediately in patients with suspected RPGN, especially when renal biopsy is not readily available. Differentiating between AAV and anti-GBM disease can be challenging in acute settings, but is clinically important, since apheresis in anti-GBM disease is globally recommended but has increasingly become under fire in AAV [Citation7].
For the detection of anti-GBM antibodies, antigen-specific or indirect immunofluorescence (IIF) assays are routinely used [Citation8–10]. The Revised 2017 international consensus on testing of ANCAs in GPA and MPA recommends the use of high-quality antigen-specific immunoassays for screening of MPO-ANCA or PR3-ANCA in patients with a clinical suspicion [Citation11]. A confirmatory immunoassay should be considered in cases of low-positive test results or in context of a high clinical suspicion but a negative ANCA test. This approach results in a high diagnostic accuracy in detecting ANCA. On the other hand, the implementation of these recommendations can potentially be challenging for clinical laboratories. Logistical problems can occur when a confirmatory test is not immediately available on site due to the lack of expertise in performing IIF or the incapacity of running two immunoassays. Additional ANCA testing may have to be performed elsewhere and may lead to a clinically relevant delay in initiating a therapy.
Several assays other than IIF or ELISA are commercially available for a rapid detection of ANCA and/or anti-GBM. For instance, dot-blot assays have been shown to be highly sensitive and specific in detecting ANCA and anti-GBM in patients with suspected AAV or anti-GBM disease [Citation12]. Drawbacks are the reduced sensitivity of 90% for ANCA detection [Citation12], in particular 80 − 86% for MPO-ANCA [Citation13], and the interpretation of the qualitative test result, leading to a relatively high inter-observer effect, especially for the detection of anti-GBM [Citation13]. An automated documentation of test results with cut-off ranges could diminish this effect. The EUROLINE MPO/PR3/GBM profile immunoblot (EUROIMMUN AG, Lübeck, Germany) allows an automated and semi-quantitative determination of MPO-ANCA, PR3-ANCA, and/or anti-GBM antibodies in patient sera within 90 min. Considering these favourable characteristics, we aimed to evaluate the diagnostic performance of the immunoblot in detecting MPO-ANCA, PR3-ANCA, and anti-GBM in different settings. First, we tested the diagnostic performance of the immunoblot when used as a confirmatory test for the presence of ANCA in patients with AAV. Furthermore, we evaluated the sensitivity and specificity when used for the detection of MPO-ANCA, PR3-ANCA, and/or anti-GBM in patients with RPGN and a biopsy-proven diagnosis of AAV and/or anti-GBM disease. We hypothesize that the immunoblot can be considered as a valuable alternative to confirm the presence of ANCA in settings when a second immunoassay is not readily available and to detect the presence of MPO-ANCA, PR3-ANCA, or anti-GBM in patients with AAV or anti-GBM disease suspected RPGN.
Materials and methods
Patients
For this retrospective study, sera of two AAV cohorts (biopsy-proven renal AAV patients and out-patient AAV patients), one control cohort (out-patient cohort with non-ANCA related vasculitides), and of one anti-GBM cohort (biopsy-proven renal anti-GBM disease and/or kidney injury in the presence of anti-GBM antibodies) from the Department of Internal Medicine, Maastricht University Medical Centre were used. The diagnosis for all AAV patients was based upon the criteria of the Chapel Hill Consensus Conference in 1992 [Citation14] because the inclusion of AAV patients ended in 2006. In detail, the biopsy-proven renal AAV cohort (n = 112) consisted of AAV patients (GPA n = 59, MPA n = 12, EGPA n = 1, and not subclassified AAV n = 40) with pauci-immune glomerulonephritis between 1989 and 2006. The patient sera were collected at the time of diagnosis. The out-patient AAV cohort (n = 74) consisted of patients diagnosed with GPA (n = 53), MPA (n = 6), EGPA (n = 12), or not subclassified AAV (n = 3) in the out-patient setting between 1997 and 2006. Samples were drawn at the time of diagnosis (n = 49) or during follow-up (n = 25). The latter samples were preferentially selected at the time that ANCA became positive again as defined by capture ELISA (n = 17), suggesting the presence of a relapse; otherwise, the first sample available was included (n = 8) for patients that remained ANCA negative over time. The anti-GBM cohort (n = 19) consisted of patients with a diagnosis of anti-GBM disease, based on renal biopsy (n = 12) and/or acute kidney injury with anti-GBM antibodies (as determined in routine clinical practice) in combination with a standard treatment for anti-GBM disease. The patient sera were collected at the time of diagnosis between 1978 and 2019. The control group consisted of 51 patient with diagnosis of a non-ANCA related vasculitis (IgA vasculitis (n = 9), cutaneous polyarteritis nodosa (n = 8), giant-cell arteritis (n = 7), cryoglobulinemic vasculitis (n = 5), idiopathic leukocytoclastic vasculitis (n = 5), idiopathic retinal vasculitis (n = 3), arteritis obliterans (n = 3), Behçet’s disease (n = 3), Takayasu arteritis (n = 2), and miscellaneous forms of vasculitis (n = 6)) between 1999 and 2006.
The study was performed in accordance with the 1997 Declaration of Helsinki. The patient sera were primarily obtained for routine clinical purposes, and since only anonymized rest-sera were used for this study, ethical approval by an ethical committee was not necessary.
Reference multi-testing algorithm
ANCA testing was performed by IIF, direct and capture ELISA for both PR3- and MPO-ANCA as published previously [Citation15]. IIF was performed on ethanol-fixed granulocytes (INOVA Diagnostics, San Diego, CA). Perinuclear (P-ANCA), fine granular cytoplasmic (C-ANCA), and diffuse homogenous cytoplasmic (atypical ANCA) staining patterns on neutrophils were categorized as ANCA positive. Before 2005 ELISA (Euro-Diagnostica, Malmö, Sweden) and after 2005 fluorescence enzyme immunoassay (EliA, Phadia, Freiburg, Germany) were used for the detection and quantification of MPO- and PR3-ANCA. Capture ELISA (Euro-Diagnostica, Malmö, Sweden) for the confirmation of MPO- or PR3-ANCA presence was performed as well [Citation15].
For the classification of MPO-ANCA, PR3-ANCA, double-positive ANCA or ANCA negative, the reference multi-testing algorithm as reported previously was used [Citation16].
Detection of MPO-ANCA, PR3-ANCA, and anti-GBM antibodies by immunoblot
MPO-ANCA and PR3-ANCA detection was performed by the EUROLINE MPO/PR3/GBM profile immunoblot (EUROIMMUN AG, Lübeck, Germany) according to the manufacturer’s instructions. It is a membrane chip printed with lines of purified antigens for MPO, PR3, and GBM. The results were automatically categorized by using the computer-based EUROLineScan Flatbed scanner test system (EUROIMMUN AG, Lübeck, Germany) according to the manufacturer’s instructions into negative (signal intensity 0–5), borderline (signal intensity 6–10) or positive (+ = signal intensity 11–25, ++= signal intensity 26–50, and +++ = signal intensity > 50).
Immunoblot as a confirmation test for ANCA positivity
For the evaluation of sensitivity and specificity of the immunoblot in confirming ANCA positivity, samples of both AAV cohorts and the control cohort were used. The diagnostic performance was calculated separately for MPO-ANCA and PR3-ANCA using two different cut-off values of positivity (signal intensity > 5 and > 10). True positivity (or negativity) was defined by a positive (or negative) immunoblot, matching the combination of clinical suspicion, and the result of the reference multi-testing algorithm. False positivity (or negativity) was defined by a mismatch between the immunoblot and the combination clinical suspicion and reference multi-testing algorithm. Because test characteristics for MPO-ANCA and PR3-ANCA were analysed separately, a true double-positive test in the immunoblot was categorized as true positive for both ANCAs. In case of double positivity in the reference algorithm, but single positivity in the immunoblot, the matching ANCA was categorized as true positive and the mismatching as false negative.
Immunoblot evaluation for ANCA and/or anti-GBM positivity in patients with the suspicion of AAV or anti-GBM disease-related renal involvement
The diagnostic performance of the immunoblot in detecting MPO-ANCA, PR3-ANCA, and/or anti-GBM antibodies was assessed by calculating the sensitivity and specificity for two cut-off values of positivity (signal intensity > 5 and > 10). For ANCA, the renal biopsy-proven renal AAV cohort was used as disease reference and the control cohort was used as disease-free reference. For GBM, the anti-GBM cohort was used as disease reference and the control cohort was used as disease-free reference.
Statistics
Data are presented in numbers (percentages). The diagnostic value of the immunoblot was assessed by calculating the sensitivity and specificity. The interval likelihood ratio (LR) was obtained by dividing the number of true positive tests within a specific range (e.g. signal intensity 6–10) by the total number of subjects with the disease and by dividing this number by the number of false-positive tests within a specific range (e.g. signal intensity 6–10) by the total number of disease-free patients. Statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY) and GraphPad version 6.0 (GraphPad Software, San Diego, CA).
Results
Diagnostic performance of the immunoblot for the confirmation of ANCA positivity
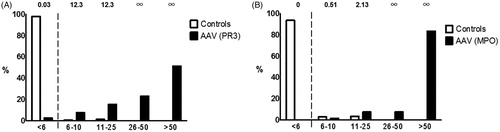
Results of the immunoblot in confirming the presence of PR3- or MPO-ANCA for different cut-offs of positivity are presented in . For sensitivity calculations, patients from the biopsy-proven renal cohort and the outpatient AAV cohort were included. For specificity, we included the disease controls and when analysing PR3-ANCA specificity the MPO-ANCA positive patients were included as negative controls for the PR3-ANCA group and vice versa for MPO-ANCA specificity. With this approach, in total 238 observations were obtained for the analysis. The sensitivity and specificity for PR3-ANCA were 97.4 and 98.1% when using a signal intensity cut-off > 5 for a positive test result, and 89.7 and 98.7% for a cut-off >10, respectively. The higher sensitivity with cut-off > 5 was explained by six true-positive results in the range of 6–10. For MPO-ANCA, a sensitivity of 100 and 98.5% and a specificity of 93.5 and 96.4% were calculated for both signal intensity cut-offs, respectively. The lower specificity of MPO-ANCA testing with a cut-off > 5 resulted from a higher number of false-positives in the range of 6–10 (n = 11) compared to a cut-off >10 (n = 6).
Table 1. Test characteristics of the EUROLINE MPO/PR3/GBM immunoblot for different cut-off values in 238 measurements in a ANCA confirmation test setting.
Interval likelihood ratios for different cut-off values of ANCA positivity
Calculating the interval LR can be helpful in assessing the diagnostic value of a test with increasing levels of result intervals. LRs of greater than 1 (or lower than 1) imply differences in the pre- to post-test probability for the presence (or absence) of a disease [Citation17]. A greater (or smaller) LR often indicates a clinically meaningful increase (or decrease) in the pre- to post-test probability [Citation18]. The results are presented in . The interval LRs for PR3-ANCA were 0.03, 12.3, and 12.3 for signal intensities between ranges of < 6, 6–10, and 11–25, respectively. For a cut-off with a signal intensity between ranges of 26–50 and > 50, the interval LRs were infinite. The interval LRs for MPO-ANCA were 0, 0.51, and 2.13 for cut-offs between < 6, 6–10, and 11–25, respectively. The interval LRs for a cut-off between 26–50 and > 50 were also for MPO-ANCA infinite.
Diagnostic performance of the immunoblot for ANCA and/or anti-GBM detection in patients with AAV or anti-GBM disease-related RPGN
Results obtained by the immunoblot in evaluating ANCA and/or GBM positivity for cut-off signal intensities of positivity > 5 and > 10 are summarized in . For ANCA detection, samples from the renal biopsy (n = 112) and control cohort (n = 51) were used. In the control cohort, two sera reacted false positive for MPO-ANCA with cut-offs between 6 and 10 (n = 1 Takayasu arteritis and n = 1 miscellaneous form of vasculitis). The sensitivity and specificity with signal intensity cut-off > 5 were 92.9 and 96.0% and for > 10 they were 90.2 and 100%, respectively. When combining a cut-off > 5 for PR3-ANCA and > 10 for MPO-ANCA, the sensitivity and specificity improved to 92.9 and 100%, respectively.
Table 2. Test characteristics of the EUROLINE MPO/PR3/GBM immunoblot for different cut-off values in patients with AAV and anti-GBM disease-related RPGN.
For anti-GBM detection, 19 samples from anti-GBM cohort and 51 samples from the control cohort were used. Renal biopsies were done in 12 (63%) out of the 19 patients. The sensitivity and specificity for both cut-off values (> 5 and > 10) were both 100%.
Discussion
This study aimed to evaluate the diagnostic performance of the EUROLINE MPO/PR3/GBM profile immunoblot (EUROIMMUN AG, Lübeck, Germany) in detecting MPO-ANCA, PR3-ANCA, and/or anti-GBM antibodies in different settings. The immunoblot performed well in terms of sensitivity and specificity when used as ANCA confirmation test in patients with AAV with a preceding positive antigen-specific immunoassay and in detecting ANCA and/or anti-GBM autoantibodies in patients with AAV or anti-GBM disease-related RPGN.
The current guidelines for ANCA testing state that confirmation of ANCA detection should be done in patients with the suspicion of AAV, in particular in cases with low-positive test results [Citation11]. The use of other ANCA specific assays, including dot- or immunoblots is not covered in this approach. However, the guideline recommends that these assays should be evaluated for their diagnostic performance based on samples from AAV patients and relevant disease controls and that the use of LRs can help to improve clinical interpretations [Citation11,Citation17]. Our study shows that by doing so, the evaluated immunoblot has good diagnostic characteristics when tested with AAV and control sera in a confirmatory setting. When using a cut-off signal intensity of positivity > 5, which is designated as “borderline” according to the manufacturer’s manual, the sensitivity and specificity (97.4 and 98.1%, respectively) for PR3-ANCA were better compared to a cut-off signal intensity > 10 (89.7 and 98.7%, respectively). Interestingly, Rutgers et al. showed previously that dot-blot assays have an excellent performance for PR3-ANCA, but they are lacking in sensitivity for MPO-ANCA [Citation13]. In our study the sensitivity of the immunoblot for MPO-ANCA was high. In contrast, more false positivity in the “borderline range” was observed. A better test performance of the immunoblot was therefore obtained when using a cut-off signal intensity of > 10 for MPO-ANCA detection (sensitivity 98.5%, specificity 96.4%, respectively). The increasing interval LRs implicate for both ANCA entities, but especially for MPO-ANCA, that a higher test result range is associated with a clinically meaningful increase in the pre- to post-test probability of disease presence. Based on the good performance of the immunoblot, it should be considered as a test for ANCA confirmation. Our results offer the opportunity to improve the diagnostic accuracy by using ANCA specific cut-off values of positivity (i.e. for PR3-ANCA a signal intensity > 5 and for MPO-ANCA a signal intensity > 10).
The diagnostic accuracy of other immunoblots in screening for the presence of ANCA or anti-GBM in patients with suspected AAV or anti-GBM disease was previously evaluated [Citation12,Citation13,Citation19,Citation20]. The positive predictive value of an assay changes when used as a screening test in routine clinical practice, since more individuals not having the disease are likely to be tested. This may result in more false positives when compared to the confirmatory setting. Nevertheless, dot-blot assays were reported to be accurate in detecting MPO-ANCA, PR3-ANCA, and anti-GBM in patients with RPGN or pulmonary haemorrhage with variances in the reported sensitivity for MPO-ANCA [Citation12,Citation13]. Zhang et al. reported a high specificity but a low sensitivity for ANCA detection for two-line immunoassays. However, the line immunoassays were semi-quantitative and qualitative, respectively, and anti-GBM was not tested [Citation20]. In our study, patient sera with biopsy-proven RPGN and with suspected AAV or anti-GBM disease were retrospectively tested with the immunoblot for the presence of autoantibodies. Compared to other techniques, the immunoblot performed well to detect PR3-ANCA, MPO-ANCA, and/or anti-GBM. The highest diagnostic performance was obtained by using different cut-offs for PR3-ANCA (signal intensity > 5) and MPO-ANCA (signal intensity > 10), resulting in a sensitivity and specificity of 92.9 and 100%, respectively. The improved accuracy with a higher cut-off for MPO-ANCA is explained by more false-positive results in the “borderline” range (n = 2; 1.2%) as this was also observed in the confirmatory setting. Of note, the diagnostic accuracy of the evaluated immunoblot is remarkably high for anti-GBM disease.
Limitations of our study are its retrospective design and relatively small number of controls. When a diagnostic test is used in a population with a lower disease prevalence, relatively more false-positive results will be found. It is therefore important that patients to be tested should be carefully selected based upon a given pre-test probability for AAV or anti-GBM disease (i.e. suspicion of RPGN or ANCA confirmatory settings). For this reason, we used disease controls with a diagnosis of non-ANCA vasculitis, which all together is a highly representative group to be tested for the presence of ANCA or anti-GBM antibodies. Moreover, the ANCA and anti-GBM cohort in this study are mainly patients with renal involvement. Patients with pulmonary haemorrhage may be underrepresented. On the other hand, 124 patients had biopsy-proven renal AAV or anti-GBM disease, which is a highly accurate diagnostic measure for active systemic vasculitis. Finally, the control group was used as disease-free reference group for the evaluation of the diagnostic accuracy of the immunoblot in the RPGN setting as well. RPGN was not primarily seen in this cohort, which differed from our disease group. This may partially explain the remarkably high diagnostic accuracy of the immunoblot for anti-GBM in our study.
Advantages of the immunoassay are the rapid testing and the automated read-out for obtaining semi-quantitative results. Detection of ANCA and anti-GBM antibodies with qualitative immunoassays can lead to variations in the interpretation of the test results, as reflected by the high inter-observer effect especially for anti-GBM antibodies [Citation13]. This can be an impairment, for instance in acute settings when experienced laboratory staff is not available. Reporting of semi-quantitative results can limit this problem and can help to improve the diagnostic accuracy as presented in our study. Finally, we observed in 4 out of 33 patients with previously negative ANCA testing a positive immunoblot for MPO-ANCA. A prospective study that evaluates whether the used immunoblot could increase the detection of ANCA autoantibodies and could allow the diagnosis of AAV in patients with previously negative ANCA testing would be interesting.
In conclusion, the diagnostic performance of the EUROLINE MPO/PR3/GBM profile immunoblot is good when used as a confirmatory test or as a detection test for the presence of ANCA and anti-GBM in an urgent setting. There are advantages over IIF, ELISA, and other assays for ANCA and anti-GBM in terms of time, costs, and interpretation of results. The immunoblot is a useful addition to current guidelines and could finally be the first test to be performed, particularly in settings where a rapid diagnosis is necessary.
Author contributions
M.H.B. and J.G.M.C.D. designed the study, carried out the experiments, and analysed the data. M.H.B., J.P.A., J.J.B.C.B., P.P., and J.G.M.C.D. drafted the paper; all authors approved the final version of the manuscript.
Acknowledgements
Euroimmun AG (Lübeck, Germany) and Biognost (Wevelgem, Belgium) are acknowledged for providing reagents free of charge.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
- Damoiseaux J, Csernok E, Rasmussen N, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann Rheum Dis. 2017;76(4):647–653.
- Kronbichler A, Shin JI, Lee KH, et al. Clinical associations of renal involvement in ANCA-associated vasculitis. Autoimmun Rev. 2020;19(4):102495.
- Rutgers A, Slot M, van Paassen P, et al. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46(2):253–262.
- McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12(7):1162–1172.
- Segelmark M, Hellmark T. Anti-glomerular basement membrane disease: an update on subgroups, pathogenesis and therapies. Nephrol Dial Transplant. 2019;34(11):1826–1832.
- Walsh M, Merkel PA, Jayne DRW. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(22):2169.
- Rutgers A, Heeringa P, Damoiseaux JG, et al. ANCA and anti-GBM antibodies in diagnosis and follow-up of vasculitic disease. Eur J Intern Med. 2003;14(5):287–295.
- Mahler M, Radice A, Sinico RA, et al. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: an international multicenter study. Nephrol Dial Transplant. 2012;27(1):243–252.
- Sinico RA, Radice A, Corace C, et al. Anti-glomerular basement membrane antibodies in the diagnosis of good pasture syndrome: a comparison of different assays. Nephrol Dial Transplant. 2006;21(2):397–401.
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017;13(11):683–692.
- de Joode AA, Roozendaal C, van der Leij MJ, et al. Performance of two strategies for urgent ANCA and anti-GBM analysis in vasculitis. Eur J Intern Med. 2014;25(2):182–186.
- Rutgers A, Damoiseaux J, Roozendaal C, et al. ANCA-GBM dot-blot: evaluation of an assay in the differential diagnosis of patients presenting with rapidly progressive glomerulonephritis. J Clin Immunol. 2004;24(4):435–440.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192.
- Damoiseaux JG, Slot MC, Vaessen M, et al. Evaluation of a new fluorescent-enzyme immuno-assay for diagnosis and follow-up of ANCA-associated vasculitis. J Clin Immunol. 2005;25(3):202–208.
- Damoiseaux J, Steller U, Buschtez M, et al. EUROPLUS ANCA BIOCHIP mosaic: PR3 and MPO antigen microdots improve the laboratory diagnostics of ANCA-associated vasculitis. J Immunol Methods. 2009;348(1–2):67–73.
- Bossuyt X, Rasmussen N, van Paassen P, et al. A multicentre study to improve clinical interpretation of proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibodies. Rheumatology (Oxford). 2017;56(9):1533–1541.
- Bossuyt X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun Rev. 2009;8(7):543–548.
- Ermens AA, Bayens AJ, Crooymans A, et al. Evaluation of a simple dot-blot method for the detection of anti-neutrophil cytoplasmic antibodies directed against proteinase 3 and myeloperoxidase. Clin Chem. 2000;46(10):1717–1719.
- Zhang W, Zheng Z, Jia R, et al. Evaluation of 12 different assays for detecting ANCA in Chinese patients with GPA and MPA: a multicenter study in China. Clin Rheumatol. 2019;38(12):3477–3483.