Abstract
Introduction
The prevalence of immune-mediated diseases has increased in the past decades and despite the use of biological treatments all patients do not achieve remission. The aim of this study was to characterise the reasons for short interruptions during treatment with two commonly used TNF-inhibitors infliximab and adalimumab and to analyse the possible effects of the interruptions on immunisation and switching the treatment.
Material and methods
This case-control study was based on retrospective analyses of patient records and a questionnaire survey to clinicians. A total of 370 patients (194 immunised cases and 172 non-immunised controls, 4 excluded) were enrolled from eight hospitals around Finland. Eleven different diagnoses were represented, and the largest patient groups were those with inflammatory bowel or rheumatic diseases.
Results
Treatment interruptions were associated with immunisation in patients using infliximab (p < .001) or adalimumab (p < .000001). Patients with treatment interruptions were more likely to have been treated with more than one biological agent compared to those without treatment interruptions. This was particularly prominent among patients with a rheumatic disease (p < .00001). The most frequent reason for a treatment interruption among the cases was an infection, whereas among the control patients it was remission. The median length of one interruption was one month (interquartile range 1–3 months).
Conclusion
Our results suggest that the interruptions of the treatment with TNF-inhibitors expose patients to immunisation and increase the need for drug switching. These findings stress the importance of careful judgement of the need for a short interruption in the biological treatment in clinical work, especially during non-severe infections.
Introduction
The prevalence of immune-mediated diseases has increased in the past decades and despite new biological treatments, it remains challenging to achieve remission in a considerable proportion of patients [Citation1–3]. Infliximab (IFX) and adalimumab (ADL) are frequently used tumour necrosis factor-alpha inhibitors (TNF-inhibitors) in the treatment of rheumatic diseases such as rheumatoid arthritis (RA), spondylarthritis or juvenile idiopathic arthritis, inflammatory bowel diseases (IBDs) and dermatological conditions such as psoriasis [Citation1–6]. Combinations of these biologicals with immunomodulators, such as methotrexate (MTX), attenuates the formation of anti-drug antibodies (ADAbs), improves the treatment response, and the efficacy of TNF-inhibitor [Citation7].
A balance must be found between effective treatment and potential adverse effects that patients may experience when receiving TNF-inhibitor and immunomodulatory therapies for immune-mediated diseases [Citation8]. Biological agents are structurally immunogenic and thus occasionally induce humoral immune responses [Citation9]. Common adverse effects of TNF-inhibitors include hypersensitivity and infusion or injection-site reactions that are associated with the presence of ADAbs [Citation10,Citation11]. Serious adverse effects like serious infections, malignancies, and neurological disorders rarely occur [Citation10].
Ever since the association of ADAbs with treatment failure was established, there have been attempts to identify the factors affecting the risk of immunisation. Several factors have been associated with insufficient clinical response and the development of ADAbs. The most widely reported of these are patient characteristics such as obesity, smoking [Citation12–14], genetic properties such as TNF-alpha polymorphism [Citation9,Citation14,Citation15], and biochemical markers such as low serum albumin level [Citation16]. In addition to these factors, constantly high plasma concentrations of a biological have been suggested to promote tolerance and prevent immunisation against the drug [Citation17].
If patients are treated for an extended time with biologicals, it is likely that they experience at least short interruptions in treatment due to various reasons. Some of the interruptions are caused by medical reasons such as vaccination with a live-attenuated vaccine, an infection, cancer therapy, surgery, and pregnancy [Citation18,Citation19]. Some treatment interruptions are initiated by the patient for reasons such as travel or financial problems. The length of the temporary discontinuation depends on the underlying reason, the biological agent used, and the local or diagnosis-associated recommendations given by different societies or associations [Citation18].
Few reports have explored the impact of discontinuing on biological treatment in relation to immunisation against biologics [Citation20–24]. Increased titres of ADAbs are more frequently detected if doses of TNF-inhibitor have not been administered as planned [Citation20,Citation21]. According to previous studies, treatment with fixed injection or infusion intervals should be preferred over episodic because of less immunogenicity and better remission rates [Citation22]. To the best of our knowledge, earlier data is lacking regarding the impact of short treatment interruptions on immunisation for biological agents. Thus, the impact of short occasional or planned treatment interruptions for immunisation is unclear.
The first aim of this study was to characterise the reasons for short treatment interruptions during biological treatment. The second aim was to analyse the possible effect of the interruptions on the immunisation of patients to the biologicals. This second aim was studied with two commonly used biological agents, IFX and ADL, separately.
Material and methods
Ethical considerations
The coordinating Ethics Committee, Helsinki and Uusimaa Hospital District, approved the study plan for adult and paediatric patients (ethics committee number 406/13/03/00/15). All case and control subjects or their caregivers (paediatric patients) gave their informed written consent to participate in this study. Permission to obtain confidential information from patient registries and documents (THL/292/5.05.00/2016) was obtained from the Finnish Institute for Health and Welfare.
Study population and data collection
Data was collected from 2016 to 2018. The study population consisted of patients that were receiving or had received ADL or IFX medication for Crohn’s disease (CD), ulcerative colitis (UC), IBD-unspecified (IBDU), juvenile idiopathic arthritis, seronegative oligoarthritis (in adults), psoriatic arthritis, RA, spondylarthritis, psoriasis, hidradenitis suppurativa, or anterior uveitis.
The case patients’ inclusion criteria were clinically significant ADAb levels for ADL (>30 AU/ml), for IFX (>12 AU/ml) detected in serum samples, or both. The ADAbs had been measured by Sanquin Diagnostics, Amsterdam, the Netherlands, using a radioimmunoassay. The control patients’ inclusion criteria were ADL or IFX therapy and no clinically significant levels of ADAbs developed after at least two years of treatment. Later use of other biological drugs during the data collection period was not an exclusion criterion.
Potential patients recruited for this case-control study were identified from the United Medix Laboratories laboratory (UML) database. Some patients were identified and recruited from clinics’ databases. The laboratory serves as one of the national centres for biological drug concentration and ADAb analyses. Data collection requests were sent to 15 hospitals in Finland and 8 participated in the study. Patients were enrolled only if clinical laboratory test results regarding an immunisation to IFX or ADL were available. The ADAb levels and the drug concentrations were obtained mainly from the UML database and partly from clinicians.
The questionnaire form for each study subject (closed-ended questions) based on patient records was completed by a local clinician. Collected data included details about the patient’s date of birth, name and date of diagnosis, TNF-inhibitor used, immunomodulatory medication from the previous 6 months in the control patients or 6 months before immunisation in the cases, and other additional medication if used, the impact of the ADAb positivity on clinical decisions, number of recorded treatment interruptions, the starting and end dates of the temporary discontinuation, and reasons for the treatment interruptions.
Patient categories and data exclusions
Patients were assigned to the case and control groups according to the inclusion criteria of ADAb levels. If there was a discrepancy between answers, for example, impacts of the ADAb positivity were mentioned but no ADAbs were observed, the patient was excluded from the analysis comparing case and control groups.
Patients were defined as “patients with one or several treatment interruptions” if the clinician had marked the patient as such in the questionnaire form. Patients with no interruptions marked were defined as “patients without treatment interruptions”. If the answer was unclear, the patient was excluded from the data analysis regarding interruptions. If the information on the dates of the patient's treatment interruption was lacking from a case patient, the patient was excluded from the subgroup analysis of IFX and ADL and was only included in the group of “All patients with treatment interruptions.” Patients with multiple diagnoses were excluded from the data when different diagnosis groups were compared. Patient categories and data exclusions are described in Supplementary Figure 1.
Statistical analysis
IBM SPSS® Statistics version 25 was used for all statistical analyses. P values less than .05 were considered significant. Distributions are described by mean and standard deviation or median and interquartile range (IQR; 25–75%) as appropriate.
Categorical data
Chi-square test and the Z-test with post-hoc Bonferroni adjustment were used to compare the case and the control groups in differences in having treatment interruptions or comparing reasons for having a treatment interruption. The effect of concomitant MTX treatment on immunisation status was obtained by the Odds ratio (OR).
Quantitative data
After confirming that the variance was equal across the groups, the Mann-Whitney U test was used to compare a number of used TNF inhibitors and having treatment interruptions. Kruskal-Wallis test was used to observe differences in different diagnosis groups between number of used TNF-inhibitors (Supplementary Figure 2) and to compare different either single actions or categories of same kind of actions on clinical management based on the IFX, IFX ADAb, ADL, and ADL ADAb levels. Student t-test was used to compare mean values of ADAbs between those with or without treatment interruptions.
Results
Altogether 370 patients from eight hospitals passed the inclusion criteria and participated in the study. Out of the 370 patients, 194 were in the group of cases (immunised patients), 172 in controls (non-immunised patients), and four were excluded. Most patients were over 18 years old (n = 251, 67.8%). The case patients’ median time for using IFX before immunisation was 11 months (IQR: 5–18 months) and for ADL 18 months (IQR: 7–41 months). Characteristics of the study cohort are described in .
Table 1. Characteristics of the study cohort
Impact of the treatment interruptions to patients’ immunisation status
Out of the 370 patients in this study, 27.3% (n = 101) had at least one interruption during their biological treatment. In total 39.2% (76/194) of cases had had interruptions during treatment while 14.5% (25/172) of controls had interruptions during treatment. When studying the impact of the interruptions on patients’ immunisation status, a statistically significant difference in having treatment interruptions between the case and the control groups was observed (Pearson Chi-Square (df = 1) 28.7, p = 8.3 × 10−8) (). In post-hoc analysis, patients having treatment interruptions were more likely to become immunised to the used biological agent. The same observation was made by studying the subgroup of those without any concomitant immunomodulatory medication (χ2(1) = 15.4, p = .000087) () and studying separately IFX users (χ2(1) = 11.8, p = .00058) and ADL users (χ2(1) = 43.3, p = 4.8 × 10−11) (). Even one interruption during the biological treatment was significantly associated with immunisation (χ2(1) = 10.1, p = .0015).
Figure 1. The number of patients with (yes) and without (no) treatment interruptions and an association between interruptions and immunisation. (A) Treatment interruptions among all the patients of the study. Patients were treated with any biological (IFX, ADL, etanercept, certolizumab pegol, golimumab, vedolizumab, ustekinumab) at the time of the possible treatment interruption. (B) Treatment interruptions among the patients using biologic monotherapy. Patients were treated with any biological (IFX, ADL, etanercept, certolizumab pegol, golimumab, vedolizumab, ustekinumab) at the time of the possible treatment interruption. (C and D) Categories of “IFX” and “ADL” consist of patients treated with IFX or ADL at the time of the possible treatment interruption. (E and F) Treatment interruptions among patients with rheumatic disease and IBD patients. Both subgroups consist of patients who were treated with any biological (IFX, ADL, etanercept, certolizumab pegol, golimumab, vedolizumab, ustekinumab) at the time of the possible treatment interruption. Graph definitions: *, p < .05; ***, p < .001.
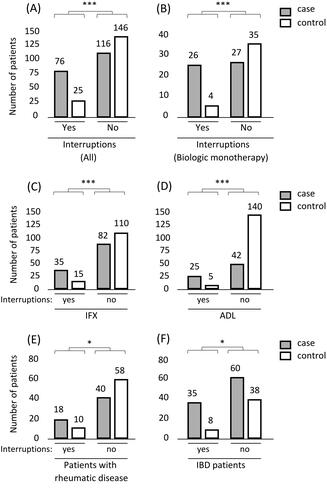
The impact of treatment interruptions on immunisation was also studied in two disease subgroups: 1) patients with a rheumatic disease (including patients with juvenile idiopathic arthritis, seronegative oligoarthritis, psoriatic arthritis, RA, or spondylarthritis); and 2) in IBD patients (CD, UC, or IBDU). There was a statistically significant difference in having treatment interruptions between the case and the control groups in both subgroups [patients with rheumatic disease p = .028 (χ2(1) = 4.8) and for IBD patients p = .019 (χ2(1) = 5.5)] (). In post-hoc analysis, the patients experiencing interruptions during the treatments were more likely to become immunised to the used biological agent in both subgroups.
Next, the impact of concomitant MTX on patients’ immunisation status was studied. A statistically significant difference was observed between concomitant MTX treatment and immunisation status in the group of patients without treatment interruptions, (χ2(2) = 10.7, p = .0047). In post-hoc analysis, patients without concomitant MTX were more likely to become immunised to the used biological agent ().
Figure 2. The number of patients with (yes) and without (no) treatment interruptions and effect of concomitant MTX on the association between treatment interruptions and immunisation. Columns on the left: Patients with interruptions in their biological treatment and with or without concomitant MTX. Columns on the right: Patients with no interruptions in their biological treatment and with or without concomitant MTX. The figure consists of patients who were treated with any biological (IFX, ADL, etanercept, certolizumab pegol, golimumab, vedolizumab, ustekinumab) at the time of the ADAb measurement. Graph definitions: ns, no statistically significant difference; **, p < .01.
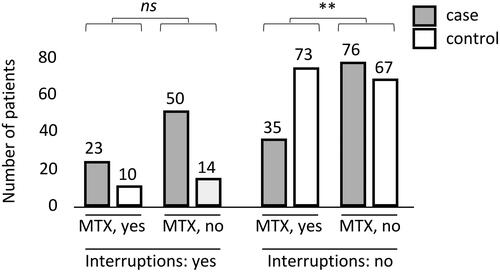
The effect of concomitant MTX treatment on the association between treatment interruptions and immunisation was next analysed in the group of all patients. In the group with concomitant MTX and no treatment interruptions, a protective effect was observed with MTX (Odds ratio, OR 2.4; 95% Cl: 1.4–4.0). In the group with MTX and treatment interruptions, the effect of MTX had an OR of 1.6 (95% Cl: 0.6–4.0).
Association between the interruptions and the number of used TNF-inhibitors
Patients who had interruptions compared to those with no interruptions during the treatment were more likely to have been treated previously with at least one other TNF-inhibitor (U = 10225.5, p = .000034, Mann-Whitney Test) or other biological drugs (U = 9918, p = 8.0 × 10−6, Mann-Whitney Test) (). The median number of different TNF-inhibitors used for those who lacked interruptions during the treatment was one, and for those with interruptions was two.
Figure 3. Association between treatment interruptions and the number of used biologicals. (A) TNF-inhibitors (IFX, ADL, etanercept, certolizumab pegol, golimumab). (B) Any biological drug (TNF-inhibitors, vedolizumab, ustekinumab, tocilizumab and abatacept). Graph definitions: bolded line, mean value; whiskers, 95% percentile; dots, extreme values; ***, p < .001.
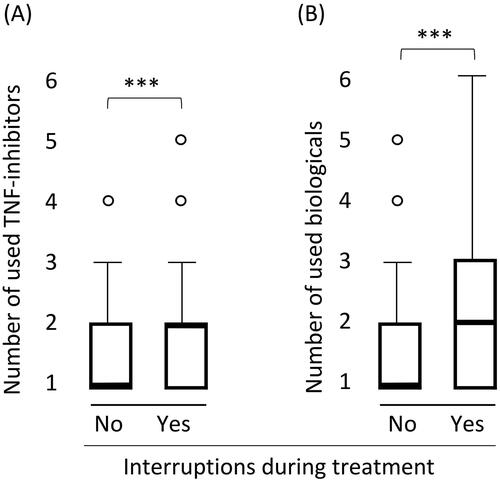
The subgroup of patients with rheumatic diseases and the subgroup of patients with IBD was studied separately. Patients with rheumatic diseases that had had interruptions during the treatment were more likely to have been treated with more than one TNF-inhibitor (U = 742, p = 9.4 × 10−6, Mann-Whitney Test), whereas among IBD patients such association was not seen (U = 2053, p = .98, Mann-Whitney Test).
The reasons for the interruptions during the biological treatment
Infection was the most frequent reason for an interruption covering 50.0% (38/76) of the case-patients that were reported with a treatment interruption (). A statistically significant difference was observed in having treatment interruptions because of infection between the case and the control groups (χ2(1)=24.5, p = 7.6 × 10−7). In post-hoc analysis, patients with interruptions because of infection were more likely to become immunised to the used biological agent. More specified data about the infections were available from 14.0% (6/43) of patients having interruptions because of infection. All these infections were minor (fever, bronchitis, or paronychia).
Table 2. The most frequent reasons for a treatment interruption in the case and the control groups
Number and duration of treatment interruptions
Out of the 76 case-patients with treatment interruptions, data regarding the number of interruptions during treatment were available for 29 patients immunised against IFX and 22 patients immunised against ADL. Out of the 25 control patients with treatment interruptions, data were available for 10 patients using IFX and 10 using ADL. In total, 47 patients were reported with one treatment interruption, 16 with two, and 5 with three or more.
Among all patients having interruptions during the treatment, the median length of one interruption was 1.0 month (IQR: 1.0–3.0 months). The median duration of one interruption among IFX immunised was 1.0 month (IQR: 1–3.3 months) and for ADL respectively 2.0 months (IQR: 1–4 months). The median duration of one interruption among control patients using IFX was 2.0 month (IQR: 1.0–3.0 months) and for ADL respectively 5.5 months (IQR: 2.5–8.5 months). Details about comparing ADAb levels are described in Supplementary Table 1.
The reasons for ADAb measurement and actions taken
The main reason for measuring ADAbs from the case patients was the secondary loss of response (LOR) (41.6% of the cases) and from the control patients were routine monitoring (41.4% of the controls) (). Case patients’ ADAb levels were measured more likely because of secondary LOR (χ2(1) = 55.3, p = 1.0 × 10−13), previously measured low level of drug concentration (χ2(1) = 40.5, p = 2.0 × 10−10), primary LOR (χ2(1) = 12.1, p = .00051), or adverse reaction (χ2(1) = 14.1, p = .00017) compared to control patients. The main action for those with significant levels of ADAb was switching the present TNF-inhibitor to another TNF-inhibitor (52.5% of the cases) (). There were no statistically significant differences in IFX or ADL concentration or IFX ADAb or ADL ADAb levels between single groups of action on clinical management. When comparing different action categories, a statistically significant difference in IFX concentrations and IFX ADAb values between the categories “Switching/discontinuing” and “Dose escalation” was observed (IFX concentration Kruskal-Wallis p = .000044 and after Bonferroni adjustment p = .00013, and IFX ADAb Kruskal-Wallis p = .00021 and after Bonferroni adjustment p = .00062) (). The number of patients using ADL in these categories was too small (n ≤ 1 in two categories) for reliable analysis of the Kruskal-Wallis test.
Table 3. Reasons for ADAb measurement
Table 4. Actions taken on clinical management after detection of significant levels of ADAbs.
Discussion
We found that interruptions during TNF-inhibitor treatment were associated with immunisation in patients using IFX or ADL. Treatment interruptions are also associated with immunisation against the given TNF-inhibitor after dividing the patients into two subgroups: patients with a rheumatic disease or those with IBD. The observation was the same in a group of patients with biological monotherapy. Similarly, in earlier reports, the formation of ADAbs has been more frequent when the drug has been given episodically compared to a scheduled treatment regimen [Citation20–22]. There have been also reports about the impact of discontinued treatment on loss of effectiveness [Citation23,Citation24]. As far as we know, our study is the first to report an association between short treatment interruptions and immunisation. This finding is, however, consistent with other studies [Citation20–24] and offers a clinically relevant reason for the generation of ADAbs. Our results suggest that encouraging patients to continuous adherence and informing patients to maintain a regular treatment dosing can be beneficial in avoiding loss of TNF-inhibitor response.
In our study population, infection was the most frequent reason (50.0%) for an interruption in TNF-inhibitor treatment among the patients with ADAbs. Among the patients without ADAbs, treatment interruptions due to infections were the second most common reason (20.0%) for treatment interruption. According to the manufacturers’ prescribing information, IFX and ADL should be discontinued if a patient develops a serious infection or sepsis [Citation25,Citation26]. A patient with a new infection during treatment with IFX or ADL should be closely monitored, and appropriate antimicrobial therapy should be initiated [Citation25–27]. However, there are no international guidelines or recommendations from drug manufacturers regarding biological treatment discontinuation during minor infections. Our study lacks detailed data on the infections, but none of the infections with detailed data (6/43) were serious. These findings warrant a need for focussing on the avoidance of unnecessary treatment interruptions, especially those due to minor infections.
To the best of our knowledge, earlier data is lacking regarding the reasons for short treatment interruptions. Other studies focus on reasons for discontinuing TNF inhibitor treatment, where the most common reasons for discontinuation were secondary LOR, primary LOR, and adverse events [Citation28–30], but financial issues were also represented [Citation24,Citation29]. In our study, secondary and primary LOR were not reasons for the interruption, but both were reasons for measuring ADAbs. However, infections and financial issues were represented in our study. In our material, the reasons for interruptions were somewhat different in the case and control groups and especially the high number of infections in the case group is interesting. The reason is unknown but, for example, the association of infections with immunisation per se or bias in retrospective reporting of infections in the study groups might explain the phenomenon.
According to our analysis, about a half of treatment interruptions could have been avoided among the case patients by not discontinuing the biological treatment during a minor infection. The lack of recommendations regarding minor infections might be one of the reasons why biological treatment interruptions are so common during such infections. Altogether up to two-thirds of the treatment interruptions could have been avoided among the case-patients during minor infections, vacations, and helping patients with their potential compliance and financial issues. Therefore, medical professionals should be critical in suggesting treatment interruptions due to minor infections, e.g. bronchitis or paronychia.
Since it is not always easy to foresee whether a minor infection will become serious or not in a real-life setting, we suggest considering the following procedure. Upon the emergence of a minor febrile infection, the next injection or infusion is delayed until the patient has been followed for at least a couple of days to reveal the course of the infection. If the infection does not become more serious, the next dose could be administered, but if a serious infection follows the minor one, the drug should be withheld until the infection is under control. A significant proportion of unnecessary interruptions could be avoided with this procedure while still protecting the patient from excessive immunosuppression in case of a life-threatening infection. This should be included in the training of clinicians and nurses and warrants the need for increased patient awareness of uninterrupted biological treatment [Citation31].
Furthermore, we observed that concomitant MTX was associated with a reduced risk of immunisation at least among patients without treatment interruptions. Previous reports have shown that concomitant immunosuppressants, especially MTX, may reduce immunogenicity and are associated with a longer duration of response [Citation21,Citation32,Citation33]. Surprisingly we noticed that concomitant MTX failed to protect patients from immunisation if the patients had interruptions during the biological treatment. Unfortunately, no data was available regarding the concomitant discontinuation of MTX during the biological treatment interruption. Because the reasons for treatment interruptions include issues such as infection and operations, concomitant discontinuation of MTX seems probable. Therefore, we cannot exclude the potential impact of concomitant MTX on immunisation after a TNF-inhibitor treatment interruption.
We found that patients with rheumatic diseases and treatment interruptions were more likely to have been treated with more than one biological drug. This is reasonable since several treatment options with various biologicals are possible for rheumatic diseases [Citation34]. Virkki et al. [Citation35] have shown that primary LOR was the most common reason to switch biological drugs to another in RA patients when biological treatment was started with ADL or etanercept. Primary and secondary LOR seemed to be equally likely reasons to switch treatment in patients treated with IFX [Citation35]. European League Against Rheumatism recommendations (2019 update) suggest that if the first TNF-inhibitor therapy fails, patients may receive an agent with another mode of action or a second TNF-inhibitor [Citation34]. Studies supporting this recommendation suggest the same in the case of primary LOR or after several TNF-inhibitor treatment failures [Citation36].
In our study population, switching to another TNF inhibitor was the most common action after immunisation (52.5% of the cases). We found that lower IFX ADAb level and simultaneous higher IFX concentration are both associated more often with dose escalation than switching to another biological drug or discontinuing biological treatment. Increasing the dose or dosing frequency are often used strategies in patients with ADAbs but the benefits of these actions have been found to be contradictory [Citation37,Citation38].
Different organisations and associations have provided recommendations for the lengths of the treatment interruptions for some patient groups in various situations [Citation18]. For example, the Finnish Institute for Health and Welfare recommends discontinuing the biological treatment of patients with rheumatic disease at least five times the half-life before taking a live-attenuated vaccine and the interruptions should continue for 2–4 weeks before starting the treatment again [Citation18,Citation39]. The European Crohn’s and Colitis Organisation recommends discontinuing IFX and ADL treatments of IBD patients 3 months before taking live-attenuated vaccines and starting treatment again one month after vaccination [Citation27]. In our study, none of the patients had an interruption of the biological therapy due to vaccination, whereas four patients interrupted biological therapy due to pregnancy. Some organisations have already provided consensus statements to suggest continuing IFX or ADL treatment during pregnancy but discontinuing MTX three months prior to planned pregnancy due to its teratogenicity [Citation19,Citation40].
One limitation of our study is that we were unable to analyse causality between the treatment interruptions and immunisation, although the association was clear. Another limitation is that the study population was affected by the usual choice of the initial biological medication since most patients were treated first with IFX and switched to ADL after immunisation. Our approach could be perceived as weak given that data on treatment interruptions was collected afterwards and therefore a bias could be caused if the patients and study physicians knew the reason to ask for details about the interruptions. To avoid such a bias, we did not emphasise the reason for various questions and thus consider the risk for a significant bias fairly small. It would be interesting to study the observed association between TNF-inhibitor treatment interruptions and immunisation in a further prospective study.
A clear strength of the study, in turn, was a dataset covering half of the hospitals in Finland. This diminishes the possible impact of a single clinic, or a few doctors, on the results of this study. The study cohort covers different areas of the country and the basic data are reliable and representative (diagnosis, ages, and sex). Our results suggest that treatment interruptions might lead to immunisation and therefore any temporary discontinuation of ADL or IFX treatment should be considered thoroughly. Unnecessary treatment interruptions should be avoided.
Conclusion
In conclusion, our results suggest that short interruptions during the studied TNF-inhibitor treatment could expose a patient to immunisation and increase the risk for drug switching. The findings motivate careful judgement of the need for a short interruption in the biological treatment in clinical work, especially during non-severe infections and warrant the need for a prospective study on this theme.
Supplemental Material
Download MS Power Point (202.4 KB)Acknowledgements
We appreciate the participation of the BLING Project Group members [Perttu Arkkila, Nina Barner-Rasmussen, Clas-Göran af Björkesten, Anja Eberl, Fredrik Forsström, Martti Färkkilä, Johanna Haapamäki, Kalle Jokelainen, Miikka Keski-Keturi, Kaisa Kosunen, Pauliina Molander, Daniela Norio, Hannu Nuutinen, Minna Takkunen (Gastroenterology, University of Helsinki and Helsinki University Hospital, Finland); Päivi Lindahl (Department of Pediatrics, University of Helsinki and Helsinki University Hospital, Finland); Tiina Levälampi (Ophthalmology, University of Helsinki and Helsinki University Hospital, Finland); Airi Jussila (Gastroenterology, Tampere University Hospital, Finland); Anna Kinnunen, Matleena Lopperi, Marja Pertovaara, Vappu Rantalaiho, Jarno Rutanen, Susanna Sihvonen, Terhi Uotila, Vladena Vinograi, Maritsa Vesalainen (Centre for Rheumatology, Tampere University Hospital, Finland); Merja Malin, Kati Markula-Patjas (Department of Pediatrics, Tampere University Hospital, Finland); Juha Asikainen, Jelena Borodina, Pekka Hannonen, Kirsi Paalanen, Anna Sillanpää, Kati Soininen, Juha Taavela, Tuomas Rannio (Rheumatology, Jyväskylä Central Hospital, Finland); Sami Turunen (Gastroenterology, Oulu University Hospital, Finland); Markku Heikkinen (Gastroenterology, Kuopio University Hospital, Finland); Markku Kauppi, Heini Pohjankoski (Rheumatology, Päijät-Häme Central Hospital, Finland); Johanna Kärki, (Kanta-Häme Central Hospital, Finland)]
We also thank the assisting personnel involved in the study, most notably Pamela Edgren, Pauli Pirinen (United Medix Laboratories, Helsinki, Finland), Virpi Pelkonen, Pirkko Tuukkala (Gastroenterology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland) and Seija Salonen (Rheumatology, Jyväskylä Central Hospital, Jyväskylä, Finland).
Disclosure statement
The following authors declare that there is no conflict of interest: Aalto K, Haapala A-M, Keskitalo P, Kokko A, Kolho K-L, Korkatti K, Koskela R, Kröger L, Lamberg T, Mikola K, Mäkinen H, Mälkönen T, Sard S, Valtanen S, Vidqvist K-L.
Eklund, K. has received lecture and consultation fees from SOBI, Pfizer, Novartis, Lilly and Janssen.
Huilaja, L. has received educational grants from Takeda, Janssen-Cilag, Novartis, AbbVie and LEO Pharma, honoraria from Sanofi Genzyme, Novartis, Abbvie and UCB Pharma for consulting and/or speaking and is an investigator for Abbvie.
Isomäki P. has received a grant from Pfizer, personal fees from Abbvie, Pfizer, Lilly and Roche and non-financial support from Abbvie and Roche.
Jokiranta, TS. has received lecture and consultation fees from the following pharmaceutical companies producing biologicals used for the patient groups mentioned in this study: Abbvie, MSD, Novartis, Pfizer, Roche, Sandoz, Takeda.
Kyllönen, M. has been on conference and congress trips sponsored by followed companies Novartis, Roche, Bristol-Myers-Squipp, Sanofi, Pfizer and UCB and has received lecture fee from Abbvie.
Lahtinen, P. has received lecture fees from Takeda.
Leinonen, S. has consultation and lecture fees from AbbVie − 2019 and lecture fees from Santen, UCBPharma, Théa Nordic.
Sipponen, T. has received consultation or lecturer fees from BMS, Janssen-Cilag.
Pfizer, Roche, and Takeda and study grants from Janssen-Cilag and Takeda.
Sokka-Isler, T. has received personal fees from Abbvie, BMS, Celgene, Medac, Merck, Novartis, Orion Pharma, Pfizer, Roche, Sandoz, UCB and Bohringer Ingelheim and non-financial support from DiaGraphIT.
Vähäsalo, P. has received lecture and consultation fees from Abbvie, SOBI, Pfizer, Novartis, Roche and MSD.
Additional information
Funding
References
- Lee TW, Fedorak RN. Tumor necrosis factor-α monoclonal antibodies in the treatment of inflammatory bowel disease: clinical practice pharmacology. Gastroenterol Clin North Am. 2010;39(3):543–557.
- Aaltonen KJ, Ylikyla S, Tuulikki Joensuu J, et al. Efficacy and effectiveness of tumour necrosis factor inhibitors in the treatment of rheumatoid arthritis in randomized controlled trials and routine clinical practice. Rheumatology. 2017;56(5):725–735.
- Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: progress and promises. Autoimmunity. 2014;47(2):77–94.
- Joensuu JT, Aaltonen KJ, Aronen P, et al. Cost-effectiveness of biologic compared with conventional synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a register study. Rheumatology. 2016;55(10):1803–1811.
- Chima M, Lebwohl M. TNF inhibitors for psoriasis. Semin Cutan Med Surg. 2018;37(3):134–142.
- Kurz K, Herold M, Winkler C, et al. Effects of adalimumab therapy on disease activity and interferon-gamma-mediated biochemical pathways in patients with rheumatoid arthritis. Autoimmunity. 2011;44(3):235–242.
- Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLOS One. 2012;7(1):e30275.
- Sozzani S, Abbracchio MP, Annese V, et al. Chronic inflammatory diseases: do immunological patterns drive the choice of biotechnology drugs? A critical review. Autoimmunity. 2014;47(5):287–306.
- Vultaggio A, Petroni G, Pratesi S, et al. How the immune system responds to therapeutic biological agents. J Int Med Res. 2016;44(1 suppl):38–42.
- Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215–226.
- Marzano AV, Borghi A, Meroni PL, et al. Immune-mediated inflammatory reactions and tumors as skin side effects of inflammatory bowel disease therapy. Autoimmunity. 2014;47(3):146–153.
- Klaasen R, Wijbrandts CA, Gerlag DM, et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63(2):359–364.
- Ottaviani S, Gardette A, Tubach F, et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(4):478–483.
- Siegel CA, Melmed GY. Predicting response to anti-TNF agents for the treatment of Crohn's disease. Therap Adv Gastroenterol. 2009;2(4):245–251.
- Blom M, Kievit W, Fransen J, et al. The reason for discontinuation of the first tumor necrosis factor (TNF) blocking agent does not influence the effect of a second TNF blocking agent in patients with rheumatoid arthritis. J Rheumatol. 2009;36(10):2171–2177.
- Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20(12):2247–2259.
- Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. 2016;7(4):289–300.
- Anttila Veli-Jukka HJ, Ritva P, Tarja T-K, et al. Biologisten lääkkeiden tauotus tulehduksellisia tauteja sairastavilla [Discontinuation of biological drugs in patients with inflammatory diseases]. Duodecim. 2016;132:369–376.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD parenthood project working group. Inflamm Bowel Dis. 2019;25(4):627–641.
- Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4(10):1248–1254.
- Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56(9):1226–1231.
- Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33(9):987–995.
- Gisbert JP, Marin AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2016;111(5):632–647.
- Wolf D, Skup M, Yang H, et al. Clinical outcomes associated with switching or discontinuation from anti-TNF inhibitors for nonmedical reasons. Clin Ther. 2017;39(4):849–862.e846.
- Janssen Biologics B.V. Remicade, Prescribing information. European Medicines Agency; 2020. [cited 2021 Aug 8]. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/REMICADE-pi.pdf.
- AbbVie Biotechnology GmbH. Humira, Precribing Information; 2021. [cited 2021 Aug 8]. Available from: https://www.rxabbvie.com/pdf/humira.pdf.
- Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):879–913.
- Titton DC, Silveira IG, Louzada-Junior P, et al. Brazilian biologic registry: Biobada Brasil implementation process and preliminary results. Rev Bras Reumatol. 2011;51(2):152–160.
- Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology. 2011;50(1):124–131.
- Ciurea A, Exer P, Weber U, et al. Does the reason for discontinuation of a first TNF inhibitor influence the effectiveness of a second TNF inhibitor in axial spondyloarthritis? Results from the Swiss Clinical Quality Management Cohort. Arthritis Res Ther. 2016;18(1):71.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106.
- Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348(7):601–608.
- Leinonen ST, Aalto K, Kotaniemi KM, et al. Anti-adalimumab antibodies in juvenile idiopathic arthritis-related uveitis. Clin Exp Rheumatol. 2017;35:1043–1046.
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699.
- Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis-a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30(11):1447–1454.
- Rubbert-Roth A, Szabo MZ, Kedves M, et al. Failure of anti-TNF treatment in patients with rheumatoid arthritis: the pros and cons of the early use of alternative biological agents. Autoimmun Rev. 2019;18(12):102398.
- Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–1468.
- Kothari MM, Nguyen DL, Parekh NK. Strategies for overcoming anti-tumor necrosis factor drug antibodies in inflammatory bowel disease: case series and review of literature. World J Gastrointest Pharmacol Ther. 2017;8(3):155–161.
- Finnish Institute for Health and Welfare. Reumalääkityksen tauottaminen tai vähentäminen [Dosage reduction and discontinuation of rheumatic medication]. 2019. [cited 2021 Jan 3]. Available from: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/tietoa-rokotuksista/eri-kohderyhmien-rokottaminen/laaketieteellisten-riskiryhmien-rokottaminen/reumatauteja-sairastavien-aikuisten-rokottaminen/reumalaakityksen-tauottaminen-tai-vahentaminen.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation: a consensus report issued by the Austrian Societies of Gastroenterology and Hepatology and Rheumatology and Rehabilitation. Wien Klin Wochenschr. 2019;131(1–2):29–44.
