Abstract
Cystic fibrosis (CF) lung disease begins early in childhood and is characterized by neutrophilic inflammation of the airways. Neutrophil extracellular traps (NETs) represent one mechanism by which neutrophils contribute to lung damage. The enzyme peptidylarginine deiminase 4 (PAD4) is required for NET formation. Our overall concept is that NET formation delivers PAD4 outside the neutrophil resulting in autoantibody generation, and this autoimmunity may be a novel mechanism contributing to CF lung disease progression. The aim of this study was to investigate clinical predictors of serum anti-PAD4 autoantibody (PAD4 Ab) levels in CF subjects with a wide range of ages from early childhood through middle age. We measured PAD4 Ab levels in sera from 104 CF subjects. PAD4 Abs were detectable among CF children as young as one year of age and elevated compared to paediatric healthy controls. PAD4 Ab levels increased significantly with age (r = 0.584, p <.001) and correlated with lower lung function (r = −0.481, n = 99, p <.001). PAD4 Abs were elevated in subjects with chronic Pseudomonas aeruginosa airways infection (p <.001), but not with other key clinical CF co-variates including sex, CFTR genotype, sweat chloride, pancreatic enzyme use, nutritional status, recent pulmonary exacerbations, Staphylococcus aureus, or CF-related diabetes. PAD4 Ab levels were also correlated with serum anti-double-stranded DNA IgA autoantibodies, which have similarly been shown to be elevated in CF subjects and associated with lung damage. In multivariable analysis, age and lung function remained correlated with PAD4 Ab levels. In summary, we describe novel findings of anti-PAD4 autoantibodies in CF that are present early in childhood, increase over time with age, and correlate with lung disease severity. Autoimmunity to antigens extruded by NETs appears to be an early event in CF lung disease, and airway autoimmunity related to NET formation is a potential mechanism of lung disease progression in CF.
Serum anti-PAD4 autoantibodies are detected in paediatric CF serum and are elevated compared to healthy paediatric controls
Anti-PAD4 autoantibodies increase with age
Anti-PAD4 autoantibodies correlate with lower lung function, Pseudomonas aeruginosa airway infection and anti-dsDNA IgA autoantibodies, but not with other key clinical CF co-variates
Age and lung function remain correlated with anti-PAD4 autoantibodies in multivariable analysis
Highlights
Introduction
Cystic fibrosis (CF) is a life-limiting disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene [Citation1]. Respiratory failure secondary to progressive pulmonary disease is the most common cause of death [Citation2]. Many risk factors for decline in lung function have been identified [Citation3], yet the reasons for the variable progression of lung disease in people with CF with the same CFTR mutations remain incompletely understood.
CF lung disease has been shown to begin early in childhood with neutrophilic inflammation detected in the bronchoalveolar lavage and airway thickening visible on chest CT at three months of age [Citation4]. Neutrophil elastase activity in bronchoalveolar lavage fluid in early life is also associated with early bronchiectasis in CF children [Citation5]. Neutrophils are abundant in CF airways and are believed to mediate lung damage by releasing intracellular contents into the airways, including oxidants and proteases [Citation6–8]. Neutrophil extracellular traps (NETs), large extracellular complexes composed of a DNA scaffold, histones, and granule-derived proteins that normally are thought to provide antibacterial defence mechanisms, may actually contribute to CF lung damage [Citation8].
NETs also appear to be a source of autoantigens in CF airways [Citation9], which could trigger an autoimmune response potentially further contributing to inflammation and airway damage. The enzyme peptidylarginine deiminase 4 (PAD4) is required for NET formation [Citation10–12], and PAD4 is released into the extracellular space when neutrophils extrude NETs [Citation13]. Autoantibodies targeting PAD4 may be important for disease progression. Our previous work in a small sample of 37 adult CF subjects showed that anti-PAD4 autoantibodies (PAD4 Abs) were elevated in CF adults compared to healthy controls and were associated with worse lung function [Citation14]. Since neutrophilic inflammation is an early pathophysiologic event in CF, we hypothesized that autoantibodies associated with NET formation would be found in very young children with CF and increase as lung disease progressed. Therefore, the aim of this study was to expand our initial study to examine a larger sample of subjects, including children as young as one year of age, and to investigate the association between serum PAD4 Ab levels and more extensive clinical variables.
Materials and methods
CF subjects
CF subjects were individuals with CF followed at the Children’s and Emory CF Care Centre who had consented to provide blood samples and clinical data for the CF Biospecimen Repository (Emory IRB00042577). A subset of CF subjects also consented to provide sputum samples. CF diagnosis was confirmed by CFTR gene mutation analysis demonstrating two disease-causing mutations and/or pilocarpine iontophoresis sweat testing. Subjects with a history of lung transplant were excluded. Specimens used for this study were collected when the subject had been clinically stable for at least three weeks without any acute illnesses or medication changes. Blood was drawn into a silicone coated tube, processed for serum collection and stored at −80 °C until shipped to the University of Georgia on dry ice for analysis. Blood samples were collected between 2010 and 2018. Sputum samples were collected from CF subjects who were able to spontaneously expectorate sputum. The sputum was placed on ice and processed to collect sputum supernatant as follows. EDTA-PBS was used to dissociate the sputum then a syringe with an 18 gauge needle was used to homogenize the sputum. Sputum supernatant was obtained following a low speed then a higher speed centrifugation. The sputum supernatant was then stored at −80 °C until shipped to the University of Georgia on dry ice for analysis.
Clinical data
Clinical variables were extracted from medical records and the centre’s CF Foundation Patient Registry data. These clinical co-variates included demographics, lung function measured by percent predicted forced expiratory volume in one second (ppFEV1), CFTR genotype, sweat chloride level, pancreatic enzyme use, CFTR modulator medication use, nutritional status, the number of CF pulmonary exacerbations requiring intravenous antibiotics, CF-related diabetes (CFRD) status, and respiratory culture (expectorated sputum or throat swab) microbiology. Raw lung function data in litres were converted to ppFEV1 using the Global Lung Function Initiative equations [Citation15]. Baseline ppFEV1 was defined as the average of the best ppFEV1 of each quarter during the 365 days prior to sample collection. Lung function data were available for subjects developmentally able to perform spirometry (generally age ≥ 6 years). Nutritional status was examined continuously as body mass index (BMI) percentile for subjects ages 2 to 19 years of age and BMI for subjects 20 years and older. Nutritional status was also examined categorically with underweight defined as BMI percentile less than the 10th percentile for ages 2 to 19 years or BMI less than 18.5 for subjects 20 years and older. Microbiologic data were collected from all clinical respiratory cultures in the 365 days prior to sample collection. Chronic Pseudomonas aeruginosa (Pa) was defined as two or more cultures collected with 50 percent or more positive for Pa in the 365 days prior to sample collection using a modification of the Leed’s criteria [Citation16]. CFRD status was assigned by one of the investigators (R.W.L.) based on information extracted from the medical record including diagnosis of CFRD and results of oral glucose tolerance tests conducted in the two years before or one year after sample collection. If a subject did not have CFRD or there was not an oral glucose tolerance test in the defined time period, then no CFRD status was assigned.
Paediatric healthy control subjects
Paediatric healthy control subjects also consented to provide blood samples to the CF Biospecimen Repository (Emory IRB00042577). Subjects had no chronic medical conditions and no acute illnesses within three weeks of specimen collection. Blood specimens were collected and processed as above in 2017.
Adult healthy control subjects
Adult healthy control subjects (n = 5) consented to provide sputum samples to the CF Biospecimen Repository (Emory IRB00042577). Subjects had no chronic medical conditions and no acute illnesses within three weeks of specimen collection. Sputum specimens were induced via a standardised sputum induction procedure with inhalation of a 3% hypertonic saline solution and collected in 2021. Sputum specimens were processed and stored as described above.
PAD4 autoantibody ELISA
Serum levels of autoantibodies to PAD4 were measured with the PAD4 Autoantibody ELISA kit following the manufacturer’s instructions (Cayman Chemical, Ann Arbour, Michigan, USA). Samples were diluted 250- to 5,000-fold and then added to a 96-well ELISA plate precoated with human PAD4. PAD4 Abs of multiple isotypes (IgG, IgA, IgM) were detected by adding horseradish peroxidase conjugated goat anti-human (H + L) antibodies. Colorimetric signal was developed using TMB substrate. The kit utilized an affinity-purified anti-PAD4 antibody from the blood of a rheumatoid arthritis patient as a standard. Results are expressed as U/ml.
Anti-double-stranded (ds)DNA IgA autoantibody ELISA
Serum levels of autoantibodies to dsDNA were measured with a commercial dsDNA Ab IgA ELISA Kit (Abnova, cat#: KA1098). Samples were diluted 25- to 100-fold and then added to microwells precoated with human recombinant dsDNA. Anti-dsDNA IgA antibodies were detected by adding horseradish peroxidase conjugated anti-human IgA antibodies. Colorimetric signal was developed using TMB substrate. Results are expressed as U/ml.
PAD4 protein-level ELISA
Sputum concentrations of PAD4 were quantitated with the help of the PAD4 (human) ELISA Kit following the manufacturer’s instructions (Cayman Chemical, Ann Arbour, MI, USA). Samples were diluted 5- to 10-fold in sterile PBS and added to wells of an ELISA microplate precoated with an anti-human PAD4 antibody. After incubation, samples were washed out and a detection anti-PAD4 antibody labelled with HRP was added. The addition of the HRP substrate TMB followed by the stop solution resulted in a yellow product. Absorbance was measured with a microplate spectrophotometer and final results were obtained with the help of a human PAD4 standard freshly prepared for each assay. Human PAD4 levels are expressed as ng/ml.
PAD4 enzymatic activity fluorescent assay
Enzymatic activities of PAD4 in sputum were measured using the fluorescent PAD4 Inhibitor Screening Assay Kit (Cayman Chemical Ann Arbour, Michigan, USA). PAD4 deiminates N-a-nenzoyl-L-arginine ethyl ester (BAEE), a non-natural substrate with similar kinetic properties to the natural substrate, producing ammonia. Ammonia reacts with a detector resulting in a fluorescent product. Fluorescence is then analysed with excitation wavelengths of 405–415 nm and an emission wavelength of 470–480 nm. A standard composed of human recombinant PAD4 is also used. Final results are expressed as percentage of the enzymatic activity of the standard.
Total protein concentration
The concentration of total protein content in sputum samples was determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Results are expressed as µg/ml.
Statistical analysis
Statistical analysis was conducted using SAS Version 9.4 (Cary, NC, USA). Descriptive statistics such as mean and standard deviations were reported for each normally distributed continuous variable, median and interquartile range (IQR) were reported for each non-normally distributed continuous variable, and frequency and its percentage were shown for categorical variables. Mann–Whitney tests, one-way ANOVA tests or Kruskal–Wallis tests were performed for numerical covariate univariate analysis when appropriate. Chi-square tests or Fisher’s exact tests were employed for categorical covariates when appropriate. Pearson correlations were calculated between log PAD4 and other continuous variables such as age and ppFEV1. Bee swarm plots are presented to show a very rich description of the underlying distribution, and scatter plots with regression analysis are presented to see whether two variables are linearly related. Multivariable linear regression was performed using the entry method to identify clinical factors associated with PAD4 Ab levels. The entry method means that all independent variables are entered into the equation at the same time, and each independent variable is assessed as though it were entered after all the other independent variables were entered. Variables included were those that were significant or marginally significant (i.e. the p value was <.1) in univariate analyses. The significance level is set at 0.05.
Results
Demographics of CF subjects
provides a summary of CF subject demographics and clinical co-variates. Of the 104 CF subjects, 62.5% were male and 96% were Caucasian. The mean age was 23.9 years ± 13.5, with a range of 1.1–60.5 years. The cohort included 44 children ages 1 through 19 years, of which 19 were under the age of 10. Clinical characteristics were fairly typical of the CF population with 93.2% requiring pancreatic enzymes for exocrine pancreatic insufficiency, 81% having at least one copy of the F508del CFTR gene mutation, and 39.4% having chronic Pa in the airways.
Table 1. Demographic and clinical characteristics of CF patients.
A secondary cohort was created with a subset of the original 104 CF patients who also had banked sputum supernatant samples available for analysis. This CF patient cohort consisted of 20 adult CF patients (50% female) with an average age of 30.1 years ± 9.0 years (range: 19.1–56.9 years) and an average ppFEV1 of 68.1 ± 21.6 (range: 35.4–117.5).
Anti-PAD4 autoantibodies are detected in paediatric CF serum and are elevated compared to healthy controls
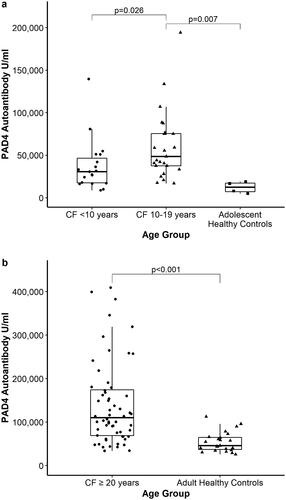
Our group previously found that serum PAD4 Ab levels were significantly higher in a small sample of adult CF patients compared to levels in adult non-CF healthy controls [Citation14]. In the current study, PAD4 Abs were detected in young CF children under 10 years of age (n = 19) with a median antibody level of 30,750 U/ml [IQR 17,059;50,929]. The median anti-PAD4 antibody level for children with CF ages 10 to 19 years (n = 25) was 48,667 U/ml [IQR 37,595;75,583], compared to 12,409 U/ml [IQR 6,478;17,881] among adolescent healthy controls aged 17–18 years (n = 4) (p = .007) (). Only one CF subject out of the cohort of 104 (a one-year old) had an undetectable level.
Anti-PAD4 autoantibodies are elevated in CF adults and increase with age
In this cohort of 104 subjects, there were 60 adult CF patients (≥20 years of age) and the median PAD4 Ab level in blood was 109,984 U/ml [IQR 67,969;175,750] which is significantly elevated (p <.001, ) compared to the level of 45,676 U/ml [IQR 35,564;65,617] found in adult healthy controls (n = 23) that we reported previously [Citation14]. In addition, PAD4 Ab levels positively correlate with age (, , Pearson correlation coefficient 0.584, p <.001).
Figure 2. Serum anti-PAD4 autoantibody levels in CF patients correlate with (a) age and (b) ppFEV1 (Pearson correlation coefficient).
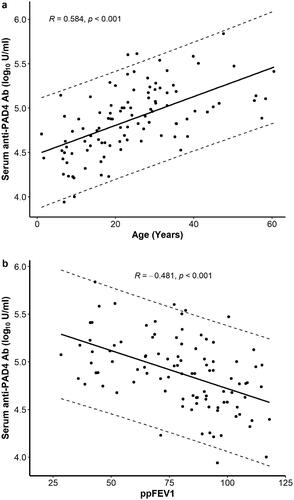
Table 2. Univariate analysis of continuous CF clinical co-variates and anti-PAD4 autoantibodies.
Anti-PAD4 autoantibodies correlate with lower lung function and Pseudomonas aeruginosa airways infection
A total of 99 subjects had lung function data; five paediatric subjects were too young or developmentally unable to perform acceptable spirometric manoeuvres. In univariate analysis, serum PAD4 Abs correlated with lower lung function (ppFEV1), a measure of airflow obstruction and lung damage (, , Pearson correlation coefficient −0.481, n = 99, p <.001). PAD4 Ab levels were also significantly higher in CF subjects with Pa on the respiratory culture collected at the time of the blood specimen (113,235 U/ml [IQR 66,500;179,000], n = 34) compared to CF subjects with Pa negative cultures (59,815 U/ml [IQR 36,464;97,140], n = 70, p = .001) (). In addition, serum PAD4 Ab levels were higher among CF subjects with chronic Pa in the airways (100,628 U/ml [IQR 66,500;168,048], n = 41) compared to CF subjects without chronic Pa (54,798 U/ml [IQR 33,432,106,802], n = 63, p < .001) (). No significant difference was observed in PAD4 Ab levels between CF subjects with mucoid compared to non-mucoid Pa respiratory cultures, or with the presence or absence of Staphylococcus aureus (S. aureus), methicillin-resistant S. aureus (MRSA), or co-infection with Pa and MRSA ().
Table 3. Univariate analysis of categorical CF clinical co-variates and anti-PAD4 autoantibodies.
Anti-PAD4 autoantibodies are not linked with other clinical variables in univariate analysis
We next examined the associations between PAD4 Abs and other potentially relevant clinical co-variates. PAD4 Abs were not associated with sex, race, ethnicity, CFTR genotype, sweat chloride level, pancreatic enzyme use, CFTR modulator use, nutritional status (body mass index or percentile or underweight status), pulmonary exacerbations requiring intravenous antibiotics in the two-year period immediately prior to obtaining the antibody level, or CF-related diabetes status ( and ).
Anti-PAD4 autoantibodies correlated with serum anti-dsDNA IgA autoantibodies
Our previous work found that serum levels of IgA autoantibodies directed against self-DNA are elevated in CF subjects and associated with lower lung function [Citation17]. In the current study, we found that serum PAD4 Abs positively correlated with anti-dsDNA IgA autoantibodies (Pearson correlation coefficient 0.259, n = 104, p = .008). There was no correlation between anti-PAD4 autoantibodies and serum absolute neutrophil count.
Age and lung function remain correlated with anti-PAD4 antibodies in multivariable analysis
Multivariable linear regression was performed among the 99 subjects developmentally able to perform spirometry. Age, sex, and the presence of chronic Pa airway infection were included in the model. After adjusting for the other co-variates, age and ppFEV1 remained significantly associated with PAD4 Ab levels in the multivariable model ().
Table 4. Multivariable linear regression model for CF clinical co-variates and log-PAD4 autoantibodies*.
CF sputum PAD4 levels are elevated but do not correlate with age
The appearance of anti-PAD4 autoantibodies assumes the extracellular presence of their autoantigen, PAD4, in CF patients. Therefore, we assessed the concentration of PAD4 protein in sputum samples of CF patients (n = 20) and five healthy controls by ELISA. Significantly higher PAD4 levels were observed in CF sputum compared to healthy control () but unlike autoantibody levels, PAD4 levels did not correlate with the age of patients (), even if normalised to the total protein content of the sputum ().
CF sputum PAD4 levels, PAD4 activity, and anti-PAD4 autoantibodies do not correlate with lung function
To further learn about PAD4 antigen presence in CF, we additionally measured enzymatic activities of PAD4 by a fluorescent assay and levels of PAD4 Ab in CF sputum and correlated these PAD4-related measures with lung disease. Lung function measured as ppFEV1 did not correlate with CF sputum levels of PAD4 (), PAD4 activity () or PAD4 Abs (). No correlation was observed when the assessed sputum levels were normalised to the total protein content measured by BCA assay (Figure S2(D–F)). Data shown in Supplementary Figures S1–2 suggest that PAD4 is present in CF sputum at higher than normal levels but PAD4 Ab concentrations seen in the sputum do not correlate with lung disease, unlike PAD4 Ab titres in the blood.
Discussion
In this article, we demonstrate that serum anti-PAD4 autoantibodies are detected in paediatric CF serum early in childhood, are elevated compared to healthy paediatric controls, increase with age, and correlate with lung disease progression. We previously found elevated anti-PAD4 antibody levels in adults with CF compared to healthy controls [Citation14]. The current findings build on this result, and for the first time, identify these antibodies in children with CF and demonstrate increasing PAD4 Ab levels with age. This finding suggests that autoimmunity to antigens extruded by NETs may be an early event in CF lung disease, as opposed to a secondary event in those with advanced disease.
In univariate analysis, PAD4 Abs also correlated with lower lung function and Pa airways infection, but not with other key clinical CF co-variates. These results build on our prior findings in a smaller cohort of adult CF patients, where PAD4 Ab levels were also associated with lower lung function and Pa airway infection [Citation14]. In this cohort of children and adults, age and lower lung function remained correlated with PAD4 Abs even in multivariable analysis. However, the presence of Pa airways infection was no longer significant in the multivariable model, suggesting the initial finding may be due to the associations between Pa and age and lung disease severity [Citation2,Citation3]. This result suggests that Pa is not required to drive NET formation and subsequent PAD4 release into the extracellular space. This is an important distinction when considering the potential relevance of NET autoantibodies as Pa is no longer the dominant CF pathogen it once was, particularly in the paediatric aged population.
Other clinical and disease characteristics were not associated with PAD4 Abs. Sweat chloride, a measure of CFTR function, was not linked to antibody levels. Other clinical factors associated with lung disease severity – including pancreatic insufficiency, poor nutritional status, frequent pulmonary exacerbations, and CFRD – also were not correlated with PAD4 Abs. These results suggest that these clinical co-variates do not likely significantly influence NET formation, PAD4 release, or the immune response to extracellular PAD4. This finding is understandable, because lung damage in CF is multifactorial, with many factors contributing to pulmonary decline, by a variety of pathologic mechanisms.
The pathophysiologic mechanism by which PAD4 Abs could lead to airway damage and lung function decline remains unknown. Neutrophils are abundant in CF airways [Citation6–8], and PAD4 is essential for NET formation [Citation10–12]. Neutrophils are thus the likely source of PAD4 in CF airways, with PAD4 release into the extracellular space when neutrophils extrude NETs as has been previously described in the joint in rheumatoid arthritis (RA) [Citation13]. We hypothesise that NET formation results in delivery of PAD4 outside the cell leading to autoantibody generation, and this autoimmunity may be a novel mechanism contributing to CF lung disease progression.
PAD4 Abs are well-characterized in RA and are thought to play an important role in driving disease pathogenesis and progression [Citation18,Citation19]. Autoantibodies against PAD4 in RA are associated with more extensive joint damage and thus may allow identification of a subset of patients with poorer prognosis [Citation18–21]. Interestingly, PAD4 Abs have been detected in serum in the preclinical phase of RA in a subset of patients [Citation22], and the presence of antibodies against PAD4 is also associated with disease duration. The specific mechanisms by which anti-PAD4 antibodies contribute to disease pathogenesis in RA have not been fully elucidated, but one postulated mechanism involves augmentation of an inflammatory response by activation of complement, NK cells, and/or induction of cytokine production [Citation18,Citation19]. Of note, PAD inhibitors that impede NET formation have shown therapeutic efficacy in mouse models of inflammatory arthritis and are being explored as a potential treatment for RA [Citation23].
Serum PAD4 Abs associated with lung disease progression in CF could similarly serve a role as a potential serum biomarker of severe disease or even as a therapeutic target to slow disease pathogenesis. Interestingly, CF patients experience acute pulmonary exacerbations with worsening of respiratory symptoms and pulmonary inflammation and the mechanism of these exacerbations is unclear. We wonder whether these flares of disease could have an immunologic basis and therefore our group is examining PAD4 Ab levels during CF exacerbations.
We previously demonstrated that serum anti-dsDNA IgA autoantibodies are elevated in CF patients and proposed a similar mechanism by which extracellular DNA from neutrophils may serve as an autoantigen in CF airways leading to airway damage [Citation17]. We also found that anti-dsDNA IgA antibodies in CF sera negatively correlated with ppFEV1. In the present article, we show a significant association between serum PAD4 Abs and anti-dsDNA IgA antibodies, supporting this overall hypothesis by demonstrating that multiple neutrophil-derived autoantigens trigger autoantibody formation and correlate with each other. These findings add to the growing literature on autoimmunity and CF, including prior studies that demonstrated associations between autoantibodies directed against bactericidal permeability-increasing protein and CF lung disease severity [Citation9].
We also explored CF sputum PAD4 levels, enzymatic activity, and PAD4 Abs. We found that sputum PAD4 protein levels are elevated in CF compared to healthy controls but do not correlate with age or lung function. Similarly, no correlation was found between PAD4 activity and autoantibody levels in sputum and lung disease. These findings suggest that it is the abnormal host response to PAD4 (as opposed to the amount or activity of PAD4 in the airway) that may contribute to the observed association between higher serum anti-PAD4 autoantibodies and lower lung function.
CF neutrophils have been shown to release more NETs than their non-CF counterparts [Citation24]. This could be due to delayed apoptosis as suggested by others [Citation24], Another possibility is that this increase in NETosis is due to higher intrinsic PAD4 or NADPH oxidase activities, both of which promote NET formation. NETs, however, do not necessarily represent the sole mechanism for PAD4 release as necrotic or necroptotic neutrophils would also be expected to release more of their intracellular content including PAD4. No matter what the mechanism of PAD4 release in CF airways from neutrophils, our data show that the strength of the autoimmune response correlates with CF lung disease and not the levels of the autoantigen in the sputum. Thus, while the extracellular presence of PAD4 as an autoantigen is very likely necessary to initiate an autoimmune response, it is the host response characterized by an exuberant production of PAD4 autoantibodies that is associated with CF lung disease progression. This contention is underscored by the detection of low levels of both sputum PAD4 antigen and serum PAD4 Abs in healthy controls.
Our study has limitations. As a cross-sectional analysis, causality cannot be inferred. Future prospective studies will be needed to evaluate longitudinal development of PAD4 Abs and to replicate these results among additional CF patients. Several of the subgroups with certain clinical characteristics were also small, potentially limiting our power to detect associations. In addition, our samples were collected before the widespread availability of highly effective modulator therapy, which may strongly impact the airway milieu for many, but not all, CF patients in the future.
Despite these limitations, we have described novel findings of anti-PAD4 autoantibodies in CF airways that are present early in childhood, increase over time with age, and correlate with lung disease progression. The role of airway autoimmunity in CF deserves further exploration as a potential mechanism of lung disease progression in CF.
Note
This manuscript contains some data that were included in a prior publication from our group [Citation14].
| Abbreviations | ||
| PAD4 Ab | = | anti-PAD4 autoantibody |
| BMI | = | body mass index |
| CF | = | cystic fibrosis |
| CFRD | = | CF-related diabetes |
| CFTR | = | cystic fibrosis transmembrane conductance regulator |
| dsDNA | = | double-stranded DNA |
| MRSA | = | methicillin-resistant Staphylococcus aureus |
| MSSA | = | methicillin-sensitive Staphylococcus aureus |
| NETs | = | neutrophil extracellular traps |
| PAD4 | = | peptidylarginine deiminase 4 |
| Pa | = | Pseudomonas aeruginosa |
| ppFEV1 | = | percent predicted forced expiratory volume in one second |
| RA | = | rheumatoid arthritis. |
Supplemental Material
Download Zip (154.2 KB)Acknowledgements
Human subject samples were provided by the CF Biospecimen Repository at the Children’s Healthcare of Atlanta and Emory University CF Discovery Core. We would like to thank Chris Driggers and Akele Carter for collecting and verifying clinical data for the CF subjects in this study.
Disclosure statement
R.W.L. reports serving on advisory boards for Vertex Pharmaceuticals Incorporated, outside of the submitted work. All other authors report no conflicts of interest.
Additional information
Funding
References
- Ratjen F, Bell SC, Rowe SM, et al. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010.
- Cystic Fibrosis Foundation. Cystic fibrosis foundation patient registry 2019 annual data report. Bethesda (MD): Cystic Fibrosis Foundation; 2019.
- Harun SN, Wainwright C, Klein K, et al. A systematic review of studies examining the rate of lung function decline in patients with cystic fibrosis. Paediatr Respir Rev. 2016;20:55–66.
- Sly PD, Brennan S, Gangell C, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF), et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152.
- Sly PD, Gangell CL, Chen L, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970.
- Cantin AM, Hartl D, Konstan MW, et al. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J Cyst Fibros. 2015;14(4):419–430.
- Witko-Sarsat V, Sermet-Gaudelus I, Lenoir G, et al. Inflammation and CFTR: might neutrophils be the key in cystic fibrosis? Mediators Inflamm. 1999;8(1):7–11.
- Khan MA, Ali ZS, Sweezey N, et al. Progression of cystic fibrosis lung disease from childhood to adulthood: neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes (Basel.). 2019;10(3):183.
- Skopelja S, Hamilton BJ, Jones JD, et al. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight. 2016;1(17):e88912.
- Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213.
- Rohrbach AS, Slade DJ, Thompson PR, et al. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360.
- Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11(3):189–191.
- Spengler J, Lugonja B, Ytterberg AJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015;67(12):3135–3145.
- Yadav R, Yoo DG, Kahlenberg JM, et al. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J Cyst Fibros. 2019;18(5):636–645.
- Quanjer PH, Stanojevic S, Cole TJ, ERS Global Lung Function Initiative, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343.
- Lee TW, Brownlee KG, Conway SP, et al. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34.
- Yadav R, Linnemann RW, Kahlenberg JM, et al. IgA autoantibodies directed against self DNA are elevated in cystic fibrosis and associated with more severe lung dysfunction. Autoimmunity. 2020;53(8):476–484.
- Curran AM, Naik P, Giles JT, et al. PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets. Nat Rev Rheumatol. 2020;16(6):301–315.
- Reyes-Castillo Z, Muñoz-Valle JF, Llamas-Covarrubias MA. Clinical and immunological aspects of anti-peptidylarginine deiminase type 4 (anti-PAD4) autoantibodies in rheumatoid arthritis. Autoimmun Rev. 2018; 17(2):94–102.
- Halvorsen EH, Pollmann S, Gilboe IM, et al. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann Rheum Dis. 2008; 67(3):414–417.
- Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008; 58(7):1958–1967.
- Kolfenbach JR, Deane KD, Derber LA, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62(9):2633–2639.
- Willis VC, Banda NK, Cordova KN, et al. Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin Exp Immunol. 2017 ;188(2):263–274.
- Gray RD, Hardisty G, Regan KH, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73(2):134–144.