Abstract
Impaired insulin secretion due to pancreatic β-cell injury is an important cause of type 2 diabetes (T2D). Regulators of guanine nucleotide binding protein (G protein) signaling proteins played a key role in regulating insulin sensitivity in vivo. To explore the role of RGS7 on palmitic acid-induced pancreatic β-cell injury, pancreatic β-cells Beta-TC-6 and Min6 were treated with palmitic acid (PA) to similar type 2 diabetes (T2D) injury in vitro. The 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT), 5-ethynyl-2′-deoxyuridine (EdU), and flow cytometry were used to analyze cell viability, proliferation, and apoptosis, respectively. Enzyme-linked immunosorbent assay (ELISA) kits were used to analyze the changes of inflammation-related cytokines. The expression of gene and protein was measured by quantitative real-time PCR (qRT-PCR) and western blot. PA modeling induced apoptosis, increased levels of inflammation-related cytokines, and suppressed cell viability and proliferation of pancreatic β-cells. RGS7 silence markedly alleviated the cell injury induced by PA. RGS7 overexpression further aggravated apoptosis and inflammatory response in PA-induced pancreatic β-cells and inhibited cell viability and proliferation. It is worth noting that RGS7 activated the chemokine signaling pathway. Silence of the key gene of the chemokine signaling pathway could eliminate the negative effect of RGS7 on PA-induced pancreatic β-cells. RGS7 silence protects pancreatic β-cells from PA-induced injury by inactivating the chemokine signaling pathway.
Introduction
Diabetes is a chronic hyperglycemia caused by pancreatic β-cell damage, deficiency of its action, or deficiency of insulin secretion caused by both [Citation1,Citation2]. Type 2 diabetes (T2D) is characterized by the dysfunction of pancreatic β-cell in elevating blood glucose levels, impaired insulin secretion, and failure of peripheral tissue response to physiological insulin levels [Citation3,Citation4]. T2D has spread rapidly around the world due to the combination of genetic and environmental factors [Citation5,Citation6] and has affected about 500 million people [Citation7,Citation8]. In addition, T2D increases the risk of a variety of diseases and results in increased morbidity and economic burden [Citation9]. In terms of treatment, the results of lifestyle-oriented and drug intervention results for T2D are often disappointing [Citation10]. Therefore, finding the effective targets for protecting pancreatic β-cells becomes an important therapeutic strategy for T2D.
Regulators of guanine nucleotide binding protein (G protein) signaling (RGSs) are originally identified as guanosine triphosphate (GTP)-activating proteins of Gα subunit of heterotrimer G protein [Citation11]. Presently, more than 30 RGS proteins have been identified [Citation12]. Of these, RGS7 is evolutionarily conservative in all animals and plays a key role in many processes and organ systems [Citation13,Citation14]. More importantly, RGS proteins were reported to play a key role in regulating insulin sensitivity in vivo [Citation11,Citation15–17]. RGS16 reconstitution substantially exacerbated insulin resistance in mice [Citation18]. RGS7 is highly expressed in the brain, especially in the hypothalamus, and may be involved in regulating the hypothalamic–pituitary–adrenal axis in response to different stresses and stimuli [Citation19]. Previous studies have provided evidence that RGS7 may constitute an obesity locus in humans [Citation19,Citation20]. Based on these findings, we speculated that RGS proteins may provide a new therapeutic opportunity for T2D.
Prolonged exposure of β-cells to high fatty acid concentrations results in increased insulin-secreting cell death [Citation21]. Palmitic acid (PA) is the most abundant long-chain saturated free fatty acid physiologically. In insulin-secreting cells, such as pancreatic β-cells, excess fatty acid accumulation leads to cellular dysfunction and damage [Citation22]. In this study, we stimulated pancreatic β-cells with PA to similar T2D injury to investigate the effect of RGS7 in T2D. We hypothesize that RGS7 silence could alleviate PA-induced injury in pancreatic β-cells.
Methods
Cell culture and treatment
Mice islet cells Beta-TC-6, NIT-1 (Procell, Wuhan, China), Min6 (BeNa Culture Collection, Beijing, China), and hamster islet cells HIT-T15 (Procell, Wuhan, China) were cultured in 90% RPMI-1640 supplemented with 10% fetal bovine serum (FBS; endotoxin levels < 5 EU/mL), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.05 mM β-mercaptoethanol medium (culture temperature, 37 °C; gas atmosphere, 5% CO2 + 95% air). When the cell density reaches 80%–90%, palmitic acid (PA, Sigma-Aldrich, St. Louis, MO, USA) was conjugated with fatty-acid-free bull serum albumin (BSA) prior to addition to cell culture. PA was dissolved in 99% ethanol to a concentration of 100 mM, and then mixed with 10% BSA in serum-free high glucose Dulbecco’s modified eagle medium (DMEM) to make a 4 mM PA stock solution. The BSA-conjugated PA was added to cells at a final concentration of 0.4 mM. Cells treated with 0.4 mM PA for 48 h were used as PA group. Cells treated with ethanol and fatty-acid free BSA without PA were used as control group. All experiments were biologically repeated three times.
Bioinformatics analysis
The data downloaded from GEO (GEO accession: GSE118230) [Citation23] was used to analyze RGS7 expression in normal and PA-treated human islet. Gene set enrichment analysis (GSEA) and Kyoto Encyclopedia of Gene and Genome (KEGG) were used to evaluate the potential mechanism underlying the involvement of RGS7 in T2D based on GEO data (GEO accession: GSE118230).
Cell transfection
The siRNA targeting RGS7 (si-RGS7 no. 1 and si-RGS7 no. 2), RAP1A (si-RAP1A no. 1 and si-RAP1A no. 2), and negative control (si-NC) were bought from Ribobio (Guangzhou, China). Beta-TC-6 cells were transfected with RGS7 siRNAs, RAP1A siRNAs, and si-NC (50 nM) using LipofectamineTM 3000 Reagent (Invitrogen) according to the manufacturer’s protocol. The sequences of siRNAs and si-NC were as follows: si-NC 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′. si-RGS7 no. 1 5′-GGCUAGAGCUAGCAGAUUAUG-3′ and antisense 5′-UAAUCUGCUAGCUCUAGCCUU-3′. si-RGS7 no. 2 5′-GACAUUGUUCAAUGGUUAAUA-3′ and antisense 5′-UUAACCAUUGAACAAUGUCUG-3′. si-RAP1A no. 1 5′-GCACUAGUUUAUUCAAUUACA-3′ and antisense 5′-UAAUUGAAUAAACUAGUGCAA-3′. si-RAP1A no. 2 5′-AGUCAAAGAUCAACGUUAAUG-3′ and antisense 5′-UUAACGUUGAUCUUUGACUUU-3′. Min6 cells were transfected with RGS7 pcDNA3.1 (2 μg, Vector, RGS7-mutant or RGS7; Invitrogen). Transfection was performed using LipofectamineTM 3000 Reagent (Invitrogen) according to the manufacturer’s protocol. After 48 h transfection, the transfection efficiency was determined by RT-qPCR. All the experiments were performed in triplicate.
Cell viability analysis
The cell viability was analyzed by using the 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay (C0009S, Beyotime). Beta-TC-6 cells and Min6 cells were seeded in 96-well plates (5 × 104 cells/well) and incubated overnight. Forty-eight hours after PA or transfection treatment, 10 μL of MTT solution was added to each well, and the cells were incubated for 4 h in a cell incubator. Add 100 μL of Formazan lysis solution to each well, mix properly, and incubate in a cell incubator until formazan is completely dissolved. The absorbance was measured at 570 nm using a microplate reader. All the experiments were performed in triplicate.
Flow cytometry analysis
The cell apoptosis was analyzed by the Annexin V-FITC Apoptosis Detection Kit (C1062S, Beyotime). Beta-TC-6 and Min6 cells were washed with phosphate buffer saline (PBS) and digested using trypsin cell digestion solution until the cells were blown down by gentle blowing, and the trypsin cell digestion solution was aspirated. Cells were then centrifuged at 1000 g for 5 min, resuspended with PBS and counted. The resuspended cells (5 × 104) were centrifuged at 1000 g for 5 min and then discarded the supernatant. Briefly, 195 μL of Annexin V-FITC binding buffer was added to resuspend the cells. Subsequently, 5 μL of Annexin V-FITC and 10 μL propidium iodide staining solution were added and incubated for 10–20 min at room temperature in the dark. The apoptosis cells were detected by flow cytometry. The sum of the proportions of early (AnnexinV+/PI–) and late (AnnexinV+/PI+) apoptotic cells is the apoptosis rate. All the experiments were performed in triplicate.
5-Ethynyl-2′-deoxyuridine analysis
The cell proliferation was analyzed by using the 5-ethynyl-2′- deoxyuridine (EdU) Cell Proliferation Kit (C0078S, Beyotime). Beta-TC-6 and Min6 cells (2 × 104 cells/well) were cultured in 24-well plates overnight. Cells were incubated with EdU solution (20 μM) for 2 h. Subsequently, 4% paraformaldehyde was added for 15 min at room temperature. After incubating with PBS for 10–15 min at room temperature, cells were incubated with endogenous peroxidase blocking solution for 20 min to inactivate endogenous peroxidase. After three times wash with PBS, 4′,6-diamidino-2-phenylindole (DAPI) was added and incubated for 10 min in a dark room at room temperature to stain cell nuclei. Finally, EdU-positive cells were observed and counted with a fluorescence microscope. All the experiments were performed in triplicate.
Quantitative real-time PCR analysis
Total RNA from Beta-TC-6 and Min6 cells was extracted with Trizol Reagent (R0016, Beyotime). The RNA concentrations were determined by measuring absorbance at 260/280 nm. The DNase I (D7076, Beyotime) was used to remove genomic DNA from RNA samples. RNA was reverse transcribed into cDNA by using the BeyoRT™II First Strand cDNA Synthesis Kit (D7178L, Beyotime). Quantitative real-time (qRT)-PCR was performed using BeyoFast™ SYBR Green qPCR Mix (D7265, Beyotime). The results were calculated using the 2–ΔΔCt method. GAPDH was used as the internal control. All the experiments were performed in triplicate. The following primers were used for amplification: RGS7 forward, 5′-GGACGAATCACCCAACATGC-3′ and reverse, 5′-TGAACAATGTCTGACCCGGAG-3′; RAP1A forward, 5′-CCGCGGACGGAGAGATTGTC-3′ and reverse, 5′-GCCTCCTGAACCAAGGACTAC-3′; GAPDH forward, 5′-GATAAGCAGGGCGGGAGG-3′ and reverse 5′-CCCAATACGGCCAAATCCGT-3′.
Western blot analysis
The protein was extracted form Beta-TC-6 and Min6 cells using the RIPA buffer, followed by separating by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to the polyvinylidene fluoride (PVDF) membranes.
The membranes were blocked with 5% skim milk and then incubated with primary antibodies (ras-associated protein 1A (RAP1A), 68125, Proteintech Group, Wuhan, China. RGS7, ab228618; AKT3, ab152157; phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3), ab235234; GAPDH, ab9485, Abcam) overnight at 4 °C and treated with secondary antibody (ab205718, ab6741 Abcam). The bands were visualized using the Beyo enhanced chemiluminescence (ECL) Moon kit (P0018FS, Beyotime) and analyzed using the ImageJ software. All the experiments were performed in triplicate.
Enzyme linked immunosorbent assay analysis
The levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6, and IL-18 were analyzed by Mouse TNF-α enzyme linked immunosorbent assay (ELISA) Kit (PT512, Beyotime), Mouse IL-1β ELISA Kit (PI301, Beyotime), Mouse IL-6 ELISA Kit (PI326, Beyotime), and Mouse IL-18 ELISA Kit (PI553, Beyotime) according to the instructions. Briefly, Beta-TC-6 and Min6 cells (1 × 107 cells/ml) were centrifuged at 2000 g at 4 °C for 5 min, and the supernatants were collected to analyze the levels of inflammatory factors. All the experiments were performed in triplicate.
Statistical analysis
All the data were presented as mean ± SD and analyzed by using GraphPad Prism 7.0 software (GraphPad Software Inc., San Diego, CA). The differences between two groups were analyzed by the Student’s t test. The differences among multiple groups were analyzed by one-way ANOVA followed by post-hoc test. p < 0.05 was considered statistically different.
Results
RGS7 is highly expressed in PA-induced pancreatic β-cells
The analysis of GEO dataset GSE118230 showed the level of R7 family members (). The top three members of the expression level are RGS4, RGS2, and RGS7 (). The role of RGS4 and RGS2 in pancreatic β-cells has been reported previously [Citation24–27]. Thus, RGS7 was selected for subsequent study. GEO dataset GSE118230 showed that the expression level of RGS7 was significantly increased after 7 days of treatment with PA (). Then, total of four pancreatic β-cell lines (Beta-TC-6, NIT-1, Min6, and HIT-T15) were employed to measure RGS7 expression. As shown in , RGS7 expression was the highest in Beta-TC-6 cells and the lowest in Min6 cells. Therefore, Beta-TC-6 and Min6 cells were selected for our study. We then treated the pancreatic β-cells Beta-TC-6 and Min6 cells, and measured the change of RGS7 expression. The results showed that the levels of RGS7 mRNA and protein in PA group were obviously upregulated compared with control group (). These results suggested that RGS7 might play a vital role in PA-induced pancreatic β-cells.
Figure 1. RGS7 is highly expressed in PA-induced pancreatic β-cells. A: Data of GSE118230 showed the expression of R7 family members. B: Data of GSE118230 showed that RGS7 was upregulated in PA-induced pancreatic β-cells. C and D: qRT-PCR and western blot showed the expression of RGS7 in four pancreatic β-cell lines. E and F: qRT-PCR and western blot showed that RGS7 was upregulated in PA-induced pancreatic β-cells. All the experiments were performed triplicate. *p < 0.05 vs. Control group.
RGS7 regulates proliferation and apoptosis of PA-induced pancreatic β-cells
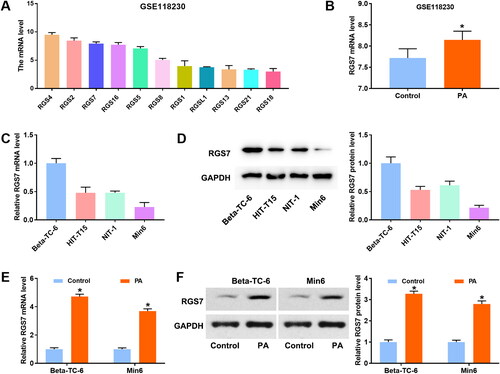
To investigate the effect of RGS7 in PA-induced pancreatic β-cells, RGS7 siRNAs were transfected into Beta-TC-6 cells. As shown in , RGS7 expression was obviously suppressed by RGS7 siRNAs. PA treatment markedly increased RGS7 expression compared with control group. CCK-8 assay showed no significant difference between si-NC group and si-RGS7 groups (). Compared with si-NC group, RGS7 expression was significantly decreased in PA + si-RGS7 groups (). Then, MTT and EdU assays were performed to detect cell viability and proliferation of pancreatic β-cells. Compared with control group, PA modeling markedly inhibited cell viability and decreased EdU-positive cells of pancreatic β-cells. RGS7 silence obviously increased the cell viability and proliferation (). In addition, cell apoptosis were measured by flow cytometry. PA modeling accelerated apoptosis, while RGS7 silence partly inhibited the increase of apoptosis induced by PA (). Then, RGS7 overexpressed plasmid was transfected into Min6 cells to further explore the effect of RGS7 on PA-induced pancreatic β-cells. As shown in , RGS7 expression was markedly increased by RGS7 overexpression. Compared with PA + Vector group, RGS7 expression in PA + RGS7 group was further increased (). We also analyzed the effect of RGS7 overexpression on cell viability, proliferation, and apoptosis. The results showed that RGS7 overexpression obviously decreased cell viability, proliferation whereas increased apoptosis of PA-induced pancreatic β-cells in comparison with PA + Vector group (). To confirm the increase in apoptosis upon RGS7 overexpression, overexpression of RGS7 and its mutant were transferred into Min6 cells. The expression of RGS7 was detected by qRT-PCR and western blot. The results showed that both RGS7 and RGS7-mutant significantly increased RGS7 expression (). The apoptosis was detected by flow cytometry. Notably, RGS7-mutant has no significant effect on apoptosis ().
Figure 2. RGS7 silence promotes proliferation and inhibits apoptosis of PA-induced pancreatic β-cells. A: RGS7 siRNAs decreased RGS7 expression in pancreatic β-cells. *p < 0.05 vs. si-NC group. B: RGS7 silence had no effect on cell viability of pancreatic β-cells. C: RGS7 siRNAs decreased RGS7 expression in PA-induced pancreatic β-cells. D and E: RGS7 silence increased cell viability and promoted proliferation of PA-induced pancreatic β-cells. F: RGS7 silence reduced apoptosis rate of PA-induced pancreatic β-cells. *p < 0.05 vs. Control group. &p < 0.05 vs. PA + si-NC group. All the experiments were performed triplicate.
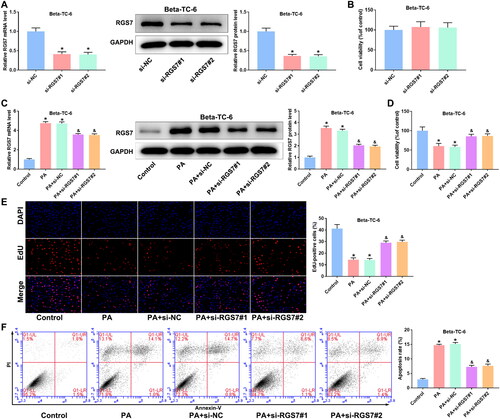
Figure 3. RGS7 overexpression inhibits proliferation and promotes apoptosis of PA-induced pancreatic β-cells. A: RGS7 overexpression increased RGS7 expression in pancreatic β-cells. *p < 0.05 vs. Vector group. B: RGS7 overexpression increased RGS7 expression in PA-induced pancreatic β-cells. C and D: RGS7 overexpression suppressed cell viability and proliferation of PA-induced pancreatic β-cells. E: RGS7 overexpression increased apoptosis rate of PA-induced pancreatic β-cells. *p < 0.05 vs. Control group. &p < 0.05 vs. PA + Vector group. F: RGS7-mutant and RGS7 overexpression significantly increased RGS7 expression in pancreatic β-cells. G: RGS7-mutant overexpression has no effect on apoptosis rate of pancreatic β-cells. *p < 0.05 vs. Vector group. All the experiments were performed triplicate.
RGS7 regulates cytokines levels of PA-induced pancreatic β-cells
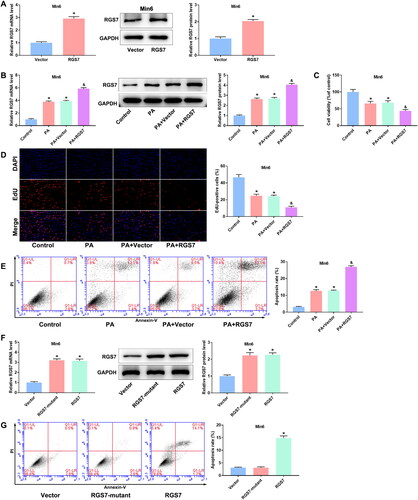
Then, we analyzed the changes of inflammation-related cytokines after transfection with RGS7 siRNAs or RGS7 overexpressed plasmid. PA modeling markedly increased the levels of TNF-α, IL-1β, IL-6, and IL-18 of Beta-TC-6 and Min6 cells. Compared with PA + si-NC group, RGS7 silence significantly reduced the levels of these inflammation-related cytokines in Beta-TC-6 cells (). Oppositely, RGS7 overexpression further increased the levels of TNF-α, IL-1β, IL-6, and IL-18 in Min6 cells compared with PA + Vector group ().
Figure 4. RGS7 silence suppressed inflammation levels of PA-induced pancreatic β-cells. A: RGS7 silence suppressed the levels of TNF-α, IL-1β, IL-6, and IL-18 of pancreatic β-cells. B: RGS7 overexpression increased the levels of TNF-α, IL-1β, IL-6, and IL-18 of pancreatic β-cells. All the experiments were performed triplicate. *p < 0.05 vs. Control group. &p < 0.05 vs. PA + si-NC/Vector group.
RGS7 activates the chemokine signaling pathway in PA-induced pancreatic β-cells
To investigate the mechanism of RGS7 in PA-induced pancreatic β-cells, KEGG enrichment analysis was performed using GSEA. The analysis result showed that RGS7 activated the chemokine signaling pathway (). The genes enriched in the chemokine signaling pathway were shown as the Blue-Pink O’ Gram (). Then, we measured the expression of chemokine signaling pathway-related proteins RAP1A, AKT3, PIK3R3, XCL2, and XCL1. PA treatment remarkably enhanced the expression of RAP1A, XCL2, and XCL1, and reduced the expression of AKT3 and PIK3R3 (). RGS7 silence markedly decreased the levels of RAP1A, XCL2, and XCL1 and increased the levels of AKT3 and PIK3R3 in Beta-TC-6 cells (). Oppositely, RGS7 overexpression markedly increased the levels of RAP1A, XCL2, and XCL1 whereas suppressed the levels of AKT3 and PIK3R3 in Min6 cells ().
Figure 5. RGS7 activates the chemokine signaling pathway in PA-induced pancreatic β-cells. A: KEGG analysis showed that RGS7 activated the chemokine signaling pathway in PA-induced pancreatic β-cells. B: RGS7 silence decreased RAP1A expression and increased the expression of AKT3 and PIK3R3 in PA-induced pancreatic β-cells. *p < 0.05 vs. si-NC group. C: RGS7 overexpression increased RAP1A expression and decreased the expression of AKT3 and PIK3R3 in PA-induced pancreatic β-cells. *p < 0.05 vs. Control group. All the experiments were performed triplicate.
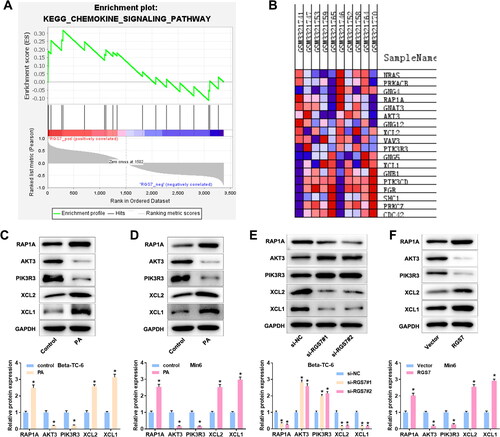
RGS7 overexpression eliminates the effect of RAP1A silence on PA-induced pancreatic β-cells
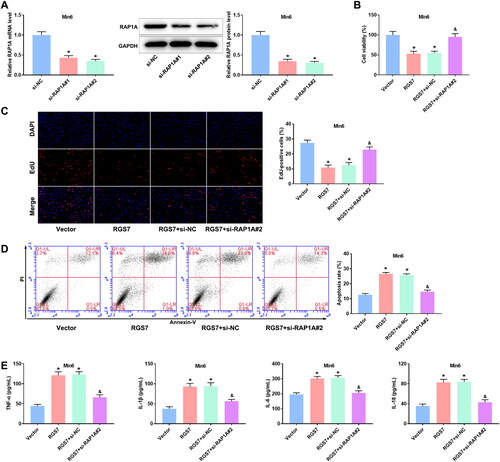
To confirm whether RGS7 functions in PA-induced pancreatic β-cells by regulating signaling pathways, RGS7 overexpression vector and RAP1A siRNA were co-transfected into Min6 cells. The data in showed that RAP1A expression was obviously suppressed by RAP1A siRNAs, especially si-RAP1A no. 2. Thus, si-RAP1A no. 2 was used for follow-up experiments. The MTT and EdU assays showed that RGS7 overexpression suppressed cell viability and proliferation of PA-induced pancreatic β-cells. However, the inhibitory effect of RGS7 overexpression was partly eliminated by RAP1A silence (). The flow cytometry data showed that RGS7 overexpression accelerated apoptosis of PA-induced pancreatic β-cells and the acceleration was eliminated by RAP1A silence (). Similarly, the increase of inflammation-related cytokines levels induced by RGS7 overexpression was eliminated by RAP1A silence in PA-induced pancreatic β-cells ().
Figure 6. RGS7 overexpression eliminates the effect of RAPIA silence on PA-induced pancreatic β-cells. A: RAP1A siRNAs decreased RAP1A expression in pancreatic β-cells. *p < 0.05 vs. si-NC group according to one-way ANOVA. B: Insulin content in PA-induced pancreatic β-cells. C: Cell viability of PA-induced pancreatic β-cells. D: The proliferation of PA-induced pancreatic β-cells. E: The apoptosis rate of PA-induced pancreatic β-cells. F: The inflammation levels of PA-induced pancreatic β-cells. *p < 0.05 vs. Vector group, #p < 0.05 vs. RGS7 + si-NC group. All the experiments were performed triplicate.
Discussion
RGS proteins are important physiologic regulators of Gαi/o and Gαq/11 signaling and have been implicated in the regulation of pancreatic function [Citation25,Citation28]. In present study, we found that RGS7 silence could promote β-cell activity and proliferation, and inhibit apoptosis and inflammatory responses of PA-treated pancreatic β-cells by regulating the chemokine signaling pathway. The findings may offer a novel therapeutic approach for T2D.
Inflammation is one of the common features of diabetes [Citation29,Citation30]. Inhibition of inflammatory response could alleviate the diabetes symptoms in vivo and in vitro [Citation29,Citation31,Citation32]. Suppression of pancreatic islet inflammation attenuates the dysfunction of pancreatic β-cells induced by lipotoxicity [Citation33]. Exacerbated islet inflammation leads to increase of β-cell apoptosis [Citation34]. In this study, RGS7 silence markedly enhanced cell viability and proliferation ability while decreased apoptosis of pancreatic β-cells. Studies have reported the changes of IL-1β, IL-6, IL-18, and TNF-α level in pancreatic β-cells [Citation35–37]. We found that RGS7 silence obviously reduced the levels of TNF-α, IL-1β, IL-6, and IL-18 in pancreatic β-cells. The contrary results were obtained in RGS7 overexpressed pancreatic β-cells. These data were consistent with previous study, suggesting that RGS7 silence may protect pancreatic β-cells from PA by reducing the levels of inflammation-related cytokines and decreasing apoptosis.
Chemokines can attract immune cells to inflammation sites due to their ability to induce directed chemotaxis of nearby responding cells [Citation38,Citation39]. Chemokines signaling participated in many physiological processes, such as cell migration [Citation40–42], angiogenesis [Citation43–45], cancer dissemination [Citation46–48], and inflammatory response [Citation49,Citation50]. XCL1 and XCL2 are members of the C-chemokine subfamily, which promote inflammatory progression and are involved in a variety of inflammatory diseases [Citation51,Citation52]. Ras-associated protein 1A (Rap1A) is a type of small GTP-binding protein that belongs to the Ras subfamily [Citation53], and plays an essential regulatory role in a variety of cellular processes, including proliferation [Citation54]. PIK3R3 is one of the regulatory subunits of phosphoinositide 3-kinase, which notably affects cell genesis, proliferation, and apoptosis [Citation55–57]. In this study, RGS7 activated the chemokine signaling pathway, showing as increased levels of RAP1A, XCL2, and XCL1, and decreased the levels of AKT3 and PIK3R3 in pancreatic β-cells. Furthermore, inhibition of RAP1A, the key gene of the chemokine signaling pathway, markedly eliminated the negative effect of RGS7 overexpression on pancreatic β-cells. These above data suggested that RGS7 silence protected pancreatic β-cells from PA by inactivating the chemokine signaling pathway.
Our findings suggested that RGS7 silence suppressed inflammatory response and apoptosis of PA-induced pancreatic β-cells. RGS7 activated the chemokine signaling pathways. RGS7 silence inhibited PA-induced inflammatory response by inactivating the chemokine signaling pathway, thereby reducing apoptosis of pancreatic β-cells.
However, there are several limitations in the present study. It is more convincing to perform RGS7 silencing and overexpression experiments in Beta-TC-6 and Min6 cells simultaneously. The regulatory effect of RGS7 should be explored in vivo. Besides, the role of chemokine signaling pathway should also be further explored in future study. Anyway, more in-depth studies are needed to explore the mechanism of RGS7 in diabetes.
Author contributions
Conception and design: Yurong Zhu; Experiment: Yurong Zhu, Jun Li and Tao Ba; Data analysis and interpretation: Yuan Sun and Xiangyun Chang; Manuscript writing: Yurong Zhu; Final approval of manuscript: All authors
Ethics approval and Consent to participate
No animal and patients
Consent for publication
Not Applicable.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
| Abbreviations | ||
| RGSs | = | regulators of G protein signaling |
| T2D | = | type 2 diabetes |
| PA | = | palmitic acid |
| MTT | = | 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide |
| EdU | = | 5-ethynyl-2′-deoxyuridine |
| qRT-PCR | = | quantitative real-time PCR |
| G protein | = | guanine nucleotide binding protein |
| GTP | = | guanosine triphosphate |
Acknowledgments
Not applicable
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Roglic G. WHO global report on diabetes: a summary. Int J Non-Commun Dis. 2016;1(1):1–10.
- Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-Cell death in type 2 diabetes. Diabetes. 2005;54(suppl_2):1–10.
- Kaviani M, Azarpira N, Karimi MH, et al. The role of microRNAs in islet β-cell development. [Cell Biol Int. 2016;40(12):1248–1255. 2016/12/01;40(12):1248–1255.
- Zheng Y, Wang Z, Zhou Z. miRNAs: novel regulators of autoimmunity-mediated pancreatic β-cell destruction in type 1 diabetes. Cell Mol Immunol. 2017;14(6):488–496.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
- Tian Y, Peng B, Fu X. New ADCY3 variants dance in obesity etiology. Trends Endocrinol Metab. 2018;29(6):361–363.
- Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584.
- Saliani N, Montazersaheb S, Montasser Kouhsari S. Micromanaging glucose tolerance and diabetes. Adv Pharm Bull. 2017;7(4):547–556.
- Salonen J. Did the North Karelia project reduce coronary mortality? Lancet. 1987;330(8553):269.
- Xu H, Du X, Xu J, et al. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020;18(2):e3000603.
- Huang X, Charbeneau RA, Fu Y, et al. Resistance to Diet-Induced obesity and improved insulin sensitivity in mice with a regulator of G protein signaling–insensitive G184S Gnai2 allele. Diabetes. 2008;57(1):77–85.
- Zheng B, De Vries L, Gist Farquhar M. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends Biochem Sci. 1999;24(11):411–414.
- Qutob N, Masuho I, Alon M, et al. RGS7 is recurrently mutated in melanoma and promotes migration and invasion of human cancer cells. Sci Rep. 2018;8(1):653–653.
- Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54(1–3):33–46.
- Huang X, Fu Y, Charbeneau RA, et al. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol. 2006;26(18):6870–6879.
- Wang Q, Pronin AN, Levay K, et al. Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion. FASEB J. 2017;31(11):4734–4744.
- Basak M, Das K, Mahata T, et al. RGS7-ATF3-Tip60 complex promotes hepatic steatosis and fibrosis by directly inducing TNFα. Antioxid Redox Signal. 2022;38(1–3):137–159.
- Zhang Y, Higgins CB, Fortune HM, et al. Hepatic arginase 2 (Arg2) is sufficient to convey the therapeutic metabolic effects of fasting [research support, N I H, extramural research support, Non-U S gov’t research support, U S gov’t, Non-P H S]. Nat Commun. 2019;10(1):1587.
- Aissani B, Wiener HW, Zhang K. Fine mapping of the body fat QTL on human chromosome 1q43. PLoS One. 2016;11(4): e0153794.
- Aissani B, Perusse L, Lapointe G, et al. A quantitative trait locus for body fat on chromosome 1q43 in french canadians: linkage and association studies. Obesity. 2006;14(9):1605–1615.
- Robertson RP, Harmon J, Tran POT, et al. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(suppl_1):S119–S124.
- Shimabukuro M, Zhou YT, Levi M, et al. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95(5):2498–2502.
- Sargsyan E, Cen J, Roomp K, et al. Identification of early biological changes in palmitate-treated isolated human islets. BMC Genomics. 2018;19(1):018–5008.
- Ruiz de Azua I, Scarselli M, Rosemond E, et al. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010;107(17):7999–8004.
- Hu L, He F, Huang M, et al. SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Sci Rep. 2020;10(1):17581.
- Sugimoto K, Katsuya T, Kamide K, et al. Promoter polymorphism of RGS2 gene is associated with change of blood pressure in subjects with antihypertensive treatment: the azelnidipine and temocapril in hypertensive patients with type 2 diabetes study. Int J Hypertens. 2010;2010:196307.
- Vazquez-Jimenez JG, Corpus-Navarro MS, Rodriguez-Chavez JM, et al. The increased expression of regulator of G-Protein signaling 2 (RGS2) inhibits Insulin-Induced akt phosphorylation and is associated with uncontrolled glycemia in patients with type 2 diabetes. Metabolites. 2021;11(2):91.
- Bastin G, Luu L, Batchuluun B, et al. RGS4-Deficiency alters intracellular calcium and PKA-Mediated control of insulin secretion in Glucose-Stimulated beta islets. Biomedicines. 2021;9(8):1008.
- Li T, Quan H, Zhang H, et al. Silencing cyclophilin a improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway. Bioengineered. 2020;11(1):1047–1057.
- Das S, Reddy MA, Senapati P, et al. Diabetes Mellitus-Induced long noncoding RNA Dnm3os regulates macrophage functions and inflammation via nuclear mechanisms. Arterioscler Thromb Vasc Biol. 2018;38(8):1806–1820.
- Ebrahimi F, Sahebkar A, Aryaeian N, et al. Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes. 2019;12:2107–2115.
- Gonzalez-Moro I, Olazagoitia-Garmendia A, Colli ML, et al. The T1D-associated lncRNA Lnc13 modulates human pancreatic β cell inflammation by allele-specific stabilization of STAT1 mRNA. Proc Natl Acad Sci USA. 2020;117(16):9022–9031.
- Wang Y, Xie T, Zhang D, et al. GPR120 protects lipotoxicity-induced pancreatic β-cell dysfunction through regulation of PDX1 expression and inhibition of islet inflammation. Clin Sci. 2019;133(1):101–116.
- Petrovic I, Pejnovic N, Ljujic B, et al. Overexpression of galectin 3 in pancreatic β cells amplifies β-Cell apoptosis and islet inflammation in type-2 diabetes in mice. Front Endocrinol (Lausanne). 2020;11:30.
- Zhang Y, Aisker G, Dong H, et al. Urolithin a suppresses glucolipotoxicity-induced ER stress and TXNIP/NLRP3/IL-1β inflammation signal in pancreatic β cells by regulating AMPK and autophagy. Phytomedicine. 2021;93:153741.
- Jiang C, Wang Y, Guo M, et al. PCB118 induces inflammation of islet beta cells via activating ROS-NLRP3 inflammasome signaling. Biomed Res Int. 2021;2021:5522578.
- Qiu A-W, Cao X, Zhang W-W, et al. IL-17A is involved in diabetic inflammatory pathogenesis by its receptor IL-17RA. Exp Biol Med (Maywood). 2021;246(1):57–65.
- Oppenheim JJ, Zachariae COC, Mukaida N, et al. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9(1):617–648.
- Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42(1):469–499.
- Lewellis SW, Knaut H. Attractive guidance: how the chemokine SDF1/CXCL12 guides different cells to different locations. Semin Cell Dev Biol. 2012;23(3):333–340.
- Mayor R, Theveneau E. The neural crest. Development. 2013;140(11):2247–2251.
- Tang J, Liao Z, Luo L, et al. CX3CL1-induced CD16(+) monocytes extravasation in myeloperoxidase-ANCA-associated vasculitis correlates with renal damage. Front Immunol. 2022;13:929244.
- Bussmann J, Wolfe SA, Siekmann AF. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development (Cambridge, England). 2011;138(9):1717–1726.
- Cha YR, Fujita M, Butler M, et al. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev Cell. 2012;22(4):824–836.
- Pennel KA, Quinn JA, Nixon C, et al. CXCL8 expression is associated with advanced stage, right sidedness, and distinct histological features of colorectal cancer. J Pathol Clin Res. 2022;25(10):290.
- Cojoc M, Peitzsch C, Trautmann F, et al. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco Targets Ther. 2013;6:1347–1361.
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348.
- Chang W, Chang Q, Lu H, et al. MicroRNA-873-5p suppresses cell malignant behaviors of thyroid cancer via targeting CXCL5 and regulating P53 pathway. Hum Vaccin Immunother. 2022;18(5):29.
- Zhang L, Yu M, Deng J, et al. Chemokine signaling pathway involved in CCL2 expression in patients with rheumatoid arthritis. Yonsei Med J. 2015;56(4):1134–1142.
- Yi L, Zhou Y, Song J, et al. A novel iridoid glycoside leonuride (ajugol) attenuates airway inflammation and remodeling through inhibiting type-2 high cytokine/chemokine activity in OVA-induced asthmatic mice. Phytomedicine. 2022;105(154345):154345.
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity [review]. Immunity. 2000;12(2):121–127.
- Stievano L, Piovan E, Amadori A. C and CX3C chemokines: cell sources and physiopathological implications. Crit Rev Immunol. 2004;24(3):205–228.
- Li Q, Xu A, Chu Y, et al. Rap1A promotes esophageal squamous cell carcinoma metastasis through the AKT signaling pathway. Oncol Rep. 2019;42(5):1815–1824.
- Sayyah J, Bartakova A, Nogal N, et al. The ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J Biol Chem. 2014;289(25):17689–17698.
- Lu S, Chen L, Tang L. Upregulation of AKT1 and downregulation of AKT3 caused by dysregulation of microRNAs contributes to pathogenesis of hemangioma by promoting proliferation of endothelial cells. J Cell Physiol. 2019;234(11):21342–21351.
- Wang L, Huang D, Jiang Z, et al. Akt3 is responsible for the survival and proliferation of embryonic stem cells. Biol Open. 2017;6(6):850–861.
- Chen Q, Sun X, Luo X, et al. PIK3R3 inhibits cell senescence through p53/p21 signaling. Cell Death Dis. 2020;11(9):798.
