Abstract
Odd-skipped related transcription factor 1 (OSR1) is implicated in various pathophysiologic processes, such as embryonic heart and urogenital formation, and functions as a tumour suppressor in diverse tumours. Regardless, the regulatory role and mechanism of OSR1 in acute myeloid leukaemia are scarce. Firstly, the CD34-positive blasts or the normal blasts were isolated from the plasma samples of acute myeloid leukaemia patients or healthy donors, respectively. Expression of OSR1 analysis by western blot and qRT-PCR showed that OSR1 was reduced in CD34-positive blasts and acute myeloid leukaemia cell lines. Secondly, acute myeloid leukaemia cell lines were transfected with pcDNA vector or shRNA for the over-expression or silence of OSR1, respectively. Functional assays demonstrated that ectopic expression of OSR1 decreased cell viability and repressed the proliferation of acute myeloid leukaemia cells, while promoted the cell apoptosis. Silence of OSR1 contributed to the proliferation of acute myeloid leukaemia cells and suppressed the cell apoptosis. Thirdly, over-expression of OSR1 decreased protein expression of leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5) and JNK phosphorylation in the acute myeloid leukaemia cells. Ectopic expression of LGR5 attenuated OSR1 over-expression-induced decrease of LGR5 and JNK phosphorylation. Lastly, ectopic expression of LGR5 attenuated OSR1 over-expression-induced decrease of cell viability and proliferation in acute myeloid leukaemia cells. In conclusion, OSR1 functioned as a tumour suppressor in acute myeloid leukaemia cells by inhibiting LGR5-mediated activation of JNK signalling.
Introduction
Acute myeloid leukaemia is a major type of leukaemia and the sixth leading cause of cancer-related death [Citation1]. Abnormal proliferation and accumulation of a large number of abnormal myeloid progenitor cells in bone marrow, peripheral blood and even other tissues is the main characteristic of acute myeloid leukaemia [Citation2]. The accumulation of myeloid progenitor cells leads to the destruction of the haematopoietic system, and the incidence and mortality of acute myeloid leukaemia are increasing every year [Citation3]. Although recent advancement in the anti-cancer therapy strategies has improved the prognosis of acute myeloid leukaemia patients, devoid of complete understanding of the pathogenesis restrains the effective treatment for acute myeloid leukaemia [Citation4]. Therefore, the identification of key genes involved in the occurrence and development of acute myeloid leukaemia would facilitate for the development of effective therapeutic targets.
Odd-skipped related transcription factor 1 (OSR1) is located on the human chromosome 2p24.1 and encodes a zinc finger transcription factor to participate in the embryonic heart and urogenital formation, as well as the endoderm development [Citation5]. The involvement of OSR1 in the progression of diverse tumours has been recently reported. For example, OSR1 was epigenetic silenced in renal cell carcinoma, and depletion of OSR1 resulted in the over-expression of oncogenic genes, thus promoting the cell proliferation and invasion [Citation6]. Reduce of OSR1 promoted breast cancer [Citation7] and colon adenocarcinoma [Citation8] cell proliferation and metastasis. Over-expression of OSR1 contributed to suppression of tongue squamous cell carcinoma progression by inhibiting the NF-κB pathway [Citation9]. Regardless, the regulatory role and mechanism of OSR1 in acute myeloid leukaemia have not been reported yet.
Focal adhesion kinase promoted activation of Akt and MAPK pathways in colon adenocarcinoma, and OSR1 repressed Akt and MAPK activation to exert the anticancer effect [Citation8]. MAPK was considered as a potential therapeutic target in acute myeloid leukaemia [Citation10]. Block of MAPK leads to promotion of acute myeloid leukaemia cell apoptosis [Citation11] and impairment of the cell proliferation and invasion [Citation12]. The understanding of regulatory mechanism of MAPK in OSR1-mediated acute myeloid leukaemia progression might contribute to effective therapies for the treatment of acute myeloid leukaemia.
This study investigated the role and mechanism of OSR1 in acute myeloid leukaemia cell lines, and identified that OSR1 functioned as a tumour suppressor in acute myeloid leukaemia in a JNK-dependent way.
Materials and methods
Cell isolation and culture
Venous blood samples were collected from healthy volunteers or patients with acute myeloid leukaemia. All the volunteers signed an informed consent that was approved by The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University and in accordance with those of the 1964 Helsinki Declaration and its later amendments for ethical research involving human subjects.
The CD34-positive blasts (1 × 106 cells) were isolated from the plasma samples via CD34 microbead kit (Miltenyi Biotec, Auburn, CA, USA). Acute myeloid leukaemia cells, including Kasumi-1, THP-1 and AML-193, were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in RPMI-1640 medium with 10% foetal bovine serum (Hyclone, South Logan, UT, USA) at a 37 °C incubator.
qRT-PCR
RNAs were isolated from the CD34-positive blasts and acute myeloid leukaemia cells via Trizol (Thermo Fisher Scientific, Waltham, MA, USA). RNAs were transcribed into cDNAs via Reverse Transcription System (Thermo Fisher Scientific), and the qRT-PCR analysis of mRNA expression of OSR1 was determined by Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) with the following primer sequences. GAPDH was used as a endogenous control ().
Table 1. Primer.
Cell transfection
Kasumi-1 and THP-1 were plated in the 96-well plates and transfected with pcDNA3.1-OSR1, pcDNA vector, shRNA-OSR1-1#, shOSR1-2#, or shNC that were constructed by RiboBio (Guangzhou, China) with Lipofectamine 2000 (Thermo Fisher Scientific). pcDNA-LGR5 was cotransfected with pcDNA3.1-OSR1 or shRNA-OSR1 into Kasumi-1 and THP-1 cells.
Cell viability and proliferation
Kasumi-1 and THP-1 with indicated transfections were plated in the 96-well plates for 24, 48, or 72 h. A total of 10 μL of CCK8 solution (Dojindo, Kumamoto, Japan) was added to each well and incubated for 2 h. Absorbance at 490 nm of each well was measured by an ELISA reader (BioTek, Winooski, VT, USA). For cell proliferation, Kasumi-1 and THP-1 with indicated transfections were plated in the 6-well plates, and cultured for two weeks. The formaldehyde-fixed cells were stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA), and counted under a light microscope (Olympus Corp. Tokyo, Japan).
Cell apoptosis
Kasumi-1 and THP-1 with indicated transfections were harvested following trypsinization and then centrifugation at 1000 g for 5 min. Cells were e resuspended in 100 μL Annexin-binding buffer with 5 μL Annexin V-FITC plus 1 μL propidium iodide (Sigma-Aldrich). The apoptotic cells were analysed under flow cytometry (Becton Dickson Immunocytometry-Systems, San Jose, CA, USA).
Caspase-3 activity assay
Kasumi-1 and THP-1 were lysed in RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific) and then centrifuged at 2000 g for 15 min to collect the supernatants. The supernatants were incubated with Ac-DEVE-pNA under the reaction buffer from Caspase-3 Colorimetric Assay Kit (Nanjing KeyGEN Biotech, Co, Ltd, Nanjing, China) for 2 h at 37 °C. Optical density at 405 nm was measured by the ELISA reader.
Western blot
The CD34-positive blasts and acute myeloid leukaemia cells were lysed in RIPA Lysis and Extraction Buffer. Protein concentration in the lysates was calculated by acid protein kit (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE, and then electro-transferred onto PVDF membrane (Millipore, Bedford, MA, USA). Membranes were blocked with 5% bovine serum albumin, and probed with primary antibodies: anti-OSR1 and anti-Bcl-2 (1:2000, Cell Signalling, Beverly, MA, USA), anti-Bax and anti-cleaved PARP (1:2500, Cell Signalling), anti-LGR5 and anti-c-myc (1:3000, Cell Signalling), anti-JNK and anti-p-JNK (1:3500, Cell Signalling), anti-β-actin (1:4000, Cell Signalling). Following incubation with corresponding horseradish peroxidase-labeled secondary antibody (1:5000; Cell Signalling), the immunoreactivities of bands in the membranes were detected by enhanced chemiluminescence (KeyGen, Nanjin, China).
Statistical analysis
Data with at least triple replicates were expressed as mean ± SEM and performed with one-way analysis of variance or student’s t-test under GraphPad Prism software. The p-value < .05 was considered statistically significant.
Results
Reduced OSR1 in acute myeloid leukaemia
To analyze the expression level of OSR1 in acute myeloid leukaemia, CD34-positive blasts were isolated from healthy volunteers and patients with acute myeloid leukaemia. mRNA expression of OSR1 was reduced in the blasts isolated from the patients compared to the healthy volunteers (). OSR1 protein was lower in blasts isolated from the patients than the healthy volunteers (). Moreover, OSR1 was also decreased in the acute myeloid leukaemia cells (Kasumi-1, THP-1 and AML-193) (), suggesting that OSR1 might be involved in acute myeloid leukaemia progression.
Figure 1. Reduced OSR1 in acute myeloid leukaemia. (A) mRNA expression of OSR1 was reduced in the blasts isolated from the patients and acute myeloid leukaemia cells (Kasumi-1, THP-1 and AML-193) compared to the healthy volunteers. (B) Protein expression of OSR1 was reduced in the blasts isolated from the patients and acute myeloid leukaemia cells (Kasumi-1, THP-1 and AML-193) compared to the healthy volunteers. ** vs. normal blasts, p < .01.
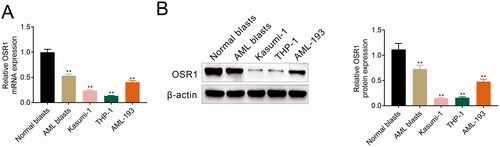
Silence of OSR1 contributed to acute myeloid leukaemia cell proliferation
pcDNA vector or shRNA were transfected into Kasumi-1 and THP-1 for the over-expression or silence of OSR1, respectively. Western blot analysis confirmed the transfection efficiency of the pcDNA vector or shRNA (). Transfection with shOSR1-2# showed lower expression of OSR1 than that of shOSR1-1# (). therefore, shOSR1-2# was used in the following functional assays and named as shOSR1. Functional assays showed that over-expression of OSR1 reduced the cell viability of Kasumi-1 and THP-1 (), and repressed the proliferation (). However, shRNA-mediated silence of OSR1 enhanced the cell viability () and promoted proliferation (), demonstrating the anti-proliferative effect of OSR1 on acute myeloid leukaemia.
Figure 2. Silence of OSR1 contributed to acute myeloid leukaemia cell proliferation. (A) Protein expression of OSR1 was increased in Kasumi-1 and THP-1 transfected with pcDNA-OSR1 and decreased by shRNA-OSR1. (B) Over-expression of OSR1 reduced the cell viability of Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 enhanced the cell viability. (C) Over-expression of OSR1 reduced the cell proliferation of Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 enhanced the cell proliferation. ** vs. control, p < .01. ## vs. shNC, p < .01.
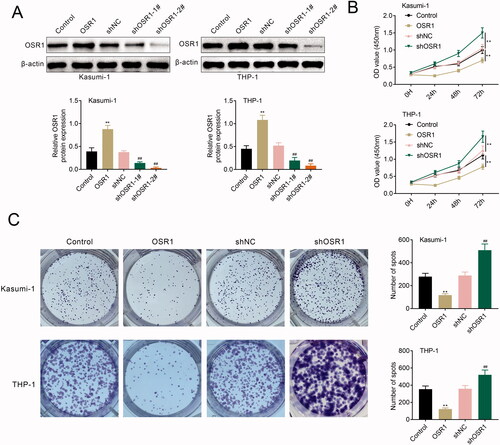
Silence of OSR1 suppressed acute myeloid leukaemia cell apoptosis
In addition to the anti-proliferative effect, flow cytometry analysis also indicated that over-expression of OSR1 promoted the cell apoptosis of Kasumi-1 and THP-1 (), while the silence of OSR1 repressed the cell apoptosis (). Caspase-3 activity was enhanced in Kasumi-1 and THP-1 transfected with pcDNA-OSR1 (), while reduced by shRNA-OSR1 (). Moreover, transfection with pcDNA-OSR1 expressed higher expression of Bax and cleaved PARP, while lower Bcl-2, than the control (). Protein expression of Bcl-2 was increased, and Bax and cleaved PARP were decreased by shRNA-OSR1 (), revealing the pro-apoptotic role of OSR1 on acute myeloid leukaemia.
Figure 3. Silence of OSR1 suppressed acute myeloid leukaemia cell apoptosis. (A) Over-expression of OSR1 promoted the cell apoptosis of Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 suppressed the cell apoptosis. (B) Over-expression of OSR1 promoted the caspase-3 activity of Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 suppressed the caspase-3 activity. (C) Over-expression of OSR1 reduced protein expression of Bcl-2, while enhanced Bax and cleaved PARP, in Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 enhanced Bcl-2, while reduced Bax and cleaved PARP. ** vs. control, p < .01. ## vs. shNC, p < .01.
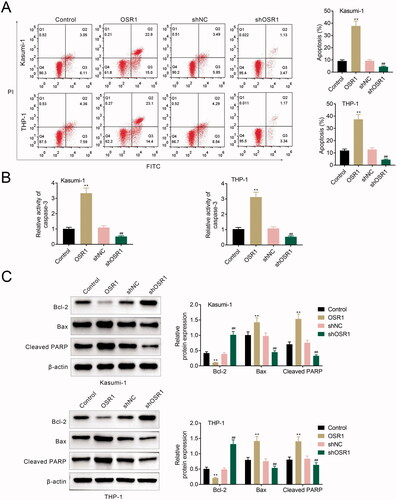
OSR1 suppressed LGR5-mediated activation of JNK pathway
OSR1 negatively regulated protein expression of LGR5 in Kasumi-1 and THP-1 (). Protein expression of JNK phosphorylation and c-myc were also reduced in Kasumi-1 and THP-1 transfected with pcDNA-OSR1 (), and enhanced by shRNA-OSR1 (). Over-expression of LGR5 attenuated OSR1 over-expression-induced decease of LGR5, JNK phosphorylation and c-myc (), while aggravated OSR1 silence-induced increase of LGR5, JNK phosphorylation and c-myc (), indicating that OSR1 suppressed LGR5-mediated activation of the JNK pathway in acute myeloid leukaemia.
Figure 4. OSR1 suppressed LGR5-mediated activation of JNK pathway. (A) Over-expression of OSR1 reduced protein expression of LGR5 in Kasumi-1 and THP-1, shRNA-mediated silence of OSR1 enhanced LGR5 expression. (B) Over-expression of LGR5 attenuated OSR1 over-expression-induced decease of LGR5, JNK phosphorylation and c-myc, while aggravated OSR1 silence-induced increase of LGR5, JNK phosphorylation and c-myc. ** vs. control + NC, p < .01. ## vs. shNC or OSR1 + NC, p < .01. && vs. shNC + NC, p < .01. $$ vs. shOSR1 + NC, p < .01.
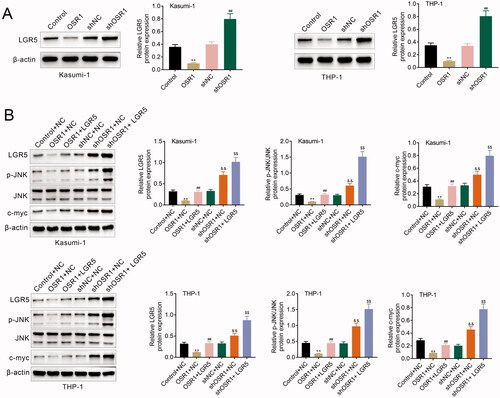
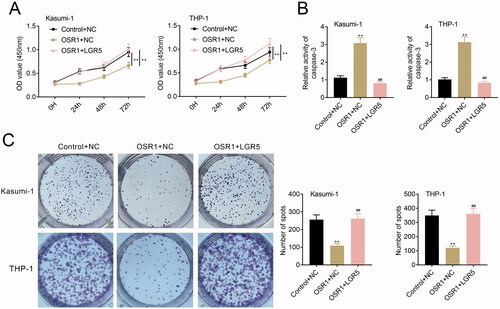
OSR1 contributed to suppression of acute myeloid leukaemia cell proliferation by inhibiting LGR5
Kasumi-1 and THP-1 were cotransfected with pcDNA-mediated over-expression of OSR1 and LGR5 to investigate the role of the OSR1/LGR5 axis on acute myeloid leukaemia. Over-expression of OSR1-induced decrease of cell viability in Kasumi-1 and THP-1 was reversed by over-expression of LGR5 (). LGR5 over-expression weakened over-expression of OSR1-induced increase of caspase-3 activity in Kasumi-1 and THP-1 (). Moreover, LGR5 over-expression counteracted the suppressive effect of OSR1 over-expression on cell proliferation of Kasumi-1 and THP-1 (), showing that OSR1 suppressed acute myeloid leukaemia cell proliferation by inhibiting LGR5.
Figure 5. OSR1 contributed to suppression of acute myeloid leukaemia cell proliferation by inhibiting LGR5. (A) Over-expression of OSR1-induced decrease of cell viability in Kasumi-1 and THP-1 was reversed by over-expression of LGR5. (B) LGR5 over-expression weakened over-expression of OSR1-induced increase of caspase-3 activity in Kasumi-1 and THP-1. (C) LGR5 over-expression counteracted the suppressive effect of OSR1 over-expression on cell proliferation of Kasumi-1 and THP-1. ** vs. control + NC, p < .01. ## vs. OSR1 + NC, p < .01.
Discussion
OSR1 is a member of the zinc finger transcription factor family and functions as a transcriptional suppressor of oncogenic genes that are involved in the cell cycle and tumour growth [Citation6]. OSR1 is generally considered as a tumour suppressor in gastric cancer due to the hypermethylation [Citation13]. OSR1 promoted p53 transcription to increase p53 and p21, while decrease cyclin D1 and cyclin-dependent kinase 4, thus suppressing the cell cycle in gastric cancer [Citation13]. Although the anti-cancer role of OSR1 has currently been investigated in diverse cancers, the regulatory role and mechanism in acute myeloid leukaemia are not completely understood.
In this study, we firstly identified the down-regulation of OSR1 in both CD34-positive blasts isolated from acute myeloid leukaemia patients and the acute myeloid leukaemia cell lines. Low expression of OSR1 has been validated in clinical tumour specimens of breast cancer [Citation7] and gastric cancer [Citation13] and the OSR1 expression predicted poor prognosis in the cancers. The clinical significance of OSR1 in acute myeloid leukaemia should be investigated to provide a potential diagnostic or prognostic biomarker in acute myeloid leukaemia.
Functional analysis in this study then demonstrated that OSR1 exerted anti-proliferative and pro-apoptotic effects in acute myeloid leukaemia. In addition to the devoid of proliferation inhibition, acquisition of migration and invasion abilities are also the common features of acute myeloid leukaemia cells [Citation14]. The invasion and epithelial-mesenchymal transition of breast cancer cells have been reported to be retarded by OSR1 (7). Therefore, OSR1 might also repress the epithelial-mesenchymal transition of acute myeloid leukaemia cells to inhibit invasiveness.
The previous study has shown that OSR1 inhibited the activity of Wnt signalling through reduction of SOX9, thereby inhibiting the proliferation and migration of lung cancer cells [Citation15]. SOX9 was involved in the tumorigenicity of cancer cells functioned as a transcription factor to enhance LGR5 expression to contribute to the glioblastoma tumorigenicity [Citation16]. Results in this study showed that OSR1 negatively regulated LGR5 expression in acute myeloid leukaemia cells. LGR5 is widely expressed on cancer stem cells [Citation17] and provides potential anti-cancer therapy in various cancers [Citation18]. Recently, LGR5 was considered as a potential therapeutic target for B cell malignancies through the promotion of tumour initiations [Citation19]. Although the involvement of LGR5 in acute myeloid leukaemia cells has not been reported, LGR5 over-expression in this study attenuated ectopic expression of OSR1-induced decrease of cell viability and proliferation, as well as the increase of cell apoptosis, in acute myeloid leukaemia cells. Our results provided the first compelling evidence that LGR5 might be involved in acute myeloid leukaemia progression.
LGR5 contributed to the activation of JNK signalling pathway to promote colon cancer cell proliferation [Citation20], inhibition of JNK reversed LGR5-mediated suppression of aldosterone secretion in human adrenal [Citation15]. JNK pathway was implicated in the antineoplastic effects of CPPTL against acute myeloid leukaemia [Citation21]. Activation of JNK promoted the suppressive effect of haem oxygenase-1 on acute myeloid leukaemia cell apoptosis [Citation22], and silence of PR/SET domain 5 suppressed activation of JNK to inhibit the acute myeloid leukaemia cell proliferation [Citation23]. Our results showed that protein expression of JNK phosphorylation was decreased by over-expression of OSR1 in acute myeloid leukaemia cells, while increased by the silence of OSR1. Therefore, OSR1 might suppress acute myeloid leukaemia cell proliferation through inhibition of LGR5-mediated JNK activation.
In conclusion, our study for the first time identified the in vitro tumour-suppressive role of OSR1 in acute myeloid leukaemia. LGR5-mediated JNK activation was implicated in the suppressive role of OSR1 in acute myeloid leukaemia, thereby providing a potential therapeutic target in acute myeloid leukaemia. However, a clinically relevant animal model should be applied to detect the role of OSR1 on acute myeloid leukaemia growth.
Ethical approval
Ethical approval was obtained from the Ethics Committee of The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University.
Statement of informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Author contributions
Lingyan Zong designed the study, supervised the data collection, Yingxin Sun analyzed the data, interpreted the data, Lingyan Zong and Yingxin Sun prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152.
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin. 2019;69(5):363–385.
- Finn L, Sproat L, Heckman MG, et al. Epidemiology of adult acute myeloid leukemia: impact of exposures on clinical phenotypes and outcomes after therapy. Cancer Epidemiol. 2015;39(6):1084–1092.
- Terashima AV, Mudumana SP, Drummond IA. Odd skipped related 1 is a negative feedback regulator of nodal-induced endoderm development. Dev Dyn. 2014;243(12):1571–1580.
- Zhang Y, Yuan Y, Liang P, et al. OSR1 is a novel epigenetic silenced tumor suppressor regulating invasion and proliferation in renal cell carcinoma. Oncotarget. 2017;8(18):30008–30018.
- Wang Y, Lei L, Xu F, et al. Reduced expression of odd-skipped related transcription factor 1 promotes proliferation and invasion of breast cancer cells and indicates poor patient prognosis. Oncol Lett. 2020;20(3):2946–2954.
- Zhang F, Jiang Z. Downregulation of OSR1 promotes colon adenocarcinoma progression via FAK-mediated AKT and MAPK signaling. Onco Targets Ther. 2020;13:3489–3500.
- Chen W, Wu K, Zhang H, et al. Odd-skipped related transcription factor 1 (OSR1) suppresses tongue squamous cell carcinoma migration and invasion through inhibiting NF-κB pathway. Eur J Pharmacol. 2018;839:33–39.
- Milella M, Kornblau SM, Estrov Z, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108(6):851–859.
- Espinoza JL, Elbadry MI, Taniwaki M, et al. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017;400:127–136.
- Huang X, Schwind S, Santhanam R, et al. Targeting the RAS/MAPK pathway with miR-181a in acute myeloid leukemia. Oncotarget. 2016;7(37):59273–59286.
- Otani K, Dong Y, Li X, et al. Odd-skipped related 1 is a novel tumour suppressor gene and a potential prognostic biomarker in gastric cancer. J Pathol. 2014;234(3):302–315.
- Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2005;2005(1):137–142.
- Wang Y, Lei L, Zheng Y-W, et al. Odd-skipped related 1 inhibits lung cancer proliferation and invasion by reducing Wnt signaling through the suppression of SOX9 and β-catenin. Cancer Science. 2018; 109(6):1799–1810.
- Hiraoka K, Hayashi T, Kaneko R, et al. SOX9-mediated upregulation of LGR5 is important for glioblastoma tumorigenicity. Biochem Biophys Res Commun. 2015;460(2):216–221.
- Hirsch D, Barker N, McNeil N, et al. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis. 2014;35(4):849–858.
- Xu L, Lin W, Wen L, et al. Lgr5 in cancer biology: functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy. Stem Cell Res Ther. 2019;10(1):219.
- Cosgun KN, Deb G, Yang X, et al. Lgr5 enables positive B-Cell selection and tumor-initiation in B-cell malignancies. Blood. 2018;132(Supplement 1):547–547.
- Kim S-H, Kim K-H, Yoo B-C, et al. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int J Oncol. 2012;41(5):1744–1750.
- Gao H-e, Sun Y, Ding Y-h, et al. Antineoplastic effects of CPPTL via the ROS/JNK pathway in acute myeloid leukemia. Oncotarget. 2017;8(24):38990–39000.
- Lin X, Fang Q, Chen S, et al. Heme oxygenase-1 suppresses the apoptosis of acute myeloid leukemia cells via the JNK/c-JUN signaling pathway. Leuk Res. 2015;39(5):544–552.
- Zhou P, Chen X, Li M, et al. Overexpression of PRDM5 promotes acute myeloid leukemia cell proliferation and migration by activating the JNK pathway. Cancer Med. 2019;8(8):3905–3917.
